Abstract
Background
Patients with recurrent ovarian cancer have limited options, especially in the context of relapse less than six months from primary platinum-based therapy. This Gynecologic Oncology Group (GOG) study was conducted to evaluate the impact of the histone deacetylase inhibitor, belinostat, in combination with carboplatin in women with platinum-resistant ovarian cancer.
Methods
Eligible patients had measurable, recurrent disease within six months of their last dose of a platinum-based combination. Belinostat was dosed at 1,000 mg/m2 daily for five days with carboplatin AUC 5 on day three of 21-day cycles. The primary endpoint was overall response rate (ORR), using a two-stage design.
Results
Twenty-nine women enrolled on study and 27 were evaluable. The median number of cycles given was two (range 1–10). One patient had a complete response and one had a partial response, for an ORR of 7.4% (95% CI, .9% - 24.3%). Twelve patients had stable disease while eight had increasing disease. Response could not be assessed in five (18.5%). Grade 3 and 4 events occurring in more than 10% of treated patients were uncommon and limited to neutropenia (22.2%), thrombocytopenia (14.8%), and vomiting (11.1%). The median progression-free survival (PFS) was 3.3 months and overall survival was 13.7 months. PFS of at least six months was noted in 29.6% of patients. Due to the lack of drug activity, the study was closed after the first-stage.
Conclusions
The addition of belinostat to carboplatin had little activity in a population with platinum-resistant ovarian cancer.
Keywords: platinum-resistant, chemotherapy, ovarian cancer, HIDAC inhibitors, carboplatin
INTRODUCTION
Ovarian cancer remains the most fatal of the gynecologic malignancies; in 2010 an estimated 21,880 cases were diagnosed and it was the cause of 13,850 deaths [1]. While over 70% of women treated with a combination of aggressive primary cytoreductive surgery and platinum/taxane-based chemotherapy experience disease remission, the vast majority will ultimately relapse and die of drug-resistant disease. It is well understood that women with platinum-free intervals (PFI) over six to12 months after their first exposure to platinum-based chemotherapy (potentially platinum-sensitive) are likely to achieve a significant benefit from re-treatment with platinum doublet regimens, such as carboplatin combined with either paclitaxel, gemcitabine, or pegylated liposomal doxorubicin [2-4]. Unfortunately, the same has not been shown in women with a PFI between zero and six months (platinum-resistant). For example, a Gynecologic Oncology Group (GOG) study evaluating cisplatin and gemcitabine in this cohort showed a 16% response rate (RR) with an overall survival (OS) of 14.9 + months [5]. These data exemplify the urgent need to identify novel therapy for platinum-resistant recurrent ovarian cancer.
Histones constitute the major proteins in chromatin, assist in DNA packaging and assembly of nucleosomes, and are important regulators of gene expression [6]. The histone tail domain is a target of enzymatic modification, including phosphorylation and acetylation, which are associated with transcriptional activities, and deacetylation, which results in transcriptional repression. Acetylation and deacetylation are controlled by histone acetyltransferases and histone deacetylases (HDAC). Epigenetic modulation (e.g. changes in acetylation levels of histone and non-histone proteins) potentially interferes with resistance mechanisms of standard chemotherapy agents like carboplatin, and may enhance the activity of such agents [6].
Belinostat (Bel, PXD101) is a low molecular weight class I and II HDAC inhibitor of the hydroxamate class which alters acetylation levels of histone and non-histone proteins rendering it a potentially important player in the epigenetic regulation of gene expression [7]. HDAC inhibitors induce the expression of many genes, some of which are involved in cell cycle arrest and tumor suppression. Combined with carboplatin, belinostat showed enhanced growth inhibitory activity over monotherapy in both carboplatin sensitive and resistant ovarian specimens grown in three-dimensional organoid culture and in the mouse A2780 tumor xenograft model [7]. Furthermore, synergy was noted in the cisplatin resistant ovarian cell line A2780/cp70 when belinostat was combined with cisplatin [7].
Single-agent belinostat treatment has been tested in three phase II studies with minimal activity seen [8-10]. However, in one study of patients with thymic carcinoma, the proportion of patients with thymoma progression-free at six months was 61% [9]. As a result, belinostat has been combined with chemotherapy in subsequent trials.
The combination of belinostat with carboplatin and paclitaxel (BelCaP) was evaluated in a Phase I study for patients with solid tumors [11]. No dose limiting toxicities were observed during dose escalation. Evaluation showed that belinostat in combination with paclitaxel and carboplatin showed similar pharmacokinetics (PK) to that observed with belinostat monotherapy. In addition, belinostat did not alter excretion of paclitaxel or carboplatin. This study showed that treatment with all three agents was feasible and the recommended phase II dose was set at Belinostat 1000 mg/m2 on days one to five with Carboplatin AUC=5 and paclitaxel 175 mg/m2 on day three, given every three weeks (BelCaP). In phase I, 23 patients were treated with a median of four cycles (range 1–32), including 10 patients completing ≥ six cycles. Two of 20 patients had a partial response and an additional 11 patients had stable disease for two to +28 cycles. The median treatment duration was 116 days (range, 43 to 592 days).
A phase II study of BelCaP was subsequently performed in a population of women with previously treated relapsed and/or refractory ovarian cancer who had received up to three prior lines of treatment in the metastatic setting [12]. Of 35 patients treated, three complete and 12 partial responses were noted for an objective response rate (ORR) of 43%. The median response duration was 5.3+ months (range, 1.2 to 12.7+ months) and the progression-free survival (PFS) rate at six months was 43%. Unfortunately, the heterogeneity of the population enrolled in the BelCAP study (14 had a platinum-free interval of six months or longer) made interpretation of these results in the context of how to treat women with recurrent ovarian cancer difficult. It was not clear if the activity of belinostat was due to its combination with carboplatin, paclitaxel, or carboplatin, plus paclitaxel. Given that the preclinical data suggests that belinostat is synergistic with platinum analogs, the GOG initiated this phase II study of belinostat and carboplatin to evaluate this combination in a homogenous group of women with specifically defined platinum-resistant ovarian cancer.
METHODS
Patient Selection
Women with recurrent ovarian cancer were eligible for the study if they satisfied the following criteria: age at entry of 18 or older; submission of the original pathology report documenting primary diagnosis of ovarian cancer; radiologic disease progression on or within six months of their last platinum- and taxane- treatment with at least one measurable “target lesion” to assess response, as defined by RECIST 1.1 [13]; not eligible for a higher priority GOG protocol; GOG Performance Status of 0, 1, or 2; recovery from effects of recent surgery, radiotherapy, or chemotherapy; discontinuation of any hormonal therapy directed at the malignant tumor at least one week prior to registration; discontinuation of prior therapy (including use of either biologic or immunologic agents) directed at the malignant tumor, at least three weeks prior to registration; up to one prior additional cytotoxic chemotherapy for management of recurrent or persistent disease; signed informed consent and authorization permitting release of personal health information; negative pregnancy test for women of childbearing potential; use of an effective form of contraception for women with preserved fertility. This study required patients to have previously been treated with both a platinum agent and a taxane. Therefore, all patients had to have a prior treatment with one chemotherapeutic regimen for management of primary disease containing carboplatin, cisplatin, or another organoplatinum compound; patients NOT treated with prior taxane-containing chemotherapy were required to receive a second regimen including paclitaxel or docetaxel. All institutions had to obtain approval by their respective Institutional Review Board (IRB) prior to enrolling patients. Patients diagnosed with recurrence by CA125 elevation on or within six months of last platinum treatment but without evidence of radiologic progression were not eligible for protocol treatment.
Other eligibility criteria included the following: absolute neutrophil count (ANC) greater than or equal to 1,500/mcl; platelets greater than or equal to 100,000/mcl; creatinine less than or equal to 1.5 × institutional upper limit normal (ULN); bilirubin less than or equal to 1.5 × ULN; SGOT (AST) less than or equal to three × ULN; alkaline phosphatase less than or equal to 2.5 × ULN; neuropathy (sensory and motor) less than or equal to the CTCAE v3.0 grade 1.
Patients were ineligible if any of the following were present: (1) prior therapy with belinostat (PXD101) or other HDAC inhibitors; (2) prior radiation to more than 25% of marrow-bearing areas; (3) prior history of other invasive malignancies, with the exception of non-melanoma skin cancer, localized breast or head/neck cancer, were excluded; (4) prior chemotherapy for any abdominal or pelvic tumor other than for the treatment of ovarian cancer are excluded. Patients may have received prior adjuvant chemotherapy for localized breast cancer, provided that it was completed more than three years prior to registration, and that the patient remains free of recurrent or metastatic disease; (5) prior radiotherapy to any portion of the abdominal cavity or pelvis other than for the treatment of ovarian cancer; prior radiation for localized cancer of the breast, head and neck, or skin was permitted, provided it was completed more than three years prior to registration, and the patient remains free of recurrent or metastatic disease; (5) use of concomitant medications on PXD infusion days that may cause Torsade de Pointes; (6) significant cardiovascular disease defined as: unstable angina pectoris, uncontrolled hypertension (blood pressure > 150/90 despite maximal medical therapy), congestive heart failure related to primary cardiac disease, any condition requiring anti-arrhythmic therapy, ischemic or severe valvular heart disease, or history of myocardial infarction within six months of trial entry; (7) pregnancy or nursing.
Treatment Plan
Belinostat (NSC#726630, IND# 729990) 1000 mg/m2 was administered as a 30-minute IV infusion for five days every three weeks. Carboplatin AUC=5 was administered two to three hours following the infusion of belinostat on cycle day three. Patients were allowed to receive up to six cycles of chemotherapy unless there was disease progression or unacceptable toxicity. However, patients clinically responding or who, in the opinion of their physician, would continue to benefit from treatment, were allowed to continue on this regimen beyond six cycles.
Subsequent cycles of therapy were initiated only when the ANC reached 1500 cells/mcl or greater and the platelet count recovered to at least 100,000/mcl. Therapy was delayed for a maximum of two weeks to allow recovery and those who did not recover adequate counts in this time-frame were removed from study. Prophylactic growth factors were not allowed unless patients experienced recurrent neutropenic complications after treatment. Erythropoietin, iron supplements, and/or transfusions were allowed as clinically indicated for management of anemia. Patients were not allowed to receive amifostine or other protective reagents.
Carboplatin was dose reduced to AUC=4 for the first occurrence of febrile neutropenia, prolonged Grade 4 neutropenia lasting seven days or longer, or Grade 4 thrombocytopenia. If patients experienced any of these as a second event, belinostat was dose reduced to 800 mg/m2. If any of these events occurred despite these dose reductions, prophylactic growth factors were added.
Grade 2 or greater non-hematologic toxicity required reduction of belinostat to 800 mg/m2 and delay in subsequent therapy for up to two weeks to allow for recovery. For Grade 2 or greater renal or hepatic toxicity, carboplatin was also reduced to AUC=4. No dose modifications were allowed for alopecia or fatigue. Patients with persistent (greater than 24 hours) Grade 3 or greater nausea, emesis, or constipation in spite of optimal medical management were dose reduced using belinostat 800 mg/m2 and carboplatin AUC=4. If events repeated despite this initial dose reduction of belinostat, then further dose reduction to 600 mg/m2 was allowed. If toxicity recurred despite this second dose reduction protocol treatment was discontinued.
Evaluation Plan
Patients were required to have measurable disease for participation in this study and RECIST 1.1 criteria were used [13]. Formal radiological evaluation was performed after every two cycles. If a response was noted, confirmation was required by a second disease assessment at least four weeks apart. Tumor markers alone were not used to assess response. However, if markers were initially above the upper normal limit, normalization was required for a patient to be considered in complete clinical response [14]. Eligible patients who received any drug were utilized in response analysis.
Toxicity Evaluation
Patients having received any amount of the study drug were evaluable for safety. Toxicity evaluations were required at baseline and throughout the study terminating four weeks after the last infusion of the study drug, unless the patient started a new anticancer regimen.
The intensity of the adverse event was graded using National Cancer Institute's Common Toxicity Criteria v. 3.0 (NCI-CTCAE v3.0). Patients with an adverse event were followed until return to baseline or ≤ grade 1, or until the patient started another type of anti-neoplastic therapy.
Statistical Design
The study employed a two-stage design that used an early stopping rule intended to limit patient accrual to inactive treatment [15]. During the first stage, the targeted accrual was 19 to 26 patients to allow for administrative coordination. If more than two out of 19-25, or more than three out of 26 patients achieved a response (complete or partial) and medical judgment indicated, accrual to the second stage would be initiated. Otherwise, the study would be stopped and the treatment regimen classified as uninteresting. If opened to the second stage, the overall study accrual would be to 44 to 51 patients. If more than six out of 44-45 patients responded, or seven out of 46-51 responded to this regimen, it would be considered worthy of additional investigation.
If the true probability of response was only 10% the average probability of stopping after completing only the first stage was 64%. On the other hand, if the true RR was 25%, then the average probability of correctly classifying the treatment as active was 90%.
RESULTS
Patient Demographics
Twenty-nine patients were enrolled on this study; one patient was never treated and one did not have measurable disease progression within six months of last platinum, as was required in eligibility. For the 27 eligible and evaluable patients, the median age was 63 (range: 48-75). The majority of patients (74%) had a performance status (PS) of 0 at entry and 24 (89%) were White. Twenty-three patients (85%) had a serous adenocarcinoma and 21 (78%), had grade 3 tumors. All patients had received prior chemotherapy. Patients received a median number of two cycles of carboplatin and belinostat on this study (range: 1-10); seven patients (26%) received more than five cycles of treatment.
Toxicity
Table 2 summarizes the hematologic and non-hematologic adverse events seen with this combination. One patient was enrolled while in hospital recovering from a small bowel obstruction and received her first cycle during that admission. Four days later she experienced respiratory distress which required intubation. Eight days after start of treatment, she experienced hematemesis, and endoscopy was inconclusive. On day 10 of cycle one her family withdrew support and she subsequently expired. This Grade 5 hemorrhagic event was reviewed by the Data Safety and Monitoring Board of the GOG and was considered related to both treatment and to disease. Grade 3-4 adverse events noted were: neutropenia (22.2%), thrombocytopenia (14.8%), vomiting (11.1%), anemia (7.4%), allergic reaction (7.4%), nausea (7.4%), other gastrointestinal (7.4%), metabolic (7.4%), and leucopenia (3.7%), and constitutional symptoms (3.7%).
Table 2.
Adverse Events
| Adverse Event | 0 | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|---|
| Leukopenia | 12 | 5 | 9 | 1 | 0 | 0 | 27 |
| Thrombocytopenia | 14 | 5 | 4 | 2 | 2 | 0 | 27 |
| Neutropenia | 13 | 2 | 6 | 4 | 2 | 0 | 27 |
| Anemia | 6 | 10 | 9 | 2 | 0 | 0 | 27 |
| Other Hematologic | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Allergy/Immunology | 23 | 0 | 2 | 1 | 1 | 0 | 27 |
| Auditory/Ear | 26 | 0 | 1 | 0 | 0 | 0 | 27 |
| Cardiac | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Coagulation | 25 | 1 | 1 | 0 | 0 | 0 | 27 |
| Constitutional | 10 | 10 | 6 | 1 | 0 | 0 | 27 |
| Dermatologic | 22 | 3 | 2 | 0 | 0 | 0 | 27 |
| Endocrine | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Nausea | 6 | 14 | 5 | 2 | 0 | 0 | 27 |
| Vomiting | 14 | 9 | 1 | 3 | 0 | 0 | 27 |
| Gastrointestinal | 10 | 8 | 7 | 2 | 0 | 0 | 27 |
| Genitourinary/Renal | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Hemorrhage | 25 | 1 | 0 | 0 | 0 | 1 | 27 |
| Lymphatics | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Metabolic | 17 | 7 | 1 | 2 | 0 | 0 | 27 |
| Musculoskeletal | 25 | 2 | 0 | 0 | 0 | 0 | 27 |
| Neurosensory | 21 | 5 | 1 | 0 | 0 | 0 | 27 |
| Other Neurological | 22 | 3 | 2 | 0 | 0 | 0 | 27 |
| Ocular/Visual | 26 | 1 | 0 | 0 | 0 | 0 | 27 |
| Pain | 15 | 8 | 4 | 0 | 0 | 0 | 27 |
| Pulmonary | 21 | 6 | 0 | 0 | 0 | 0 | 27 |
| Vascular | 25 | 0 | 2 | 0 | 0 | 0 | 27 |
Clinical Activity
Table 3 summarizes the clinical activity seen in this study. One patient (3.7%) had a complete response, and a second had resolution of measurable disease for a radiologic complete response; however, her CA-125 did not normalize on treatment, and thus, she was classified as having a partial response. Therefore, the overall response rate (ORR) was 7.4% (range, 0.9-24.3%). Twelve patients (44.4%) had stable disease (SD) as their best response and 8 (29.7%) had progressive disease (PD) on treatment. Four patients received only one cycle of protocol treatment before it was discontinued due to either an allergic reaction (1) and toxicity (4). Despite receiving two cycles of treatment, one patient was not evaluable because she opted to discontinue protocol treatment secondary to Grade 2 fatigue. She did not undergo repeat disease assessment.
Table 3.
Treatment outcomes
| Response Category | Number of cases | % |
|---|---|---|
| Complete Response | 1 | 3.7 |
| Partial Response | 1 | 3.7 |
| Stable Disease | 12 | 44.4 |
| Increasing Disease | 8 | 29.7 |
| Inevaluable | 5 | 18.5 |
| Total | 27 | 100.0 |
The observed response rate is 7.4% (95% confidence interval .9%-24.3%).
Survival Analysis
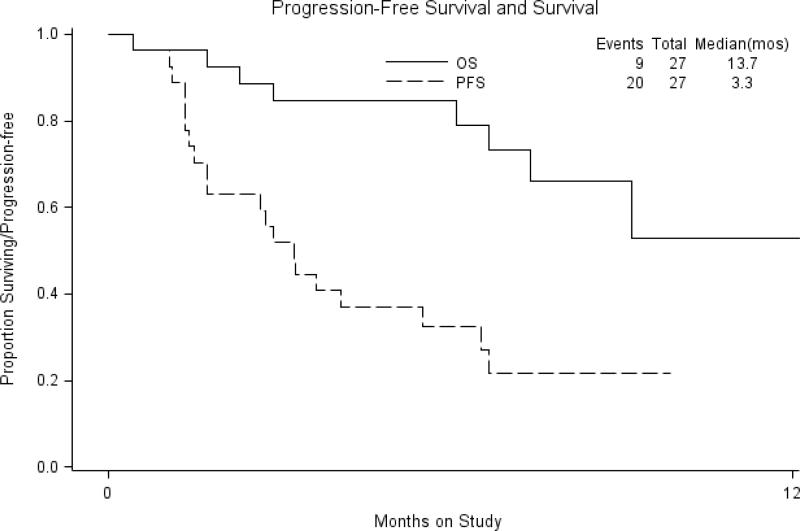
Median PFS was 3.3 months, while median OS in this platinum-resistant cohort was 13.7 months. The rate of PFS at six months was 29.6%. Kaplan-Meier analysis is demonstrated in Figure 1.
Figure 1.
Survival Analysis
DISCUSSION
Histones play an integral role in the storage of DNA and enable the tight regulation of genetic transcription [16]. This mechanism of genetic regulation is felt to play an important role in tumor genesis and propagation and to be an important modulator of cancer initiation and metastases [16]. The family of histone deacetylators (HDs) constitute 18 isoenzymes, structurally classified into 4 classes. Class I HDAC was found to be expressed in high levels in ovarian carcinoma nuclei in a recent study by Weichert, et al, and most commonly in over 50% of mucinous, serous, and clear cell carcinomas [17]. Coupled with preclinical data demonstrating that HDAC inhibitors increase the activity of platinums, this seemed a sound rationale to evaluate the effect of belinostat on the response to carboplatin, specifically in a cohort with platinum-resistant ovarian cancer.
The ORR in this study was 7.4%, which did not meet the criteria for further development in the GOG as established by the study; hence, the study was closed at the first stage. The population of women in this study was enrolled with strict criteria in terms of defining platinum-resistance; they were required to progress radiographically, and not solely on the basis of either a persistently abnormal CT scan at the end of primary treatment (which might indicate persistent disease) or an isolated rise in the CA-125. Thus, we enrolled a homogenous population with clearly defined platinum- and paclitaxel resistant disease, and as such, a potentially more challenging population to demonstrate clinical benefit from a platinum re-challenge compared to prior populations similarly studied in the GOG. Furthermore, the PFS at six months, which is routinely used to document activity of biologic agents and not cytotoxic therapies, was less than 30%, which is inferior to bevacizumab in a previously treated population [18].
The strict eligibility criteria enabled us to evaluate this novel agent with the investment of a minimal number of participants in clinical research, and a much smaller number than would have been required for a randomized phase II. It requires investigators to demonstrate apparent benefit in specific situations before randomized comparison, rather than prematurely move to randomized phase II trials based on preliminary data extrapolated from other settings, which is increasingly popular [19]. In counterpoise, a very high bar for clinical benefit does not mean that belinostat may not have merit in other settings of relatively less chemotherapy resistance, such as in women with relapse within six to 12 months.
Whether further evaluation of HIDAC inhibitors in ovarian cancer is warranted remains to be seen. As in other solid tumors, some data suggest it has limited activity as a single agent. For example, a phase II trial evaluated single-agent belinostat in women with platinum-resistant ovarian cancer (n=18) and micropapillary ovarian tumors (n=14) [8]. In this study, platinum-resistance was defined as progression within six months of platinum therapy and allowed up to three prior lines of treatment. There were no responses among platinum-resistant patients although nine patients had stable disease. The median progression free survival was 2 months and 13% were progression-free at six months.
The combination of belinostat and chemotherapy has shown promise in one prior study of women with recurrent ovarian cancer using the triple-drug combination of belinostat, carboplatin, and paclitaxel (BelCaP) in women with recurrent ovarian cancer. In this study, the overall response rate was 43% [12]. It is probable the different trial designs and different patient eligibility utilized explains the discrepant results seen between the BelCAP study and our trial. However, our results suggest that the activity of BelCaP may reflect the synergy between paclitaxel and belinostat, which has been demonstrated in preclinical studies [7].
A recent study in breast cancer suggests that patient selection may be of importance with the use of HIDAC inhibitors. Yardley, et al. recently presented the results of another HIDAC inhibitor, the orally available agent entionstat, in combination with exemestane for women with metastatic hormone-receptor positive breast cancer [20]. As part of this trial, patients were tested for protein lysine acetylation in peripheral blood mononuclear cells to determine if this correlated with outcome. Of 49 patients tested, hyperacetylation was seen in 27%, and was associated with a 77% reduction in the risk of disease progression as well as improvement in PFS. Future trials may be able to select patients for this biomarker in attempts to further define a future of HIDAC inhibitors in ovarian (and other gynecologic) cancers.
In conclusion, belinostat does not appear to improve the activity of carboplatin in a platinum-resistant population. Development of this particular combination does not warrant further investigation in these women.
HIGHLIGHTS.
The combination of belinostat and carboplatin did not have clinically meaningful activity for women with platinum-resistant ovarian cancer.
Novel therapeutic strategies to overcome platinum resistance in ovarian cancer are critically needed.
Table 1.
Patient demographics
| Characteristic | Number of Cases | |
|---|---|---|
| Age | 40-49 | 2 |
| 50-59 | 8 | |
| 60-69 | 15 | |
| 70-79 | 2 | |
| Performance | 0 | 20 |
| Status | 1 | 7 |
| Race | White | 24 |
| African American | 1 | |
| Asian | 1 | |
| Unspecified | 1 | |
| Cell Type | Serous | 23 |
| Clear Cell | 2 | |
| Mixed epithelial | 1 | |
| Adenocarcinoma | 1 | |
| Tumor Grade | 1 | 1 |
| 2 | 3 | |
| 3 | 21 | |
| Unspecified | 2 | |
| Prior Radiotherapy | 0 | |
| Prior Chemotherapy | 27 | |
| Courses | 1 | 4 |
| 2 | 11 | |
| 3 | 0 | |
| 4 | 5 | |
| 5 | 0 | |
| >5 | 7 |
ACKNOWLEDGEMENT
The authors wish to thank Dr. Carol Aghajanian for her mentorship during this trial and Wendy Young, NP at Women & Infants’ Hospital of Rhode Island for her assistance in the conduct and management of this study. We also wish to acknowledge the role of our collaborators at the National Cancer Institute's Cancer Therapeutics Evaluation Branch and Sandra Dascomb, Diana Blade, and Julie Kremzier, of the Gynecologic Oncology Group Administrative Office and Statistical and Data Center for administrative and data support.
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517). The following Gynecologic Oncology Group member institutions participated in this study: Roswell Park Cancer Institute, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Mississippi Medical Center, University of Pennsylvania Cancer Center, University of North Carolina School of Medicine, Rush-Presbyterian-St. Luke's Medical Center, The Cleveland Clinic Foundation, Cooper Hospital/University Medical Center, MD Anderson Cancer Center, University of Massachusetts Medical School, University of Oklahoma, Women and Infants Hospital, The Hospital of Central Connecticut and Community Clinical Oncology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Dr. Richard T. Penson received research funding for PXD-Cancer & Leukemia Group B/belinostat studies sponsored by Curagen and Topotarget. All other co-authors have no conflicts of interest to declare.
REFERENCES
- 1.American Cancer Society Cancer Facts & Figures 2010. 2010.
- 2.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 3.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 4.Pujade-Lauraine E, Wagner U, Aavall-Lundquist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010;28:3323–39. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 5.Brewer CA, Blessing JA, Nagourney RA, Morgan M, Hanjani P. Cisplatin plus gemcitabine in platinum-refractory ovarian or primary peritoneal cancer: a phase II study of the Gynecologic Oncology Group. Gynecol. Oncol. 2006;103:446–50. doi: 10.1016/j.ygyno.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Rasheed WK, Johnstone RW, Prince HM. Histone deacetylase inhibitors in cancer therapy. Expert Opin Investig Drugs. 2007;16:659–78. doi: 10.1517/13543784.16.5.659. [DOI] [PubMed] [Google Scholar]
- 7.Qian X, LaRochelle WJ, Ara G, Wu F, Petersen KD, Thougaard A, et al. Activity of PXD101, a histone deacetylase inhibitor, in preclinical ovarian cancer studies. Mol. Cancer Ther. 2006;5:2086–95. doi: 10.1158/1535-7163.MCT-06-0111. [DOI] [PubMed] [Google Scholar]
- 8.Mackay HJ, Hirte H, Colgan T, Covens A, MacAlpine K, Grenci P, et al. Phase II trial of the histone deacetylase inhibitor Belinostat in women with platinum resistant epithelial ovarian cancer and micropapillary (LMP) ovarian tumors. Eur J Cancer. 2010;46:1573–9. doi: 10.1016/j.ejca.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giaccone G, Rajan A, Berman A, Kelly RJ, Szabo E, Lopez-Chavez A, et al. Phase II Study of Belinostat in Patients With Recurrent or Refractory Advanced Thymic Epithelial Tumors. J Clin Oncol. 2011;29:2052–9. doi: 10.1200/JCO.2010.32.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramalingam SS, Belani CP, Ruel C, Frankel P, Gitlitz B, Koczywas M, et al. Phase II study of Belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J Thorac Oncol. 2009;4:97–101. doi: 10.1097/JTO.0b013e318191520c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassen U, Molife LR, Sorensen M, Engelholm SA, Vidal L, Sinha R, et al. A phase I study of the safety and pharmacokinetics of the histone deacetylase inhibitor belinostat administered in combination with carboplatin and/or paclitaxel in patients with solid tumours. Br J Cancer. 2010;103:12–7. doi: 10.1038/sj.bjc.6605726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkler NJ, et al. Phase II multicenter trial of the histone deactylase inhibitor (HDACi) belinostat, carboplatin and paclitaxel (BelCaP) in patients (pts) with relapsed epithelial ovarian cancer (EOC). J Clin Oncol. 2008:26. 15 (abstr 5519) [Google Scholar]
- 13.Eisenhauer EA. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, et al. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). J Natl Cancer Inst. 2004;96:487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 15.Chen TT, et al. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Graham JS, Kaye SB, Brown R. The promises and pitfalls of epigenetic therapies in solid tumours. Eur. J. Cancer. 2009;45:1129–36. doi: 10.1016/j.ejca.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Weichert W, Denkert C, Noske A, Darb-Esfahani S, Dietel M, Kalloger SE, et al. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia. 2008;10:1021–7. doi: 10.1593/neo.08474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 19.Cannistra SA. Phase II trials in Journal of Clinical Oncology. J Clin Oncol. 2009;27:3073–6. doi: 10.1200/JCO.2009.23.1811. [DOI] [PubMed] [Google Scholar]
- 20.Yardley DA, Ismail-Khan R, Klein P. Results of ENCORE 301, a randomized, phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive (ER+) breast cancer progressing on a nonsteroidal aromatase inhibitor (AI). J Clin Oncol. 2011;29 doi: 10.1200/JCO.2012.43.7251. abstr 268. [DOI] [PMC free article] [PubMed] [Google Scholar]