Abstract
Chikungunya virus (CHIKV) recently emerged as a global threat to public health through its adaptation to the cosmopolitan mosquito Aedes albopictus Skuse. Aedes albopictus is highly susceptible to the emergent strain of CHIKV, relative to the historical vector of CHIKV, Aedes aegypti (L.). We hypothesized that the high susceptibility of Ae. albopictus to CHIKV may have a cost in terms of longevity and fecundity among infected vs non-infected mosquitoes, relative to Ae. aegypti. We performed a longevity experiment comparing Ae. aegypti and Ae. albopictus exposed to the emergent strain of CHIKV (LR-2006OPY1). We found a small but significant decrease in longevity of Ae. albopictus, but not Ae. aegypti, in response to exposure to CHIKV. We did not observe significant differences in numbers of eggs laid by either species in response to exposure. Longevity and body titer of infected Ae. albopictus were significantly negatively correlated, such that individuals that lived longer had lower viral body titers when they died. The cost of exposure, while not high, suggests there may be physiological constraints in the evolution of viral infectiousness in its insect vector.
Keyword Index: Vector, evolution, epidemic, mosquito, arbovirus
INTRODUCTION
From 2004–2007, chikungunya virus (CHIKV) emerged as a global pathogen, with outbreaks in Africa, the Indian Ocean basin, India, and Italy. Historically, Aedes albopictus (Skuse) (Diptera: Culicidae) had not been considered an important vector of this alphavirus (Monath 1988), but in the recent outbreaks, Ae. albopictus was implicated as the primary vector in epidemics on La Réunion Island and in Italy (Enserink 2006, Reiter et al. 2006, Delatte et al. 2008). Possibly due to the abundance of Ae. albopictus and the lack of alternative vectors, strains of chikungunya isolated later in the La Réunion outbreak (LR-2006OPY1) acquired a mutation making them highly infectious to Ae. albopictus, with no effect on their ability to infect Aedes aegypti (L.), the historical epidemic vector of CHIKV (Monath 1988, Schuffenecker et al. 2006, Tsetsarkin et al. 2007). The high susceptibility of Ae. albopictus relative to Ae. aegypti may be one reason for the speed and depth of the outbreak on La Réunion; however, this same degree of infectiousness may have a detrimental effect on the longevity or fecundity of infected Ae. albopictus mosquitoes.
For a virus that circulates exclusively in humans during epidemics, such as chikungunya virus, the highly anthropophilic Ae. aegypti may be a better vector than the less anthropophilic Ae. albopictus due to a higher host-biting rate, although the degree of anthropophilly may depend on local conditions (McClelland and Weitz 1963, Ponlanwat and Harrington 2005, Richards et al. 2006). The large outbreak of CHIKV attributable to Ae. albopictus suggests that CHIKV may have overcome the lower host specificity of Ae. albopictus, or that Ae. albopictus on La Réunion are more anthropophilic, as other Indian Ocean island populations are (Bagny et al. 2009). The outbreak on La Réunion means that CHIKV had a positive population growth rate in areas where Ae. albopictus is the sole vector (Delatte et al. 2008), and therefore the intrinsic rate of increase (R0) must have been greater than one. One way chikungunya virus may have maintained an R0 > 1 is to be highly transmittable in this species by being infectious at a very low host viremia and disseminating quickly in the vector. Although the importance of infectiousness at low host viremias has not been examined for disease transmission, the abundance of hosts that can achieve a viremia high enough to infect vectors has been shown to be a critical factor in models of disease transmission dynamics, suggesting that increased susceptibility of a vector at low viremias may have a similar effect (Lord et al. 2006).
To examine how viruses evolve species specific characteristics, comparative studies between Ae. albopictus and Ae. aegypti need to be conducted in a “common garden” of viral infection and adult conditions (Weaver 2006). When challenged with the same viral strain, the two vectors may respond differently in terms of the replication of virus in their bodies, and the subsequent deleterious effects of that infection on the vector. Numerous studies have documented variation between Aedes species and within both Ae. albopictus and Ae. aegypti strains in vector competence for CHIKV (Mangiafico 1971, Tesh et al. 1976, Turell 1992, Reiskind et al. 2008). Comparative studies of CHIKV infection have shown higher infection and dissemination or transmission rates in Ae. albopictus relative to Ae. aegypti (Mangiafico 1971, Tsetsarkin et al. 2007, Reiskind et al. 2008). There is no literature on the effects of CHIKV infection on the behavior or life history of adults of either species, although one study did note CHIKV infection had negative effects on larval survival in Ae. taeniorhynchus, a species not known to be a vector of this arbovirus (Turell et al. 1992).
There has been considerable work on the fitness effects of arboviruses on mosquito adults, usually showing a deleterious (or no) effect on female longevity or fecundity [e.g., Rift Valley fever virus on Culex pipiens L. (Faran et al. 1987), eastern equine encephalomyelitis virus on Culiseta melanura (Coquillet) (Scott and Lorenz 1998), western equine encephalomyelitis virus or West Nile virus on Culex tarsalis Coquillet (Weaver et al. 1992, Lee et al. 2000, Mahmood et al. 2004, Styer et al. 2007), and sindbis virus on Ae. albopictus (Bowers et al. 2003)]. Although most studies focused on a single mosquito species, one study documented negative effects of eastern equine encephalomyelitis virus on Coquillettidia perturbans (Walker), but not Anopheles quadrimaculatus Say nor Ae. albopictus (Moncayo et al. 2000). Overall, nearly every published study of viral effects on vector fitness has documented at least some pathology of infection in some of the vectors examined (Lambrechts and Scott 2009). Studies of the effects of the emergent CHIKV on adult mosquitoes have not been done, nor have studies comparing ecologically similar and phylogenetically related vectors. The sensitivity of arbovirus epidemiology to small changes in adult longevity suggests that even a small negative impact of viral infection on mosquitoes may be significant by increasing adult mortality among infected mosquitoes (Dye 1986). Indeed, Anderson and May (1991) note that a major assumption of mathematical models of vectorborne parasites is a lack of effect on the vector, and that this assumption, if relaxed, may have important implications in the spread of disease.
The empirical observations of increased susceptibility, higher dissemination rates, and higher body titer after infection in Ae. albopictus compared to Ae. aegypti when challenged with the La Réunion strain of CHIKV (LR2006-OPY1) suggest there may be differential effects of viral infection on adults of these two species (Tsetsarkin et al. 2007, Reiskind et al. 2008). Therefore, we hypothesize that Ae. albopictus exposed to CHIKV have lower rates of daily survival and have lower lifetime fecundity than unexposed Ae. albopictus, and that the size of this effect will be larger in Ae. albopictus compared to Ae. aegypti. We tested these hypotheses in a common garden experiment by comparing longevity and egg production in Ae. aegypti and Ae. albopictus exposed and not exposed to CHIKV under controlled conditions, with the a priori expectation that exposure to CHIKV will have negative effects on mosquito longevity and fecundity.
MATERIALS AND METHODS
Mosquitoes
Mosquitoes of both species used were F1 progeny of larvae and eggs collected between 1 January 2008 and 25 March 2008 in Palm Beach County, FL. F1 eggs were hatched using standard procedures (Reiskind et al. 2008), and reared to adults under optimal conditions of 50 larvae/liter with 0.3 g of 1:1 yeast:albumin as a food resource, provided at the beginning of the growth period. Pupae were collected over a three-day period, and emerged adults (both males and females) were kept for seven days after the last adult emerged, in order to obtain adults of a standardized age (seven to ten days) at the initiation of the experiment. Because females and males were kept together during the period before infection, we assumed all females were mated. Adults were housed at 26° C and 95% RH until being exposed to virus in a single screened cage (total volume: 300 cm3; BugDorm, BioQuip Products, Rancho Dominguez, CA) and were provided 20% sucrose solution ad libitum until 24 h prior to the start of the experiment.
Virus
We used CHIK LR2006-OPY1 passaged once in green monkey cells (Vero cells) after isolation from a febrile patient in France who had been infected on La Réunion Island (Parola et al. 2006). The stock titer had been previously determined by plaque assay (Gargan et al. 1983) to be 7.2 pfu/ml, with aliquots of the stock stored at −80° C until use. To infect mosquitoes, freshly grown virus was used. A T-75 tissue culture flask, with a confluent monolayer of Vero cells was inoculated with 100 μl of stock virus and incubated in a 5% CO2 atmosphere for 24 h. After incubation, the supernatant was removed from the flask. The supernatant was mixed with blood at a 1:5 dilution.
Oral infection of mosquitoes
Mosquitoes were randomly taken from the single holding cage and exposed in groups of 100 to citrated bovine blood (Hemostat Laboratories, Covella, CA) mixed with supernatant (infected group) or blood mixed with BA-1 buffer (control) in water-jacketed glass membrane feeders with Edicol collagen film (Devro Inc., Sandy Run, SC). Blood meal titer was determined by rt-PCR (see below) to be 6.3 log10 pfu equivalents/ml. Mosquitoes were given the opportunity to feed for one h, after which each group of mosquitoes was cold anesthetized for 45 s, and only fully engorged mosquitoes were kept. Engorgement was determined by visual inspection. Aedes albopictus had low feeding rates (≈5%), and only 22 control mosquitoes were fully engorged. The Ae. aegypti control group had 31 mosquitoes; the two groups containing exposed Ae. albopictus and Ae. aegypti had 32 engorged mosquitoes each. Altogether, 117 blood-engorged mosquitoes were used in the study. Each blood-engorged female was placed in a separate 500 ml cardboard container (Dade Container, inc., Miami, FL). For each exposed group (Ae. albopictus and Ae. aegypti), and the unexposed Ae. aegypti, three additional mosquitoes were immediately frozen and subsequently tested for CHIKV using the qRT-PCR protocol described below to confirm ingestion of virus (for the infected group) or not (for the control group). Exposed mosquitoes of each species had similar average titers of 5.96 log10 pfu equivalents/ml (n=3, Ae. aegypti) and 5.74 log10 pfu equivalents/ml (n=3, Ae. albopictus), while unexposed Ae. aegypti did not show any evidence of viral RNA. The low numbers of unexposed Ae. albopictus precluded the sacrifice of any in this group to test the initial viral concentration. However, as these were the control group, and were fed the same blood as the control Ae. aegypti, testing these mosquitoes was unnecessary.
Maintenance of adults
Adults were kept in a single incubator (Percival Inc, Perry, IA) maintained at 24° C and 99% RH with a 14:10 L:D cycle, which included one-h dusk/dawn periods. Containers with a single blood-fed female were placed in blocks of four, such that each block had one container from each treatment to control for any within incubator factors that might impact longevity or egg production. As there were fewer control Ae. albopictus, some blocks only had three containers (treatments) in them. In each container, mosquitoes were provided an oviposition cup with tap water and a strip of seed germination paper. The water level of the cup was checked daily and replenished to keep the water level approximately halfway up the germination paper. Every other day, cotton soaked in 10% sucrose was placed on the top of each container, providing the mosquitoes with a carbohydrate source. For the first four weeks of the experiment, mosquitoes were offered a blood meal via cotton soaked in citrated bovine blood (Hemostat Laboratories, Covella, CA). A fresh blood meal was provided weekly. However, only five were noted blood-fed, and these were all Ae. aegypti. Consequently, we stopped providing blood meals after four weeks.
Daily mortality and oviposition
Mosquitoes were checked daily for survival between 15:30 and 16:30. If a mosquito appeared dead, the container was removed to a sealed glove-box and examined carefully. The arrangement of cups was retained in the incubator. If the mosquito showed any signs of movement, she was returned to the incubator in her assigned spot and not counted dead. After a mosquito was discovered dead, she was immediately placed in a −80° C freezer to be processed at a later date. The oviposition paper was removed. All eggs on the oviposition paper and in the oviposition cup were counted. The container was examined for eggs not laid in the oviposition cup, although we never observed any eggs outside of the oviposition cup. For the purposes of determining eggs laid, all mosquitoes that died before three days post-exposure (one unexposed Ae. albopictus and one exposed Ae. aegypti) were removed from the analysis of data, as this period was shorter than their gonotrophic cycle (Christophers 1960, Hawley 1988).
Determination of infection status and size
Individuals that had been frozen in order to confirm the presence of the virus were removed from the freezer and kept on ice while being processed. Legs and wings were removed from each individual, while the head, thorax, and abdomen were placed in 1 ml of Trizol™ reagent (Invitrogen Corporation, Carlsbad, CA) with eight to ten glass beads. The body was then homogenized at 25 hz for 3 min in a Tissuelyzer homogenizer (Qiagen Inc., Valencia, CA) and clarified by centrifugation (3,128×g for 4 min at 4° C). Total RNA was extracted following the manufacturer’s directions.
Wings were mounted on slides and measured, as an index of body size, from the alula to the wing tip excluding any fringe scales. Legs were placed in 900 μl of BA-1 media for subsequent total RNA extraction using Trizol-LS™ reagent (Invitrogen Corporation, Carlsbad, CA) to determine viral dissemination.
One-step qRT-PCR was used to determine infection status and body titer of samples. Primers were designed from the E1 gene (Genbank accession no. DQ443544) and had the following sequences: forward: 5′-ACC CGG TAA GAG CGA TGA ACT-3′; reverse: 5′AGG CCG CAT CCG GTA TGT-3′; and probe: 5′-/5cy5/CCG TAG GGA ACA TGC CCA TCT CCA/3BHQ_2/-3′ (IDT DNA, Coralville, IA). Reactions were performed in a 96-well reaction plate, with each reaction containing: 0.4 μl SuperScript III RT/Platinum Taq mix (Invitrogen, Carlsbad, CA), 10 μl 2x reaction mix (a buffer system, MgSO4, dNTPs and stabilizers), 1 μl forward primer (10 μmol/liter), 1 μl reverse primer (10 μmol/liter), 0.4μl fluorogenic probe (10 μmol/liter), 2.2 μl DEPC-treated H2O, and 5 μl test sample. RNA was quantified using a Roche LC480 light-cycler (Roche Applied Sciences, Indianapolis, IN) with the following thermal conditions: 20 min at 48° C and 2 min at 95° C, followed by 40 cycles of PCR, 10 s at 95° C and 15 s at 60° C followed by a cool down for 30 s at 50° C. A negative control (DEPC-treated water in place of sample) and a positive control (CHIKV stock virus, 10−2 dilution) were included in each reaction run. PFU equivalents were calculated based upon a standard curve of a 10-fold serial dilution of viral supernatant comparing crossing point values with plaque assay (Cp = −3.455*Log10(PFU) + 32.2, n=6, p<0.0001, r2 =0.9985)(Bustin 2000, Reiskind et al. 2008).
Individuals were considered positive if they had a body titer crossing point value of less than 30. Legs were tested of those considered positive for infection. Mosquitoes were considered disseminated if the crossing point of virus in legs was less than 35. A lower threshold was used for the legs because of the small initial amount of tissue used.
Statistical analysis
To test the hypothesis that infection had an effect on longevity in each species, Kaplan-Meier survival curves were calculated and compared with log-rank statistics (PROC LIFETEST, SAS 9.1.3, SAS Institute, Cary, NC). Significance was evaluated using one-tailed probabilities, as we had a clear a priori expectation of increased mortality in infected mosquitoes. As egg counts could not be transformed to achieve normality, Wilcoxon two-sample tests were used to compare exposed vs unexposed mosquitoes within species, with one-tailed probabilities to detect lower fecundity in exposed mosquitoes. Linear regression was used to examine the correlations among days to death and body titer, leg titer, wing length, and fecundity (SAS 9.1.3, SAS Institute, Cary, NC). Location in the incubator was not significant and was not used in any subsequent analyses.
RESULTS
Infection and dissemination rates
Three-quarters (24/32) of exposed Ae. albopictus were considered infected by qRT-PCR, and of those, 91% were disseminated (22/24). Almost two-thirds (63%) of exposed Ae. aegypti were considered infected by qRT-PCR (20/32), and of those, 80% were disseminated (16/20).
Survival analysis
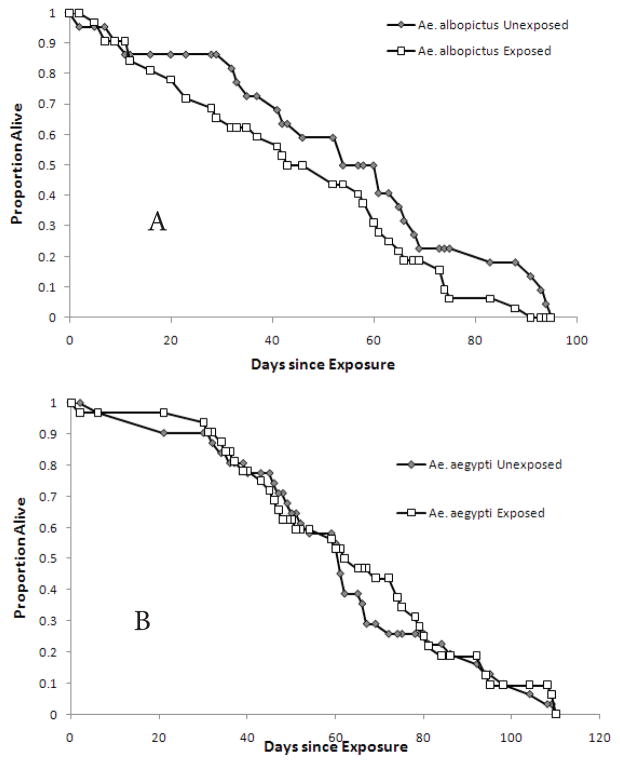
Average days to death and maximum life-spans for each group are given in Table 1. Exposure to virus significantly reduced the longevity in Ae. albopictus (Figure 1a, log-rank χ2 = 3.6347, p=0.028, one-tailed), but not Ae. aegypti (Figure 1b, log-rank χ2 = 0.327, p=0.29, one-tailed). Between the species, Ae. aegypti survived significantly longer (logrank χ2 = 7.7128, p=0.005, two-tailed). When interspecific longevity was compared within exposed vs non-exposed groups, exposed Ae. aegypti lived significantly longer than exposed Ae. albopictus (log-rank χ2 = 7.7128, p=0.0018, twotailed), while there was no difference between unexposed Ae. aegypti and Ae. albopictus (log-rank χ2 = 2.0, p=0.5, two-tailed). If the data set is limited to those mosquitoes that were positive by qRT-PCR (had a body titer crossing point value > 30.00), there was no significant difference in longevity between infected and non-infected mosquitoes of either species (for Ae. albopictus log-rank χ2 = 1.79, p=0.09, one-tailed; for Ae. aegypti log-rank χ2 = 1.42, p=0.12, onetailed). Three mosquitoes survived to day 110 post-infection (one Ae. aegypti uninfected and two Ae. aegypti infected), and were right censored in the survival analyses.
Table 1.
Average lifespans, standard deviations, and maximum lifespans of exposed and unexposed Ae. albopictus and Ae. aegypti to chikungunya virus.
| Group | Average Lifespan in Days | Standard Deviation | Maximum Lifespan, in Days (n) |
|---|---|---|---|
| Ae. albopictus | |||
| exposed | 45.19 | 25.09 | 91 (1) |
| unexposed | 54.77 | 27.62 | 95 (1) |
| Ae. aegypti | |||
| exposed | 63.75 | 26.52 | 110 (2)* |
| unexposed | 61.29 | 26.33 | 110 (1)* |
The experiment was ended at day 110.
Figure 1.
Survival curve of exposed and unexposed female Ae. albopictus (A) and Ae. aegypti (B).
Oviposition
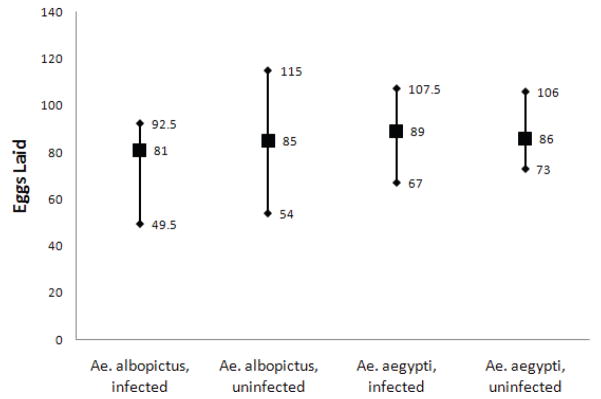
There was no significant effect of exposure on the median number of eggs laid by either species, although there was a trend for fewer eggs laid by exposed females compared to unexposed females in Ae. albopictus (Figure 2, Wilcoxon Two-sample Test: Z-approximation = 1.47, p = 0.0703, one-tailed).
Figure 2.
Median number of eggs laid in each group (filled squares) with 75 and 25 quartiles (diamonds). Numbers of eggs are to the right of each appropriate symbol. No differences were significant.
Body titer and days to death
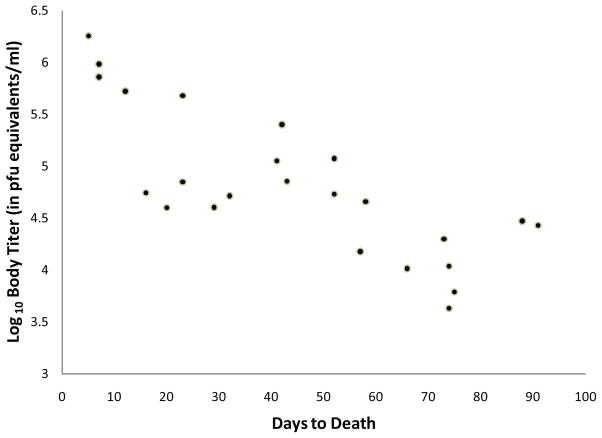
There was a significant negative, linear correlation between the days to death and the body titer of exposed Ae. albopictus mosquitoes (r = 0.3918, p<0.05). When the data set was reduced to only include mosquitoes with unequivocal infection (crossing point < 30.0), the correlation became stronger (Figure 3, r = 0.7871, p < 0.0001). There were no significant correlations for exposed or infected Ae. aegypti between body titer and days to death (data not shown).
Figure 3.
Correlation between body titer and days to death in infected Ae. albopictus with detectable levels of CHIK RNA (crossing point> 30) (r=0.7871, p<0.0001).
DISCUSSION
The results suggest a small but significant negative effect of CHIKV exposure on longevity of Ae. albopictus, but not of Ae. aegypti. This supports the prediction from the first part of our hypothesis that the LR2006-OPY1 strain of CHIKV has more deleterious effects on Ae. albopictus than on Ae. aegypti. The second part of our hypothesis, that CHIKV infection would have an effect on mosquito fecundity, was not supported. Although we observed a trend towards decreased numbers of eggs laid by Ae. albopictus exposed to CHIKV, relative to unexposed mosquitoes, this difference was not significant. We also found significantly longer-lived females in Ae. aegypti than Ae. albopictus, a result seen under a variety of conditions in previous studies (Mogi et al. 1996, Reiskind and Lounibos, 2009).
This study was complicated by the unexpectedly small sample sizes. In particular, the poor feeding of the Ae. albopictus control group limited the strength of conclusions about differences in fecundity between exposed and unexposed Ae. albopictus. A larger sample size may also eliminate the need for non–parametric statistical tests in favor of more powerful parametric tests. Furthermore, the lack of complete infection in Ae. albopictus was unexpected, as previous studies in our laboratory using similar or lower titers of CHIKV achieved 100% infection rates (Reiskind et al. 2008, Pesko et al. 2009). The lower than expected infection rates may be due to differences in the handling of mosquitoes in this study compared to previous work. The nature of this study meant that instead of sacrificing live mosquitoes and immediately preserving their RNA, mosquitoes found dead may have been dead for up to 24 h. In some cases, the carcass was found in the oviposition water, which may have had a negative effect on the stability of viral RNA, increasing the probability of finding false negatives. Although we present this as a possible explanation for finding exposed mosquitoes that were negative for virus, previous studies of dengue virus infected mosquito carcasses in harsh conditions suggests PCR is sufficiently sensitive to detect infection even under these challenging conditions (Bangs et al. 2007). Sample size was satisfactory for Ae. aegypti, and the lack of significant differences between the exposed and unexposed group in Ae. aegypti suggests no deleterious effect of infection with CHIKV (LR2006-OPY1) on that species.
The conditions in which the adults were maintained may have lessened the impact of arboviral infection. Although the temperature (24° C) is within the range of temperatures a mosquito experiences in Florida, it may have extended the lifespan of the mosquitoes relative to a warmer temperature, masking the detection of negative effects by decreasing the effect size. The average effect size infection had on longevity (longevity of infected/longevity of controls) in previous studies was approximately a 15% reduction in lifespan, although there is considerable variation around this estimated effect size depending upon a number of factors (Lambrechts and Scott 2009). Lambrechts and Scott (2009) demonstrated the importance of both viral family and mosquito genus, but only found a single study (Moncayo et al. 2000) that examined an Alphavirus-Aedes interaction in terms of mosquito longevity. Moncayo et al. (2000) found no effect of infection with eastern equine encephalitis virus on Ae. albopictus. Our results suggest a modest reduction of longevity by 18% in Ae. albopictus and no longevity reduction in Ae. aegypti.
The negative effect on Ae. albopictus but not Ae. aegypti suggests that the increased susceptibility and dissemination observed in previous studies may have a cost to the vector (Tsetsarkin et al. 2007, Reiskind et al. 2008). For a population of virions to persist, each infected host must infect at least one naïve host (e.g., R0>1). Therefore, the negative effects of infection on individual Ae. albopictus must have been outweighed by the higher vectorial capacity of the population of Ae. albopictus for chikungunya through increased susceptibility of mosquitoes in the La Réunion outbreak. Furthermore, our results suggest that the increased mortality may occur after a mosquito has fed twice (10–12 days after initial blood meal; Figure 1) and thus after a potential transmission event.
The correlation between body titer and days to death may suggest that a high viral load is detrimental and leads to earlier death. However, simple correlation prevents any clear interpretation, as it is equally possible that longer-lived mosquitoes begin to clear the infection or the number of virions within the mosquito body declines through immune function or natural death of virions. The difference between Ae. aegypti and Ae. albopictus in decline of viral titer over time, however, suggests some kind of species specific interaction with the virus. Previous work with arboviruses suggests transient viral replication early after exposure (days 2–5), followed by little viral replication, slow decline in total body viral titer, and tissue specific viral clearance, although this may be very specific to the virus-vector system studied (Bowers et al. 1995, Girard et al. 2004). These studies were conducted over shorter periods than the results reported here, also complicating any inferences.
We found a small impact of LR2006-OPY1 exposure on Ae. albopictus but not Ae. aegypti, and we suggest this interspecific difference in response to exposure to be a cost to Ae. albopictus associated with its higher susceptibility. To determine if this interspecific difference in cost of exposure is associated with the emergent strain, longevity studies should be conducted that compare CHIK LR2006-OPY1 to strains of CHIKV isolated before the recent outbreak in the Indian Ocean.
Acknowledgments
The authors thank Drs. Deborah Jaworski and George Opit at Oklahoma State University, and Dr. George O’Meara at the Florida Medical Entomology Laboratory, for reading prior drafts of this work and two anonymous reviewers for their comments. This work was supported by NIH 5R01-047793 to LPL and Hatch project #2702 to MHR through the Oklahoma Agricultural Experiment Station.
Footnotes
The authors declare no competing financial interests with the research presented in this manuscript.
REFERENCES CITED
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford University Press; NY: 1991. p. 768. [Google Scholar]
- Bagny L, Delatte H, Elissa N, Quilici S, Fontenille D. Aedes (Diptera: Culicidae) Vectors of Arboviruses in Mayotte (Indian Ocean): Distribution Area and Larval Habitats. J Med Entomol. 2009;46:198–207. doi: 10.1603/033.046.0204. [DOI] [PubMed] [Google Scholar]
- Bangs MJ, Pudiantari R, Gionar YR. Persistence of dengue virus RNA in dried Aedes aegypti (Diptera: Culicidae) exposed to natural tropical conditions. J Med Entomol. 2007;44:163–167. doi: 10.1603/0022-2585(2007)44[163:podvri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bowers DF, Abell BA, Brown DT. Replication and tissue tropism of the alphavirus sindbis in the mosquito Aedes albopictus. Virol. 1995;212:1–12. doi: 10.1006/viro.1995.1447. [DOI] [PubMed] [Google Scholar]
- Bowers DF, Coleman CG, Brown DT. Sindbis virus-associated pathology in Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2003;40:698–705. doi: 10.1603/0022-2585-40.5.698. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Christophers SR. Aëdes aegypti (L.), The Yellow Fever Mosquito; Its Life History, Bionomics, and Structure. University Press; Cambridge, UK: 1960. [Google Scholar]
- Delatte H, Dehecq JS, Thiria J, Domerg C, Paupy C, Fontenille D. Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a Chikungunya epidemic event. Vector-Borne Zoonotic Dis. 2008;8:25–34. doi: 10.1089/vbz.2007.0649. [DOI] [PubMed] [Google Scholar]
- Dye C. Vectorial capacity: must we measure all its components? Parasitol. Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Enserink M. Infectious diseases - Massive outbreak draws fresh attention to little-known virus. Science. 2006;311:1085. doi: 10.1126/science.311.5764.1085a. [DOI] [PubMed] [Google Scholar]
- Faran ME, Turell MJ, Romoser WS, Routier RG, Gibbs PH, Cannon TL, Bailey CL. Reduced survival of adult Culex pipiens infected with Rift-Valley fever virus. Am J Trop Med Hyg. 1987;37:403–409. doi: 10.4269/ajtmh.1987.37.403. [DOI] [PubMed] [Google Scholar]
- Gargan TP, Bailey CL, Higbee GA, Gad A, Elsaid S. The effect of laboratory colonization on the vector-pathogen interactions of Egyptian Culex pipiens and Rift Valley fever virus. Am J Trop Med Hyg. 1983;32:1154–1163. doi: 10.4269/ajtmh.1983.32.1154. [DOI] [PubMed] [Google Scholar]
- Girard YA, Klingler KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector-Borne Zoonotic Dis. 2004;4:109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- Hawley W. The biology of Aedes albopictus. J Am Mosq Contr Assoc Supplement. 1988;1:1–40. [PubMed] [Google Scholar]
- Lambrechts L, Scott TW. Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proc Roy Soc B. 2009;276:1369–1378. doi: 10.1098/rspb.2008.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Rowley WA, Platt KB. Longevity and spontaneous flight activity of Culex tarsalis (Diptera: Culicidae) infected with western equine encephalomyelitis virus. J Med Entomol. 2000;37:187–193. doi: 10.1603/0022-2585-37.1.187. [DOI] [PubMed] [Google Scholar]
- Lord CC, Rutledge CR, Tabachnick WJ. Relationships between host viremia and vector susceptibility for arboviruses. J Med Entomol. 2006;43:623–630. doi: 10.1603/0022-2585(2006)43[623:rbhvav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood F, Reisen WK, Chiles RE, Fang Y. Western equine encephalomyelitis virus infection affects the life table characteristics of Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2004;41:982–986. doi: 10.1603/0022-2585-41.5.982. [DOI] [PubMed] [Google Scholar]
- Mangiafico J. Chikungunya virus infection and transmission in 5 species of mosquito. Am J Trop Med Hyg. 1971;20:642–645. doi: 10.4269/ajtmh.1971.20.642. [DOI] [PubMed] [Google Scholar]
- McClelland GAH, Weitz B. Serological identification of natural hosts of Aedes aegypti (L.) And some other mosquitoes (Diptera, Culicidae) caught resting in vegetation in Kenya and Uganda. Ann Trop Med Parasitol. 1963;57:214–224. doi: 10.1080/00034983.1963.11686176. [DOI] [PubMed] [Google Scholar]
- Mogi M, Miyagi I, Toma T, Hasan M, Abadi K, Syafruddin Occurrence of Aedes (Stegomyia) spp. mosquitoes (Diptera: Culicidae) in Halmahela villages, Indonesia. J Med Entomol. 1996;33:169–172. doi: 10.1093/jmedent/33.1.169. [DOI] [PubMed] [Google Scholar]
- Monath TP. The Arboviruses: Epidemiology and Ecology. CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- Moncayo AC, Edman JD, Turell MJ. Effect of eastern equine encephalomyelitis virus on the survival of Aedes albopictus, Anopheles quadrimaculatus, and Coquillettidia perturbans (Diptera: Culicidae) J Med Entomol. 2000;37:701–706. doi: 10.1603/0022-2585-37.5.701. [DOI] [PubMed] [Google Scholar]
- Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, Brouqui P, Flahault A, Raoult D, Charrel RN. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Inf Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesko K, Westbrook C, Mores C, Lounibos L, Reiskind M. Effects of infectious virus dose and blood meal delivery method on susceptibility of Aedes aegypti and Aedes albopictus to chikungunya virus. J Med Entomol. 2009;46:395–399. doi: 10.1603/033.046.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponlanwat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Lounibos LP. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Med Vet Entomol. 2009;23:62–68. doi: 10.1111/j.1365-2915.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind MH, Pesko K, Westbrook CJ, Mores CN. Susceptibility of Florida mosquitoes to infection with chikungunya virus. Am J Trop Med Hyg. 2008;78:422–425. [PMC free article] [PubMed] [Google Scholar]
- Reiter P, Fontenille D, Paupy C. Aedes albopictus as an epidemic vector of chikungunya virus: another emerging problem? Lancet Infect Dis. 2006;6:463–464. doi: 10.1016/S1473-3099(06)70531-X. [DOI] [PubMed] [Google Scholar]
- Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central north Carolina. J Med Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Brehin AC, Cubito N, Despres P, Kunst F, Rey FA, Zeller H, Brisse S. Genome microevolution of Chikungunya Viruses causing the Indian Ocean outbreak. Plos Med. 2006;3:1058–1070. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TW, Lorenz LH. Reduction of Culiseta melanura fitness by eastern equine encephalomyelitis virus. Am J Trop Med Hyg. 1998;59:341–346. doi: 10.4269/ajtmh.1998.59.341. [DOI] [PubMed] [Google Scholar]
- Styer LM, Meola MA, Kramer LD. West Nile virus infection decreases fecundity of Culex tarsalis females. J Med Entomol. 2007;44:1074–1085. doi: 10.1603/0022-2585(2007)44[1074:wnvidf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Gubler DJ, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. Am J Trop Med Hyg. 1976;25:326–335. doi: 10.4269/ajtmh.1976.25.326. [DOI] [PubMed] [Google Scholar]
- Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. Plos Pathogens. 2007;3:1895–1906. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ. Virus-dependent mortality in Rift-Valley fever, eastern equine encephalomyelitis, and chikungunya virus-inoculated mosquito (Diptera, Culicidae) larvae. J Med Entomol. 1992;29:792–795. doi: 10.1093/jmedent/29.5.792. [DOI] [PubMed] [Google Scholar]
- Weaver SC. Evolutionary influences in arboviral disease. Curr Top Microbiol Immunol. 2006;299:285–314. doi: 10.1007/3-540-26397-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Lorenz LH, Scott TW. Pathological-changes in the midgut of Culex tarsalis following infection with western equine encephalomyelitis virus. Am J Trop Med Hyg. 1992;47:691–701. doi: 10.4269/ajtmh.1992.47.691. [DOI] [PubMed] [Google Scholar]