Abstract
Although the calcium-sensing receptor (CaSR) and parathyroid hormone (PTH) may each exert skeletal effects, it is uncertain how CaSR and PTH interact at the level of bone in primary hyperparathyroidism (PHPT). Therefore, we simulated PHPT with 2 wk of continuous PTH infusion in adult mice with deletion of the PTH gene (Pth−/− mice) and with deletion of both PTH and CaSR genes (Pth−/−-Casr −/− mice) and compared skeletal phenotypes. PTH infusion in Pth−/− mice increased cortical bone turnover, augmented cortical porosity, and reduced cortical bone volume, femoral bone mineral density (BMD), and bone mineral content (BMC); these effects were markedly attenuated in PTH-infused Pth−/−-Casr−/− mice. In the absence of CaSR, the PTH-stimulated expression of receptor activator of nuclear factor-κB ligand and tartrate-resistant acid phosphatase and PTH-stimulated osteoclastogenesis was also reduced. In trabecular bone, PTH-induced increases in bone turnover, trabecular bone volume, and trabecular number were lower in Pth−/−-Casr−/− mice than in Pth−/− mice. PTH-stimulated genetic markers of osteoblast activity were also lower. These results are consistent with a role for CaSR in modulating both PTH-induced bone resorption and PTH-induced bone formation in discrete skeletal compartments.
Keywords: hyperparathyroidism, bone resorption, calcium
parathyroid hormone (PTH) and extracellular calcium ([Ca2+]e) play important roles in modulating systemic calcium homeostasis. To defend against a decrease in [Ca2+]e, PTH, acting on a G protein-coupled receptor, the type 1 PTH/PTH-related protein receptor (PTH1R), increases renal reabsorption of calcium (Ca2+), enhances the conversion of 25 hydroxyvitamin D to the active metabolite 1,25 dihydroxyvitamin D[1,25(OH)2D], thus indirectly augmenting intestinal Ca2+ absorption, and also produces net bone resorption with resultant mobilization of skeletal Ca2+ (14). To defend against an increase in [Ca2+]e, [Ca2+]e can itself activate a cation-sensing G protein-coupled receptor [calcium-sensing receptor (CaSR)] to inhibit release of PTH and diminish renal calcium reabsorption (3). Both PTH and [Ca2+]e, acting via CaSR, can exert complex effects in bone. The actions of PTH in bone ensue from its binding to the PTH1R on bone marrow stromal cells (BMSC) (40) and their osteoblastic progeny (4, 34), resulting in increased bone turnover in both cortical and trabecular bone and producing either net bone loss or net bone gain. In the fetus (27) and neonate (52), endogenous PTH is required for normal trabecular bone formation, and in both neonates and adult animals, exogenous PTH, when administered intermittently, can have an anabolic effect predominantly by increasing trabecular bone formation, although it may also concomitantly produce a small reduction in cortical bone (9, 29, 48). Primary hyperparathyroidism (PHPT) and continuous rather than intermittent PTH treatment induce cortical bone loss at least in part by enhancing endosteal bone resorption and intracortical bone resorption (17, 25, 36). Although severe, persistent elevations of PTH levels may also lead to trabecular bone loss (17), both PHPT and continuous PTH treatment often produce a modest increase in trabecular bone (33, 44, 54). The catabolic effect of PTH has been shown to be mediated in part by enhanced production of the cytokine receptor activator of nuclear factor-κB ligand (RANKL) and by decreased production of the RANKL decoy receptor osteoprotegerin (OPG) by BMSC and osteoblasts (26, 49).
CaSR has been reported to function in vitro in a variety of skeletal cells, including BMSC, osteoblasts, monocytes/macrophages, and osteoclasts (6). By in situ hybridization, CaSR transcripts have been found mainly in osteoblasts, osteocytes, and bone marrow cells but rarely in mature osteoclasts. In vivo studies have shown that transgenic mice, with a constitutively active mutant CaSR targeted to mature osteoblasts, demonstrate enhanced bone resorption due to an associated increase in RANKL (10). Although bone formation rates were unchanged in this model, bone mineral density (BMD) and bone volume decreased. In contrast, mice with osteoblast-specific deletion of CaSR exhibit undermineralized skeletons (5). CaSR also mediates alterations in bone turnover in murine neonates in response to changes in dietary calcium and modulates PTH-stimulated bone turnover, which is required for bone anabolism (43). In adult mouse models we recently provided evidence that the absence of [Ca2+]e-stimulated CaSR activity reduces bone resorption (37), and we (30) and others (11) have provided evidence in vivo in rodent models for the effect of an allosteric activator of CaSR, cinacalcet, to increase the number and activity of osteoclasts.
Mice homozygous for the CaSR-null mutation (Casr−/− mice) die shortly after birth because of PHPT and hypercalcemia (15), thus precluding the analysis of PTH and CaSR interactions in adult animals in this model. However, ablating the PTH gene can “rescue” Casr−/− mice (20, 47). Pth−/−-Casr−/− mice survive to adulthood and therefore provide a valuable tool to study the role of CaSR in tissues. To delineate the interaction of PTH and CaSR on cortical and trabecular bone in a controlled model simulating hyperparathyroidism, we examined the consequences of continuous PTH infusion on mineral and skeletal homeostasis in adult mice with targeted deletion of only Pth (Pth−/−) and compared this with the effects of PTH infusion in adult animals with deletion of both PTH and CaSR (Pth−/−-Casr−/−) genes.
MATERIALS AND METHODS
Mice.
All animal experiments were reviewed and approved by the McGill University Animal Care Committee. The derivation of the two parental strains of CaSR homozygous (Casr−/−) and PTH homozygous (Pth−/−) mice by homologous recombination in embryonic stem cells was described previously by Ho et al. (15) and Miao et al. (27), respectively. The wild-type (WT), Pth−/−, and Pth−/−-Casr−/− mice were generated by breeding PTH and CaSR double-heterozygous mice (Pth+/−-Casr+/−). Mice were housed individually and exposed to a 12:12-h light-dark cycle. No differences in the growth of WT, Pth −/−, or Pth−/−-Casr−/− mice were observed over the course of the study.
Experimental design.
To reduce the profound hypocalcemia of hypoparathyroidism, mice were provided water containing 1% calcium gluconate after weaning (19). Ten-week-old WT, Pth−/−, and Pth−/−-Casr−/− mice (n = 16/genotype, 8 males and 8 females) were implanted with Alzet pumps (Durect, Cupertino, CA). One day prior to implantation, Alzet pumps were filled aseptically with appropriate amounts of human (h)PTH(1–34) (60 μg·kg−1·day−1; Bachem Americas, Torrance, CA) or vehicle (equivalent volume of sterile PBS with 2% cysteine, adjusted to pH 4.3, using 10 mM acetic acid) and stored at 4°C until use. The PTH dose used was intermediate to the highest (120 μg·kg−1·day−1) and lowest doses (20 μg·kg−1·day−1) used previously by us and others to simulate hyperparathyroidism (13, 19, 38, 42). The pumps were implanted subcutaneously into the back of the necks of mice under anesthesia (100 mg/kg ketamine and 3 mg/kg xylazine), and the incision was closed according to the manufacturer's instructions. All procedures were performed aseptically. Mice were euthanized 2 wk later. Serum was prepared for analysis of calcium, phosphorus, hPTH(1–34), and 1,25(OH)2D levels. Urine was taken directly from the bladder for assessment of the calcium/creatinine ratio. Humeri were harvested for analysis of gene expression, as described subsequently. Femora were harvested, fixed in 2% paraformaldehyde containing 0.075 mM lysine and 0.01 mM sodium periodate (PLP) for 12 h, and stored in 75% ethanol at 4°C until the samples were processed.
Serum and urine analysis.
Serum and urine calcium and serum phosphorus levels were determined by QuantiChrom calcium or phosphorus assay kits (BioAssay Systems, Hayward, CA). Serum 1,25(OH)2D was measured by ELISA (ImmunoDiagnostic Systems, Fountain Hills, AZ). Serum hPTH(1–34) was measured by ELISA (Immunotopics, San Clemente, CA). Urine creatinine was determined by QuantiChrom creatinine assay kit (BioAssay Systems, Hayward, CA).
Histology and histomorphometry.
Fixed femora were processed histologically, as described elsewhere (51). In brief, decalcified femora were dehydrated and embedded in paraffin, after which 5-μm sections were cut on a rotary microtome. The sections were stained with hematoxylin and eosin (H & E) for osteoblasts or histochemically for tartrate-resistant acid phosphatase (TRACP) activity as an osteoclast marker. Calcified femora were embedded in low-temperature methyl methacrylate (Sigma Chemical), and 4-μm sections were cut with a diamond knife. The sections were stained histochemically for alkaline phosphatase (ALP) and for mineral using the Goldner trichrome staining procedure.
Slides were inspected visually under a bright field using a Zeiss microscope (Carl Zeiss Canada, Toronto, ON, Canada). The digital images of three nonoverlapping fields per slide were collected with a Retiga 1300 digital camera (Q Imaging, Burnaby, BC, Canada). Images were processed using Image J 1.38 software from the National Institutes of Health (http://rsb.info.nih.gov). ALP-positive area was determined as the percentage of ALP-positive area relative to trabecular bone area or cortical bone area. Osteoid volume was calculated as a percentage of trabecular or cortical bone volume from undecalcified Goldner trichrome-stained sections, as described previously (51). The number of osteoblasts was determined from H & E-stained sections, and the number of osteoclasts was determined from TRACP-stained sections. Cells were identified visually, traced manually, counted automatically, and reported as cell numbers per millimeter of bone perimeter in trabecular bone or per millimeter squared of tissue area in cortical bone. Quantitative histomorphometry was used to measure bone parameters in the metaphyseal region 0.5–2.0 mm proximal to the metaphyseal side of the growth plate of the femur for trabecular bone or in the diaphyseal region for cortical bone.
Microcomputed tomography and dual-energy X-ray absorptiometry.
Microcomputed tomography (μCT) scans were performed on fixed femora using a high-resolution μCT scanner (SkyScan-1072, Aartselaar, Belgium), and the subsequent three-dimensional architecture analyses were completed using the associated software applications, as described previously (45). This scanner is equipped with a sealed microfocus X-ray tube, with operating power ranging from 20 to 100 kV (0–250 μA), and X-ray charge-coupled device (CCD) camera with a cooled 1,024 × 1,024 pixels 12-bit CCD sensor. Briefly, the samples were scanned at X-ray source power of 45 kV/222 μA, with spatial resolution of 5.63 μm/pixel. The rotation was set at 0.9°/step for 180°. The exposure time was set at 2.2 s/step. The samples were wrapped with Parafilm (Pechiney Plastic Packaging, Chicago, IL) to prevent them from dehydration during scanning. The NRecon (version 1.6.1.3) was employed to perform the reconstruction following the scans. The CT-Analyzer (version 1.10.0.1) was used for analyses of three-dimensional architectural parameters and creation of three-dimensional models. To measure trabecular bone volume/tissue volume (BV/TV), trabecular number (Tb.N), trabecular bone pattern factor (Tb.Pf), and structure model index, slices with 11.25-μm resolution, corresponding to the volume from 0.26 to 2.26 mm below the growth plate in the femur, were reconstructed and analyzed using 3D analysis. Cortical bone volume/tissue volume, cortical bone volume, closed porosity, and open porosity were measured in slices with 11.25-μm resolution corresponding to the volume from 2.62 to 3.39 mm below the growth plate in the femur.
For BMD (g/cm2) and bone mineral content (BMC; g) measurement, the anesthetized mice were scanned using a PIXImus densitometer (Lunar; GE Healthcare, Madison, WI) at 0 and 2 wk after vehicle or PTH infusion. After scanning, the right femur and lumbar spine (L4–L6) were measured separately for BMD and BMC.
Bone marrow cell culture.
Mouse bone marrow cells were isolated as described previously (16). Briefly, tibias and femurs from 8-wk-old WT, Pth−/−, and Pth−/−-Casr−/− mice were dissected free of soft tissue. The bone ends were removed, and the marrow cavity was flushed with α-minimal essential medium (α-MEM; Gibco, Grand Island, NY) without serum by slowly injecting medium into one end of the bone using a sterile 25-gauge needle. Marrow cells were collected into tubes, washed twice with α-MEM, and cultured in α-MEM containing 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT). Cultures were performed in 5-cm culture dishes (3 × 106 cells·5 ml−1·dish−1). Cultures were fed every 2–3 days by replacing 80% of the medium with fresh medium. hPTH(1–34) (10 nM) was added at the beginning of the culture and with each medium change. Cells for TRACP staining were fixed on day 7 of culture with PLP fixation for 10 min. Cells for testing gene expression were collected in TRIzol Reagent (Invitrogen Life Technologies, Calsbad, CA). TRACP staining and real-time PCR were performed as described later. Osteoclast-like cells were defined as TRACP-positive multinucleated cells that contained greater than three nuclei.
Real-time RT-PCR.
Total RNA was isolated with TRIzol reagent from humeri of 12-wk-old WT, Pth−/−, and Pth−/−-Casr−/− mice infused with PTH or vehicle and from PTH or vehicle treated-bone marrow cells from WT, Pth−/−, and Pth−/−-Casr−/− mice. RNA was reverse-transcribed into complementary DNA (cDNA), as described previously (50). Real-time RT-PCR analyses were performed in a final volume of 20 μl in a StepOnePlus Real-Time PCR System (ABI, Carlsbad, CA) for gene expression. The reaction mixtures contained 10 μl of Fast SYBR Green Master Mix (ABI), 1 ng/μl of each primer, and 5 μl of cDNA template and were amplified in a StepOnePlus Real-Time PCR System. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as a reference transcript for quantitative analysis of mRNA encoding runt-related transcription factor-2 (Runx2), the Wnt target gene naked cuticle 2 homolog (Nkd2), PTH1R, TRACP, OPG, and RANKL mRNAs. Primers and PCR cycle conditions were as reported previously for mouse Runx2 (41), NKD2 (2), TRACP, RANKL (18), OPG (28), and GAPDH (50).
Statistical analysis.
All data are presented as means ± SE. Studies were analyzed by two-way ANOVA using Sigmaplot 11.0 (Systat Software, Chicago, IL), including the main effects for animal genotypes (Pth−/− and Pth−/−-Casr−/−) and treatment (vehicle and PTH infusion) plus interaction between animal genotypes (Pth−/− and Pth−/−-Casr−/−) and PTH treatment. And then one-way ANOVA (SPSS statistical program version 15.0; SPSS, Chicago, IL) was used to compare the differences among treatment (vehicle and PTH infusion) within each genotype (WT, Pth−/−, and Pth−/−-Casr−/−) or the differences among genotypes (WT, Pth−/−, and Pth−/−-Casr−/−) within treatment (vehicle or PTH treatment). Differences among PTH treatment within each genotype or differences among genotypes within vehicle or PTH treatment were determined by the Bonferoni post hoc test when variances were equal and by Tamhane's T2 post hoc test when variances were unequal. Differences were considered significant when the P value was <0.05.
RESULTS
Biochemical changes in WT, Pth−/−, and Pth−/−-Casr−/− mice.
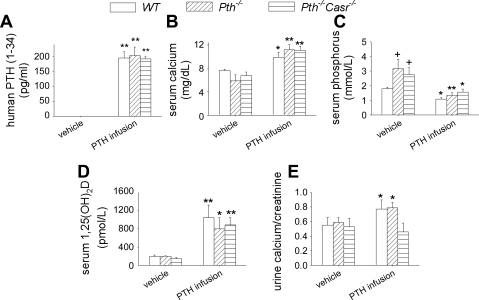
After infusion of hPTH(1–34) for 2 wk, circulating levels of hPTH(1–34) reached ∼200 pg/ml, with no significant difference among the three genotypes (Fig. 1A). We have reported previously that endogenous mouse PTH(1–84) in wild-type mice, using a two-site enzyme-linked immunosorbent assay, is ∼30 pg/ml (37), whereas endogenous PTH in Pth−/− and Pth−/−-Casr−/− is undetectable (43). Serum calcium was increased significantly in WT, Pth−/−, and Pth−/−-Casr-−/− mice after PTH infusion. Compared with vehicle-infused WT and Pth−/−-Casr−/− mice, serum calcium was slightly lower in vehicle-infused Pth−/− mice even after augmentation of the drinking water with 1% calcium gluconate, but the difference was not significant (Fig. 1B). Basal serum phosphorus was 1.8-fold higher in vehicle-infused Pth−/− and Pth−/−-Casr−/− mice than in vehicle-infused WT mice. PTH infusion markedly decreased serum phosphorus in WT, Pth−/−, and Pth−/−-Casr−/− mice (Fig. 1C). After PTH infusion, serum 1,25(OH)2D was increased eightfold in all genotypes (Fig. 1D). Urine calcium excretion was increased after PTH infusion in WT and Pth−/− mice but not in Pth−/−-Casr-−/− mice (Fig. 1E).
Fig. 1.
Serum and urine biochemistry. Serum hPTH(1–34) (A), calcium (B), phosphorus (C), 1,25(OH)2D (D), and urine calcium/creatinine ratio (E) in 12-wk-old WT, Pth−/−, and Pth−/−-Casr −/− mice infused with 60 μg·kg−1·day−1 hPTH(1–34) or vehicle for 2 wk. Each value is the mean ± SE (n = 8/genotype). Symbols indicate significant changes, using 1-way ANOVA as described in Statistical analysis. *P < 0.05 and **P < 0.01 compared with vehicle-infused mice of the corresponding genotype. +P < 0.05 compared with vehicle-infused WT mice. WT, wild type; hPTH, human PTH; Casr, calcium-sensing receptor. Results of 2-way ANOVA using genotype (Pth−/− and Pth−/−-Casr−/−) and parathyroid hormone (PTH) infusion treatment (vehicle and PTH infusion) as main effects: serum hPTH(1–34): genotype, P = 0.573; PTH, P < 0.001; genotype × PTH, P = 0.573. Serum calcium: genotype, P = 0.583; PTH, P < 0.01; genotype × PTH, P = 0.466. Serum phosphorus: genotype, P = 0.952; PTH, P < 0.001; genotype × PTH, P = 0.435. Serum 1,25(OH)2D3: genotype, P = 0.977; PTH, P < 0.001; genotype × PTH, P = 0.792. Urine calcium/creatine: genotype, P = 0.027: PTH, P = 0.442; genotype × PTH, P = 0.107.
Bone turnover in cortical and trabecular bone.
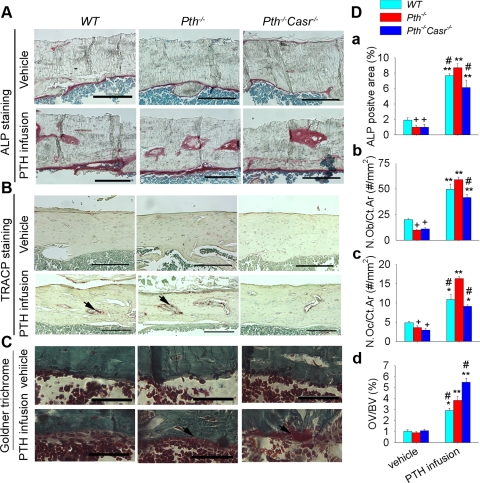
In cortical bone, the ALP-positive area, osteoblast numbers, and osteoclast numbers were significantly lower in the vehicle-treated Pth−/− and Pth−/−-Casr−/− mice than in the vehicle-treated WT mice (Fig. 2). After PTH infusion, ALP-positive area, osteoblasts, and osteoclasts were increased in all genotypes, but PTH infusion increased most parameters to a greater extent in Pth−/− mice than in WT and Pth−/−-Casr −/− mice (Fig. 2D a–c). Osteoid volume was increased in all three genotypes after PTH infusion, although increases were most prominent in Pth−/−-Casr−/− mice [Fig. 2D (d)].
Fig. 2.
Effects of PTH infusion on histomorphometric parameters in cortical bone. Representative photomicrograph sections stained histochemically for alkaline phosphatase (ALP) activity (A), tartrate-resistant acid phosphatase (TRACP) activity (B), and bone mineralization by Goldner trichrome (C) from 12-wk-old WT, Pth−/−, and Pth−/−-Casr−/− mice infused with hPTH(1–34) or vehicle for 2 wk. Histomorphometric analysis for ALP-positive area from histochemical staining (D, a), osteoblast number (N.Ob/Ct.Ar, cell no./mm2 cortical area) from hematoxylin and eosin (H & E)-stained sections (D, b), osteoclast number (N.Oc/Ct.Ar, cell no./mm2 cortical area) from TRACP staining (D, c), and osteoid volume (OV/BV) from Goldner trichrome staining (D, d). Each value is the mean ± SE (n = 8/genotype). Symbols indicate significant changes using 1-way ANOVA, as described in Statistical analysis. *P < 0.05 and **P < 0.01 compared with vehicle-infused mice of the corresponding genotype. +P < 0.05 compared with vehicle-infused WT mice. #P < 0.05 compared with PTH-infused Pth−/− mice. The scale bars represent 500 (A and B) and 125 μm (C). Arrows point to osteoclast (B) and osteoid (C). Results of 2-way ANOVA using genotype (Pth−/− and Pth−/−-Casr−/−) and PTH infusion treatment (vehicle and PTH infusion) as main effects: ALP: genotype, P = 0.003; PTH, P < 0.001; genotype × PTH, P = 0.003. N.Ob/Ct.Ar: genotype, P = 0.001; PTH, P = 0.001; genotype × PTH, P = 0.001. N.Oc/Ct.Ar: genotype, P = 0.001; PTH, P = 0.001; genotype × PTH, P = 0.001. OV/BV: genotype P < 0.001; PTH, P < 0.001; genotype × PTH, P < 0.001.
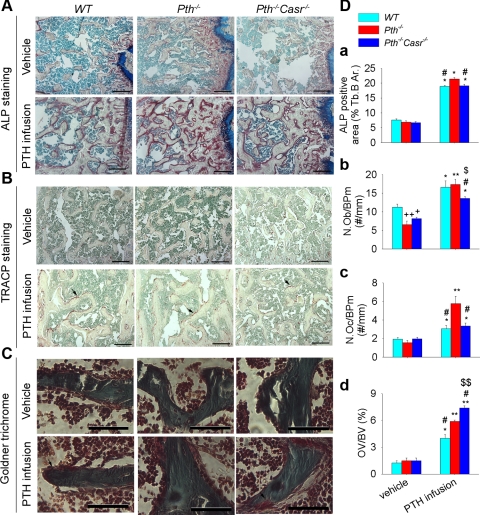
ALP-positive area and osteoblast numbers were also increased significantly by continuous PTH infusion in trabecular bone of WT and Pth−/− mice. Additionally, continuous PTH infusion increased ALP-positive area and osteoblast numbers in trabecular bone of Pth−/−-Casr−/− mice, but the increase in osteoblast numbers was significantly lower in Pth−/−-Casr−/− mice than in WT and Pth−/− mice [Fig. 3, A and D (a and b)]. Osteoclast numbers were also increased by continuous PTH infusion in trabecular bone in all three genotypes. However, the osteoclast numbers were significantly higher in Pth−/− mice than in WT and Pth−/−-Casr−/− mice after continuous PTH infusion [Fig. 3, B and D (c)]. In trabecular bone, PTH infusion also induced an increase in the osteoid volume in all three genotypes, which was most prominent in Pth−/−-Casr−/− mice [Fig. 3, C and D (d)].
Fig. 3.
Effects of continuous PTH infusion on histomorphometric parameters in trabecular bone. Representative photomicrograph sections stained histochemically for ALP activity (A), TRACP activity (B), and bone mineralization (C) by Goldner trichrome from 12-wk-old WT, Pth−/−, and Pth−/−Casr-−/− mice infused with hPTH(1–34) or vehicle for 2 wk. Histomorphometric analysis for ALP-positive area from histochemical staining (D, a), osteoblast number (N.Ob/BPm, cell no./mm bone perimeter) from H & E-stained sections (D, b), osteoclast number (N.Oc/BPm, cell no./mm bone perimeter) from TRACP staining (D, c), and bone mineralization from Goldner trichrome (D, d). Each value is the mean ± SE (n = 8/genotype). Symbols indicate significant changes using 1-way ANOVA, as described in Statistical analysis. *P < 0.05 and **P < 0.01 compared with vehicle-infused mice of the corresponding genotype. +P < 0.05 and ++P < 0.01 compared with vehicle-infused WT mice. #P < 0.05 compared with PTH-infused Pth−/− mice. $P < 0.05 and $$P < 0.01 compared with PTH-treated WT mice. The scale bars represent 500 (A and B) and 125 μm (C). Arrows in B point to osteoclast and osteoid. Results of 2-way ANOVA using genotype (Pth−/− and Pth−/−-Casr−/−) and PTH infusion treatment (vehicle and PTH infusion) as main effects: ALP: genotype, P = 0.001; PTH, P < 0.001; genotype × PTH, P = 0.003. N.Ob/BPm: genotype, P = 0.125; PTH, P = 0.001; genotype × PTH, P = 0.001. N.Oc/BPm: genotype, P = 0.01; PTH, P < 0.001; genotype × PTH, P = 0.001. OV/BV: genotype P < 0.001; PTH, P < 0.001; genotype × PTH, P < 0.001.
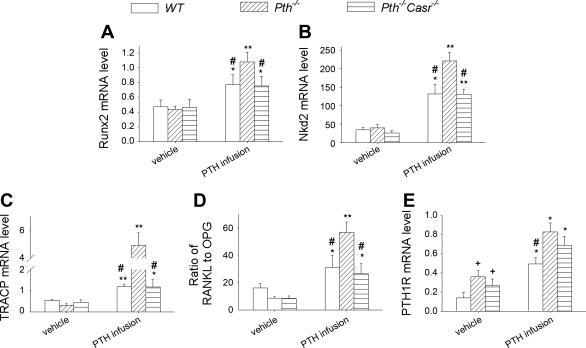
Expression of genes related to bone formation, i.e., Runx2 and the Wnt target gene Nkd2, and expression of genes related to bone resorption, i.e., TRACP, RANKL, and OPG, were examined ex vivo. The results showed that, after PTH infusion, the expression of Runx2 and Nkd2 was increased 1.5- and 3.8-fold, respectively, in both WT and Pth−/−-Casr−/− mice and 2.5- and 5.5-fold, respectively, in Pth−/− mice (Fig. 4, A and B). TRACP mRNA was increased 1.5-fold in both WT and Pth−/−-Casr-−/− mice and 10-fold in Pth−/− mice after PTH infusion (Fig. 4C). The ratio of RANKL to OPG mRNA levels also showed increases in all three genotypes after PTH infusion, but the ratio of RANKL to OPG increased two- to threefold in WT and Pth−/−-Casr −/− mice and sevenfold in Pth−/− mice (Fig. 4D).
Fig. 4.
Effects of continuous PTH infusion on gene expression in bone. Humeral mRNA was obtained from 12-wk-old WT, Pth−/−, and Pth−/−-Casr−/− mice that had been infused with 60 μg·kg−1·day−1 hPTH(1–34) or vehicle for 2 wk and was analyzed by real-time RT-PCR for runt-related transcription factor-2 (Runx2; A), naked cuticle 2 homolog (Nkd2; B), TRACP (C), the ratio of receptor activator of nuclear factor-κB ligand (RANKL) to osteoprotegerin (OPG; D), and type 1 PTH/PTH-related protein receptor (PTH1R; E), as described in materials and methods. Messenger RNA expression was calculated as a ratio relative to the GAPDH mRNA level. Each value is the mean ± SE (n = 8/genotype). Symbols indicate significant changes using 1-way ANOVA, as described in Statistical analysis. *P < 0.05 and **P < 0.01 compared with vehicle-infused mice of the corresponding genotype. +P < 0.05 compared with vehicle-infused WT mice. #P < 0.05 compared with PTH-infused Pth−/− mice. Results of 2-way ANOVA using genotype (Pth−/− and Pth−/−-Casr−/−) and PTH infusion treatment (vehicle and PTH infusion) as main effects: Runx2: genotype, P = 0.049; PTH, P < 0.001; genotype × PTH, P = 0.023. Nkd2: genotype, P < 0.001; PTH, P < 0.001; genotype × PTH, P < 0.001. TRACP: genotype, P < 0.001; PTH, P < 0.001; genotype × PTH, P < 0.001. RANKL/OPG: genotype P < 0.001; PTH, P < 0.001; genotype × PTH, P < 0.001. PTH1R: genotype, P = 0.059: PTH, P < 0.001; genotype × PTH, P = 0.70.
PTH1R gene expression was increased ∼2.5- and twofold in vehicle-treated Pth−/− and Pth−/−-Casr−/− mice, respectively, compared with vehicle-treated WT mice. After PTH infusion, PTH1R mRNA levels were further increased about two- to threefold (Fig. 4E).
μCT analyses and BMD.
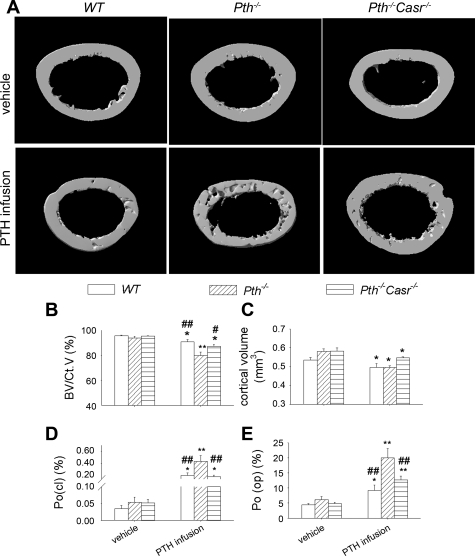
After continuous PTH infusion, μCT analysis of the cortical bone of the femur (Fig. 5A) revealed that cortical bone volume relative to cortical volume was decreased 5 and 8% in WT and Pth−/−-Casr−/− mice, respectively, but was decreased more dramatically (15%) in Pth−/− mice (Fig. 5B). Moreover, the absolute cortical volume was decreased 6 and 7% in WT and Pth−/−-Casr −/− mice, respectively, but was decreased more dramatically (14%) in Pth−/− mice (Fig. 5C). In cortical bone, continuous PTH infusion increased both closed porosity, reflecting intracortical resorption, and open porosity, reflecting endosteal resorption. The increase of porosity in cortical bone induced by PTH infusion was higher in Pth−/− than in WT and Pth−/−-Casr-−/− mice (closed porosity: Pth−/− vs. WT and Pth−/−-Casr−/− mice, 0.43 vs. 0.21 and 0.19%, respectively; open porosity: Pth−/− vs. WT and Pth−/−-Casr−/− mice, 20 vs. 9.23 and 12%, respectively; Fig. 5, D and E).
Fig. 5.
Analysis by microcomputed tomography (μCT) of effects of continuous PTH infusion on cortical bone parameters. A: representative μCT reconstructed cross-sections of femurs from 12-wk-old WT, Pth−/−, and Pth−/−-Casr−/− mice infused with 60 μg·kg−1·day−1 hPTH(1–34) or vehicle for 2 wk. Analysis by μCT of femur cortical bone showing bone volume/cortical volume (BV/CtV; B), cortical volume (C), closed porosity [Po(cl); D], and open porosity [Po(op); E]. Each value is the mean ± SE (n = 8/genotype). Symbols indicate significant changes using 1-way ANOVA, as described in Statistical analysis. *P < 0.05 and **P < 0.01 compared with vehicle-infused mice of the corresponding genotype. #P < 0.05 and ##P < 0.01 compared with PTH-infused Pth−/− mice. Results of 2-way ANOVA using genotype (Pth−/− and Pth−/−-Casr−/−) and PTH infusion treatment (vehicle and PTH infusion) as main effects: BV/CtV: genotype, P = 0.001; PTH, P < 0.001; genotype × PTH, P = 0.016. Cortical volume: genotype, P = 0.029; PTH, P < 0.001; genotype × PTH, P = 0.049. Po(cl): genotype, P = 0.003; PTH, P < 0.001; genotype × PTH, P = 0.003. Po(op): genotype P = 0.002; PTH, P < 0.001; genotype × PTH, P = 0.021.
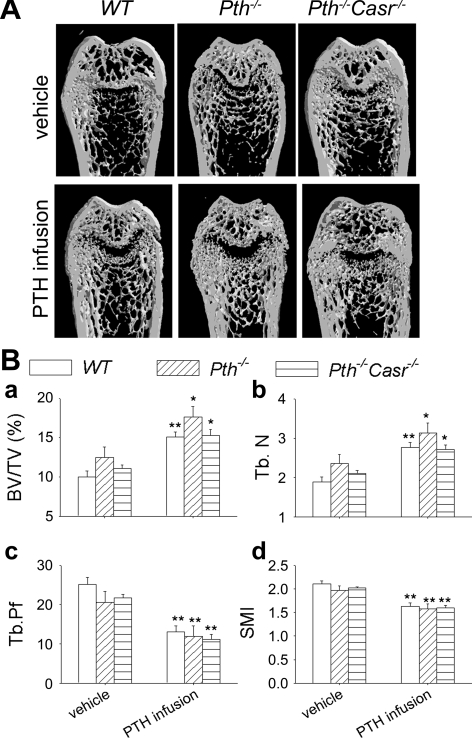
In trabecular bone of the distal femur, both trabecular bone volume relative to tissue volume (BV/TV; Fig. 6B, a) and Tb.N (Fig. 6B, b) were increased significantly in PTH-infused WT, Pth−/−, and Pth−/−-Casr−/− mice compared with vehicle-treated mice of the same genotype. Levels were slightly but not significantly lower in PTH-infused Pth−/−-Casr−/− mice than in PTH-infused Pth−/− mice. Both Tb.Pf, an inverse index of trabecular bone connectivity, and structure model index, an estimate of plate-rod characteristics, were decreased after PTH infusion (Fig. 6B, c and d, respectively), implying less disconnected trabecular bone and a more plate-like trabecular structure, respectively.
Fig. 6.
Analysis by μCT of the effects of continuous PTH infusion on the trabecular bone phenotype. A: representative longitudinal μCT reconstructed sections of femurs from 12-wk-old WT, Pth −/−, and Pth −/−-Casr −/− mice infused with 60 μg·kg−1·day−1 hPTH(1–34) or vehicle for 2 wk. B: μCT analysis of femoral trabecular bone showing bone volume/tissue volume (BV/TV; a), trabecular number (Tb.N; b), trabecular pattern factor (Tb.Pf; c), and structure model index (SMI; d) Each value is the mean ± SE (n = 8/genotype). Symbols indicate significant changes using 1-way ANOVA, as described in Statistical analysis. *P < 0.05 and **P < 0.01 compared with vehicle-infused mice of the corresponding genotype. Results of 2-way ANOVA using genotype (Pth−/− and Pth−/−-Casr−/−) and PTH infusion treatment (vehicle and PTH infusion) as main effects: BV/TV: genotype, P = 0.04; PTH, P < 0.001; genotype × PTH, P = 0.603. Tb.N: genotype, P = 0.051; PTH, P < 0.001; genotype × PTH, P = 0.615. Tb.Pf: genotype, P = 0.939; PTH, P < 0.001; genotype × PTH, P = 0.597. SMI: genotype P < 0.572; PTH, P < 0.001; genotype × PTH, P = 0.82.
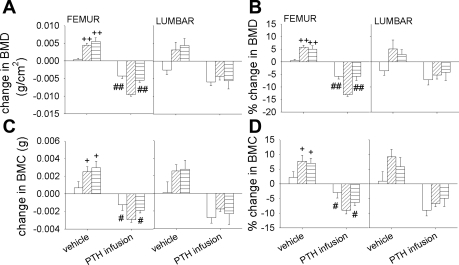
In the vehicle-infused control mice, increases in both absolute and relative femoral BMD and BMC changes were observed in Pth−/− and Pth−/−-Casr−/− mice after 2 wk. Continuous PTH infusion decreased femoral BMD and BMC significantly in all three groups; however, the decrease in both absolute and relative femoral BMD and BMC was 2.5-fold higher in Pth−/− mice than in WT and Pth−/−-Casr-−/− mice. In the lumbar spine, absolute and relative BMD and BMC changes were also increased in vehicle-infused Pth−/− and Pth−/−-Casr−/− mice, although absolute and relative BMD changes were decreased in vehicle-infused WT mice. The decreases in both absolute and relative BMD and BMC changes in WT, Pth−/−, and Pth−/−-Casr−/− mice observed after PTH infusion were not significantly different from each other (Fig. 7).
Fig. 7.
Effects of continuous PTH infusion on bone mineral density (BMD) and bone mineral content (BMC). Femoral and lumbar spine BMD were determined using dual-energy X-ray absorptiometry in WT, Pth −/−, and Pth−/−-Casr −/− mice before (in 10-wk-old mice) and after (at 12 wk of age) infusion of 60 μg·kg−1·day−1 hPTH(1–34) or vehicle. Bars represent means ± SE. Both change and %change in BMD (A and B) and BMC (C and D) represent the difference between between 2 and 0 wk after PTH or vehicle infusion (n = 8/genotype). Symbols indicate significant changes using 1-way ANOVA, as described in Statistical analysis. +P < 0.05 and ++P < 0.01 compared with vehicle-infused WT mice. ##P < 0.01 and #P < 0.05 compared with PTH-infused Pth−/− mice. Results of 2-way ANOVA using genotype (Pth−/− and Pth−/−-Casr−/−) and PTH infusion treatment (vehicle and PTH infusion) as main effects: Femur BMD change: genotype, P = 0.001; PTH, P < 0.001; genotype × PTH, P = 0.028. (%change: genotype, P < 0.019; PTH, P < 0.001; genotype × PTH, P = 0.002). Femur BMC change: genotype, P = 0.042; PTH, P < 0.001; genotype × PTH, P = 0.032 (%change: genotype, P = 0.059; P < 0.001; genotype × PTH, P = 0.014). Lumbar BMD changes: genotype, P = 0.73; PTH, P < 0.001; genotype × PTH, P = 0.650; (%change: genotype × P = 0.825, PTH, P = 0.001; genotype × PTH, P = 0.505). Lumbar BMC change: genotype, P = 0.878; PTH, P < 0.001; genotype × PTH, P = 0.691 (%change: genotype, P = 0.686; PTH, P < 0.001; genotype × PTH, P = 0.0.273).
Interaction of PTH and CaSR in vitro.
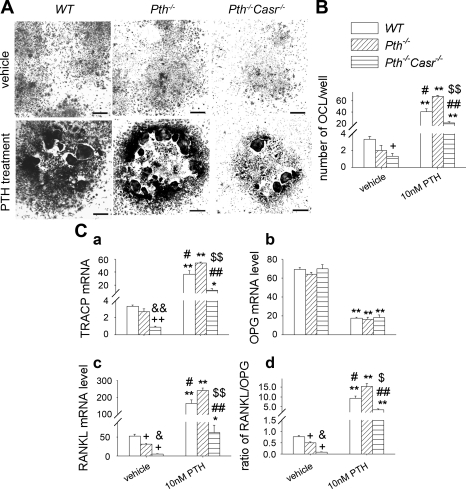
To examine the interaction of PTH effects and CaSR effects in vitro, we assessed the efficacy of PTH on osteoclastogenesis in bone marrow cultures in the presence or absence of CaSR. Bone marrow cells were isolated from WT, Pth−/−, and Pth−/−-Casr−/− mice and treated with vehicle or 10 nM hPTH(1–34) for 7 days. The results showed that PTH increased osteoclast-like cell number and TRACP mRNA levels 10–15% in bone marrow cell cultures from WT and Pth−/−-Casr−/− mice and >30-fold in bone marrow cell cultures from Pth−/− mice [Figs. 8, A, B, and C (a)]. PTH decreased OPG mRNA levels by ∼75% in all three genotypes (Fig. 8C, b), and RANKL mRNA was increased significantly by PTH treatment (Fig. 8C, c). RANKL mRNA was 1.5- and fourfold higher in PTH-treated bone marrow cell cultures from Pth−/− mice than from WT and Pth−/−-Casr−/− mice, respectively (Fig. 8C, c). The ratio of RANKL to OPG mRNA was 1.7- and 4.7-fold higher in bone marrow cell cultures from Pth−/− mice than in those from WT and Pth−/−-Casr−/− mice, respectively, after PTH treatment (Fig. 8C, d).
Fig. 8.
Role of CaSR in PTH-stimulated osteoclast differentiation. A: photomicrograph of TRACP-stained cells in bone marrow cultures from 8-wk-old WT, Pth−/−, and Pth−/−-Casr−/− mice treated with 10 nM hPTH(1–34) or vehicle for 7 days. B: no. of osteoclast-like cells (OCL) in each well. C: levels from WT, Pth−/−, and Pth−/−-Casr−/− mice of mRNA encoding TRACP (a), OPG (b), and RANKL (c) and of the ratio of mRNA of RANKL/OPG in bone marrow cultures treated in vitro with 10 nM hPTH(1–34) or vehicle (d). Values are means ± SE for 3 determinations/group. Symbols indicate significant changes, using 1-way ANOVA as described in Statistical analysis. *P < 0.05 and **P < 0.01 compared with vehicle-treated bone marrow cell cultures from mice of the corresponding genotype. +P < 0.05 compared with vehicle-treated bone marrow cell cultures from WT mice. #P < 0.05 and ##P < 0.01 compared with PTH-treated bone marrow cell cultures from Pth−/− mice. $P < 0.05 and $$P < 0.01 compared with PTH-treated bone marrow cell cultures from WT mice. &P < 0.05 and &&P < 0.01 compared with vehicle-treated bone marrow cell cultures from Pth−/− mice. Scale bars, 1 mm. Results of 2-way ANOVA using genotype (Pth−/− and Pth−/−-Casr−/−) and PTH infusion treatment (vehicle and PTH infusion) as main effects: no of OCL: genotype, P < 0.001; PTH, P < 0.001; genotype × PTH, P < 0.001. TRACP: genotype, P = 0.0259; PTH, P = 0.003; genotype × PTH, P < 0.001. OPG: genotype, P = 0.47; PTH, P < 0.001; genotype × PTH, P = 0.066. RANKL: genotype P = 0.042; PTH, P < 0.015; genotype × PTH, P = 0.001. RANKL/OPG: genotype, P < 0.001: PTH, P < 0.001; genotype × PTH, P < 0.001.
DISCUSSION
Continuous PTH administration has been shown to produce a primarily catabolic effect on bone and, in this way, to mimic the effects of excess circulating PTH in primary hyperparathyroidism. The predominant catabolic action of PTH has been reported to occur in cortical bone. In view of the fact that the majority of skeletal calcium resides in cortical bone, this is consistent with the role of chronically elevated PTH to mobilize calcium from bone to maintain calcium homeostasis. Our studies show that continuous PTH administered to wild-type and hypoparathyroid animals produced increased turnover in cortical bone, as demonstrated by histomorphometry. This increased turnover resulted in increased cortical porosity and loss of cortical bone volume, as demonstrated by microcomputed tomography. Inasmuch as femoral bone is comprised to a major extent of cortical bone, this was reflected in a decrease in femoral bone mineral content and density. This loss of cortical bone undoubtedly contributed to the increased serum calcium concentration observed at the end of the PTH infusion. The magnitude of the effects of continuous PTH administration on cortical bone turnover, increased cortical porosity, and loss of cortical bone volume in Pth−/− mice appeared to exceed the magnitude of the corresponding responses to continuous PTH infusion in the wild-type littermates. In previous studies with short-term infusions of PTH in humans, enhanced acute renal responses, including increases in nephrogenous cyclic AMP and phosphaturia, have been reported in hypoparathyroid vs. normal individuals (22). However, systematic study of skeletal responses to PTH infusions in hypoparathyroid vs. normal humans or animals has not been reported previously. Our data showing that infusion of the same quantities of PTH(1–34) in all genotypes produced very similar steady-state levels of serum PTH(1–34) strongly suggests that altered clearance of PTH would not account for differences in responsiveness of bone to PTH in the different genotypes. We did not measure endogenous PTH in WT mice, which may be a limitation in assessing total PTH levels in our studies. Nevertheless, others have found that endogenous PTH is significantly but not totally suppressed by raising serum calcium in WT mice with a protocol similar to the one we used (17). If total endogenous plus exogenous PTH was higher in WT mice than in the hypoparathyroid models, then this would further emphasize the increased sensitivity of the Pth−/− mice to PTH administration. Our data showed that PTH1R expression was increased in the hypoparathyroid mice, which may have contributed to increased skeletal sensitivity to PTH following chronic deficiency, although this does not exclude other receptor or postreceptor events that may contribute to this increased sensitivity. Furthermore, consistent with previous reports (24, 32), PTH1R expression was increased further after PTH infusion and was more markedly elevated in Pth−/− mice, possibly reflecting this increased sensitivity in the hypoparathyroid state. Increases in RANKL expression ex vivo and in vitro in Pth−/− relative to WT mice after PTH infusion are also in keeping with altered molecular events that may be a manifestation of increased skeletal sensitivity.
Although the major consequence of continuous PTH infusion (and of primary hyperparathyroidism), at least in adult bone, is enhanced resorption of cortical bone, several studies have also demonstrated a modest increase in trabecular bone (33, 44, 54). Furthermore, osteoblastic expression of a constitutively active PTH1R induced a substantial increase in trabecular bone volume in addition to a decrease in cortical bone thickness (4). This trabecular bone increase is most consistent with the fetal and neonatal skeletal functions of PTH. Thus, under conditions where mechanisms for maintenance of calcium homeostasis are very different than in the adult animal, i.e., in the fetus and suckling neonate, PTH appears to be primarily anabolic and is required for normal bone formation. Indeed, it is this function that is generally mimicked by intermittent PTH administration. In our current studies, increased turnover in trabecular bone along with increases in bone volume and trabecular number were observed, consistent with the anabolic effect of PTH. The absence of a significant decrease in lumbar spine BMD in Pth−/− mice after PTH infusion that was observed in femur may reflect the relatively large trabecular compared with cortical compartment in bones of the axial skeleton. Therefore, these observations reinforce the concept that PTH may simultaneously exert distinct effects on distinct skeletal compartments. In our studies, PTH also increased osteoid in the infused mice, and this might have been caused by the reduction in serum phosphorous induced by abnormally high levels of PTH (53). Although the osteomalacia could have contributed to the reduction in BMD observed in these animals, BMD reductions in the femur were greater in Pth−/− mice than in Pth−/−-Casr−/− mice despite the fact that osteomalacia appeared to be more pronounced in the latter model. Consequently, increased bone resorption likely contributed very significantly to the reduced BMD in these models. Furthermore, it seems likely that the increased intracortical porosity that occurred within mineralized cortical bone represented excess resorption rather than reduced mineralization. Increased bone resorption is also supported by the increased bone resorption noted histomorphometrically and in vitro and by the hypercalcemia that ensued after PTH infusion in vivo.
CaSR has been associated primarily with maintenance of calcium homeostasis, as has PTH. However, increasing evidence points to the role of important skeletal functions of this receptor. In our studies, genetic deletion of CaSR reduced PTH-stimulated bone turnover in cortical bone, impeded PTH-induced reductions of cortical bone volume, and reduced PTH-induced increases in cortical porosity. PTH-induced reductions in femoral BMD were thus mitigated. Ex vivo and in vitro studies demonstrated reductions of PTH-stimulated RANKL expression, and in vitro studies demonstrated reductions in PTH-stimulated osteoclastogenesis. Whether the in vitro decrease in RANKL is due to decreased numbers of bone marrow stromal cells or to decreased production of RANKL by such cells remains to be determined. In view of the fact that CaSR is expressed in many calciotropic organs outside the skeleton, including parathyroid glands, kidneys, and the gastrointestinal tract, deficiency in CaSR signaling and PTH responses in these tissues could have had significant impact on the skeletal phenotypes seen in the CaSR-deficient mice. However, our in vitro studies demonstrating reductions of PTH-stimulated RANKL expression and reductions in PTH-stimulated osteoclastogenesis were performed after 7 days in culture and are consistent with a cell-autonomous role for skeletal CaSR in modulating PTH-induced bone resorption. Furthermore, although CaSR deficiency in our model appropriately alters parathyroid and renal function, it has been reported that an exon 5-truncated, spliced-variant CaSR variant in our model could be partly functional in lung development (12), in epidermal keratinocytes (31), and in growth plate chondrocytes (39). Our studies suggest that if a similar splice variant is partly functional in the CaSR in bone, complete elimination of this variant would produce an even more severe phenotype, i.e., more pronounced reduction in PTH-stimulated bone resorption.
Osteoid volume induced by PTH infusion was greater in Pth−/−-Casr−/− mice than in WT and Pth−/− mice, which might suggest a role for CaSR in regulating bone mineralization, and indeed, mice homozygous for targeted deletion of the CaSR gene manifest osteomalacia (5, 15, 45). Nevertheless, elimination of the hyperparathyroidism in Casr−/− mice has been reported to normalize the skeletal phenotype (47). Consequently, the precise role of CaSR in mineralization of bone remains to be clarified.
Our studies are in keeping with previous studies in CaSR-depleted adult mice and in studies using an allosteric activator of CaSR in adult mice, which indicated that CaSR could induce bone resorption. In view of the fact that [Ca2+]e is a natural ligand for CaSR, [Ca2+]e that is increased by PTH via enhanced renal reabsorption of calcium and via 1,25(OH)2D-induced gut absorption of calcium might amplify PTH-induced cortical bone resorption. Thus, the degree of cortical bone resorption (a primary determinant of long bone fractures) in primary hyperparathyroidism may be modulated by the level of serum calcium per se acting via CaSR. This would be consistent with clinical reports that suggest that, when compared with primary hyperparathyroidism, bone loss and fractures may be less prominent in familial hypocalciuric hypercalcemia, a disease associated with increased circulating PTH and inactivating mutations of CaSR (1, 7, 23). They may also bear on the efficacy of exogenous PTH as an anabolic agent in osteoporosis since the relative anabolic efficacy of intermittent exogenous PTH may be tempered by the degree and/or duration of hypercalcemia that ensues after administration. Finally, our studies may also be consistent with the clinical reports that suggest that calcium supplementation may predispose elderly individuals to hip fractures (8, 35, 46).
In addition to its role in facilitating transplacental entry of calcium in fetal animals (21), previous genetic studies have also suggested a direct requirement for CaSR in normal fetal and neonatal skeletal development (5). Previously, we reported a skeletal anabolic effect for CaSR in neonates in association with daily exogenous PTH administration (43). These studies suggest a skeletal anabolic role for CaSR in the fetus and neonate independent of its role of maintaining calcium homeostasis in adult animals. In the present studies, we found that genetic deletion of CaSR reduced PTH-stimulated bone turnover in trabecular bone and tended to impede PTH-induced increases in trabecular bone. The absence of an effect of CaSR deletion on PTH-induced changes in spine BMD may reflect the fact that lumbar spine BMD comprises the composite actions of PTH and CaSR on a relatively large trabecular compartment but nevertheless a substantial cortical compartment. Increases in expression of Runx2 and of Nkd2, a marker of Wnt activity, were lower in Pth−/−-Casr−/− mice than in Pth−/− mice. Consequently, PTH-induced anabolic effects in trabecular bone were modestly impeded in CaSR-deficient mice. Therefore, these results appear to be consistent with a role for CaSR in modulating PTH-induced bone formation, although additional studies in other models examining primarily the anabolic effects of PTH will be required.
GRANTS
This work was supported by operating grants to D. Goltzman and A. C. Karaplis from the Canadian Institutes of Health Research.
DISCLOSURES
All authors state that they have no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
Y. Xue and D.G. did the conception and design of the research; Y. Xue, Y. Xiao, J.L., and D.G. performed the experiments; Y. Xue, Y. Xiao, and D.G. analyzed the data; Y. Xue, Y. Xiao, A.C.K., M.R.P., E.M.B., D.M., and D.G. interpreted the results of the experiments; Y. Xue and D.G. prepared the figures; Y. Xue and D.G. drafted the manuscript; Y. Xue, Y. Xiao, A.C.K., M.R.P., E.M.B., D.M., and D.G. edited and revised the manuscript; Y. Xue, Y. Xiao, J.L., A.C.K., M.R.P., E.M.B., D.M., and D.G. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Jun-li Liu and Xiaoquan Xiong (McGill University) for collection of histological images.
REFERENCES
- 1.Abugassa S, Nordenstrom J, Jarhult J. Bone mineral density in patients with familial hypocalciuric hypercalcaemia (FHH). Eur J Surg 158: 397–402, 1992 [PubMed] [Google Scholar]
- 2.Amantea CM, Kim WK, Meliton V, Tetradis S, Parhami F. Oxysterol-induced osteogenic differentiation of marrow stromal cells is regulated by Dkk-1 inhibitable and PI3-kinase mediated signaling. J Cell Biochem 105: 424–436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest 107: 277–286, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal 1: ra1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang W, Tu C, Chen TH, Komuves L, Oda Y, Pratt SA, Miller S, Shoback D. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 140: 5883–5893, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Christensen SE, Nissen PH, Vestergaard P, Heickendorff L, Rejnmark L, Brixen K, Mosekilde L. Skeletal consequences of familial hypocalciuric hypercalcaemia vs. primary hyperparathyroidism. Clin Endocrinol (Oxf) 71: 798–807, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Cumming RG, Nevitt MC. Calcium for prevention of osteoporotic fractures in postmenopausal women. J Bone Miner Res 12: 1321–1329, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev 14: 690–709, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Dvorak MM, Chen TH, Orwoll B, Garvey C, Chang W, Bikle DD, Shoback DM. Constitutive activity of the osteoblast Ca2+-sensing receptor promotes loss of cancellous bone. Endocrinology 148: 3156–3163, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Finch JL, Tokumoto M, Nakamura H, Yao W, Shahnazari M, Lane N, Slatopolsky E. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol 298: F1315–F1322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finney B, Wilkinson WJ, Searchfield L, Cole M, Bailey S, Kemp PJ, Riccardi D. An exon 5-less splice variant of the extracellular calcium-sensing receptor rescues absence of the full-length receptor in the developing mouse lung. Exp Lung Res 37: 269–278, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Wu X, Terauchi M, Li JY, Grassi F, Galley S, Yang X, Weitzmann MN, Pacifici R. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab 8: 132–145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goltzman D. Emerging roles for calcium-regulating hormones beyond osteolysis. Trends Endocrinol Metab 21: 512–518, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, Brown EM, Seidman JG, Seidman CE. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet 11: 389–394, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Hurley MM, Lee SK, Raisz LG, Bernecker P, Lorenzo J. Basic fibroblast growth factor induces osteoclast formation in murine bone marrow cultures. Bone 22: 309–316, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Iida-Klein A, Lu SS, Kapadia R, Burkhart M, Moreno A, Dempster DW, Lindsay R. Short-term continuous infusion of human parathyroid hormone 1–34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol 186: 549–557, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Ju JH, Cho ML, Moon YM, Oh HJ, Park JS, Jhun JY, Min SY, Cho YG, Park KS, Yoon CH, Min JK, Park SH, Sung YC, Kim HY. IL-23 induces receptor activator of NF-kappaB ligand expression on CD4+ T cells and promotes osteoclastogenesis in an autoimmune arthritis model. J Immunol 181: 1507–1518, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kantham L, Quinn SJ, Egbuna OI, Baxi K, Butters R, Pang JL, Pollak MR, Goltzman D, Brown EM. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab 297: E915–E923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, Guise TA, Pollak MR. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest 111: 1021–1028, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs CS, Ho-Pao CL, Hunzelman JL, Lanske B, Fox J, Seidman JG, Seidman CE, Kronenberg HM. Regulation of murine fetal-placental calcium metabolism by the calcium-sensing receptor. J Clin Invest 101: 2812–2820, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law WM, Jr, Heath H., 3rd Increased renal responses to exogenous parathyroid hormone in postsurgical hypoparathyroidism. J Clin Endocrinol Metab 59: 394–397, 1984 [DOI] [PubMed] [Google Scholar]
- 23.Law WM, Jr, Wahner HW, Heath H., 3rd Bone mineral density and skeletal fractures in familial benign hypercalcemia (hypocalciuric hypercalcemia). Mayo Clin Proc 59: 811–815, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Li X, Liu H, Qin L, Tamasi J, Bergenstock M, Shapses S, Feyen JH, Notterman DA, Partridge NC. Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem 282: 33086–33097, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lotinun S, Evans GL, Bronk JT, Bolander ME, Wronski TJ, Ritman EL, Turner RT. Continuous parathyroid hormone induces cortical porosity in the rat: effects on bone turnover and mechanical properties. J Bone Miner Res 19: 1165–1171, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ma YL, Cain RL, Halladay DL, Yang X, Zeng Q, Miles RR, Chandrasekhar S, Martin TJ, Onyia JE. Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology 142: 4047–4054, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest 109: 1173–1182, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SS, Bracey JW, Hogue WR, Wong DH, Ritchie RO, Suva LJ, Derynck R, Guise TA, Alliston T. Pharmacologic inhibition of the TGF-beta type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One 4: e5275, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434–1441, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Nguyen-Yamamoto L, Bolivar I, Strugnell SA, Goltzman D. Comparison of active vitamin D compounds and a calcimimetic in mineral homeostasis. J Am Soc Nephrol 21: 1713–1723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oda Y, Tu CL, Chang W, Crumrine D, Komuves L, Mauro T, Elias PM, Bikle DD. The calcium sensing receptor and its alternatively spliced form in murine epidermal differentiation. J Biol Chem 275: 1183–1190, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Onyia JE, Helvering LM, Gelbert L, Wei T, Huang S, Chen P, Dow ER, Maran A, Zhang M, Lotinun S, Lin X, Halladay DL, Miles RR, Kulkarni NH, Ambrose EM, Ma YL, Frolik CA, Sato M, Bryant HU, Turner RT. Molecular profile of catabolic versus anabolic treatment regimens of parathyroid hormone (PTH) in rat bone: an analysis by DNA microarray. J Cell Biochem 95: 403–418, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Parisien M, Silverberg SJ, Shane E, de la Cruz L, Lindsay R, Bilezikian JP, Dempster DW. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab 70: 930–938, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab 15: 60–65, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Reid IR, Bolland MJ, Grey A. Effect of calcium supplementation on hip fractures. Osteoporos Int 19: 1119–1123, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, Olivos N, Passeri G, O'Brien CA, Bivi N, Plotkin LI, Bellido T. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J Bone Miner Res 26: 1035–1046, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richard C, Huo R, Samadfam R, Bolivar I, Miao D, Brown EM, Hendy GN, Goltzman D. The calcium-sensing receptor and 25-hydroxyvitamin d-1alpha-hydroxylase interact to modulate skeletal growth and bone turnover. J Bone Miner Res 25: 1627–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Robling AG, Kedlaya R, Ellis SN, Childress PJ, Bidwell JP, Bellido T, Turner CH. Anabolic and catabolic regimens of human parathyroid hormone 1–34 elicit bone- and envelope-specific attenuation of skeletal effects in Sost-deficient mice. Endocrinology 152: 2963–2975, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez L, Tu C, Cheng Z, Chen TH, Bikle D, Shoback D, Chang W. Expression and functional assessment of an alternatively spliced extracellular Ca2+-sensing receptor in growth plate chondrocytes. Endocrinology 146: 5294–5303, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Rouleau MF, Mitchell J, Goltzman D. Characterization of the major parathyroid hormone target cell in the endosteal metaphysis of rat long bones. J Bone Miner Res 5: 1043–1053, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145: 401–406, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samadfam R, Xia Q, Miao D, Hendy GN, Goltzman D. Exogenous PTH and endogenous 1,25-dihydroxyvitamin D are complementary in inducing an anabolic effect on bone. J Bone Miner Res 23: 1257–1266, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Shu L, Ji J, Zhu Q, Cao G, Karaplis A, Pollak MR, Brown E, Goltzman D, Miao D. The calcium sensing receptor mediates bone turnover induced by dietary calcium and parathyroid hormone in neonates. J Bone Miner Res 26: 1057–1071, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV, Lindsay R, Clemens TL, Bilezikian JP. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 4: 283–291, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Sun W, Liu J, Zhou X, Xiao Y, Karaplis A, Pollak MR, Brown E, Goltzman D, Miao D. Alterations in phosphorus, calcium and PTHrP contribute to defects in dental and dental alveolar bone formation in calcium-sensing receptor-deficient mice. Development 137: 985–992, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang BM. Does calcium supplementation really cause more hip fractures? Osteoporos Int 20: 833–834, author reply 835–836, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest 111: 1029–1037, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1–34) on rat bone. Bone 16: 477–484, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Weir EC, Lowik CW, Paliwal I, Insogna KL. Colony stimulating factor-1 plays a role in osteoclast formation and function in bone resorption induced by parathyroid hormone and parathyroid hormone-related protein. J Bone Miner Res 11: 1474–1481, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology 136: 1317–1327, e1311–e1312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xue Y, Karaplis AC, Hendy GN, Goltzman D, Miao D. Genetic models show that parathyroid hormone and 1,25-dihydroxyvitamin D3 play distinct and synergistic roles in postnatal mineral ion homeostasis and skeletal development. Hum Mol Genet 14: 1515–1528, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Xue Y, Zhang Z, Karaplis AC, Hendy GN, Goltzman D, Miao D. Exogenous PTH-related protein and PTH improve mineral and skeletal status in 25-hydroxyvitamin d-1alpha-hydroxylase and PTH double knockout mice. J Bone Miner Res 20: 1766–1777, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C, Xie Y, Gao T, Drezner MK, Bonewald LF, Feng JQ. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res 26: 1047–1056, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou H, Shen V, Dempster DW, Lindsay R. Continuous parathyroid hormone and estrogen administration increases vertebral cancellous bone volume and cortical width in the estrogen-deficient rat. J Bone Miner Res 16: 1300–1307, 2001 [DOI] [PubMed] [Google Scholar]