Abstract
Phenylketonuria (PKU) is caused by a mutation in the phenylalanine (phe) hydroxylase gene and requires a low-phe diet plus amino acid (AA) formula to prevent cognitive impairment. Glycomacropeptide (GMP) contains minimal phe and provides a palatable alternative to AA formula. Our objective was to compare growth, body composition, and energy balance in Pahenu2 (PKU) and wild-type mice fed low-phe GMP, low-phe AA, or high-phe casein diets from 3–23 wk of age. The 2 × 2 × 3 design included main effects of genotype, sex, and diet. Fat and lean mass were assessed by dual-energy X-ray absorptiometry, and acute energy balance was assessed by indirect calorimetry. PKU mice showed growth and lean mass similar to wild-type littermates fed the GMP or AA diets; however, they exhibited a 3–15% increase in energy expenditure, as reflected in oxygen consumption, and a 3–30% increase in food intake. The GMP diet significantly reduced energy expenditure, food intake, and plasma phe concentration in PKU mice compared with the casein diet. The high-phe casein diet or the low-phe AA diet induced metabolic stress in PKU mice, as reflected in increased energy expenditure and intake of food and water, increased renal and spleen mass, and elevated plasma cytokine concentrations consistent with systemic inflammation. The low-phe GMP diet significantly attenuated these adverse effects. Moreover, total fat mass, %body fat, and the respiratory exchange ratio (CO2 produced/O2 consumed) were significantly lower in PKU mice fed GMP compared with AA diets. In summary, GMP provides a physiological source of low-phe dietary protein that promotes growth and attenuates the metabolic stress induced by a high-phe casein or low-phe AA diet in PKU mice.
Keywords: indirect calorimetry, energy balance, splenomegaly, cytokines, amino acid metabolism
phenylketonuria (pku) is an autosomal recessive inborn error of metabolism caused by a deficiency of hepatic phenylalanine hydroxylase (PAH; EC 1.14.16.1), resulting in hyperphenylalaninemia due to an inability to convert phenylalanine (phe) to tyrosine (14). The primary therapy for PKU is life-long adherence to a diet that limits phe intake to the minimum amount needed to support growth and protein turnover (40). The low-phe PKU diet requires extensive restriction of foods containing natural protein (often only 5–10 g protein per day are allowed) and supplementation with amino acids (AA), usually in the form of a formula, to achieve adequate intake of nitrogen, indispensable AA, and micronutrients (31). Compliance with the low-phe diet is often poor after 10 yr of age, despite the detrimental neuropsychological consequences of hyperphenylalanemia (23, 57).
Glycomacropeptide (GMP), the only known naturally occurring dietary protein that, in its pure form, contains no phe (25), provides a new nutritional paradigm for PKU. Our studies in the murine model of PAH deficiency, the Pahenu2 mouse (PKU mouse) (32, 39), which mimics the phenotype of human PKU, including elevated phe concentrations and cognitive defects, indicate that a GMP diet reduces phe concentrations in plasma and brain compared with an AA diet (39). Our studies in humans with PKU (30, 38, 54) indicate that GMP improves protein retention, phe concentrations, and satiety and palatability of the low-phe diet. However, questions remain about the ability of GMP to support long-term growth, physiological function, and energy balance in those with PKU, especially given the rising incidence of overweight in children and adults with PKU treated with conventional AA diets (3, 33).
GMP is a 64-AA glycophosphopeptide, corresponding to AA 106–169 of κ-casein, which is released in the human stomach after ingestion of bovine milk or yogurt (11). For commercial use, GMP is obtained from cheese making when κ-casein is cleaved by rennet chymosin into para-κ-casein, which remains with the cheese curd, and GMP, which remains with the whey (8, 17). GMP, the third most abundant protein in cheese whey, is highly polar, with an isoelectric point below 3.8, and is glycosylated by galactosamine, galactose, and ο-sialic acid at threonine sites (25). Moreover, GMP has a unique AA profile, including an absence of aromatic AA, phe, tryptophan, and tyrosine, as well as arginine, cysteine, and histidine, and concentrations of isoleucine and threonine that are two- to threefold greater, respectively, than those found in other dietary proteins (17). GMP supplemented with limiting AA provides a low-phe source of dietary protein that is an acceptable alternative to AA formula and can be made into an array of foods and beverages to enhance variety in the low-phe diet required for those with PKU (28, 54).
In addition to its unique application to the nutritional management of PKU, GMP demonstrates a number of interesting biological activities, as summarized in current reviews (8, 24). In vitro studies indicate that GMP binds cholera and E. coli enterotoxins and inhibits bacterial and viral adhesions (36), inhibits adhesion of cariogenic bacteria with usage in toothpaste (2), and modulates immune response (27, 47). In vivo studies indicate that oral administration of GMP shows substantial anti-inflammatory effects in rat models of colitis and ileitis (29, 46), increases zinc absorption in rhesus monkeys (22), and may promote satiety in humans (9, 26, 30, 56). The mechanisms underlying these observations of GMP action are largely unknown, although thought to be linked with the unique chemical structure of GMP.
Given the potential of dietary GMP as an acceptable alternative to AA formula in the nutritional management of PKU, it is important to examine the metabolic and physiological effects of GMP relative to an AA-based diet. Moreover, characterization of the metabolic phenotype of the PKU mouse will facilitate the design and interpretation of PKU research using this important and well-accepted genetic mouse model. Thus our objective was to compare growth, body composition, and energy balance in weanling PKU and wild-type (WT) mice fed diets containing GMP, AA, or casein from weaning through young adulthood. Systemic inflammation as a contributor to metabolic stress was explored by assessing spleen mass and histology and plasma cytokine concentrations. We observed a significant increase in energy expenditure in PKU mice, and attenuation of parameters reflecting metabolic stress in PKU mice fed a low-phe diet containing GMP compared with a high-phe casein diet or a low-phe AA diet.
MATERIALS AND METHODS
Animals and experimental design.
The animal facilities and protocols reported were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee. A PKU mouse colony was established using the murine model of PAH deficiency, the Pahenu mouse, on a C57Bl/6 background (20, 32). PKU heterozygous mice were bred to produce PKU homozygous mice and WT control mice. Genotyping for the presence of the Pahenu2 mutation was performed by PCR analysis of toe biopsy DNA on an amplified region of exon 7 of the PAH gene at 7–10 days of age (TaqMan Gene Expression assay ID AHAAB1X, Applied Biosystems, Foster City, CA). The experimental design included 12 treatment groups in a 2 × 2 × 3 factorial design, which included 3 main effects and interactions, genotype, PKU homozygous, or WT; sex, male or female; and 3 semipurified diets, GMP, AA, and casein control. Mice were randomized to the three diets by stratified randomization at weaning (21 days), separated by sex, and housed within their litters and dietary treatment groups in shoe-box cages in a room maintained at 22°C on a 12:12-h light-dark cycle with free access to water. The mice were weighed between 0900–1100 five times per week from 21 to 35 days of age, and then three times per week through the end of the study.
Mice were fed the experimental diets from weaning through young adulthood for a mean of 20.4 ± 0.11 wk (range 17–23 wk, n = 180 mice). Fat mass and lean mass of each mouse were assessed by dual-energy X-ray absorptiometry (DXA) utilizing PIXImus (GE/Lunar Corp, Madison, WI). Principally, this quantification is possible by comparing the differential attenuation of two X-ray beams of different intensities through tissue (44). The scans were analyzed by a single individual blinded to the treatment groups with PIXImus software version 2.10, as previously reported, and shown to be strongly correlated with proximate biochemical analysis of soft tissue composition (12, 43). Following anesthesia with isoflurane via an anesthesia machine (IsoFlo, Abbott Laboratories, North Chicago, IL), mice were placed prone on the scanner bed with the limbs and tail stretched away from the body. One scan per mouse, requiring 4 min, was performed at four different time points, at 6–9, 10–13, 14–17, and 18–21 wk of age. The analysis of each scan excluded the head and provided a serial assessment of lean and fat tissue masses for each mouse throughout the growth cycle.
After consuming the experimental diets for 20 wk, mice were anesthetized using isoflurane via an anesthesia machine and euthanized by exsanguination between 0800 and 1000 in a fed state. Blood was collected by cardiac puncture into syringes containing a final concentration of 2.7 mmol/l EDTA, and plasma was isolated by centrifugation at 4°C. The profile of AA in plasma was determined in the Wisconsin State Laboratory of Hygiene using a Hitachi L-8900 AA analyzer equipped with an ion chromatography system using postcolumn ninhydrin derivatization (52). The samples were deproteinized with sulfosalicylic acid, centrifuged, and passed through a 0.2-μm filter before addition of an internal standard and injection into the column.
Plasma cytokines were quantified according to manufacturer specifications using the Bio-Plex Pro Mouse Cytokine Assay and read using the Bio-Rad Bio-Plex 200 system using flow cytometry to count fluorescently coded beads conjugated to antibodies (Bio-Rad, Hercules, CA). Liver, kidney, and spleen were dissected and weighed. Spleen samples (n = 22) were fixed in 10% formalin, paraffin embedded, cut into sections, and stained with hematoxylin and eosin for histological inspection at ×10 magnification.
Diets.
The three experimental diets were formulated to provide equivalent amounts of vitamins, minerals, macronutrients (18% kcal protein, 64–66% kcal carbohydrate, and 16–17% kcal fat), and energy (3.8 kcal/g metabolizable energy) and differed only in the source of protein (Harlan Teklad, Madison, WI; TD.09667-TD.09669). The composition of the diets is provided in Table 1. The sole source of protein in the diets was provided by 20% (wt/wt) casein plus 0.3% l-cystine, 17.5% free AA (48), or 20% GMP (BioPURE GMP, Davisco Foods International, LeSueur, MN). The GMP diet was supplemented with 1.5 times the National Research Council suggested requirement (equivalent to a total supplementation of 2.8% AA) for the following, limiting AA to compensate for faster absorption and degradation of AA compared with intact protein (39, 41): arginine, histidine, leucine, methionine, tryptophan, and tyrosine. The AA diet was patterned after the AA composition reported by Rogers and Harper (48) to support maximal growth in rodents and does not mimic the profile of AA found in a specific intact protein. Complete AA analysis of the diets was conducted in the Experiment Station Chemical Laboratories, University of Missouri-Columbia (Columbia, MO) (Table 2). The phe content of the diets expressed per kilogram diet was casein, 9.2 g phe; AA, 2.2 g phe; and GMP, 2.6 g phe. The low-phe AA and GMP diets provided the minimum level of phe needed to support growth (39).
Table 1.
Experimental diets
| Ingredient | Casein | GMP | Amino Acid |
|---|---|---|---|
| Protein | |||
| Casein | 200 | ||
| BioPure GMP* | 200 | ||
| l-Arginine HCl | 4.6 | 12.1† | |
| l-Cystine | 3.0 | 3.5 | |
| l-Histidine, HCl-H2O | 3.2 | 4.5 | |
| l-Leucine | 6.8 | 11.1 | |
| l-Methionine | 7.0 | 8.2 | |
| l-Phenylalanine | 1.64 | 2.5 | |
| l-Tyrosine | 5.0 | 5.0 | |
| l-Tryptophan | 1.3 | 1.8 | |
| Carbohydrate | |||
| Sucrose | 250 | 250 | 250 |
| Cornstarch | 241 | 225 | 268 |
| Maltodextrin | 130 | 130 | 130 |
| Cellulose | 50 | 50 | 50 |
| Fat | |||
| Soybean oil | 70 | 70 | 70 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Vitamins and Minerals | |||
| Vitamin mix AIN-93-VX | 13 | 13 | 13 |
| Mineral mix, 98057‡ | 35 | 35 | 35 |
| Sodium chloride | 4.2 | 3.6 | |
| Sodium phosphate, dibasic | 7.2 | 8.0 | |
| Potassium phosphate, dibasic | 2.0 | 2.0 | |
| Calcium phosphate, dibasic | 5.25 | ||
| Calcium phosphate, monobasic | 9.5 | ||
| Calcium carbonate | 13.7 | 6.75 | 9.9 |
| Antioxidant | |||
| t-Butylhydroquinone | 0.014 | 0.014 | 0.014 |
Values are in g/kg. GMP, glycomacropeptide.
BioPURE GMP; Davisco Foods International, Inc., LeSueur, MN.
In addition, the following l-amino acids were included for a total of 175 g amino acids/kg diet: alanine, 3.5; asparagine, 6.0; aspartic acid, 3.5; glutamic acid, 40; glycine, 23.3; isoleucine, 8.2; lysine HCl, 18.0; proline, 3.5; serine, 3.5; threonine, 8.2; and valine, 8.2, based on Rogers and Harper (48).
Teklad Custom Diets, Madison, WI; mineral mix without calcium and phosphorus.
Table 2.
Complete amino acid analysis of the diets
| Amino Acid | Casein | GMP | Amino Acid |
|---|---|---|---|
| Alanine | 5.5 | 9.3 | 3.2 |
| Arginine | 6.5 | 4.8 | 8.8 |
| Aspartic acid | 12.5 | 14.2 | 9.0 |
| Cysteine | 3.3 | 0.3 | 3.3 |
| Glutamic acid | 40.0 | 33.0 | 36.5 |
| Glycine | 3.3 | 1.9 | 22.2 |
| Histidine* | 5.5 | 3.1 | 3.1 |
| Isoleucine* | 9.4 | 15.8 | 7.9 |
| Leucine* | 17.2 | 11.4 | 10.5 |
| Lysine* | 14.5 | 10.2 | 14.5 |
| Methionine* | 4.9 | 8.6 | 5.9 |
| Phenylalanine* | 9.2 | 2.6 | 2.2 |
| Proline | 19.0 | 18.5 | 3.8 |
| Serine | 8.9 | 10.2 | 2.4 |
| Threonine* | 7.4 | 24.0 | 7.6 |
| Tryptophan* | 2.7 | 1.6 | 1.9 |
| Tyrosine* | 8.4 | 4.8 | 4.4 |
| Valine* | 11.9 | 13.5 | 7.7 |
Values are in g/kg diet.
Indispensable amino acids.
Metabolic phenotyping.
Acute energy balance was assessed in a subset of 120 mice at 23 wk of age with a metabolic phenotyping system utilizing indirect calorimetry (LabMaster modular animal monitoring system, TSE Systems, Chesterfield, MO), as previously reported (37). Food and water intake, oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were continuously measured over a 48-h period, and the respiratory exchange ratio (RER, CO2/O2) was calculated. Mice were acclimated to the individual housing in the metabolic cages before data collection, as indicated by characteristic circadian rhythms of V̇o2 and V̇co2 (37). Indirect calorimetry measurements (V̇o2 and V̇co2) and the RER from the same time for the 48-h measurement period were averaged, and the area under the curve was calculated and expressed per 24 h or during the light (0600–1800) or dark (1801–0559) cycles for each mouse. V̇o2 data were expressed per kilogram of lean mass (21).
Statistical analyses.
The primary method of analysis for data obtained at one time point was three-way ANOVA using PROC MIXED, with random effects for animal identification, to identify the main treatment effects of genotype, sex, diet, and their interactions, followed by the protected least significant differences technique to identify differences among treatment groups (SAS Institute, 2007, Cary, NC). Plasma cytokines were analyzed by one-way ANOVA. Changes in body weight were analyzed separately for male and female mice using a repeated-measures model within PROC MIXED. The model included the fixed effects of diet, genotype, and sex, and all of their interactions, as well as the factor time, and all of the two-way interactions with the other main effects. A random effect of animal nested within the diet × genotype × sex interaction was also included. To account for autocorrelated errors, an autoregressive error structure was included, and the Kenward-Roger method was used to compute the denominator degrees of freedom for the tests of the fixed effects. Analysis of longitudinal body composition data and V̇o2 were each done using a three-way ANOVA that also included age and lean mass, respectively, as a covariate with interaction terms. Backward elimination was used to remove nonsignificant terms involving age or lean mass. Statistics were performed on transformed data for results showing unequal variances among groups; actual data are presented when transformations were required. Data are presented as means ± SE; P < 0.05 was considered statistically significant. When significant main effects without significant interaction were observed, data presented in Figs. 1–7 were pooled across corresponding treatment groups. Sample size is indicated on the tables and figures.
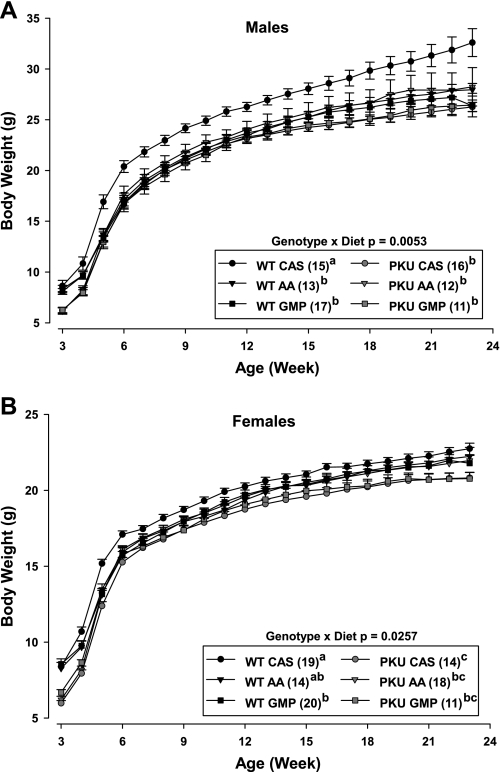
Fig. 1.
Change in body weight of male (A) and female (B) phenylketonuria (PKU) and wild-type (WT) mice fed casein (CAS), amino acid (AA), or glycomacropeptide (GMP) diets from weaning (3 wk) through 23 wk of age. Repeated-measures analysis indicated significant genotype × diet interaction for both male and female mice. Values are means ± SE; n = sample sizes (in parentheses). a,b,cMeans with different superscripted letters are significantly different (P < 0.05), as shown in the legend.
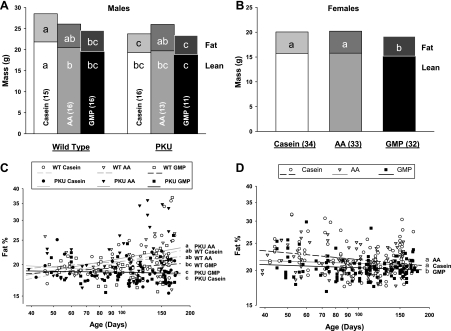
Fig. 2.
Body composition of lean mass and fat mass determined by dual-energy X-ray absorptiometry in male (A and C) and female (B and D) mice fed CAS, AA, or GMP diets. Final composition of lean and fat mass was assessed (A and B). a,b,cMeans with different superscripted letters are significantly different (P < 0.05). The percentage of body fat was assessed over time (C and D). a,b,cMeans with different superscripted letters indicate significant differences in mean %body fat at the midpoint of time (P < 0.05). Male mice showed significant genotype × diet interaction, as reflected in presentation of the six groups (A and C). Female mice showed a significant main effect for dietary treatment without significant interaction with genotype, as reflected in combining the female WT and PKU dietary groups (B and D). Nos. in parentheses indicate sample size.
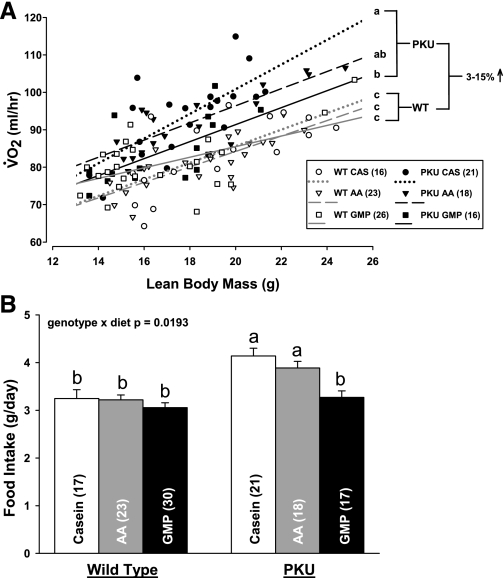
Fig. 3.
Oxygen consumption (V̇o2; A) and food intake (B) in PKU and WT mice fed CAS, AA, and GMP diets. V̇o2 was assessed at 23 wk of age over a 48-h period and analyzed with lean body mass as a covariate. Mean V̇o2 showed a significant effect of genotype (P < 0.0001) and significant interaction of genotype and diet (P = 0.0180) at the mean of lean body mass. Energy expenditure as reflected in V̇o2 was significantly increased by 3–15% in PKU mice compared with WT mice (2.55% increase bGMP; 12.88% increase a,bAA, and 14.82% increase aCAS). a,bDifferent superscripted letters indicate significant differences in mean V̇o2 and food intake based on significant genotype × diet interaction. Male mice consumed significantly more food than female mice without diet interaction (P = 0.04). Values are means + SE; nos. in parentheses indicate sample size.
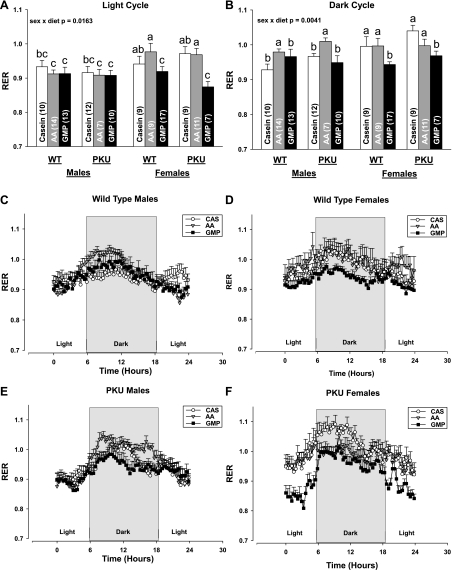
Fig. 4.
The respiratory exchange ratio (RER; CO2 produced/O2 consumed) during the light (A) and dark cycles (B) in male (WT, C; PKU, E; time course over 24 h) and female (WT, D; PKU, F; time course over 24 h) mice fed CAS, AA, and GMP diets. Significant interaction of sex and diet was observed. Values are means + SE; nos. in parentheses indicate sample size. a,b,cMeans with different superscripted letters reflecting the significant interaction of sex and diet are significantly different (P < 0.05). During the dark cycle, there was a significant effect of genotype with higher RER in PKU vs. WT mice (P = 0.0380).
Fig. 5.
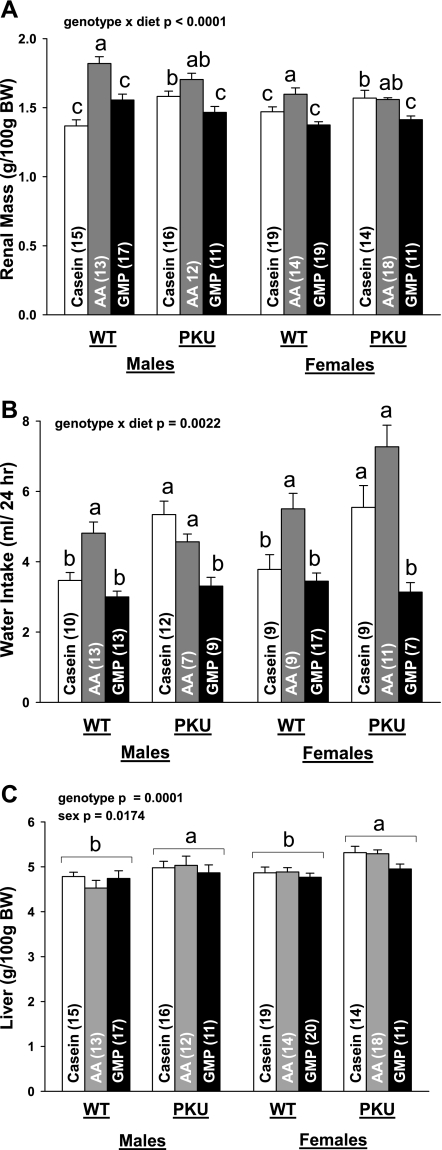
Relative renal mass (A), 24-h water intake (B), and relative liver mass (C) in PKU and WT mice fed CAS, AA, and GMP diets. Values are means + SE; nos. in parentheses indicate sample size. a,b,cMeans with different superscripted letters are significantly different (P < 0.05), reflecting the significant interaction of genotype and diet for kidney and water intake. Relative mass of liver showed a significant effect of genotype (P = 0.0001) and sex (P = 0.0174) without interaction; greater liver mass was observed in PKU vs. WT mice and female vs. male mice. BW, body weight.
Fig. 6.
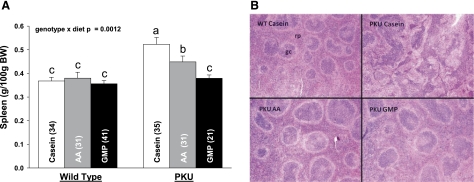
Relative mass of spleen (A) and representative spleen histology at ×10 magnification (B) from PKU and WT mice fed CAS, AA, and GMP diets. rp, red pulp; gc, germinal center. Values are means + SE; nos. in parentheses indicate sample size. a,b,cMeans with different superscripted letters are significantly different (P < 0.05). Relative spleen mass was significantly greater in female vs. male mice without interaction (P = 0.0001).
Fig. 7.
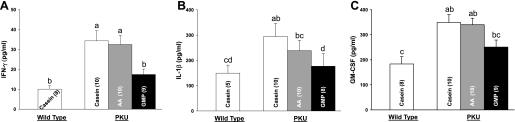
Plasma cytokine concentrations for interferon-γ (IFN-γ; A), interleukin-1β (IL-1β; B), and granulocyte macrophage colony-stimulating factor (GM-CSF; C) in male PKU and WT mice fed CAS, AA, and GMP diets. Values are means + SE; nos. in parentheses indicate sample size. a,b,c,dMeans with different superscripted letters are significantly different (P < 0.05).
RESULTS
Plasma AA profile.
Consistent with an absence of PAH activity, the plasma concentration of phe was 15- to 49-fold higher in PKU compared with WT mice (Table 3). The low-phe AA and low-phe GMP diets reduced plasma phe concentration in PKU mice by 65% and in WT mice by 24% compared with the high-phe casein control diets. The plasma concentration of tyrosine was over 50% lower in PKU compared with WT mice, and the AA and GMP diets reduced plasma tyrosine concentration by 30–62% in WT and PKU mice compared with the casein diet.
Table 3.
Concentrations of amino acids in plasma of WT and PKU mice fed diets containing casein, GMP, or amino acids
| WT Mice |
PKU Mice |
ANOVA P Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acid | Casein | Amino Acid | GMP | Casein | Amino Acid | GMP | Genotype | Diet | Sex | Genotype × Diet |
| N | 15 | 13 | 13 | 14 | 15 | 14 | ||||
| Alanine | 754 ± 62 | 1,021 ± 97 | 874 ± 110 | 755 ± 67 | 952 ± 112 | 716 ± 63 | 0.0364 | |||
| Arginine | 95 ± 4 | 93 ± 12 | 83 ± 6 | 73 ± 5 | 87 ± 3 | 79 ± 4 | 0.0087 | 0.0063 | ||
| Aspartate | 14 ± 2 | 11 ± 1 | 13 ± 2 | 10 ± 1 | 7 ± 1 | 8 ± 1 | 0.0033 | |||
| Citrulline | 76 ± 5 | 68 ± 6 | 75 ± 6 | 78 ± 5 | 70 ± 3 | 64 ± 5 | 0.0350 | |||
| Cysteine | 17 ± 5 | 6 ± 3 | 5 ± 3 | 9 ± 4 | 5 ± 3 | 2 ± 2 | 0.0244 | |||
| Glutamate | 29 ± 2 | 32 ± 5 | 41 ± 8 | 28 ± 2 | 27 ± 2 | 40 ± 3 | 0.0009 | 0.0333 | ||
| Glutamine | 474 ± 16 | 533 ± 42 | 661 ± 82 | 434 ± 27 | 475 ± 26 | 541 ± 25 | 0.0337 | <0.0001 | ||
| Glycine | 214 ± 10 | 886 ± 126 | 188 ± 8 | 179 ± 7 | 785 ± 127 | 183 ± 8 | 0.0119 | <0.0001 | ||
| Histidine | 73 ± 3 | 67 ± 4 | 81 ± 14 | 82 ± 4 | 69 ± 5 | 76 ± 2 | ||||
| Isoleucine | 110 ± 8c,d | 83 ± 9d | 243 ± 30a | 134 ± 12c | 95 ± 7c,d | 185 ± 24b | <0.0001 | 0.0355 | ||
| Leucine | 162 ± 12 | 113 ± 11 | 148 ± 11 | 186 ± 20 | 134 ± 10 | 127 ± 5 | 0.0003 | |||
| Lysine | 437 ± 25 | 447 ± 51 | 372 ± 28 | 415 ± 35 | 470 ± 39 | 339 ± 24 | 0.0187 | 0.0032 | ||
| Methionine | 77 ± 6 | 114 ± 16 | 148 ± 46 | 75 ± 7 | 89 ± 12 | 88 ± 13 | ||||
| Ornithine | 61 ± 4 | 75 ± 9 | 65 ± 9 | 67 ± 5 | 59 ± 4 | 55 ± 4 | ||||
| Phenylalanine | 51 ± 2c | 36 ± 4d | 41 ± 4d | 2,103 ± 92a | 722 ± 26b | 766 ± 18b | <0.0001 | <0.0001 | 0.0397 | |
| Proline | 157 ± 20 | 90 ± 12 | 242 ± 53 | 203 ± 32 | 81 ± 12 | 167 ± 28 | <0.0001 | |||
| Serine | 165 ± 10 | 271 ± 24 | 205 ± 35 | 180 ± 11 | 252 ± 13 | 157 ± 12 | <0.0001 | |||
| Taurine | 500 ± 30 | 589 ± 69 | 561 ± 40 | 417 ± 24 | 488 ± 36 | 489 ± 35 | 0.0097 | 0.0245 | ||
| Threonine | 317 ± 23 | 409 ± 53 | 971 ± 125 | 305 ± 24 | 384 ± 37 | 807 ± 98 | <0.0001 | |||
| Tryptophan | 61 ± 4 | 75 ± 10 | 72 ± 7 | 64 ± 25 | 52 ± 4 | 65 ± 4 | 0.0033 | 0.0040 | ||
| Tyrosine | 102 ± 11 | 57 ± 12 | 71 ± 17 | 50 ± 7 | 22 ± 3 | 19 ± 3 | <0.0001 | <0.0001 | ||
| Valine | 315 ± 23 | 219 ± 17 | 401 ± 42 | 352 ± 28 | 227 ± 14 | 333 ± 31 | <0.0001 | |||
| BCAA | 587 ± 42 | 415 ± 32 | 792 ± 80 | 672 ± 60 | 456 ± 28 | 644 ± 55 | <0.0001 | |||
Values are means ± SE in μmol/l; n, no. of mice. WT, wild type; PKU, phenylketonuria; BCAA, branched chain amino acid. There was a significant genotype effect where WT mice had higher plasma concentrations of arginine, aspartate, glutamine, glycine, taurine, tryptophan, and tyrosine compared with homozygous mice. The amino acid diet resulted in higher values for alanine, glycine, and serine compared with casein and GMP. Both amino acid and casein diets had higher values for lysine compared with GMP. The casein diet resulted in higher plasma cysteine than GMP, but not amino acid diet. GMP had higher plasma tryptophan than casein, but not amino acid diet. Casein resulted in higher plasma leucine and tyrosine than both GMP and amino acid diets. Both casein and GMP diets resulted in higher plasma proline, valine, and BCAA than the amino acid diet. The GMP diet resulted in higher plasma glutamate, glutamine, and threonine compared with both the casein and amino acid diets. Females have higher plasma levels of arginine, citrulline, lysine, and taurine, and males have higher plasma glutamate. There was a significant genotype by diet interaction for isoleucine and phenylalanine. a,b,c,dMeans in a row with superscripted letters without a common letter differ, P < 0.05. BCAA, sum of isoleucine, leucine, and valine. P values not listed are nonsignificant.
There were alterations in the plasma AA profiles due to diet, consistent with our laboratory's previous report and the unique AA profiles of the AA, GMP, and casein diets (39) (Table 2). In both PKU and WT mice, plasma concentrations of glutamine, isoleucine, and threonine were significantly higher, and plasma concentration of lysine was significantly lower with ingestion of the GMP compared with the AA and casein diets. Likewise, the plasma concentrations of glycine and serine were significantly higher with ingestion of the AA compared with the GMP and casein diets. Both the casein and GMP diets induced higher plasma concentrations of the large neutral AA, proline, valine, and the sum of the branched-chain AA, leucine, isoleucine, and valine. Female WT and PKU mice showed higher plasma concentrations of arginine, citrulline, lysine, and taurine, but lower concentrations of glutamate compared with male mice.
Growth rate.
Growth curves showing change in body weight for male and female mice from weaning through 23 wk of age are shown in Fig. 1. At weaning when the experimental diets were initiated, PKU mice weighed 34% less than WT mice (6.2 ± 0.10 vs. 8.4 ± 0.15 g; P < 0.0001). As expected, male mice showed significantly greater accretion of body weight and lean body mass over time than female mice, and this response was independent of genotype and diet.
Male WT mice fed the casein diet showed a greater rate of gain in body weight, beginning at week 5 and continuing through the end of the study compared with the other treatment groups. Comparing the entire growth curve of each male treatment group, the only significant difference was that WT males fed the casein diet weighed, on average, 14% more than the other five groups.
In female mice, the PKU genotype modulated the growth response, such that WT mice weighed significantly more from 14 to 23 wk than did PKU mice. The final body weight of WT female mice was ∼3% greater compared with PKU female mice (22.2 ± 0.21 vs. 21.6 ± 0.21 g body wt, P = 0.0312). Moreover, PKU female mice fed casein weighed the least from weeks 4–6, weeks 8–13, and during the second half of the growth curve compared with the other five treatment groups. With respect to the entire growth curve of each female treatment group, the findings are similar to the male data in that the growth trajectory of WT and PKU mice fed the AA and GMP diets was similar. In addition, WT mice fed casein compared with the low-phe diets showed a greater rate of gain in body weight in association with higher phe intake and plasma phe concentration. Interestingly, female PKU mice did not show a rebound in body weight from weaning while eating the casein diet, as noted in male PKU mice.
Body composition.
Composition of lean mass and fat mass determined by DXA are shown in Fig. 2. Coincident with greater body weight, male WT mice fed casein showed a significantly greater amount of lean mass compared with the other five male groups, whereas the amount of lean mass in WT and PKU female mice was not significantly different due to diet. Both male and female PKU mice showed a catch-up in body weight from weaning and a similar amount of final lean mass compared with their respective WT littermates fed the GMP or AA diets. Thus the low-phe AA and GMP diet formulations support growth to a similar extent in both WT and PKU mice.
Diet had a significant effect on final body fat content and the percent of body weight as fat assessed over time. Male PKU mice showed significantly lower final body fat content, as well as percent body fat when fed the GMP or casein diets compared with the AA diet (Fig. 2, A and C). In contrast, percent body fat was not significantly different among male WT mice fed the casein, AA, or GMP diets. Female WT and PKU mice fed GMP showed a significantly lower final fat mass and percent body fat compared with both the AA and casein diets (Fig. 2, B and D). Overall, both male and female PKU mice fed the GMP diet showed a significantly lower percentage of body fat compared with mice fed the AA diet, despite similar lean mass and gain in body weight.
PKU mice show increased energy expenditure.
Energy expenditure determined by mean V̇o2 over 48 h was significantly increased by 3–15% in PKU mice compared with WT mice (Fig. 3A). In PKU mice, the GMP diet significantly attenuated the increase in V̇o2 induced by the high-phe casein diet (2.55 vs. 14.82% increase, P < 0.05), although energy expenditure remained significantly higher compared with WT mice. The AA diet did not alter energy expenditure compared with the casein diet in PKU mice (12.88% increase AA diet vs. 14.82% increase casein diet compared with WT mice). PKU mice also showed an increase in mean 24-h energy expenditure compared with WT mice based on V̇o2 per kilogram of lean mass (data not shown). Consistent with greater energy expenditure and yet similar growth, food intake was significantly greater in PKU mice fed the casein or AA diets compared with WT mice (Fig. 3B). The GMP diet normalized food intake in PKU mice, such that the amount of food eaten by PKU mice to support growth when fed GMP was significantly lower than the amount eaten when fed casein or AA, but not significantly different from that observed in WT mice. Taken together, the V̇o2 and food intake data demonstrate that the GMP diet supported similar growth and attenuated the metabolic stress that was reflected in increased energy expenditure in PKU mice fed the casein or AA diets.
GMP diet reduces the RER compared with the AA diet.
The RER (CO2 produced/O2 consumed) during the dark cycle when mice were eating was significantly lower in both WT and PKU mice fed the GMP diet compared with the AA diet; significant interaction of sex and diet was observed (Fig. 4). The reduction in RER with ingestion of the GMP diet was most apparent in female mice, both WT and PKU, where the RER was significantly reduced during both the light and dark cycles compared with the AA and casein diets. The reduction in RER for male mice, both WT and PKU, fed GMP was only observed during the dark cycle. The lower RER suggests that the GMP diet increased fat oxidation compared with the AA diet. Moreover, it is consistent with the significant reduction in final fat mass observed in female WT and PKU mice fed GMP compared with both the AA and casein diets, and in male PKU mice fed GMP compared with the AA diet.
GMP diet decreases renal workload in WT and PKU mice compared with the AA diet.
To assess the impact of diet and the PKU genotype on kidney workload, we determined mean 24-h water intake (a surrogate for urine output) and renal mass. Water intake and renal mass were significantly greater in both WT and PKU mice fed the AA diet compared with the GMP diet; the magnitude of increase in renal mass with the AA diet was greater in male compared with female mice (sex × diet, P = 0.0002) (Fig. 5). In PKU mice, the casein diet increased renal mass to a level similar to the AA diet, most likely due to the excretion of organic acids derived from the highly elevated levels of phe in blood. These findings suggest that the GMP diet reduced renal metabolic activity and energy expenditure compared with both the casein and AA diets in PKU mice. Moreover, consistent with greater energy expenditure for metabolic activities, such as urea synthesis, relative liver mass was increased by 7% in PKU compared with WT mice with a nonsignificant trend in female PKU mice (P = 0.0730) for lower liver mass in PKU mice fed GMP (Fig. 5C).
PKU mice fed casein or AA diets show systemic inflammation.
Surprisingly, spleen mass was significantly elevated in both male and female PKU mice, fed either the casein diet (38% increase) or AA diet (18% increase) compared with the GMP diet (Fig. 6). In contrast, the GMP diet normalized spleen mass to the level observed in WT mice. Systemic inflammation was suggested by splenomegaly and reinforced by histology. Evaluation of histological sections from spleen indicated that PKU mice fed the casein diet, and to a lesser extent the AA diet, showed more prominent germinal centers where interactions between antigens and lymphocytes occur, compared with WT mice fed casein or PKU mice fed GMP. Consistent with systemic inflammation, plasma levels of the inflammatory cytokines, interleukin-1β (IL-1β), interferon-γ (IFN-γ), and granulocyte macrophage colony-stimulating factor (GM-CSF), were elevated in male PKU mice fed the casein or AA diets compared with WT mice; the GMP diet normalized this response (Fig. 7). Female mice showed generally higher plasma cytokine levels than male mice, and there were no significant diet effects (data not shown). Taken together, this suggests that the casein and AA diets induced systemic inflammation in PKU mice associated with splenomegaly and prominent germinal centers, which was normalized by the GMP diet.
DISCUSSION
PKU is the first human genetic disease to have an effective therapy. The essential feature of therapy for PKU is adherence to a low-phe diet that includes AA formula as the primary source of nitrogen and indispensable AA. Initiation of a low-phe diet shortly after birth prevents the devastating PKU phenotype of severe cognitive impairment; however, lifelong adherence to a low-phe, AA-based diet is extremely difficult (23, 40, 57). We have developed a new paradigm for the PKU diet utilizing low-phe foods made from the intact whey protein GMP, supplemented with limiting AAs to provide a palatable alternative to AA formula. This study extends our research in humans with PKU (30, 38, 54), as well as PKU mice (39) by assessing the impact of GMP, AA, and casein diets fed from weaning through adulthood on growth, body composition, energy balance, and systemic inflammation in PKU and WT mice. When fed the high-phe casein diet or the low-phe AA diet, PKU mice exhibited metabolic stress, or altered homeostasis induced by the absence of PAH activity, as reflected in increased energy expenditure and intake of food and water, increased renal mass, and systemic inflammation compared with WT mice. When PKU mice were fed the low-phe GMP diet, all of these adverse effects were significantly attenuated in association with similar growth and reductions in plasma phe concentration. Our findings suggest that GMP provides a more physiological source of low-phe dietary protein for PKU because it reduces the metabolic stress associated with ingestion of casein- or AA-based diets in PKU mice.
Absence of hepatic PAH activity in PKU mice resulted in a dramatic, diet-dependent increase in mean 24-h energy expenditure and a corresponding increase in food intake with ingestion of casein or AA, but not GMP, compared with WT mice. Increased energy expenditure is consistent with greater metabolic activity in PKU mice, as reflected in greater liver mass (58), the cost of metabolizing excess phe to phenylketones and other organic acids, and the energy lost from urinary excretion of phe-derived organic acids. In support of increased energy needs to metabolize excess phe, PKU mice fed casein showed the highest plasma phe concentrations and the largest 15% increase in energy expenditure, and when plasma phe concentrations were reduced with the low-phe diets, the increase in energy expenditure was attenuated. Parallel to these observations in PKU mice, two studies found that resting energy expenditure is, on average, 5–10% higher in female children with PKU than that predicted by standard equations used to estimate energy needs (4, 42). Higher energy expenditure due to the absence of PAH activity, especially when phe levels are poorly controlled, may help explain the reduced growth observed in children with PKU who struggle with the AA-based diet (6, 16).
Greater nervous physical activity may also help to explain why PKU mice show higher energy expenditure compared with WT mice. Brain sections from PKU mice show two- to sevenfold higher levels of orexin, a hormone found to increase physical activity (53), compared with WT mice. When ambulatory activity was measured by infrared sensors in PKU mice, researchers found hypoactivity in PKU mice at 10 mo of age (35); however, PKU mice at any age were noted to display a tremor (personal communication, A. Kume), which may contribute to greater overall energy expenditure.
Ingestion of an AA meal rapidly increases plasma AA concentrations, resulting in increased ureagenesis and reduced protein retention compared with intact protein (13, 18, 34, 54). Our data suggest that the AA diet increased renal workload to facilitate nitrogen excretion in both PKU and WT mice, as reflected in increased water intake and renal mass compared with the casein diet in WT mice. In contrast, the GMP diet did not induce an increase in water intake or renal mass. Taken together with the findings on energy expenditure and food intake, the GMP diet supports growth and reduces plasma phe concentration to a similar extent as the AA diet, and yet it is more physiological based on normalization of energy expenditure, food and water consumption, as well as renal mass.
Another interesting finding from our study is that PKU mice fed the casein diet, and to a lesser extent the AA diet, showed clear evidence of systemic inflammation based on splenomegaly, prominent germinal centers, and elevated plasma concentrations of the inflammatory cytokines IFN-γ, IL-1β, and GM-CSF. Strikingly, GMP normalized these inflammatory responses. The mechanism by which systemic inflammation occurs in PKU mice fed casein or AA diets remains unknown and requires further research. We speculate that increased concentrations of phe, associated organic acids, or alterations in the plasma AA profile may play a role (50, 51). The human condition of PKU is not associated with increased risk of infectious disease; however, skin irritation often occurs when blood phe concentrations are poorly controlled (14).
Our finding that the GMP diet normalized inflammatory responses observed in PKU mice is consistent with evidence that GMP modulates innate immunity, as reflected in increased cytokine production in human monocytes (45), and suppresses adaptive immunity, as reflected in decreased stimulation of T helper 1 (Th1) lymphocytes (47). Moreover, oral gavage of GMP shows significant anti-inflammatory effects in rat models of colitis and ileitis, resulting in normalization of spleen mass and reduced expression of IL-1β in ileum and colon (29, 46), similar to our observations in PKU mice. GMP inhibits the induction of IFN-γ, and, to a lesser extent TNF-α, in concanavalin A-stimulated rat splenocytes (47), also consistent with our observation that the GMP diet normalized the elevated plasma levels of IFN-γ noted in PKU mice fed casein or AA diets. Conversely, stimulation of Th1 lymphocytes in spleen and higher plasma concentration of IFN-γ in PKU mice fed the casein diet may help explain the increased size and number of germinal centers noted in PKU mice fed casein, but not GMP (15). Finally, normalization of the plasma concentration of GM-CSF in PKU mice fed GMP supports the hypothesis that GMP suppresses inflammation and Th1 lymphocytes (10, 47).
Ingestion of whey protein, a complex mixture of three major proteins including β lactoglobulin (35–50%), α lactalbumin (20–25%), and GMP (15–20%) (25) has shown promise in human studies to promote satiety (5, 19, 55) and facilitate loss of body fat mass (1, 7, 49). Studies that have isolated the specific impact of GMP demonstrate that GMP promotes satiety and modulates food intake in both control subjects (8, 26, 56) and those with PKU (30); this response has been linked with the ability of GMP to stimulate cholecystokinin release (9) and inhibit ghrelin release after eating (30) and the high content of branched chain AA in GMP. Evidence in the present study demonstrates that GMP, compared with an AA diet, significantly lowers RER during the dark cycle in both WT and PKU mice, consistent with increased fat oxidation and supported by a significantly lower percentage of body fat in PKU mice fed GMP. Interestingly, female compared with male mice showed a more pronounced response to the GMP diet in lowering RER and percentage of body fat. Consistent with our findings, a study in male Wistar rats indicates that GMP limits expansion of fat mass while maintaining lean mass (49). Given the rising incidence of overweight in children and adults with PKU (3, 33), further investigation is warranted to determine the relevance of our findings to the treatment and/or prevention of obesity in patients with PKU.
In summary, we report for the first time that PKU mice exhibit increased energy expenditure and that PKU mice fed high-phe casein or low-phe AA diets show evidence of systemic inflammation. Both phenotypes are attenuated by the GMP diet. Our findings support the view that a GMP diet provides a more physiological source of low-phe protein compared with the usual AA diet because it reverses the metabolic stress reflected in increased renal workload and immune stimulation that is observed in PKU mice fed an AA diet. Further research is needed to investigate the application of these novel findings to the human condition of PKU.
GRANTS
This work was supported by the National PKU Alliance, US Department of Agriculture HATCH Grant WISO1517, and National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-088210 and T32-DK-07665.
DISCLOSURES
D. Ney is an inventor for the provisional patent application serial no. 61/186690, titled Glycomacropeptide (GMP) medical foods for nutritional management of phenylketonuria (PKU) and other metabolic disorders, that was filed June 12, 2009 by the Wisconsin Alumni Research Foundation. A license was executed by Cambrooke Foods LLC on March 8, 2010, and a percentage of all royalty payments is awarded to the inventors.
AUTHOR CONTRIBUTIONS
Author contributions: P.M.S., S.G.M., and D.W.N. performed experiments; P.M.S., S.G.M., A.S.B., D.W.N., M.K.C., and D.M.N. analyzed data; P.M.S., D.W.N., M.K.C., C.-L.E.Y., and D.M.N. interpreted results of experiments; P.M.S., S.G.M., and A.S.B. prepared figures; P.M.S., S.G.M., A.S.B., D.W.N., M.K.C., C.-L.E.Y., and D.M.N. edited and revised manuscript; P.M.S., S.G.M., A.S.B., D.W.N., M.K.C., C.-L.E.Y., and D.M.N. approved final version of manuscript; D.M.N. conception and design of research; D.M.N. drafted manuscript.
ACKNOWLEDGMENTS
We thank Michael Grahn and Ricky Coleman for assistance with tissue processing and DXA analyses; undergraduate students Wing Pun, Therese Breunig, Samantha Peterson, and Margaret Habib for assistance with the PKU mouse colony; Peter Crump for assistance with statistical models; Philip J. Williams for performing analysis of the plasma AA concentrations in the Wisconsin State Laboratory of Hygiene; and PhD candidate Joseph Pierre for valuable input regarding the spleen and cytokine data.
REFERENCES
- 1. Acheson KJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Emady-Azar S, Ammon-Zufferey C, Monnard I, Pinaud S, Nielsen-Moennoz C, Bovetto L. Protein choices targeting thermogenesis and metabolism. Am J Clin Nutr 93: 525– 534, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Aimutis WR. Bioactive properties of milk proteins with particular focus on anticariogenesis. J Nutr 134: 989S– 995S, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Albersen M, Bonthuis M, de Roos NM, van den Hurk DA, Carbasius Weber E, Hendriks MM, de Sain-van der Velden MG, de Koning TJ, Visser G. Whole body composition analysis by the BodPod air-displacement plethysmography method in children with phenylketonuria shows a higher body fat percentage. J Inherit Metab Dis June 24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen JR, McCauley JC, Waters DL, O'Connor J, Roberts DC, Gaskin KJ. Resting energy expenditure in children with phenylketonuria. Am J Clin Nutr 62: 797– 801, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Anderson GH, Tecimer SN, Shah D, Zafar TA. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J Nutr 134: 3011– 3015, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Arnold GL, Vladutiu CJ, Kirby RS, Blakely EM, Deluca JM. Protein insufficiency and linear growth restriction in phenylketonuria. J Pediatr 141: 243– 246, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Baer DJ, Stote KS, Paul DR, Harris GK, Rumpler WV, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr 141: 1489– 1494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brody EP. Biological activities of bovine glycomacropeptide. Br J Nutr 84, Suppl 1: S39– S46, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Burton-Freeman BM. Glycomacropeptide (GMP) is not critical to whey-induced satiety, but may have a unique role in energy intake regulation through cholecystokinin (CCK). Physiol Behav 93: 379– 387, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Campbell IK, van Nieuwenhuijze A, Segura E, O'Donnell K, Coghill E, Hommel M, Gerondakis S, Villadangos JA, Wicks IP. Differentiation of inflammatory dendritic cells is mediated by NF-kappaB1-dependent GM-CSF production in CD4 T cells. J Immunol 186: 5468– 5477, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Chabance B, Marteau P, Rambaud JC, Migliore-Samour D, Boynard M, Perrotin P, Guillet R, Jolles P, Fiat AM. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 80: 155– 165, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Colman RJ, Nam G, Huchthausen L, Mulligan JD, Saupe KW. Energy restriction-induced changes in body composition are age specific in mice. J Nutr 137: 2247– 2251, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, Ballevre O, Beaufrere B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab 280: E340– E348, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Donlon J, Levy H, Scriver C. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In: Metabolic and Molecular Basis of Inherited Disease, edited by Scriver C, Beaudet A, Sly W, Valle D. New York: McGraw-Hill, 2007, chapt. 77, p. 1– 150 [Google Scholar]
- 15.Egle A, Harris AW, Bath ML, O'Reilly L, Cory S. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood 103: 2276– 2283, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Enns GM, Koch R, Brumm V, Blakely E, Suter R, Jurecki E. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol Genet Metab 101: 99– 109, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Etzel MR. Manufacture and use of dairy protein fractions. J Nutr 134: 996S– 1002S, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Gropper SS, Acosta PB. Effect of simultaneous ingestion of l-amino acids and whole protein on plasma amino acid and urea nitrogen concentrations in humans. JPEN J Parenter Enteral Nutr 15: 48– 53, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr 89: 239– 248, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Harding CO, Gillingham MB, Hamman K, Clark H, Goebel-Daghighi E, Bird A, Koeberl DD. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther 13: 457– 462, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaiyala KJ, Schwartz MW. Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60: 17– 23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelleher SL, Chatterton D, Nielsen K, Lonnerdal B. Glycomacropeptide and alpha-lactalbumin supplementation of infant formula affects growth and nutritional status in infant rhesus monkeys. Am J Clin Nutr 77: 1261– 1268, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Koch R, Burton B, Hoganson G, Peterson R, Rhead W, Rouse B, Scott R, Wolff J, Stern AM, Guttler F, Nelson M, de la Cruz F, Coldwell J, Erbe R, Geraghty MT, Shear C, Thomas J, Azen C. Phenylketonuria in adulthood: a collaborative study. J Inherit Metab Dis 25: 333– 346, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr 26: 713S– 723S, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Laclair CE, Ney DM, MacLeod EL, Etzel MR. Purification and use of glycomacropeptide for nutritional management of phenylketonuria. J Food Sci 74: E199– E206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lam SM, Moughan PJ, Awati A, Morton HR. The influence of whey protein and glycomacropeptide on satiety in adult humans. Physiol Behav 96: 162– 168, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Li EW, Mine Y. Immunoenhancing effects of bovine glycomacropeptide and its derivatives on the proliferative response and phagocytic activities of human macrophagelike cells, U937. J Agric Food Chem 52: 2704– 2708, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Lim K, van Calcar SC, Nelson KL, Gleason ST, Ney DM. Acceptable low-phenylalanine foods and beverages can be made with glycomacropeptide from cheese whey for individuals with PKU. Mol Genet Metab 92: 176– 178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopez-Posadas R, Requena P, Gonzalez R, Suarez MD, Zarzuelo A, Sanchez de Medina F, Martinez-Augustin O. Bovine glycomacropeptide has intestinal antiinflammatory effects in rats with dextran sulfate-induced colitis. J Nutr 140: 2014– 2019, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Macleod EL, Clayton MK, van Calcar SC, Ney DM. Breakfast with glycomacropeptide compared with amino acids suppresses plasma ghrelin levels in individuals with phenylketonuria. Mol Genet Metab 100: 303– 308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacLeod EL, Ney DM. Nutritional management of phenylketonuria. Ann Nestle 68: 58– 69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McDonald JD, Bode VC, Dove WF, Shedlovsky A. Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proc Natl Acad Sci U S A 87: 1965– 1967, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McStravick N, Robertson LV, Ripley S, Weetch E, Adams D, Micciche A. Body mass index in adult patients with diet treated phenylketonuria (Abstract). J Inherit Metab Dis 33: S107, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Metges CC, El-Khoury AE, Selvaraj AB, Tsay RH, Atkinson A, Regan MM, Bequette BJ, Young VR. Kinetics of l-[1-(13)C]leucine when ingested with free amino acids, unlabeled or intrinsically labeled casein. Am J Physiol Endocrinol Metab 278: E1000– E1009, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Mochizuki S, Mizukami H, Ogura T, Kure S, Ichinohe A, Kojima K, Matsubara Y, Kobayahi E, Okada T, Hoshika A, Ozawa K, Kume A. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther 11: 1081– 1086, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Nakajima K, Tamura N, Kobayashi-Hattori K, Yoshida T, Hara-Kudo Y, Ikedo M, Sugita-Konishi Y, Hattori M. Prevention of intestinal infection by glycomacropeptide. Biosci Biotechnol Biochem 69: 2294– 2301, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Nelson DW, Gao Y, Spencer NM, Bahn T, Yen CL. Deficiency of the monoacylglycerol acyltransferase MGAT2 increases energy expenditure without high-fat feeding and protects genetically obese mice from excessive weight gain. J Lipid Res 52: 1723– 1732, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ney DM, Gleason ST, van Calcar SC, Macleod EL, Nelson KL, Etzel MR, Rice GM, Wolff JA. Nutritional management of PKU with glycomacropeptide from cheese whey. J Inherit Metab Dis 32: 32– 39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ney DM, Hull AK, van Calcar SC, Liu X, Etzel MR. Dietary glycomacropeptide supports growth and reduces the concentrations of phenylalanine in plasma and brain in a murine model of phenylketonuria. J Nutr 138: 316– 322, 2008 [DOI] [PubMed] [Google Scholar]
- 40. NIH Phenylketonuria (PKU): Screening and Management. NIH Consensus Statement. Bethesda, MD: National Institutes of Health, 2000, vol. 17, p. 1– 33 [PubMed] [Google Scholar]
- 41. NRC Nutrient Requirements of the Mouse. In: Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy Press, 1995, p. 80– 102 [Google Scholar]
- 42.Quirk ME, Schmotzer BJ, Singh RH. Predictive equations underestimate resting energy expenditure in female adolescents with phenylketonuria. J Am Diet Assoc 110: 922– 925, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raff H, Bruder ED, Jankowski B, Oaks MK, Colman RJ. Growth hormone therapy during neonatal hypoxia in rats: body composition, bone mineral density, and insulin-like growth factor-1 expression. Endocrine 16: 139– 143, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Raff H, Bruder ED, Jankowski BM, Colman RJ. Effect of neonatal hypoxia on leptin, insulin, growth hormone and body composition in the rat. Horm Metab Res 33: 151– 155, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Requena P, Daddaoua A, Guadix E, Zarzuelo A, Suarez MD, Sanchez de Medina F, Martinez-Augustin O. Bovine glycomacropeptide induces cytokine production in human monocytes through the stimulation of the MAPK and the NF-kappaB signal transduction pathways. Br J Pharmacol 157: 1232– 1240, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Requena P, Daddaoua A, Martinez-Plata E, Gonzalez M, Zarzuelo A, Suarez MD, Sanchez de Medina F, Martinez-Augustin O. Bovine glycomacropeptide ameliorates experimental rat ileitis by mechanisms involving downregulation of interleukin 17. Br J Pharmacol 154: 825– 832, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Requena P, Gonzalez R, Lopez-Posadas R, Abadia-Molina A, Suarez MD, Zarzuelo A, de Medina FS, Martinez-Augustin O. The intestinal antiinflammatory agent glycomacropeptide has immunomodulatory actions on rat splenocytes. Biochem Pharmacol 79: 1797– 1804, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Rogers QR, Harper AE. Amino acid diets and maximal growth in the rat. J Nutr 87: 267– 273, 1965 [DOI] [PubMed] [Google Scholar]
- 49. Royle PJ, McIntosh GH, Clifton PM. Whey protein isolate and glycomacropeptide decrease weight gain and alter body composition in male Wistar rats. Br J Nutr 100: 88– 93, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Santos K D, Rocha M, Wannmacher C M, Wajner M. The influence of organic acids on the proliferation of human peripheral lymphocytes activated by concanavalin A and pokeweed mitogen. Int J Immunopharmacol 18: 761– 769, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, Yoshiya K, Matsushima A, Sumi Y, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H. Altered gut flora and environment in patients with severe SIRS. J Trauma 60: 126– 133, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Slocum RH, Cummings JG. Amino acid analysis of physiological samples. In: Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual, edited by Hommes FA. New York: Wiley-Liss, 1991, p. 87– 126 [Google Scholar]
- 53.Surendran S, Campbell GA, Tyring SK, Matalon K, McDonald JD, Matalon R. High levels of orexin A in the brain of the mouse model for phenylketonuria: possible role of orexin A in hyperactivity seen in children with PKU. Neurochem Res 28: 1891– 1894, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Van Calcar SC, Macleod EL, Gleason ST, Etzel MR, Clayton MK, Wolff JA, Ney DM. Improved nutritional management of phenylketonuria by using a diet containing glycomacropeptide compared with amino acids. Am J Clin Nutr 89: 1068– 1077, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Dose-dependent satiating effect of whey relative to casein or soy Physiol Behav 96: 675– 682, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Effects of complete whey-protein breakfasts versus whey without GMP-breakfasts on energy intake and satiety Appetite 52: 388– 395, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Walter JH, White FJ, Hall SK, MacDonald A, Rylance G, Boneh A, Francis DE, Shortland GJ, Schmidt M, Vail A. How practical are recommendations for dietary control in phenylketonuria? Lancet 360: 55– 57, 2002 [DOI] [PubMed] [Google Scholar]
- 58. Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Heller M, Later W, Heymsfield SB, Muller MJ. Evaluation of specific metabolic rates of major organs and tissues: comparison between men and women. Am J Hum Biol 23: 333– 338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]