Abstract
Amenorrhea is common in young athletes and is associated with low fat mass. However, hormonal factors that link decreased fat mass with altered gonadotropin pulsatility and amenorrhea are unclear. Low levels of leptin (an adipokine) and increased ghrelin (an orexigenic hormone that increases as fat mass decreases) impact gonadotropin pulsatility. Studies have not examined luteinizing hormone (LH) secretory dynamics in relation to leptin or ghrelin secretory dynamics in adolescent and young adult athletes. We hypothesized that 1) young amenorrheic athletes (AA) would have lower LH and leptin and higher ghrelin secretion than eumenorrheic athletes (EA) and nonathletes and 2) higher ghrelin and lower leptin would be associated with lower LH secretion. This was a cross-sectional study. We examined ghrelin and leptin secretory patterns (over 8 h, from 11 PM to 7 AM) in relation to LH secretory patterns in AA, EA, and nonathletes aged 14–21 yr. Ghrelin and leptin were assessed every 20 min and LH every 10 min. Groups did not differ for age, bone age, or BMI. However, fat mass was lower in AA than in EA and nonathletes. AA had lower LH and higher ghrelin pulsatile secretion and AUC than nonathletes and lower leptin pulsatile secretion and AUC than EA and nonathletes. Percent body fat was associated positively with LH and leptin secretion and inversely with ghrelin. In a regression model, ghrelin and leptin secretory parameters were associated independently with LH secretory parameters. We conclude that higher ghrelin and lower leptin secretion in AA related to lower fat mass may contribute to altered LH pulsatility and amenorrhea.
Keywords: amenorrhea, adolescents, luteinizing hormone, energy
amenorrhea in athletes ranges between 3.4 and 66%, depending on the type, intensity, and duration of exercise as well as nutritional status (20, 27, 28), compared with only 2–5% in the general population. Although low energy availability has been implicated as a cause of disruption of patterns of luteinizing hormone (LH) pulsatility in normally cycling adult women (21), specific endocrine factors that link suboptimal energy availability in athletes to hypothalamic amenorrhea and impaired gonadotropin-releasing hormone (GnRH) secretion have not been well characterized. These determinants of menstrual disruption need to be better elucidated, particularly given the impact of hypogonadism on bone density during the adolescent and young adult years (1, 7) and the potential impact on peak bone mass.
We have previously reported markedly lower fat mass and fasting leptin and higher fasting ghrelin in another cohort of normal-weight adolescent amenorrheic athletes (AA) compared with eumenorrheic athletes (EA) and nonathletic controls despite similar activity levels in AA and EA (6, 7). Leptin is secreted by adipocytes and is regulated by energy status; levels are low in conditions of low fat mass (6, 24) and increased physical activity (13). Low leptin levels are associated with hypogonadism in both animals and humans, and overexpression of leptin in mice causes early-onset puberty (10, 34). The impact of leptin on GnRH secretion is independent of kisspeptin and appears to be mediated by interneurons (9). In addition, leptin administration induces ovulation in GnRH-deficient mice (4) and can reverse hypothalamic amenorrhea in at least some adult women (33). Low leptin levels in AA consequent to low fat mass may contribute to altered GnRH pulsatility (as reflected by LH pulse parameters) and thus to hypothalamic amenorrhea in these young women. However, leptin secretory parameters in relation to LH secretory parameters have not been studied in adolescent and young adult AA compared with EA and nonathletes.
Ghrelin is another hormone that reflects energy status and is high in conditions of low weight (23) and in conditions associated with low fat mass despite overall normal weight (7). Ghrelin levels are associated inversely with fat mass (23) and may be regulated by fat. Ghrelin administration leads to a decrease in gonadotropin pulsatility in both animals and humans (17, 18, 32). Thus it is possible that high ghrelin levels in AA (consequent to low fat mass) contribute to altered GnRH and LH pulsatility.
We examined ghrelin and leptin secretory patterns (over an 8-h period, from 11 PM to 7 AM) in relation to LH secretory patterns in AA, EA, and nonathletes 14–21 yr old. We hypothesized that AA would have lower LH and leptin and higher ghrelin basal and pulsatile secretion and total area under the curve (AUC) than EA and nonathletes and that higher ghrelin and lower leptin secretory parameters related to lower fat mass would be associated with lower levels of LH secretory parameters. These data would suggest that alterations in hormones that are either secreted (leptin) or regulated by fat (ghrelin) are a potential link between low fat mass and altered LH secretion in conditions such as athletic amenorrhea.
SUBJECTS AND METHODS
Subject selection.
We enrolled 59 adolescents and young adult women (21 AA, 18 EA, and 20 nonathletes) between 14 and 21 yr of age for this study. We defined amenorrhea as absence of menses for 3 mo within a 6-mo period of oligo/amenorrhea (cycle length >6 wk) or absence of menses at ≥16 yr. We defined EA as those who had had ≥9 menses in the previous year. Inclusion criteria for athletes included ≥4 h of aerobic weight-bearing activity or 20 miles of running weekly for the preceding 6 mo. Inclusion criteria for nonathletes included <2 h/wk of weight-bearing activities. The study was approved by the Institutional Review Board of the Partners HealthCare system. Informed consent was obtained from subjects ≥18 yr old and parents of subjects <18 yr old. Informed assent was obtained from subjects <18 yr old.
Experimental protocol.
Subjects were seen in the Clinical Research Center of Massachusetts General Hospital. The screening visit included a history and physical examination and labs to rule out conditions other than excessive exercise that may cause hypothalamic amenorrhea. Thus, we ruled out hyperprolactinemia, primary ovarian failure, polycystic ovarian syndrome (PCOS; based on clinical/biochemical findings) (3), and hypothyroidism. For PCOS, subjects were assessed using androgen and also sex hormone globulin levels (to obtain estimates of free testosterone) (5). LH/FSH ratios were available; however, we relied primarily on androgen levels to rule out PCOS (3). The Ferriman Gallwey score was used to quantify hirsutism when present, and a score of 8 or more was considered significant. Subjects were also excluded if they were taking hormonal medications that could affect the hypothalamic-pituitary-ovarian axis. All girls (except for 1 AA) were postmenarchal, and the one premenarchal girl had a bone age of 16 yr and was Tanner 5 for breasts, indicating that amenorrhea was not from failure of maturation of the hypothalamic-pituitary-gonadal (HPG) axis.
Qualifying subjects were admitted for an overnight stay at the Clinical Research Center (CRC) of Massachusetts General Hospital. This visit included a history and physical examination, bone age assessment using the methods of Greulich and Pyle (14), body composition assessment using dual energy X-ray absorptiometry [coefficient of variation (CV) for fat mass 1.7%; Hologic 4500A, Waltham, MA], and frequent blood sampling overnight between 11 PM and 7 AM. All subjects had dinner at 8 PM, an intravenous catheter was introduced at 10 PM, and subjects were encouraged to go to bed by 10 PM. Blood sampling was performed every 10 min for LH and every 20 min for ghrelin and leptin. EA and nonathletes were assessed in the early to midfollicular phase of their cycles (based on menstrual history). In addition, energy intake was assessed using a 4-day food record [Minnesota Nutrition Data System software (version 2.93, nutrient database 29; and version 4.03, nutrient database 31)] and energy expenditure using the Bouchard 3-day activity record (16).
Analysis of LH, ghrelin, and leptin secretory dynamics.
We used deconvolution analysis and techniques described previously by Veldhuis and Johnson (31) to determine LH, ghrelin, and leptin basal secretion (basal secretion rate × duration of sampling), number of secretory pulses over the sampling period, interval between secretory pulses, pulse amplitude, pulse mass, total pulsatile secretion (no. of secretory pulses × pulse mass), and total AUC.
Biochemical analysis.
LH levels were assessed in the Harvard Catalyst Core Laboratory using an Access chemiluminescent immunoassay (limit of detection 0.2 mIU/ml, precision 4.3–6.4%; Beckman Coulter, Fullerton, CA). Leptin levels were assayed using a radioimmunoassay (limit of detection 0.1 ng/ml, intra-assay CV 5.2–7.5%, interassay CV 3.2–8.9%; Linco Research, St. Charles, MO). Total ghrelin was measured with an ELISA (limit of detection 100 pg/ml, intra-assay CV 1.32%, interassay CV 6.62%; Millipore). Androgen and sex hormone-binding globulin levels were measured by Labcorp using standard methods. All samples were stored at −80°C until analysis and were tested in duplicate.
Statistical analysis.
We used JMP Software (version 8; SAS Institute, Cary, NC) for data analysis. Results are reported as means ± SE, and a P value <0.05 is considered significant. To determine differences between AA, EA, and nonathletes, we used analysis of variance, followed by the Tukey Kramer test (to control for multiple comparisons). Secretory parameters of LH and leptin (but not ghrelin) pulsatility required logarithmic transformation to approximate a normal distribution. We also used Pearson's correlations to determine associations between covariates and regression modeling to control for potential confounders and to determine independent associations of LH secretory parameters with covariates such as ghrelin and leptin secretory parameters and body mass index (BMI; P = 0.10 to enter and leave the model).
RESULTS
Clinical characteristics.
Clinical characteristics for our subjects are reported in Table 1. The groups did not differ for age, bone age, height, weight, or BMI. Age of menarche was later in AA compared with nonathletes, and fat mass was lower in AA than in EA and nonathletes. Percent body fat was lower in AA than in nonathletes. Energy intake (as assessed using 4-day food records) did not differ among the groups (data not shown). Energy expenditure, as assessed using the Bouchard 3-day questionnaire, was overall higher in athletes than in nonathletes (2,805 ± 169, 3,177 ± 310, and 2,263 ± 87 calories/day in AA, EA, and nonathletes, P = 0.01). Estradiol levels did not significantly differ between the groups.
Table 1.
Clinical characteristics in AA, EA, and nonathletes
| AA (n = 21) | EA (n = 18) | Nonathletes (n = 20) | P Value | |
|---|---|---|---|---|
| Age, yr | 20.0 ± 0.4 | 18.7 ± 0.4 | 19.1 ± 0.4 | NS |
| Bone age, yr | 17.7 ± 0.2 | 17.5 ± 0.2 | 17.6 ± 0.2 | NS |
| Age of menarche, yr | 13.9 ± 0.5 | 13.1 ± 0.3 | 12.2 ± 0.4 | 0.02* |
| Height, cm | 165.9 ± 1.5 | 167.5 ± 2.0 | 164.1 ± 1.3 | NS |
| Weight, kg | 57.5 ± 1.6 | 63.8 ± 2.7 | 58.3 ± 1.7 | NS |
| BMI, kg/m2 | 20.9 ± 0.4 | 22.6 ± 0.6 | 21.7 ± 0.6 | NS |
| Fat, kg | 12.5 ± 0.7 | 15.7 ± 1.1 | 15.7 ± 1.2 | 0.02*† |
| %Body fat | 21.0 ± 1.0 | 23.5 ± 1.0 | 26.3 ± 1.2 | 0.003* |
| Estradiol, pg/ml | 22.2 ± 3.2 | 27.3 ± 5.8 | 32.0 ± 6.5 | NS |
Values are means ± SE.
AA, amenorrheic athletes; EA, eumenorrheic athletes; NAC, nonathletic controls; NS, not significant; BMI, body mass index.
P < 0.05, AA vs. nonathletes;
P < 0.05, AA vs. EA.
Ghrelin, leptin, and LH secretory dynamics.
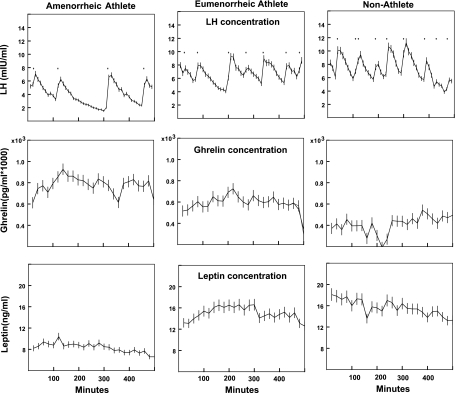
Secretory parameters for our subjects are described in Table 2. AA had lower LH pulse amplitude, total pulsatile secretion, and AUC than nonathletes. Pulse mass trended lowest in AA (P = 0.08). Representative data from an AA, EA, and nonathlete are shown in Fig. 1. Ghrelin secretory pulse parameters were highest in AA. Ghrelin secretory pulse amplitude, pulse mass, total pulsatile secretion, and AUC were higher in AA than in nonathletes, and ghrelin secretory pulse amplitude, pulse mass, and total pulsatile secretion trended higher in AA than in EA. Leptin secretory pulse amplitude, pulse mass, total pulsatile secretion, and AUC were the lowest in AA and significantly lower in AA than in the other two groups.
Table 2.
LH in AA, EA, and nonathletes
| AA (n = 21) | EA (n = 18) | Nonathletes (n = 20) | P Value | |
|---|---|---|---|---|
| LH secretory dynamics | ||||
| Total basal secretion, mIU·ml−1·8 h−1 | 13.66 ± 7.22 | 6.7 ± 1.51 | 12.46 ± 4.94 | NS* |
| Secretory pulse height, mIU/ml | 0.45 ± 0.11 | 0.66 ± 0.11 | 0.87 ± 0.16 | 0.005*† |
| Secretory pulse mass, mIU/ml | 3.06 ± 0.59 | 3.5 ± 0.45 | 4.93 ± 1.95 | NS* |
| Total pulsatile secretion, mIU·ml−1·8 h−1 | 17.44 ± 4.89 | 20.02 ± 2.66 | 29.53 ± 11.72 | 0.02*† |
| AUC, mIU·ml−1·8 h | 1,786 ± 409 | 2,626 ± 391 | 4,008 ± 1676 | 0.02*† |
| Ghrelin secretory dynamics | ||||
| Total basal secretion, pg·ml−1·8 h−1 | 0.060 ± 0.003 | 0.052 ± 0.002 | 0.053 ± 0.003 | NS |
| Secretory pulse height, pg/ml | 30.4 ± 3.3 | 23.1 ± 2.2 | 19.1 ± 1.3 | 0.006†§ |
| Secretory pulse mass, pg/ml | 761.5 ± 83.0 | 578.4 ± 56.1 | 463.2 ± 37.3 | 0.004†§ |
| Total pulsatile secretion, pg·ml−1·8 h−1 | 9,899.7 ± 1078.9 | 7,486.5 ± 729.4 | 6,021.7 ± 484.3 | 0.004†§ |
| AUC, pg·ml−1·8 h | 374,914 ± 44,151 | 290,177 ± 28,719 | 242,735 ± 15,918 | 0.015† |
| Leptin secretory dynamics | ||||
| Total basal secretion, ng·ml−1·8 h−1 | 0.06 ± 0.01 | 5.90 ± 5.85 | 19.09 ± 15.63 | NS* |
| Secretory pulse height, ng/ml | 0.46 ± 0.07 | 0.69 ± 0.08 | 0.69 ± 0.07 | 0.005*†‡ |
| Secretory pulse mass, ng/ml | 11.45 ± 1.81 | 17.26 ± 2.10 | 17.34 ± 1.68 | 0.005*†‡ |
| Total pulsatile secretion, ng·ml−1·8 h−1 | 148.8 ± 23.57 | 223.88 ± 27.39 | 224.24 ± 22.04 | 0.006*†‡ |
| AUC, ng·ml−1·8 h | 4,473.24 ± 691.62 | 7,572.53 ± 902.31 | 8,047.35 ± 820.65 | 0.0006*†‡ |
Values are means ± SE. AUC, area under the curve.
P values and pairwise comparisons reported for log-converted data;
P < 0.05, AA vs. nonathletes;
P < 0.05, AA vs. EA;
P < 0.05–0.10, AA vs. EA.
Fig. 1.
LH, ghrelin, and leptin concentrations over the sampling period in an amenorrheic athlete, an eumenorrheic athlete, and a nonathlete.
Associations of LH, ghrelin, and leptin secretory parameters with BMI and body fat.
Associations of LH, ghrelin, and leptin secretory dynamics with BMI and body fat are shown in Table 3. For the group as a whole, LH pulse amplitude, mass, and total pulsatile secretion were associated positively with percent body fat. These associations were driven primarily by AA, in whom pulse mass and total pulsatile secretion correlated positively with BMI, fat mass, and percent body fat.
Table 3.
Correlations of LH, ghrelin, and leptin secretory parameters with body composition parameters for all subjects and within AA
| Pulse Height |
Pulse Mass |
Total Basal Secretion |
Total Pulsatile Secretion |
AUC |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| LH secretory parameters | ||||||||||
| BMI, kg/m2 | ||||||||||
| All subjects | 0.13 | NS | 0.19 | NS | 0.06 | NS | 0.32 | 0.02 | 0.16 | NS |
| AA | 0.35 | NS | 0.45 | 0.04 | 0.21 | NS | 0.62 | 0.003 | 0.34 | NS |
| Fat mass, kg | ||||||||||
| All subjects | 0.21 | NS | 0.25 | 0.07 | 0.14 | NS | 0.36 | 0.004 | 0.15 | NS |
| AA | 0.40 | 0.07 | 0.49 | 0.02 | 0.28 | NS | 0.63 | 0.002 | 0.24 | NS |
| %Body fat | ||||||||||
| All subjects | 0.30 | 0.03 | 0.28 | 0.04 | 0.21 | NS | 0.37 | 0.005 | 0.13 | NS |
| AA | 0.34 | NS | 0.45 | 0.04 | 0.31 | NS | 0.52 | 0.02 | 0.17 | NS |
| Ghrelin secretory parameters | ||||||||||
| BMI, kg/m2 | ||||||||||
| All subjects | −0.27 | 0.04 | −0.25 | 0.07 | −0.10 | NS | −0.25 | 0.07 | −0.26 | 0.06 |
| AA | −0.32 | NS | −0.32 | NS | 0.06 | NS | −0.32 | NS | −0.26 | NS |
| Fat mass, kg | ||||||||||
| All subjects | −0.29 | 0.04 | −0.29 | 0.04 | 0.01 | NS | −0.29 | 0.03 | −0.26 | 0.06 |
| AA | −0.34 | NS | −0.27 | NS | 0.13 | NS | −0.34 | NS | −0.26 | NS |
| %Body fat | ||||||||||
| All subjects | −0.31 | 0.03 | −0.31 | 0.02 | −0.01 | NS | −0.32 | 0.02 | −0.27 | 0.05 |
| AA | −0.33 | NS | −0.33 | NS | 0.28 | NS | −0.33 | NS | −0.24 | NS |
| Leptin secretory parameters | ||||||||||
| BMI, kg/m2 | ||||||||||
| All subjects | 0.51 | <0.0001 | 0.52 | <0.0001 | 0.22 | 0.10 | 0.52 | <0.0001 | 0.55 | <0.0001 |
| AA | 0.59 | 0.006 | 0.59 | 0.006 | 0.59 | 0.006 | 0.59 | 0.006 | 0.51 | 0.02 |
| Fat mass, kg | ||||||||||
| All subjects | 0.60 | <0.0001 | 0.60 | <0.0001 | 0.36 | 0.006 | 0.59 | <0.0001 | 0.69 | <0.0001 |
| AA | 0.76 | 0.0001 | 0.76 | 0.0001 | 0.38 | NS | 0.76 | 0.0001 | 0.74 | 0.0003 |
| %Body fat | ||||||||||
| All subjects | 0.63 | <0.0001 | 0.63 | <0.0001 | 0.31 | 0.002 | 0.62 | <0.0001 | 0.73 | <0.0001 |
| AA | 0.78 | 0.0001 | 0.78 | 0.0001 | 0.02 | NS | 0.78 | 0.0001 | 0.76 | 0.0001 |
Significant associations are in boldface.
Ghrelin pulse amplitude, pulse mass, total pulsatile secretion, and AUC correlated inversely with fat mass and percent body fat. Leptin secretory dynamics correlated positively and strongly with BMI, fat mass, and percent body fat for the group as a whole and within AA. There were no associations of age, energy intake, or energy expenditure with secretory parameters (data not shown).
Associations of ghrelin and leptin with LH secretory parameters.
Overall, we observed significant inverse associations of ghrelin and positive associations of leptin with LH secretory parameters for the group as a whole and also within AA.
All subjects.
There were inverse associations of ghrelin pulse amplitude with LH pulse amplitude (r = −0.41, P = 0.003), ghrelin pulse mass with LH pulse mass (r = −0.47, P = 0.0004), and total ghrelin pulsatile secretion with total LH pulsatile secretion (r = −0.46, P = 0.0005). There was a weaker association of ghrelin AUC with LH AUC (r = −0. 25, P = 0.07).
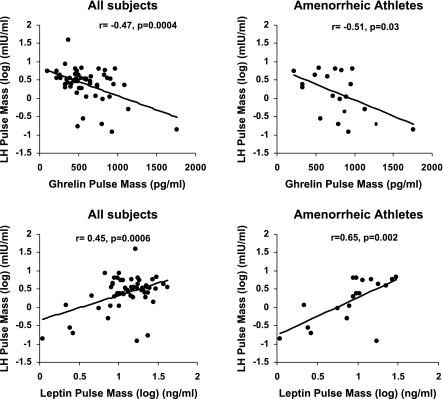
There were positive associations of leptin pulse amplitude with LH pulse amplitude (r = 0.37, P = 0.005), leptin pulse mass with LH pulse mass (r = 0.45, P = 0.0006), total leptin pulsatile secretion with total LH pulsatile secretion (r = 0.54, P < 0.0001), and leptin AUC with LH AUC (r = 0.32, P = 0.02). Figure 2 shows the inverse and positive associations of ghrelin and leptin pulse mass, respectively, with LH pulse mass.
Fig. 2.
Associations between ghrelin and leptin pulse mass with LH pulse mass for the group as a whole and within amenorrheic athletes. Top: inverse association of ghrelin pulse mass with LH pulse mass for all subjects (left) and amenorrheic athletes (right). Bottom: positive associations of leptin pulse mass with LH pulse mass for all subjects (left) and amenorrheic athletes (right). Correlation coefficients and P values are indicated in the figure.
There were also inverse associations of ghrelin pulse amplitude with leptin pulse amplitude (r = −0.53, P < 0.0001), ghrelin pulse mass with leptin pulse mass (r = −0.53, P < 0.0001), ghrelin total pulsatile secretion with leptin total pulsatile secretion (r = −0.53, P < 0.0001), and ghrelin AUC with leptin AUC (r = −0.49, P = 0.0002).
AA.
There was an inverse association of ghrelin pulse mass with LH pulse mass (r = −0.51, P = 0.03) and total ghrelin pulsatile secretion with total LH pulsatile secretion (r = −0.51, P = 0.03). A weaker inverse association of ghrelin pulse amplitude with LH pulse amplitude was observed (r = −0.43, P = 0.07). There were positive associations of leptin pulse amplitude with LH pulse amplitude (r = 0.51, P = 0.02), leptin pulse mass with LH pulse mass (r = 0.65, P = 0.002), and total leptin pulsatile secretion with total LH pulsatile secretion (r = 0.77, P = 0.0001). The inverse and positive associations of ghrelin and leptin pulse mass, respectively, with LH pulse mass in AA are shown in Fig. 2.
As with the larger group, ghrelin pulse amplitude correlated inversely with leptin pulse amplitude, ghrelin pulse mass with leptin pulse mass, and ghrelin pulsatile secretion with leptin pulsatile secretion (r = −0.48, P = 0.046 for all).
EA and nonathletes.
We found no associations of ghrelin or leptin with LH secretory parameters within EA or nonathletes.
Regression modeling to determine independent predictors of LH secretory parameters.
To determine independent predictors of LH secretory parameters, we performed regression modeling with the corresponding ghrelin and leptin secretory parameter and BMI entered into the model. Because of the strong positive associations of fat mass and percent body fat with leptin, we did not add fat mass to the model.
All subjects.
On regression modeling, with ghrelin and leptin pulse amplitude and BMI entered into the model, LH pulse amplitude was independently associated with ghrelin pulse amplitude (but not with leptin pulse amplitude; P = 0.003, r2 = 0.16). Similarly, with ghrelin and leptin pulse mass and BMI entered into the model, LH pulse mass was associated independently and inversely with ghrelin pulse mass (P = 0.03) and positively with leptin pulse mass (P = 0.05) (28% of the total variability explained by ghrelin and leptin pulse mass). For LH total pulsatile secretion, leptin pulsatile secretion (P = 0.002) and ghrelin pulsatile secretion (P = 0.08) were independent positive and negative predictors, respectively (35% of the total variability explained), and for LH AUC, leptin AUC (P = 0.02, r2 = 0.11) was an independent positive predictor. When we replaced BMI with percent body fat in the regression model, these associations remained significant. Additionally, percent body fat was an independent predictor of LH AUC in this model, accounting for an additional 6% of the variability.
AA.
On regression modeling, LH pulse amplitude was independently and positively associated with leptin pulse amplitude (P = 0.06, r2 = 0.20), LH pulse mass with leptin pulse mass (P = 0.004, r2 = 0.41), LH total pulsatile secretion with BMI (P = 0.04) and leptin pulsatile secretion (P = 0.006, 70% of the total variability explained), and LH AUC with BMI (P = 0.08, r2 = 0.21). These associations were not observed within EA and nonathletes. Associations between LH and leptin held even when we replaced BMI with percent body fat in the model.
DISCUSSION
This is the first study to examine LH secretory parameters in relation to ghrelin and leptin secretory parameters in amenorrheic and eumenorrheic adolescent and young adult athletes and also nonathletes. We report lower levels of LH and leptin secretory parameters and higher levels of ghrelin secretory parameters in AA compared with EA and nonathletes. We also report inverse associations of ghrelin and positive associations of leptin with LH secretion.
LH secretory parameters (pulse amplitude, total pulsatile secretion, and AUC) were the lowest in AA. This was despite the fact that EA and controls were assessed in the early to midfollicular phase of their cycles, consistent with low estradiol levels in both groups.
Leptin is an anorexigenic hormone secreted by adipocytes, and adult athletes and overexercisers have low leptin levels (19, 29), which is thought to contribute to reduced LH pulsatility. Adolescence is uniquely characterized by maturation of the HPG axis and changes in fat distribution that may affect adipokine levels. We have previously reported lower fat mass and fasting leptin levels (6) in adolescent AA compared with EA and controls. However, associations between leptin and LH pulsatility in adolescent athletes have not been examined previously. In this study, we observed significantly lower leptin pulse amplitude, pulse mass, total pulsatile secretion, and AUC in AA compared with EA and nonathletes. We also found strong positive associations of most leptin secretory parameters with BMI and fat mass for the group as a whole and within AA. These data suggest that relatively lower BMI and particularly lower fat mass in AA may contribute to lower leptin levels in this group. Although BMI did not differ among the three groups studied, fat mass was significantly lower in AA than in EA and nonathletes, consistent with our previous study (6) and with lower leptin levels in AA.
We found very strong positive associations of leptin secretory parameters with the corresponding LH secretory parameters for the group as a whole and within AA, consistent with our hypothesis that lower leptin levels in AA may contribute to impaired LH pulsatility. Although causality cannot be inferred in this cross-sectional study, a study in adult women with hypothalamic amenorrhea did demonstrate that recombinant human leptin administration improves LH pulsatility patterns and may induce ovulation in at least some women (33). However, not all of the women in that study resumed menses despite administration of robust doses of leptin, and the women in this study were low-weight and not normal-weight overexercisers. More interventional studies are necessary to confirm the contribution of low leptin levels to hypothalamic amenorrhea in normal-weight athletes.
We have reported previously that increases in fat mass predict menstrual recovery better than increases in leptin in adolescent anorexia nervosa, another condition of hypothalamic amenorrhea (25). These data, together with a study of leptin administration in women with hypothalamic amenorrhea in whom only three of the eight women resumed ovulatory menses (33), suggest that fat-related factors other than leptin may also impact the normal functioning of the HPG axis. Interestingly, an animal study reported that re-placing leptin to physiological levels in a state of caloric restriction did not prevent suppression of LH secretion, although re-placing leptin to pharmacological levels was successful in maintaining LH levels (30). The authors concluded that although leptin may be permissive for normal functioning of the HPG axis, additional factors may contribute to hypogonadism in energy deficit states.
Ghrelin, an orexigenic hormone secreted by gastric oxyntic cells, decreases LH pulsatility when administered to animals (26, 32) and humans (17), likely by downregulating kisspeptin expression (12). Ghrelin increases with weight loss (15, 23), and we have reported higher fasting ghrelin in normal-weight adolescent AA compared with EA and controls (6). Similarly, adult overexercisers have an increase in ghrelin (8). Our studies have demonstrated that fat mass is an important determinant of ghrelin (6). An important question is whether higher ghrelin in athletes contributes to decreased LH pulsatility and whether ghrelin is another mediator of the association of low fat mass and a state of hypothalamic amenorrhea in athletes. In this study, we found inverse associations of various ghrelin pulse parameters with fat mass for the group as a whole. These associations were similar in AA but did not reach statistical significance. Ghrelin secretory parameters were associated inversely with LH secretory parameters for the group as a whole and also in AA, suggesting that high ghrelin levels may contribute to decreased LH pulsatile secretion. However, as indicated previously, causality cannot be inferred from these associations in a cross-sectional study.
Consistent with previous studies (24), we observed inverse associations between ghrelin and leptin for the group as a whole and within AA. This raises the question of whether ghrelin or leptin is independently associated with LH secretion after controlling for each other and also for overall energy status as reflected by BMI. In a regression model that included corresponding ghrelin and leptin secretory parameters and BMI, we found that ghrelin and leptin contributed to the variability in LH secretory parameters for the group as a whole independently of each other. Therefore, it appears that in addition to low leptin levels, high ghrelin in AA may contribute to decreased LH pulsatility.
Of note, underlying PCOS can also be a cause of amenorrhea in athletes. The condition is diagnosed using clinical and biochemical criteria, both of which have potential limitations (reviewed in Ref. 3). Hirsutism is quantified by the Ferriman-Gallwey score; however, familial and racial/ethnic factors may also account for some degree of hirsutism. Quantification of acne can be difficult, and some degree of menstrual irregularity is not uncommon in the first 2 yr postmenarche. Testosterone assays are unreliable at levels found in women, and measurements of free and bioavailable testosterone are derived from measurements of testosterone and sex hormone-binding globulin and are thus also prone to error (3). Ruling out lean PCOS thus requires an assessment of clinical and biochemical features in athletes and can be particularly challenging.
A limitation of this study is its cross-sectional nature. Also, we measured total ghrelin in this study, which includes the active acylated and the inactive desacylated forms. However, both the acylated and desacylated forms are effective in suppressing gonadotropins in humans and in animals (22). Thus, total ghrelin is the optimal test when the effects of ghrelin on the HPG axis are assessed. Data for LH, ghrelin, and leptin pulsatility are robust, and differences among the groups are strong. It will be important to assess the impact of menses resumption through increased food intake or reduced energy expenditure or administration of a ghrelin antagonist on LH secretory parameters in AA. A possible limitation is that excessive athletic activity may cause delayed maturation of the HPG axis and thus delayed menarche. However, the only girl in this study who was premenarchal had a bone age of 16 yr and was fully pubertal for breast development. Finally, we did not perform 24-h pulse analysis in our subjects to limit the amount of blood drawn. Nighttime LH pulsatility is suppressed in the early follicular phase in normally cycling women (11), and LH pulse amplitude is higher at night than during the day, whereas LH pulse frequency is higher during the day during the midfollicular phase in late puberty (2). Thus, differences between groups may have been even more marked if we had sampled our subjects during the daytime as well as nighttime hours.
To conclude, this is the first report of LH secretory parameters in adolescent and young adult AA compared with EA and nonathletes. We demonstrate lower pulsatile LH secretion and AUC in AA compared with EA and nonathletes associated with lower fat mass, lower levels of leptin, and higher levels of ghrelin. Our study provides additional data supporting an association between low fat mass, low leptin, and the HPG axis in young women and new information implicating increases in ghrelin in AA (in association with low fat mass) with disruptions in GnRH pulsatility. Studies assessing the impact of a ghrelin antagonist on LH pulse parameters in hypothalamic amenorrhea may be necessary to confirm causality.
GRANTS
This study was supported by National Institutes of Health Grants 1-UL-1-RR-025758-01 and 1-R01-HD-060827-01A1 (Clinical Trial Registration No. NCT00946192).
DISCLOSURES
The authors have no conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
K.E.A., K.S., G.G., L.P., M.S., N.M.E., D.B.H., and M.M. performed the experiments; K.E.A., K.S., G.G., L.P., M.S., N.M.E., and M.M. interpreted the results of the experiments; K.E.A. prepared the figures; K.E.A. drafted the manuscript; K.E.A., K.S., G.G., L.P., M.S., N.M.E., D.B.H., and M.M. approved the final version of the manuscript; K.S. and M.M. analyzed the data; M.M. did the conception and design of the research; M.M. edited and revised the manuscript.
ACKNOWLEDGMENTS
We thank the CRC dieticians for their help with analyzing the food records and exercise questionnaires and the CRC nurses for performing the frequent blood sampling required for this study. We also thank Anne Breggia from Maine Medical Center for analyzing the ghrelin samples. Finally, we thank our study subjects, without whom this study would not have been possible.
REFERENCES
- 1.Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, Bouxsein ML, Misra M. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab 96: 3123–3133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apter D, Butzow TL, Laughlin GA, Yen SS. Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab 76: 940–949, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91: 456–488, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Barkan D, Hurgin V, Dekel N, Amsterdam A, Rubinstein M. Leptin induces ovulation in GnRH-deficient mice. FASEB J 19: 133–135, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Buggs C, Rosenfield RL. Polycystic ovary syndrome in adolescence. Endocrinol Metab Clin North Am 34: 677–705, x, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christo K, Cord J, Mendes N, Miller KK, Goldstein MA, Klibanski A, Misra M. Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: a cross-sectional study. Clin Endocrinol (Oxf) 69: 628–633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christo K, Prabhakaran R, Lamparello B, Cord J, Miller KK, Goldstein MA, Gupta N, Herzog DB, Klibanski A, Misra M. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics 121: 1127–1136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Souza MJ, Leidy HJ, O'Donnell E, Lasley B, Williams NI. Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab 89: 3536–3542, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest 121: 355–368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooqi IS. Leptin and the onset of puberty: insights from rodent and human genetics. Semin Reprod Med 20: 139–144, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Filicori M, Santoro N, Merriam GR, Crowley WF., Jr Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 62: 1136–1144, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Forbes S, Li XF, Kinsey-Jones J, O'Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett 460: 143–147, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Franks PW, Farooqi IS, Luan J, Wong MY, Halsall I, O'Rahilly S, Wareham NJ. Does physical activity energy expenditure explain the between-individual variation in plasma leptin concentrations after adjusting for differences in body composition? J Clin Endocrinol Metab 88: 3258–3263, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Greulich W, Pyle S. Radiographic Atlas of Skeletal Development of the Hand and Wrist, 2nd ed. Stanford, CA: Stanford University, 1959 [Google Scholar]
- 15.Harada T, Nakahara T, Yasuhara D, Kojima S, Sagiyama K, Amitani H, Laviano A, Naruo T, Inui A. Obestatin, acyl ghrelin, and des-acyl ghrelin responses to an oral glucose tolerance test in the restricting type of anorexia nervosa. Biol Psychiatry 63: 245–247, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc 43: 449–456, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab 92: 3202–3205, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated ghrelin inhibits spontaneous luteinizing hormone pulsatility and responsiveness to naloxone but not that to gonadotropin-releasing hormone in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrinol Metab 93: 3633–3639, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Laughlin GA, Yen SS. Hypoleptinemia in women athletes: absence of a diurnal rhythm with amenorrhea. J Clin Endocrinol Metab 82: 318–321, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Loucks AB, Horvath SM. Athletic amenorrhea: a review. Med Sci Sports Exerc 17: 56–72, 1985 [PubMed] [Google Scholar]
- 21.Loucks AB. The response of luteinizing hormone pulsatility to 5 days of low energy availability disappears by 14 years of gynecological age. J Clin Endocrinol Metab 91: 3158–3164, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Martini AC, Fernández-Fernández R, Tovar S, Navarro VM, Vigo E, Vazquez MJ, Davies JS, Thompson NM, Aguilar E, Pinilla L, Wells T, Dieguez C, Tena-Sempere M. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology 147: 2374–2382, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Misra M, Miller Kk, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289: E347–E356, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, Herzog DB, Klibanski A. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab 289: E373–E381, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Misra M, Prabhakaran R, Miller KK, Tsai P, Lin A, Lee N, Herzog DB, Klibanski A. Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res 59: 598–603, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Ogata R, Matsuzaki T, Iwasa T, Kiyokawa M, Tanaka N, Kuwahara A, Yasui T, Irahara M. Hypothalamic Ghrelin suppresses pulsatile secretion of luteinizing hormone via beta-endorphin in ovariectomized rats. Neuroendocrinology 90: 364–370, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Otis C. Exercise-associated amenorrhea. Clin Sports Med 11: 351–362, 1992 [PubMed] [Google Scholar]
- 28.Shangold M, Rebar R, Wentz A, Schiff I. Evaluation and management of menstrual dysfunction in athletes. JAMA 263: 1665–1669, 1990 [PubMed] [Google Scholar]
- 29.Thong FS, McLean C, Graham TE. Plasma leptin in female athletes: relationship with body fat, reproductive, nutritional, and endocrine factors. J Appl Physiol 88: 2037–2044, 2000 [DOI] [PubMed] [Google Scholar]
- 30.True C, Kirigiti MA, Kievit P, Grove KL, Smith MS. Leptin is not the critical signal for kisspeptin or luteinising hormone restoration during exit from negative energy balance. J Neuroendocrinol 23: 1099–1112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veldhuis JD, Johnson ML. Deconvolution analysis of hormone data. Methods Enzymol 210: 539–575, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Vulliémoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M. Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab 89: 5718–5723, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351: 987–997, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fujii S, Nakao K. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest 105: 749–755, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]