Abstract
To discover hypothalamic genes that might play a role in regulating energy balance, we carried out a microarray screen for genes induced by a 48-h fast in male C57Bl/6J mouse hypothalamus. One such gene was Fkbp51 (FK506 binding protein 5; Locus NP_034350). The product of this gene is of interest because it blocks glucocorticoid action, suggesting that fasting-induced elevation of this gene in the hypothalamus may reduce glucocorticoid negative feedback, leading to elevated glucocorticoid levels, thus promoting obese phenotypes. Subsequent analysis demonstrated that a 48-h fast induces Fkbp51 in ventromedial, paraventricular, and arcuate hypothalamic nuclei of mice and rats. To assess if hypothalamic Fkbp51 promotes obesity, the gene was transferred to the hypothalamus via an adeno-associated virus vector. Within 2 wk following Fkbp51 overexpression, mice on a high-fat diet exhibited elevated body weight, without hyperphagia, relative to mice receiving the control mCherry vector. Body weight remained elevated for more than 8 wk and was associated with elevated corticosterone and impaired glucose tolerance. These studies suggest that elevated hypothalamic Fkbp51 promotes obese phenotypes.
Keywords: obesity, glucose tolerance, negative feedback, glucocorticoids, corticosterone
glucocorticoids generally predispose toward obesity and the metabolic syndrome. For example, Cushing's disease, characterized by pathologically elevated cortisol, also causes obesity that is largely resolved after normalizing cortisol by adrenalectomy (2). Similarly, obese phenotypes can be produced by simply administering corticosterone orally (8). Conversely, genetic obesity in mice caused by mutations in the leptin gene or leptin receptors is also characterized by elevated corticosterone, and the obese phenotypes are largely resolved by adrenalectomy (12). The physiological significance of these observations is suggested by the observation that nutritional deprivation reduces plasma leptin and increases corticosterone as well as neuroendocrine responses (e.g., increased appetite and hypothalamic gene expression that predispose toward other obese phenotypes) that are prevented by leptin replacement (1). Of particular interest, adrenalectomy also prevents, and elevation of glucocorticoids mimics, many of these neuroendocrine responses to nutritional deprivation (11).
A key question raised by these studies is what sustains the elevated glucocorticoid secretion characterizing some forms of genetic obesity as well as nutritional deprivation, since glucocorticoids ordinarily exert powerful negative feedback effects in the hypothalamic-pituitary-adrenal (HPA) axis that would be expected to prevent prolonged elevation. Indeed, some evidence supports that obesity in humans entails some reduction in the negative feedback of cortisol (14), suggesting that reduced feedback in the HPA axis may predispose toward obesity. This hypothesis is strongly supported by the observation that reducing negative feedback by direct inhibition of the Type 2 glucocorticoid receptor produces profound obesity (17). However, it has remained unclear how leptin deficiency or nutritional deprivation might reduce negative feedback of the HPA axis and the extent to which this mediates neuroendocrine and obese phenotypes.
In a follow-up screen for hypothalamic genes regulated by nutritional deprivation (13), a DNA microarray study suggested the induction of one hypothalamic gene that might mediate the reduction of negative feedback in the HPA axis. This gene, denoted FK506 binding protein 5 (Fkbp51), was first characterized as coding for a protein product that binds the immunosuppressant FK506 (3). Subsequently, the protein was shown to inhibit glucocorticoid action, and elevated activity of Fkbp51 was shown to cause chronically elevated glucocorticoid secretion in squirrel monkeys (4, 5, 19, 20). These observations suggest that fasting-induced elevation of hypothalamic Fkbp51 expression might mediate sustained hyperactivity of the HPA axis by blocking negative feedback response, and that enhanced hypothalamic expression of this gene might, therefore, predispose to diet-induced obesity. The present studies assessed this hypothesis.
MATERIALS AND METHODS
Animals.
All studies were approved by the appropriate institutional animal review board (Institutional Animal Care and Use Committee). Male C57BL/6J male retired breeders were obtained from The Jackson Laboratory (Bar Harbor, ME) and singly housed with free access to food and water under 12:12-h light-dark cycle (lights on at 7:00 AM). Retired breeders were used because, at that age, body weight is stable on a chow diet, so changes in body weight provide a more reliable indicator of metabolic perturbations. In addition, the metabolic syndrome is primarily of concern in middle-aged rather than young adult individuals (15). Young adult male Sprague-Dawley rats were obtained from Charles River.
Adeno-associated viral vectors.
Murine Fkbp51 open reading frame was PCR amplified from brain cDNA library with a COOH-terminal FLAG tag and subcloned into an adeno-associated virus (AAV) expression plasmid to generate AAV.Fkbp51. A control vector (AAV.mCherry) was designed to express mCherry-FLAG. The expression of both transgenes is regulated by a hybrid cytomegalovirus/chicken β-actin promoter. Virus stocks were prepared by packaging the vector plasmids into AAV serotype 2 (AAV2) particles using a helper-free plasmid transfection system. The vectors were purified using heparin affinity chromatography, dialyzed against PBS supplemented with 2 mM MgCl2, and diluted to 1012 genomic particles per milliliter.
Stereotaxic surgery.
Mice were anesthetized with avertin (Tribromoethanol) and fixed into a stereotaxic frame. Small burr holes were drilled through the skull and a 25-G Hamilton syringe was used to deliver bilaterally 1 μl of 1 × 109 genomic particles of AAV.Fkbp51 or AAV.mCherry to the hypothalamus (n = 6–7/genomic particle). Infusions were delivered to the coordinates: anteroposterior, −1.5; mediolateral, ± 0.4; dorsoventral −6.0 over 10 min using a microsyringe. The needle was left in for an additional 5 min and then withdrawn. Transfer of small hairpin RNA (shRNA) directed to Fkbp51 in rats was accomplished as previously described (9).
In situ hybridization.
In situ hybridization was carried out in rat brains using a riboprobe directed to bases 112–1,452 of the Fkbp51 gene (Genbank NM_001012174), as described (6).
Responses to high-fat diet.
Body weight and food intake on a high-fat diet (4.73 kcal/g; diet composition: 20% protein, 35% carbohydrate, and 45% fat) were measured from day of infusion and recorded weekly for 11 wk. Diet was then switched to standard chow (3.40 kcal/g; diet composition: 23% protein, 64% carbohydrate, and 11% fat), and body weight and food intake were measured weekly for an additional 4 wk.
Hypothalamic microdissection.
Mice were killed by brief exposure to carbon dioxide and then decapitated. Whole brain was quickly dissected, and 1 mm sections were isolated using a brain matrice and razor blades. Sections containing arcuate nucleus (ARC), ventromedial nucleus (VMN), and paraventricular nucleus (PVN) were transferred into RNAlater to stabilize RNA (Applied Biosystems, Foster City, CA) and incubated at 4°C overnight. Regions of interest were microdissected, and tissue was stored in Eppendorf tubes at −80°C.
Extraction of RNA, cDNA synthesis, and quantitative RT-PCR.
RNA was extracted using MagMax-96 Total RNA Isolation Kit, and protocol was according to manufacturer's instructions (Applied Biosystems, Foster City, CA). Using the Superscript Choice system (Invitrogen, Carlsbad, CA), first-strand cDNA synthesis was carried out at 42°C for 60 min using Superscript II reverse transcriptase. Reaction volumes were 20 μl in a 40-cycle three-step PCR using the ABI Prism 7900 thermocycler (Applied Biosystems, Foster City, CA). The PCR master mix contained 1× PCR buffer (20 mM Tris, pH 8.4, 50 mM KCl), 5 mM MgCl2, 200 μM dNTPs, 0.5% (0.05 μl of 100×) SYBRgreen (Molecular Probes, Eugene, OR), 200 μM for each primer pair, and 0.25 units of Platinum Taq (Invitrogen, Carlsbad, CA). Samples were normalized using a primer set for a control transcript (Cyclophilin). Primers for quantitative RT-PCR were designed using the software program MacVector 7.0 (Accelrys). Primer sets incorporated the 3′ end of transcript, guanine-cytosine content between 45 and 55%, and a single amplicon between 100 and 300 bp. All primers were tested with nonquantitative PCR, and products were run on a 1% agarose gel to optimize reaction conditions.
Glucose tolerance test.
Mice were fasted overnight by removing food before lights out (6 PM). The next day, mice were intraperitoneally injected with glucose (2 g/kg body wt). Blood was extracted from the tail vein before glucose injection and 15, 30, 60, and 120 min after injection. Blood glucose levels were determined with Contour blood glucose meter (Bayer).
Corticosterone ELISA.
Blood corticosterone levels were measured using an ELISA from Assay Designs (Ann Arbor, MI).
Immunocytochemical localization of Fkbp51-flag or mcherry-flag.
Mice were perfused with 4% paraformaldehyde and stored at 4°C in 30% sucrose until brains were cut into 30-μm sections. Sections were incubated at 4°C overnight with primary flag antibody (Sigma rabbit, 1:1,000), washed, then incubated with goat anti-rabbit secondary labeled with Alexa Fluor 488 (In Vitrogen, 1:200). A Zeiss upright confocal laser scanning microscope (CLSM710) was used for imaging. A total of 5 × 5 overlapping tiles at a zoom setting of 0.7 were collected (×10 objective, 458-nm excitation laser, image average × four frames) and exported to photoshop, in which minimal adjustments were made to optimize contrast and brightness. Scale bar = 500 μm.
RESULTS
Fkbp51 expression in hypothalamic PVN, VMN, and ARC after fasting in C57BL/6J mice and Sprague-Dawley rats.
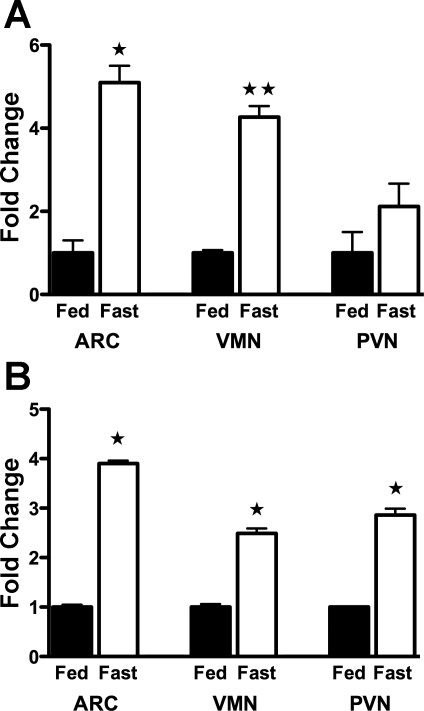
Fkbp51 expression was quantified by quantitative PCR in the PVN, VMN, and ARC. Mice and rats underwent a 48-h fast, while controls were ad libitum fed. The PVN, VMN, and ARC hypothalamus were microdissected, and total RNA was extracted for quantitative PCR. Fkbp51 was induced in the selected hypothalamic regions for both mice and rats (Fig. 1), demonstrating the robustness of Fkbp51 induction by fasting across two rodent species.
Fig. 1.
FK506 binding protein 5 (Fkbp51) mRNA levels in mice and rats by quantitative PCR. C57BL/6J mice fasted for 48 h (A), and Sprague-Dawley rats fasted for 48 h (B). ARC, arcuate nucleus; VMN, ventromedial nucleus; PVN, paraventricular nucleus. Data are calculated as fold change to ad libitum-fed mice. Values are means ± SE (n = 4–6). *P < 0.05 and **P < 0.01 (by Student's t-test).
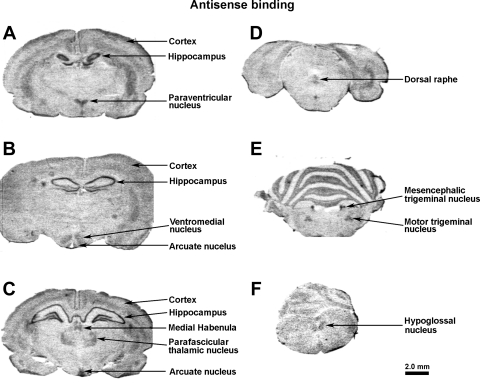
Detailed mapping by in situ hybridization demonstrated that Fkbp51 is highly expressed in several hypothalamic nuclei and in many other brain regions, including the hippocampus (Fig. 2).
Fig. 2.
Localization of Fkbp51 mRNA in rat brain by in situ hybridization. In situ hybridization by riboprobe is directed toward rat Fkbp51 [112–1452 of the Fkbp51 gene (Genbank NM_001012174)], in rostral to caudal order (A–F), as indicated.
Overexpression of Fkbp51 promotes obesity without changing food intake.
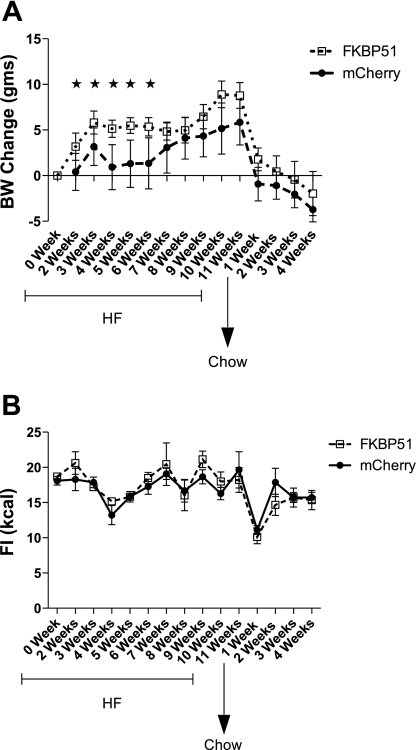
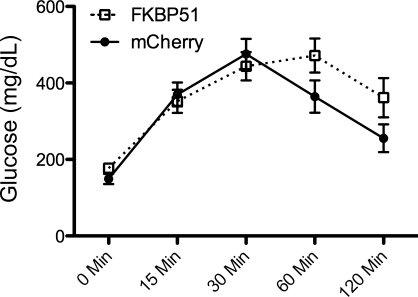
To assess if hypothalamic Fkbp51 plays a role in promoting obesity, the gene was delivered to the hypothalamus (directed toward the VMN and ARC) via an AAV vector. Transfer of Fkbp51 resulted in increased body weight on a high-fat diet relative to mice with the mCherry control vector (Fig. 3A). This increased body weight was not accompanied by increased food intake. Transferring mice to a low-fat diet largely reversed the obesity (Fig. 3B). Consistent with weight gain on the high-fat diet, transfer of Fkbp51 to the hypothalamus impaired glucose tolerance (Fig. 4). In contrast, transferring an shRNA construct that significantly reduced Fkpb51 expression in vitro did not significantly reduce obese phenotypes in a rat model of diet-induced obesity, although the level of expression was only reduced ∼25% (Table 1).
Fig. 3.
Delivery of AAV.Fkbp51 increases body weight (BW) but not food intake (FI). Mice were placed on a high-fat (HF) diet for 11 wk and switched to chow diet for 4 wk. BW (A) and FI (B) were monitored on a weekly basis. Values are means ± SE (n = 6 all groups). *P < 0.05 (by Student's t-test).
Fig. 4.
Fkbp51 overexpression decreases glucose tolerance. Blood samples were collected before injection and 15, 30, 60, and 120 min after injection. Values are means ± SE (n = 6 all groups).
Table 1.
Effect of hypothalamic Fkbp51 shRNA AAV on body weight, intake, adiposity, and plasma glucose in 6-wk-old male Sprague-Dawley rats injected in the hypothalamus with control vs. Fkbp51 shRNA AAV and then fed chow for 28 days and HE diet for 63 days
| Control | Fkbp51 shRNA AAV | shRNA/Control, % | |
|---|---|---|---|
| Body weight, initial, g | 230 ± 6 | 233 ± 3 | NS |
| Body weight gain, 28-day chow, g | 179 ± 7 | 195 ± 7 | NS |
| Intake chow 28 days, kcal | 29,655 ± 86 | 3,009 ± 94 | NS |
| Body weight gain, 63-day HE diet, g | 317 ± 21 | 268 ± 29 | NS |
| Intake HE diet 63 days, kcal | 8,060 ± 234 | 7,573 ± 317 | NS |
| Body weight gain total, g | 501 ± 24 | 499 ± 49 | NS |
| Intake total 91 days, kcal | 47,084 ± 1,664 | 4,592 ± 3,098 | NS |
| Total fat pad/body weight, % | 15.2 ± 0.5 | 13.8 ± 1.1 | NS |
| Trunk glucose, mg/dl | 183 ± 4 | 177 ± 10 | NS |
| VMN Fkbp51 mRNA | 1.21 ± 0.06 | 0.94 ± 0.05 | 75 |
Values are means ± SE.
Fkbp51, FK506 binding protein 5; shRNA, small hairpin RNA; AAV, adeno-associated virus; HE, high energy; VMN, ventromedial nucleus; NS, no significant difference. %Difference P = 0.0006.
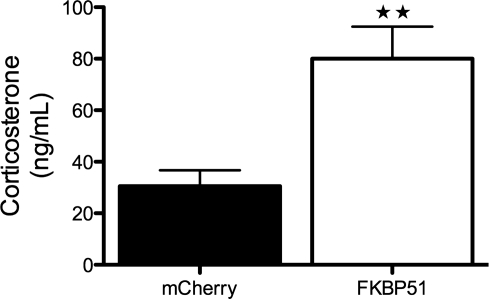
Fkbp51 overexpression induces corticosterone.
One purpose of this study was to determine whether Fkbp51 overexpression would increase corticosterone, consistent with impaired negative feedback in the HPA axis. As shown in Fig. 5, transfer of Fkbp51 to the hypothalamus led to a twofold increase in baseline corticosterone, supporting reduced HPA-negative feedback in this model.
Fig. 5.
Fkbp51 overexpression induces serum corticosterone. Values are means ± SE (n = 6 all groups). **P < 0.015 (by Student's t-test).
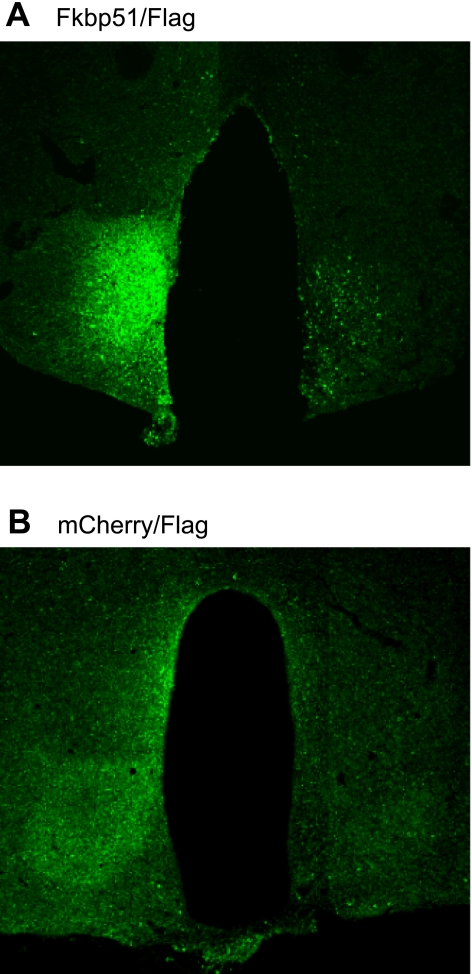
Detailed histological mapping of the expression of Fkpb51 after AAV-mediated gene transfer indicated that the transferred gene was generally expressed in the VMN, less reliably in the ARC, and not appreciably in any other hypothalamic nucleus or brain area, consistent with our laboratory's previous studies (16) (Fig. 6). Consistent with the known tropism of the AAV serotype used in these studies (AAV2), the vast majority of the flag immunoreactivity appeared to be in neurons based on the morphology of the immunoreactive cells.
Fig. 6.
Visualization of Fkbp51-flag (A) or mCherry-flag (B) by flag immunocytochemistry. Flag immunoreactivity was generally observed in the VMN, sometimes in the ARC, and rarely in any other hypothalamic nuclei or other parts of the brain.
DISCUSSION
Our initial screen assessed gene expression in a hypothalamic dissection that includes the VMN and the ARC, a portion of the PVN, but relatively little lateral hypothalamus (18). Extending these studies to microdissected nuclei, we observed that fasting induced Fkbp51 in all of these hypothalamic nuclei in both mice and rats (although not significantly in mouse PVN).
To assess the functional significance of the induction of Fkbp51, we assessed the metabolic and endocrine effects of enhancing expression of the gene using AAV targeted to the medial basal hypothalamus (mainly the VMN and ARC), at the same time switching mice to a high-fat diet. Within 2 wk (about as long as it takes for AAV to express maximally), hypothalamic overexpression of Fkbp51 enhanced weight gain on the high-fat diet, relative to mice given the mCherry control vector in the absence of significant increase in food intake. This elevated weight gain persisted during the entire period the mice were maintained on a high-fat diet. When mice were switched to a low-fat diet, all mice lost weight, and the difference between experimental and control mice, while maintaining the previous trend, was no longer significant. The enhanced weight in the Fkbp51-expressing mice was associated with impaired glucose tolerance and, of particular interest, persistently elevated plasma corticosterone levels. Although we did not observe that transfer of an shRNA construct to the hypothalamus reduced diet-induced obesity, it has been reported that Fkbp51−/− mice resist diet-induced obesity (21).
These results are consistent with the hypothesis that the effects of nutritional deprivation to persistently increase HPA activity is due, at least in part, to elevation of Fkbp51 expression in the medial basal hypothalamus. However, this conclusion is subject to several caveats. First, although overexpression of Fkbp51 targeted to the whole hypothalamus did increase baseline levels of plasma corticosterone, as anticipated, we have not yet demonstrated that reducing plasma corticosterone would reverse sensitivity to diet-induced obesity. However, our laboratory has previously demonstrated that, while adrenalectomy would reverse obese phenotypes in genetic obesity due to leptin deficiency (12), it does not prevent diet-induced obesity in normal wild-type mice (10). Whether adrenalectomy would prevent the excess weight gain on a high-fat diet caused by overexpression of Fkbp51 remains to be determined. A second caveat is that, although the serotype of AAV (AAV2) is thought to transfect neurons almost exclusively (7), we did not demonstrate this conclusively in the present study, so some of the effects may have been mediated via glial expression of Fkbp51. Nevertheless, the present studies strongly support that elevated expression of Fkbp51 in the hypothalamus does enhance sensitivity to diet-induced obesity and impaired glucose tolerance, without reducing food intake, in association with increased plasma corticosterone levels.
GRANTS
These studies were supported by R24 National Institute of Diabetes and Digestive and Kidney Diseases Grant DK045735-17S1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.Y., F.I., S.P.K., W.J., and A.D.-M. performed experiments; L.Y. and C.V.M. analyzed data; L.Y. and C.V.M. interpreted results of experiments; L.Y. and C.V.M. prepared figures; L.Y. and C.V.M. drafted manuscript; L.Y., K.Y., and C.V.M. edited and revised manuscript; L.Y. and C.V.M. approved final version of manuscript; K.Y., X.F., J.M., B.E.L., R.M., R.S., S.M., and C.V.M. conception and design of research.
ACKNOWLEDGMENTS
We appreciate the advice and discussions from Dr. Vanessa Routh.
REFERENCES
- 1. Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Ashizawa N, Takagi M, Seto S, Suzuki S, Yano K. Serum adiponectin and leptin in a patient with Cushing's syndrome before and after adrenalectomy. Intern Med 46: 383–385, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Baughman G, Wiederrecht GJ, Campbell NF, Martin MM, Bourgeois S. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol 15: 4395–4402, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denny WB, Prapapanich V, Smith DF, Scammell JG. Structure-function analysis of squirrel monkey FK506-binding protein 51, a potent inhibitor of glucocorticoid receptor activity. Endocrinology 146: 3194–3201, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology 141: 4107–4113, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51: 2056–2065, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, During MJ. Long-term gene expression and phenotypic correction using adeno- associated virus vectors in the mammalian brain. Nat Genet 8: 148–154, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 151: 2117–2127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin BE, Becker TC, Eiki J, Zhang BB, Dunn-Meynell AA. Ventromedial hypothalamic glucokinase is an important mediator of the counterregulatory response to insulin-induced hypoglycemia. Diabetes 57: 1371–1379, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Makimura H, Mizuno TM, Beasley J, Silverstein JH, Mobbs CV. Adrenalectomy stimulates hypothalamic proopiomelanocortin expression but does not correct diet-induced obesity. BMC Physiol 3: 4, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makimura H, Mizuno TM, Isoda F, Beasley J, Silverstein JH, Mobbs CV. Role of glucocorticoids in mediating effects of fasting and diabetes on hypothalamic gene expression. BMC Physiol 3: 5, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makimura H, Mizuno TM, Roberts J, Silverstein J, Beasley J, Mobbs CV. Adrenalectomy reverses obese phenotype and restores hypothalamic melanocortin tone in leptin-deficient ob/ob mice. Diabetes 49: 1917–1923, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Mastaitis JW, Wurmbach E, Cheng H, Sealfon SC, Mobbs CV. Acute induction of gene expression in brain and liver by insulin-induced hypoglycemia. Diabetes 54: 952–958, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Mattsson C, Reynolds RM, Simonyte K, Olsson T, Walker BR. Combined receptor antagonist stimulation of the hypothalamic-pituitary-adrenal axis test identifies impaired negative feedback sensitivity to cortisol in obese men. J Clin Endocrinol Metab 94: 1347–1352, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Mizuno T, Shu IW, Makimura H, Mobbs C. Obesity over the life course. Sci Aging Knowledge Environ 2004: re4, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A 104: 2501–2506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pepin MC, Pothier F, Barden N. Impaired type II glucocorticoid-receptor function in mice bearing antisense RNA transgene. Nature 355: 725–728, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Poplawski MM, Mastaitis JW, Yang XJ, Mobbs CV. Hypothalamic responses to fasting indicate metabolic reprogramming away from glycolysis toward lipid oxidation. Endocrinology 151: 5206–5217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scammell JG. Steroid resistance in the squirrel monkey: an old subject revisited. ILAR J 41: 19–25, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Scammell JG, Westberry JM, Sadosky PW, Hubler TR, Williams LE, Gibson SV, Singh RJ, Taylor RL, Shackleton CH. Cortisol metabolism in the Bolivian squirrel monkey (Saimiri boliviensis boliviensis). Comp Med 56: 128–135, 2006 [PubMed] [Google Scholar]
- 21. Warrier M. Role of FKBP51 and FKBP52 in Glucocorticoid Receptor Regulated Metabolism. Toledo, OH: University of Toledo, 2008 [Google Scholar]