Abstract
Chronic high flow can induce arterial remodeling, and this effect is mediated by endothelial cells (ECs) responding to wall shear stress (WSS). To assess how WSS above physiological normal levels affects ECs, we used DNA microarrays to profile EC gene expression under various flow conditions. Cultured bovine aortic ECs were exposed to no-flow (0 Pa), normal WSS (2 Pa), and very high WSS (10 Pa) for 24 h. Very high WSS induced a distinct expression profile compared with both no-flow and normal WSS. Gene ontology and biological pathway analysis revealed that high WSS modulated gene expression in ways that promote an anti-coagulant, anti-inflammatory, proliferative, and promatrix remodeling phenotype. A subset of characteristic genes was validated using quantitative polymerase chain reaction: very high WSS upregulated ADAMTS1 (a disintegrin and metalloproteinase with thrombospondin motif-1), PLAU (urokinase plasminogen activator), PLAT (tissue plasminogen activator), and TIMP3, all of which are involved in extracellular matrix processing, with PLAT and PLAU also contributing to fibrinolysis. Downregulated genes included CXCL5 and IL-8 and the adhesive glycoprotein THBS1 (thrombospondin-1). Expressions of ADAMTS1 and uPA proteins were assessed by immunhistochemistry in rabbit basilar arteries experiencing increased flow after bilateral carotid artery ligation. Both proteins were significantly increased when WSS was elevated compared with sham control animals. Our results indicate that very high WSS elicits a unique transcriptional profile in ECs that favors particular cell functions and pathways that are important in vessel homeostasis under increased flow. In addition, we identify specific molecular targets that are likely to contribute to adaptive remodeling under elevated flow conditions.
Keywords: high shear stress, microarray, vascular remodeling, intracranial aneurysm initiation, outward remodeling
in the throats of stenoses (38), in arteries feeding arteriovenous fistulae (21), and in collateral arteries secondary to arterial blockages (39, 48), flowing blood exerts particularly high levels of frictional drag, or fluid wall shear stress (WSS), on the vessel wall. High WSS usually results in expansive remodeling of the artery to compensate the elevated flow and restore WSS back to physiological normal levels. However, little is known about how endothelial cells (ECs), which form the layer that is in direct contact with flowing blood, respond to WSS that exceeds the values in normal straight arterial segments (1.5–2.5 Pa) (17, 24). To this date, most studies of endothelial responses to flow have focused on ECs exposed to lower WSS (0.1–0.05 Pa), because such hemodynamics are believed to play an important role in atherogenesis (16, 51). Low to normal WSS (up to 2.5 Pa) has been shown to modulate EC morphology, cytoskeletal structure, ion channel activation, and gene expression, but very little is known about the effects of genuinely high WSS; i.e., WSS > 5 Pa.
In the intracranial vasculature, even higher WSS (ranging from ∼10 to 30 Pa) exists chronically at specific locations that are prone to aneurysm formation; i.e., at the apices of bifurcations and on the outer sides of curvatures (1, 23). We have recently shown aneurysmal remodeling and aneurysm initiation induced by increased flow at the basilar terminus in rabbits (11, 27, 31). The remodeling occurs specifically where there is a positive spatial gradient in WSS and the local WSS exceeds a certain threshold. This indicates that high WSS plays a key role in inducing the maladaptive remodeling associated with intracranial aneurysms (31). Because only the endothelium is in direct contact with flowing blood, this process is almost certainly mediated by EC responses to high WSS.
While there is scarce knowledge about the effects of very high WSS on ECs, WSS is known to modulate the expression levels of a number of endothelial genes involved in vascular reactivity. For example, when ECs are exposed to laminar WSS of 1.5–2.5 Pa, as opposed to either low or disturbed flow, they exhibit reduced expression of genes that code for several proatherogenic factors and enter a more quiescent, anti-inflammatory, anti-thrombotic, and anti-proliferative state (5, 24). However, the effects of very high WSS, i.e., well above physiologically normal, have not been examined. In the absence of a fundamental understanding of how ECs respond to very high WSS and how this may be related to adaptive or maladaptive vascular remodeling, we conducted the present study to elucidate the transcriptional responses of vascular ECs to high WSS on the order of 10 Pa.
MATERIALS AND METHODS
Cell culture.
Bovine aortic ECs were cultured as previously described (29, 43). These cells maintain their endothelial phenotype, as indicated by expression of Factor VIII, platelet endothelial cell adhesion molecule-1 (PECAM-1), endothelial nitric oxide synthase (eNOS), and VEGF receptor; binding of low-density lipoprotein; formation of monolayers that maintain barrier function as demonstrated by electric resistance and protein permeability assays; tube formation on matrigel; and angiogenesis-like migration in collagen gels (18, 22, 25, 29). ECs were seeded on sterilized uncoated Fisherbrand Microscope Cover Glass (Fisher Scientific) and allowed to grow to confluence for 5 days before exposure to flow. Twenty-four hours before the flow experiment commenced, the culture medium was replaced with culture medium containing a lower percentage of fetal bovine serum (5%) and to which dextran 70 (Sigma) was added to increase the viscosity of the media to 3.5 cP at 37°C, equivalent to that of blood. This medium was used as the perfusion fluid in all flow experiments.
Shear stress exposure.
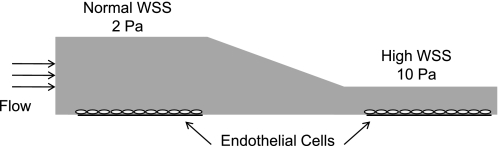
To study the effect of high WSS compared with normal WSS on ECs in time-matched experiments, a modified version of the flow chamber originally described in Metaxa et al. (29) was designed and employed. The analytic solution for WSS, τ, in a channel of constant height is given by τ = 6μQ/wh2, where μ is viscosity, Q is volumetric flow rate, w is channel width, and h is channel height (2). This relationship, valid for Newtonian laminar flow in a high aspect ratio (w >> h) channel, was used to estimate chamber dimensions, and the exact WSS distributions were then determined by computational fluid dynamic (CFD) simulations, as described in Szymanski et al. (43), except that extruded quad meshes were used as computational grids. The chamber (Fig. 1) used a steady flow rate of 918 ml/min, constant chamber width of 22 mm, and a varying height, with an initial parallel section (h = 2.8 mm) followed by a tapering height into a narrower second parallel section (h = 1.2 mm). This created a normal WSS of 2 Pa in the first parallel section and a high WSS of 10 Pa in the downstream parallel section. Flow through the entire chamber and especially in the high-WSS test section was laminar, as calculated by the low Reynolds number and CFD simulations. Specifically, the Reynolds number was 356 in the first parallel section and increased to 380 in the second parallel section. Simulations of the fluid streamlines through the chamber were highly uniform and parallel to the long axis of the channel and gave no indication of flow instability as occurs during turbulent flow. Pressure dropped ∼8 mmHg through the chamber. The materials and fabrication steps used for this chamber, as well as the setup of the flow loop systems, are described in Dolan et al. (9).
Fig. 1.
Schematic of the flow chamber. Flow enters a parallel section with uniform wall shear stress (WSS) of 2 Pa and then tapers to a second parallel section with uniform WSS of 10 Pa.
In each flow experiment, a total of six coverslips were used for three different flow conditions: two coverslips were placed under normal WSS, two under high WSS, and two were kept as static (no flow) controls. At the end of 24-h shear exposure, the coverslips were observed at ×10 magnification under phase contrast to confirm that cells remained adherent. Culture medium was then aspirated, and total RNA was extracted from each set of coverslips using RNeasy Mini kit (Qiagen) with on-column genomic DNA elimination treatment (Qiagen) for microarray and PCR analysis. Each set of isolated total RNA was separated into two portions: one for microarray analysis, and one for quantitative PCR (qPCR). A total of three independent flow experiments were performed following this method, creating nine samples for microarray analysis (from 3 separate runs for each flow condition).
Microarray analysis.
The concentration and purity of total RNA samples were determined using a NanoDrop Spectrophotometer (Thermo Scientific) and integrity verified using an Agilent Bioanalyzer 2100 (Agilent Technologies). Microarray processing of samples was conducted at the University at Buffalo Next-Generation Sequencing and Expression Analysis Core Facility, located at the New York State Center of Excellence in Bioinformatics and Life Sciences. RNA was transcribed to cDNA and biotin-labeled using GeneChip 3′ IVT Express Kit (Affymetrix). Since Affymetrix is a single-channel microarray platform, each of the nine samples was hybridized to its own GeneChip Bovine Genome Array (Affymetrix) containing 24,072 probe sets. Hybridized arrays were stained with streptavidin phycoerythrin conjugate and scanned using Affymetrix GeneChip Scanner and software. The raw data has been deposited into NCBI Gene under accession number GSE29376.
All analyses were performed in R/Bioconductor (12). Full analysis code is available on request (fjsim@buffalo.edu). Affymetrix bovine CEL files were preprocessed and normalized using RMA (15). Informative probe sets were determined using Factor Analysis for Robust Microarray Summarization (FARMS), which uses probe level information as repeated measures to quantify the signal-to-noise ratio of each given probe set (44). Probe sets are called as informative when many of the probes within a probe set correlate with one another with respect to changes in expression across unclassified samples. This method has been shown to be a robust means by which to exclude poor-quality data that does not rely on subjective determination of thresholds of, for example, relevant present/absent calls. Using FARMS, 7,565 probe sets or ∼31% were classified as informative and were used in all further analyses. Sample-to-sample data exploration was performed using unsupervised hierarchical clustering and principle component analysis. Normal-flow and high-flow samples clustered distinctly from one another and from the no-flow samples. Differential gene expression analysis was performed using a linear model approach and employing an empirical Bayes method for calculation of statistical significance (Bioconductor, limma package) (41).
Pathway-based functional analysis.
We employed two distinct types of functional analysis. First, using the set of genes identified as differentially expressed, annotated genes were subdivided into five functional categories based on Gene Ontology (GO)-based molecular function annotations. These annotations were further refined manually using PubMed and other literature-based sources and include ligand (GO:0005102), receptor (GO:0004872), transcription factor (GO:0006355, GO:0030528, GO:0003677), extracellular matrix (ECM) and cell adhesion (GO:0005578, GO:0007155), and enzyme (GO:0003824, GO:0006096, GO:0016125). Hypergeometric testing of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was performed using the GOstats package (P < 0.05). Second, gene set enrichment analysis (GSEA) was performed using parametric GSEA (PGSEA package) (17a) using both GO and KEGG databases. The linear model, described above, was then used to generate a moderated t-test statistic and false discovery rate (FDR) corrected P values associated with specific gene set enrichment (5% FDR).
qPCR.
cDNA was synthesized from samples using QuantiTect Reverse Transcriptase Kit (Qiagen), according to the manufacturer's instructions. qPCR was performed with primers specific to bovine PLAT (tissue plasminogen activator), PLAU (urokinase plasminogen activator), ADAMTS1 (a disintegrin and metalloproteinase with thrombospondin motif-1), IL-8 (interleukin-8), TIMP3 (tissue inhibitor of matrix metalloproteinases-3), CXCL5 [chemokine (C-X-C motif) ligand-5], THBS1 (thrombospondin-1), and 18SrRNA. Primers were designed using Primer 3 software (MIT Whitehead and Howard Hughes Medical Institute, http://frodo.wi.mit.edu/primer3/primer3_code.html) and manufactured by Invitrogen (Grand Island, NY). The MyiQ Single-Color Real-time PCR Detection System (Bio-Rad) was implemented to detect PCR products using SYBR Green Supermix (Bio-Rad). The conditions for qPCR reaction included an initial denaturing at 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s. Three replicate amplifications were used for each sample, and a melting-curve analysis was performed to confirm the absence of primer dimers and other nonspecific PCR products. The amplification efficiency of each of the primer sets was tested by performing serial dilutions on a single DNA sample, and the number of cycles required to reach threshold for each dilution was determined. Primer sequences and efficiencies are shown in Table 1. The relative abundance of transcript expression was calculated by the 2−ΔΔCt (cycle threshold) method, and the expression data were normalized to 18srRNA. Results are presented as either the ratio of the concentration of mRNA relative to 18SrRNA mRNA (2−ΔCt) or as ΔΔCt values. Statistical analysis was performed on ΔCt values, and P values (P ≤ 0.05) were calculated using a paired t-test.
Table 1.
Primer sequences and efficiencies
| Gene | Primer Sequence (5′ to 3′) | Efficiency |
|---|---|---|
| ADAMTS1 | tgaatggcgacttcactctg | 0.93 |
| gaaagtagggatggcgttga | ||
| TIMP3 | gcttgggttgaccagatgtt | 1.05 |
| tccaggaggaaatcttgtgg | ||
| PLAT | tgctgcacctgaaatcagac | 0.97 |
| tgttggtgacggtcctgtta | ||
| PLAU | gaggtggttttgcttgtggt | 0.93 |
| tgggtctccttccacctatg | ||
| THBS1 | acacgactgcaacaagaacg | 1.01 |
| ggttggggcaattatccttt | ||
| CXCL5 | agacaagaagcaagggctca | 1.01 |
| agcaaatacggagcaggaaa | ||
| IL-8 | gaagagagctgagaagcaagatcc | 1.01 |
| acccacacagaacatgaggc | ||
| 18srRNA | agaaacggctaccacatcca | 0.97 |
| caccagacttgccctcca |
See text for definitions of gene acronyms.
In vivo high-flow model.
Adult female New Zealand White rabbits underwent bilateral common carotid artery (CCA) ligation to increase flow through the basilar artery (BA), as previously described (11, 14, 27). In rabbits, this manipulation results in a 2.5-fold increase in WSS through the BA that does not return to normal until 14–28 days after surgery (14). In sham control rabbits, the CCAs were exposed but not ligated. Rabbits were euthanized by intravenous administration of 100 mg/kg of pentobarbital sodium 5 days after the operation. All care and procedures were performed in accordance with institutional guidelines, as approved by the local Institutional Animal Care and Use Committee.
Immunohistochemistry.
Following death, the vertebral arteries were perfused with phosphate-buffered saline and pressure fixed at 150 mmHg with 10% formalin for 30 min. The entire brain was then removed and placed in 10% formalin for 24 h. The BA was excised, embedded in paraffin, and sectioned longitudinally into 4-μm-thick sections. For immunofluorescent staining, sections were deparaffinized, rehydrated, and boiled in citrate buffer for antigen retrieval. Sections were incubated with goat anti-human urokinase (American Diagnostica) or goat anti-human ADAMTS1 (R&D Systems), followed by a bovine anti-goat Dylight 649 conjugated secondary. Sections were costained with a mouse monoclonal antibody to PECAM-1 (a gift from Dr. Peter Newman) and visualized with donkey anti-mouse rhodamine-conjugated secondary to aid in identifying the EC layer. Sections were mounted with media containing 4,6-diamidino-2-phenylindole for staining cell nuclei. Fluorescent images were taken at ×20 using a Zeiss Axio Imager Z1 microscope.
Quantification of staining in ECs was performed using National Institutes of Health ImageJ software as follows. The background was subtracted from each image, then ECs were identified based on nuclei and PECAM-1 staining, and manually traced in the ADAMTS1 and urokinase plasminogen activator (uPA) images to segment them from the adjacent medial layer. The mean intensity in the separated EC layer was then determined and averaged across samples. An unpaired t-test was used to determine if a statistically significant difference (P ≤ 0.05) in mean intensity existed between the BA of sham-operated control and 5-day ligated animals.
RESULTS
High WSS induces a distinct gene expression profile.
To form an initial understanding of the effect of high WSS on EC gene expression, we used microarray analysis for gene expression profiling of ECs exposed to static (no flow), normal WSS (2 Pa), and high WSS (10 Pa) conditions for 24 h. Using a linear model approach, we identified differentially expressed genes as those genes that had at least twofold change in expression between compared samples and were statistically significantly regulated using a FDR adjusted P value of 1%. A complete list of the regulated genes between high WSS and static controls, normal WSS and static controls, and normal WSS and high WSS is provided in the Supplementary Materials (Supplemental Tables S1, S2, and S3 respectively; the online version of this article contains supplemental data).
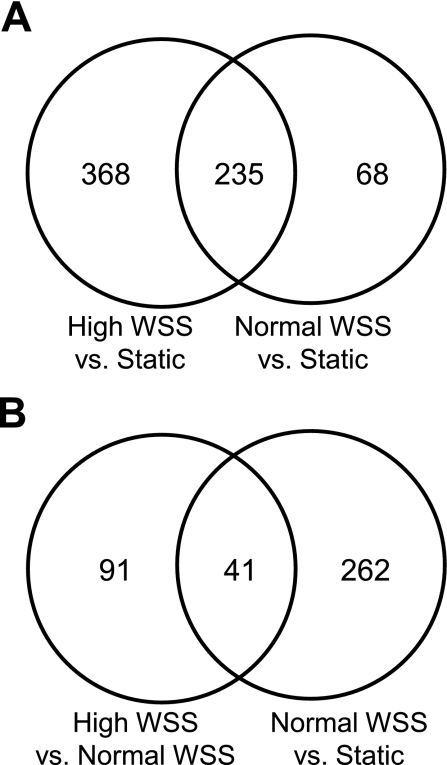
We first compared ECs exposed to high WSS and those exposed to static conditions. Initial microscopic examination of ECs under high WSS indicated that cells elongated and aligned their long axes perpendicular to the flow direction, whereas static cells were polygonal and unaligned (data not shown). Our finding is an agreement with previous studies demonstrating perpendicular alignment of ECs under high WSS of 10 and 12.8 Pa (29, 49). Gene expression analysis revealed that 603 genes were differentially expressed between high WSS and static conditions (Table 2). Since it is well recognized that normal WSS affects EC morphology and gene expression (5, 24, 33), we then compared normal WSS to static conditions. Microscopic assessment of EC morphology indicated that normal WSS induced cell elongation and alignment to the flow direction (data not shown) as expected (24). Furthermore, the comparison of normal WSS to static WSS indentified 303 differentially expressed genes (Table 2). Consistent with previous reports, genes involved in regulating cytokine-mediated signaling and endothelial proliferation were downregulated by normal WSS relative to static conditions, while genes involved in maintaining endothelial integrity (20), NOS3 (eNOS) and TEK (TIE2), were upregulated (Supplemental Table S2). Venn analysis indicated that the 603 genes affected by high WSS included 235 genes that were differentially regulated between normal WSS and static conditions, leaving 368 genes that were affected by high WSS but not normal WSS (Fig. 2A). The 235 shared genes were regulated in the same direction by normal and high WSS and may represent genes whose expression is modulated when ECs experience flow, regardless of whether WSS is normal or high.
Table 2.
Number of genes that are differentially expressed between flow conditions
| High WSS vs. Static | Normal WSS vs. Static | High WSS vs. Normal WSS | |
|---|---|---|---|
| Upregulated | 322 | 145 | 68 |
| Downregulated | 281 | 158 | 64 |
| Total | 603 | 303 | 132 |
Significantly enriched genes were identified by a linear model approach employing an empirical Bayes method at 1% false discovery rate (FDR) and more than twofold change between groups. The full list of differentially expressed genes between these comparisons (from left to right) is available in Supplemental Tables S1, S2, and S3, respectively. WSS, wall shear stress.
Fig. 2.
Venn diagrams of the number of genes that are differentially regulated by various flow conditions. A: genes that are differentially expressed when endothelial cells (ECs) under high WSS (10 Pa) or normal WSS (2 Pa) are compared with static cultures. The overlap of the two circles represents genes whose expression is altered when static ECs encounter flow, regardless of whether WSS is normal or high. B: genes that are expressed when ECs under high WSS or maintained in static culture are compared with normal WSS. The overlap represents genes that are affected when static ECs encounter normal WSS, but also change further, or in the opposite direction, when normal WSS is increased to 10 Pa.
To identify responses that are elicited specifically by high WSS, as opposed to flow in general, we compared ECs under high WSS to those under normal WSS. This yielded 132 differentially regulated genes (Table 2). Venn analysis showed that only 41 of these genes overlapped with those that were differentially expressed in normal WSS vs. static flow conditions (Fig. 2B). Thus 91 genes were uniquely affected by high WSS. Furthermore, of the 41 genes that were regulated by both high and normal WSS, only 25 had expression changes in the same direction (i.e., the effects of high and normal WSS differed only in magnitude). The remaining 16 genes (Supplemental Table S4) were regulated in opposite directions between the two comparisons; i.e., genes that were upregulated in high vs. normal WSS were downregulated in normal vs. static, and vice versa. Thus, high WSS and normal WSS had completely different effects on these 16 genes. In sum, high WSS elicited responses in 107 genes (91 + 16) (Table 3) that were not simply a quantitative variation of the response to normal WSS.
Table 3.
The 107 genes differentially expressed between high and normal WSS, grouped by Gene Ontology molecular functions
| Genes | Expression Ratio (Log2 Fold Change) | Adjusted P value |
|---|---|---|
| Enzymes | ||
| ENPP1 | +1.90 | 0.00003 |
| PLAT | +1.67 | 0.00001 |
| PTPRB | +1.54 | 0.00003 |
| HYAL2 | +1.40 | 0.00003 |
| GFPT2 | +1.38 | 0.00018 |
| LOC537017 | +1.22 | 0.00063 |
| DCK | +1.18 | 0.00017 |
| PLAU | +1.08 | 0.00027 |
| PAH | −1.30 | 0.00144 |
| THUMPD3 | −1.20 | 0.00007 |
| NRBP2 | −1.10 | 0.00027 |
| AXL | −1.07 | 0.00042 |
| NQO2 | −1.04 | 0.00032 |
| Ligands | ||
| CXCL5 | −3.03 | 0.00150 |
| TTR | −2.69 | 0.00004 |
| CSF2 | −2.05 | 0.00006 |
| CXCL2 | −2.01 | 0.00071 |
| ADM | −1.61 | 0.00017 |
| BMP4 | −1.59 | 0.00005 |
| SST | −1.50 | 0.00084 |
| IL8 | −1.40 | 0.00074 |
| Receptors | ||
| PTPRR | +2.64 | 0.00001 |
| PROCR | +1.75 | 0.00002 |
| PLAUR | +1.13 | 0.00006 |
| SCARA5 | −2.66 | 0.00006 |
| GHR | −1.60 | 0.00011 |
| NTRK2 | −1.53 | 0.00018 |
| CCRL1 | −1.13 | 0.00388 |
| CXCR4 | −1.02 | 0.00889 |
| Transcription Factors | ||
| BHLHB2 | +1.34 | 0.00024 |
| HOPX | +1.00 | 0.00248 |
| ASCL1 | −2.83 | 0.00047 |
| CITED2 | −1.04 | 0.00158 |
| ECM/cell adhesion | ||
| ADAMTS1 | +3.87 | 0.00001 |
| ADAMTS6 | +1.53 | 0.00005 |
| LYVE1 | +1.33 | 0.00178 |
| TIMP3 | +1.30 | 0.00006 |
| JUP | +1.03 | 0.00021 |
| THBS1 | −1.74 | 0.00053 |
| COL12A1 | −1.39 | 0.00005 |
| TSC1 | −1.17 | 0.00053 |
| Other | ||
| AIF1L | +2.16 | 0.00001 |
| AKAP12 | +1.67 | 0.00017 |
| LOC511659 | +1.49 | 0.00025 |
| LOC538402 | +1.39 | 0.00044 |
| RCAN3 | +1.33 | 0.00139 |
| NPHP1 | +1.32 | 0.00006 |
| MLLT11 | +1.32 | 0.00418 |
| MYOZ2 | +1.30 | 0.00003 |
| RPS6KA3 | +1.26 | 0.00015 |
| LOC529423 | +1.21 | 0.00311 |
| TMEM131 | +1.20 | 0.00012 |
| SERPINB8 | +1.16 | 0.00015 |
| HSPA12B | +1.15 | 0.00017 |
| HMCN1 | +1.14 | 0.00859 |
| RGS2 | +1.14 | 0.00019 |
| CLIC2 | +1.13 | 0.00006 |
| DPYSL5 | +1.13 | 0.00039 |
| FAM134B | +1.12 | 0.00014 |
| MRAS | +1.12 | 0.00007 |
| FXYD3 | +1.10 | 0.00053 |
| ZDHHC23 | +1.06 | 0.00115 |
| LOC788353 | +1.05 | 0.00021 |
| KLF4 | +1.04 | 0.00158 |
| PLN | +1.04 | 0.00072 |
| ARHGEF15 | +1.02 | 0.00014 |
| LOC512304 | +1.02 | 0.00086 |
| HSPA12A | +1.02 | 0.00053 |
| ORAI1 | +1.02 | 0.00024 |
| SIRPA | +1.01 | 0.00011 |
| CLEC14A | +1.01 | 0.00030 |
| INPP4B | +1.01 | 0.00282 |
| DBNDD2 | +1.01 | 0.00093 |
| RSF1 | −1.98 | 0.00016 |
| ARHGEF11 | −1.90 | 0.00077 |
| RPRM | −1.60 | 0.00053 |
| LOC538778 | −1.53 | 0.00011 |
| CCL26 | −1.47 | 0.00046 |
| CXCL2 | −1.46 | 0.00078 |
| LOC539374 | −1.42 | 0.00002 |
| SRPX2 | −1.40 | 0.00142 |
| CLCA3P | −1.38 | 0.00013 |
| LOC783538 | −1.28 | 0.00007 |
| FBXO32 | −1.27 | 0.00002 |
| MYLIP | −1.26 | 0.00041 |
| HIVEP1 | −1.24 | 0.00003 |
| WISP2 | −1.23 | 0.00007 |
| RBM3 | −1.22 | 0.00191 |
| RNF183 | −1.22 | 0.00009 |
| ZRANB2 | −1.20 | 0.00011 |
| SEPP1 | −1.16 | 0.00034 |
| RND3 | −1.13 | 0.00006 |
| FAT1 | −1.12 | 0.00015 |
| KBTBD6 | −1.12 | 0.00245 |
| TAGLN | −1.12 | 0.00060 |
| NFKBIZ | −1.12 | 0.00094 |
| PDLIM4 | −1.12 | 0.00009 |
| LOC534839 | −1.06 | 0.00027 |
| LOC781795 | −1.06 | 0.00006 |
| NPC3 | −1.03 | 0.00015 |
| CYB561 | −1.02 | 0.00253 |
| NRG1 | −1.01 | 0.00023 |
| CBLB | −1.01 | 0.00183 |
| PDE4B | −1.00 | 0.00047 |
| FRMD6 | −1.00 | 0.00079 |
| SLC17A9 | −1.00 | 0.00037 |
| MXRA5 | −1.00 | 0.00199 |
Genes are grouped based on Gene Ontology molecular functions with their corresponding expression ratio (log2 fold change) shown. All P values are ≤ 0.009, with gene descriptions given in Supplemental Tables S3 and S4.
High WSS regulates ECM genes.
Having identified a set of genes that are differentially expressed between high WSS and normal WSS, we investigated what functional responses these genes might indicate by classifying regulated genes according to GO-based molecular functions (Table 3). Because WSS is recognized to modulate vascular remodeling, it was particularly notable that several genes upregulated by high WSS code for ECM modulators, including the enzymes PLAT [tissue plasminogen activator (tPA)] and PLAU (uPA) and the receptor for PLAU, PLAU receptor (PLAUR, uPAR). The proteins encoded by PLAU and PLAT directly and indirectly lead to degradation of ECM components. Furthermore, high WSS upregulated members of the ADAMTS family, ADAMTS1 and ADAMTS6. As their names suggest, these are metallopeptidases involved in turnover of the ECM. Also upregulated by high WSS was TIMP3. TIMP3 localizes to the ECM and inhibits ADAMTS1 and members of the matrix metalloproteinases (MMP) family (37).
We also sought to identify EC functional responses by determining what regulatory pathways were particularly represented among the genes responding to high WSS vs. normal WSS. An unbiased pathways analysis for overrepresented KEGG pathways (minimum 2 genes included and 5% FDR) identified the a priori pathways listed in Table 4. Among the pathways suppressed under high WSS is ECM-receptor interactions. Genes encoding both THBS1 (TSP1) and SDC2 (syndecan-2), proteins that mediate cell-to-matrix interactions and adhesion, were downregulated. Decreased adhesion between ECs and the matrix may facilitate cell rearrangement during tissue remodeling.
Table 4.
Pathways that are particularly regulated by high WSS relative to normal WSS
| KEGG Pathway | Hypergeometric P Value |
|---|---|
| Upregulated | |
| Complement and coagulation cascades | 0.001 |
| Amino sugar and nucleotide sugar metabolism | 0.032 |
| Chemokine signaling pathway | 0.0003 |
| Cytokine-cytokine receptor interaction | 0.0004 |
| Downregulated | |
| PPAR signaling pathway | 0.017 |
| TGF-β signaling pathway | 0.027 |
| ECM-receptor interaction | 0.038 |
| Jak-STAT signaling pathway | 0.044 |
Up- and downregulated Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways identified from the set of differentially expressed genes between high-WSS- and normal-WSS-treated cells are given. Hypergeometric testing of KEGG pathways was performed using the GOstats package (minimum 2 genes included and 5% FDR).
PPAR, peroxisome proliferator-activated receptor; TGF-β, transforming growth factor-β; ECM, extracellular matrix; Jak-STAT, Janus kinase-signal transducer and activator of transcription.
High WSS induces anti-inflammatory and anticoagulation pathways.
KEGG pathway analysis also indicated that genes involved in mobilizing innate and adaptive inflammatory defenses were significantly underexpressed in cells exposed to high WSS. Specifically, the chemokine signaling and the cytokine-cytokine receptor interaction pathways were both downregulated by high WSS. Downregulated proinflammatory genes (Supplemental Table S3) include IL-8, GM-CSF (granulocyte-macrophage colony-stimulating factor), CXCL2, BMP4 (bone morphogenetic protein-4), and also CXCL5, which was the gene with the greatest fold decrease between high and normal WSS conditions. The major signaling mechanism for cytokines, the Janus kinase-signal transducer and activator of transcription pathway, was also downregulated by high WSS. Furthermore, the gene coding for endothelial protein C receptor (PROCR, EPCR) was upregulated by high WSS (Table 3). The binding of protein C to this receptor is recognized to elicit anti-inflammatory and cytoprotective signaling responses (35).
KEGG analysis indicated upregulation of genes involved in the complement and coagulation cascade. Specifically, genes involved in the fibrinolytic system were represented and include PLAT, PLAUR, and PLAU. In addition to the role PLAU and PLAT play in matrix degradation, the proteins encoded by these genes are essential to the plasminogen-plasmin activation system. Both PLAU and PLAT convert latent plasminogen into active plasmin, which directly dissolves fibrin clots. PLAUR serves to localize PLAU through ligand-receptor interaction.
High WSS stimulates cell proliferation genes.
To further examine the pathways influenced by exposure to high WSS, we employed parametric GSEA, which considers expression changes across the complete profile of all genes in the microarray (17a). The main benefit of this approach is that it identifies pathways whose genes are coordinately regulated independent of whether the individual genes are otherwise selected as genes of interest. GO-based GSEA (summarized in Table 5, full results in Supplemental Table S5) indicated that high WSS upregulated multiple categories involved in cell proliferation, including cell cycle phase, M-phase, mitotic cell cycle, and mitosis (each significantly <5% FDR). KEGG-based GSEA further confirmed that high WSS induced gene sets involved in cell cycle, DNA replication, and the MAPK signaling pathways (Supplemental Table S6).
Table 5.
Gene Ontology-based gene set enrichment analysis functional annotations for high-WSS endothelial cells relative to normal-WSS endothelial cells
| Functional Category | Log2 Expression Ratio (High WSS/Normal WSS) | Adjusted P Value |
|---|---|---|
| Chromosome | 9.98 | 0.011 |
| Cell cycle phase | 9.70 | 0.012 |
| M phase | 9.70 | 0.012 |
| Metalloendopeptidase activity | 9.24 | 0.009 |
| Chromosomal part | 9.18 | 0.009 |
| Mitotic cell cycle | 8.53 | 0.012 |
| Microtubule cytoskeleton | 8.25 | 0.043 |
| M phase of mitotic cell cycle | 8.24 | 0.012 |
| Mitosis | 8.14 | 0.012 |
| Chloride ion binding | 7.89 | 0.012 |
Representative functional gene categories identified from the high-WSS vs. normal-WSS microarray profile using gene set enrichment analysis and the Gene Ontology database. For the complete list, see Supplemental Table S5.
Additionally, GO-based GSEA indicated enrichment of metalloendopeptidase (Table 5) and metallopeptidase (Supplemental Table S5) activity by high WSS, lending further support of increased matrix processing under high WSS.
Quantitative real-time PCR confirmation.
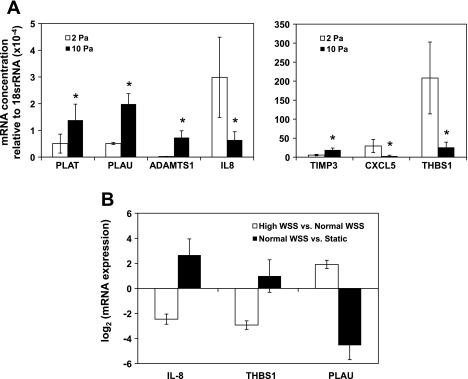
To confirm the differences in gene expression observed in the microarray experiments between high WSS and normal WSS, quantitative real-time PCR was performed. We selected the following seven genes because the above analyses highlighted their possible role in the endothelial response to high WSS: PLAT, PLAU, ADAMTS1, IL-8, TIMP3, CXCL5, and THBS1 (Fig. 3A). Significant differences between high WSS and normal WSS were found for all seven of the genes (PLAT, P = 0.047; PLAU, P = 0.028; ADAMTS1, P = 0.017; IL-8, P = 0.026; TIMP3, P = 0.035; CXCL5, P = 0.011; and THBS1, P = 0.013). Furthermore, there was very close agreement (R2 = 0.9774, P < 0.0001) between the array and qPCR results: PLAT, PLAU, ADAMTS1, and TIMP3 were upregulated, whereas IL-8, CXCL5, and THBS1 were downregulated by high WSS. Furthermore, IL-8, THBS1, and PLAU were 3 of the 16 genes regulated in opposite directions between the high WSS vs. normal WSS and the normal WSS vs. static comparison (Supplemental Table S4) indicated above. Indeed, qPCR demonstrated this switch in the direction of regulation between the two comparisons, since IL-8 and THBS1 were upregulated, while PLAU was downregulated, between normal WSS and static controls (Fig. 3B). Thus quantitative real-time PCR confirmed the accuracy of the microarray data in all cases examined, supporting the validity of the overall genomics dataset.
Fig. 3.
Quantitative PCR validation of microarray results. A: the mRNA concentration of tissue plasminogen activator (PLAT), urokinase plasminogen activator (PLAU), a disintegrin and metallopeptidase with thrombospondin type 1 motif, 1 (ADAMTS1), interleukin-8 (IL-8), tissue inhibitor of matrix metalloproteinase 3 (TIMP3), chemokine (C-X-C motif) ligand 5 (CXCL5), and thrombospondin 1 (THBS1) relative to the housekeeping gene 18SrRNA. B: the mRNA expression ratio (log2 fold change) of IL-8, THBS1, and PLAU under normal vs. static conditions and high vs. normal WSS. Error bars are means ± SE; n = 3. *Statistically significant difference for delta cycle threshold values (P < 0.05, paired t-test).
High flow increases uPA and ADAMTS1 proteins in vivo.
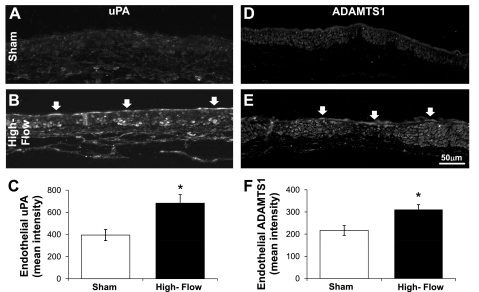
We next tested if matrix remodeling genes were upregulated at the protein level in endothelium exposed to high WSS in vivo. Our laboratory previously demonstrated that bilateral ligation of the CCAs in rabbits increases WSS in the BA by an average of 1.7 times when measured 4 days after surgery (14). Using immunohistochemistry, we examined uPA and ADAMTS1 protein expression in the BA of sham-operated animals (which experienced a baseline WSS) and high-flow rabbits 5 days after surgery when WSS was still high (14). In sham animals, uPA staining was weak and at similar levels in all cell layers of the BA (Fig. 4A). In arteries experiencing increased flow, the endothelial layer displayed much stronger uPA staining (Fig. 4B). The average intensity of staining in the ECs of 5-day ligated animals was significantly higher than that in sham animals (Fig. 4C; P = 0.028). Increased uPA staining was also observed in irregularly distributed patches in the smooth muscle cell layer in the high-flow arteries. Expression of ADAMTS1 was similarly increased by high WSS in vivo. Sham-operated rabbits stained very weakly for ADAMTS1 in all cell layers of the BA, including the ECs (Fig. 4D). In contrast, 5 days after contralateral ligation, ADAMTS1 stained prominently throughout the basilar artery, with widespread distribution in many different cells (Fig. 4E). Measurement of staining specifically in the ECs showed significantly higher intensity in the ECs of 5-day ligated animals compared with the ECs of sham controls (Fig. 4F; P = 0.021). Thus, not only are the genes for uPA and ADAMTS1 upregulated by high WSS in vitro, but expression of the ADAMTS1 and uPA proteins increases in arterial ECs exposed to high WSS in vivo.
Fig. 4.
Stimulation of urokinase plasminogen activator (uPA) and ADAMTS1 expression by WSS in the basilar artery. The basilar artery of either sham rabbits (A and D) or rabbits 5 days after carotid artery ligation (B and E) were stained for uPA (A and B) and ADAMTS1 (D and E) by indirect immunofluorescence. In sham animals, both uPA (A) and ADAMTS1 (D) staining was weak in ECs and the other cells of the basilar artery. uPA staining was intense in 5-day animals and strongly localized to the endothelium (B; open arrow). ADAMTS1 staining increased not only in ECs (E; open arrows), but also in other cells in the 5-day ligated animals. Staining intensity specifically within the ECs was measured as described in materials and methods for uPA (C) and ADAMTS1 (F). Bars represent the average mean intensity ± SE, (n = 5 for both ligated groups and for the uPA sham group; n = 4 for the sham control group for ADAMTS1). *Statistically significant difference between sham and ligated groups (unpaired t-test, P < 0.03).
DISCUSSION
The present study shows that very high WSS (10 Pa) causes ECs to express a set of genes that is quite distinct from both the expression pattern observed under physiological normal WSS of 2 Pa and that elicited by low-WSS conditions that are associated with atherosclerosis. The high-WSS responses we observed could contribute significantly to the regulation of adaptive expansive remodeling that occurs in response to elevated flow in arteries throughout the vascular tree and may also contribute to the natural history of aneurysmal remodeling within the intracranial vasculature, as discussed below.
Flow conditions are known to induce at least two well-recognized phenotypes in ECs. Normal WSS (1.5–2.5 Pa) promotes a quiescent EC state, suppressing proliferation, inflammation, and apoptosis, while promoting a protective anti-thrombotic and selective permeability barrier (5, 13, 24). In our study, we observe a similar result when normal WSS conditions were compared with static culture; i.e., decreased proliferation and cytokine-mediated signaling, but increased expression of genes involved in maintaining vascular homeostasis (NOS3 and TEK). The other phenotype exhibited by ECs occurs under low or no flow (0–0.4 Pa) and bears traits associated with atherogenic events in vivo. Under low or no flow, ECs enter into a high cell turnover, permeable, and procoagulant/prothrombrotic state, coupled with the upregulation of inflammatory mediators, adhesion molecules, and reactive oxygen species and simultaneous downregulation of antioxidant enzymes and vasodilatory molecules (7, 24, 42). The EC responses we saw under very high WSS were substantially different from both the low-flow and normal-flow phenotypes. Specifically, high WSS induced a promatrix remodeling, proliferative, anticoagulant, and anti-inflammatory state.
Induction of matrix remodeling by high WSS may facilitate flow-induced arterial expansion. Exposure of a straight vessel segment to chronically elevated flow, and thus high WSS, typically results in an adaptive expansion of the vessel to accommodate the increased flow and bring WSS back to normal levels. Flow-induced adaptive expansion is characterized by increased luminal diameter with relatively small changes in wall thickness, and temporary and minute fragmentation of the internal elastic lamina (IEL) that is believed to allow the artery to enlarge (40, 46). In the present study, we demonstrated that uPA was significantly increased in ECs when the rabbit basilar artery was exposed to higher than baseline WSS to induce adaptive expansion. uPA degrades fibrin, laminin, and fibronectin, whereas both uPA and tPA generate plasmin to activate MMPs to degrade elastin and collagen. We also observed that high WSS in vitro upregulated genes for ADAMTS1 and ADAMTS6, which share structural homology to MMPs and can directly bind and digest matrix components (34, 37). Furthermore, in vivo ADAMTS1 protein was also significantly increased in ECs during flow-induced expansion of the basilar artery. Tronc et al. (46) used doxycycline and BB-94 to inhibit MMPs and concluded that MMP-2 and -9 were involved in degrading the IEL during flow-induced outward remodeling. Although unknown at the time of their study, those drugs also inhibit ADAMTS proteins (37). Thus ADAMTS1, and perhaps ADAMTS6, might also be important contributors to IEL degradation during expansive remodeling.
A proliferative endothelium under high WSS would also facilitate adaptive remodeling in response to elevated flow. In vivo, Sho et al. (39, 40) have shown that raising WSS to ∼8.5 Pa in rabbit carotid arteries leads to adaptive expansion of the vessels, accompanied by an increase in the number of proliferating ECs. Increased proliferation presumably occurs to maintain endothelial coverage over the larger surface. Our laboratory previously reported that WSS of 3–10 Pa (29) and as high as 28.4 Pa (9) stimulates proliferation in cultured ECs. Thus the upregulation of genes related to proliferation by high WSS reported here is consistent with observed proliferative responses of ECs in other systems, both in vivo and in vitro.
The anticoagulant and anti-inflammatory changes induced in ECs under high WSS may be important in preventing damage to an arterial wall when the vessel is exposed to elevated flow conditions. An essential function of the endothelium under normal circumstances is to prevent platelet aggregation and clot formation. However, increasing WSS can cause platelet activation (32). Upregulation of an anticoagulation gene set (PLAU, PLAT, and PLAUR) may serve as a protective mechanism that inhibits coagulation when flow is unusually high. Likewise, anti-inflammatory behavior could help prevent the attraction and anchorage of inflammatory cells in the event that high WSS causes minor mechanical damage to the endothelium. While an influx of inflammatory cells is beneficial for repairing large wounds or damage from infection, it can also cause damage by creating gaps between ECs, degrading ECM, and producing oxidative stress. Therefore, downregulating the cytokines IL-8, GM-CSF, CXCL-5, and CXCL-2, while upregulating anti-inflammatory molecules (PROCR) under high WSS may help preserve the endothelium and vessel wall from inflammatory cell-associated damage.
The anti-inflammatory, anti-coagulation effects of high WSS may protect against the destructive vascular remodeling that occurs during the formation of intracranial aneurysms, which tend to develop specifically at locations where WSS is very high (27, 31). Aneurysms may form when there is a breakdown in the protective responses as a result of EC dysfunction. Two of the major risk factors for intracranial aneurysm development, hypertension and smoking, cause significant EC dysfunction (3, 36). In addition, our laboratory has observed dysfunctional characteristics in ECs when they are exposed to a positive spatial gradient in WSS (9, 19, 50), which is another hemodynamic condition found at sites where aneurysms preferentially form (28, 31, 50). The relationship between EC dysfunction and the hemodynamic environment associated with intracranial aneurysm warrants continued investigation.
The mechanosensory mechanism that allows ECs to respond specifically to high WSS is unknown. WSS exerts a tangential force that causes a net stretching across the endothelial layer that may be exacerbated when WSS increases in magnitude. Tzima et al. (47) have shown that a complex of PECAM-1, vascular EC cadherin, and VEGFR2 can make fibroblasts responsive to WSS, and it has been proposed that the shear stress changes the conformation of the complex so that a key regulatory site on PECAM-1 becomes accessible to phosphorylation, thereby initiating various intracellular signaling pathways. Higher forces may produce different conformations and/or different levels of activation in the downstream signaling pathways. Alternatively, high WSS may act on a different mechanosensor that requires a greater mechanical force to become activated. Stretch-activated ion channel (SACs), which regulate calcium influx into ECs, have been specifically implicated in high WSS sensing. Our laboratory recently showed that the SAC inhibitor, streptomycin, attenuated expansive remodeling of the CCA in mice following ligation of the contralateral CCA (45). In the same study, we showed that SAC inhibition did not affect eNOS protein expression in cultured ECs under baseline WSS (1.5–2.5 Pa), but did substantially inhibit a flow-induced increase in eNOS expression when WSS was between 2.5 and 10 Pa. These results suggest that SACs are activated in ECs by higher WSS than normal physiological WSS conditions.
It should be noted that ECs from different vascular beds display different patterns of gene expressions, both in vivo and in vitro (4, 6, 52). We do not know if ECs from other sources, such as venous or capillary endothelia, are similar to aortic ECs in their responses to high WSS. However, we expect that many of the responses we observed here are widely shared by other arterial ECs, because chronic increases in WSS above physiological baseline generally cause arteries to remodel. The increased matrix processing and EC proliferation indicated by gene expression analysis in vitro are both important components of the arterial remodeling process in vivo. In this regard, in future studies we wish to examine the responses of ECs from intracranial arteries, because there are dramatic differences in wall structure and thickness between intra- and extracranial arteries, and this may require very different remodeling events.
In conclusion, very high WSS, such as occurs with compensatory flow increase in collateral arteries or at apices of bifurcations and outer sides curvatures, induces a unique gene expression profile indicative of an anticoagulant, anti-inflammatory, proliferative, and promatrix remodeling phenotype. These responses are significantly different from what has been previously reported in normal- and low-WSS conditions. The unique gene expression responses have potentially important implications in maintaining homeostasis when accommodating a chronic flow increase and in maladaptive responses, such as the initiation of intracranial aneurysms.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01NS064592 (H. Meng) and the American Society for Quality Biomedical Division Dr. Richard J. Schlesinger Grant (J. M. Dolan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.M.D., H.M., and J.K. conception and design of research; J.M.D. performed experiments; J.M.D. and F.J.S. analyzed data; J.M.D., F.J.S., H.M., and J.K. interpreted results of experiments; J.M.D. prepared figures; J.M.D. drafted manuscript; J.M.D., F.J.S., H.M., and J.K. edited and revised manuscript; J.M.D., F.J.S., H.M., and J.K. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jianping Xiang and Sukhjinder Singh for assistance with CFD, Nicholas Liaw and Max Mandelbaum for conducting the animal surgeries and for stimulating discussions, and Scott Woodward for technical assistance in the chamber design. We thank Dr. Song Liu for initial input and guidance in getting these studies off the ground. We also acknowledge Dr. Te-Chung Lee for use of laboratory equipment and the assistance of Wade Sigurdson and the Confocal Microscope and Flow Cytometry Core Facility at the University of Buffalo.
REFERENCES
- 1.Alnaes MS, Isaksen J, Mardal KA, Romner B, Morgan MK, Ingebrigtsen T. Computation of hemodynamics in the circle of Willis. Stroke 38: 2500–2505, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bacabac RG, Smit TH, Cowin SC, Van Loon JJ, Nieuwstadt FT, Heethaar R, Klein-Nulend J. Dynamic shear stress in parallel-plate flow chambers. J Biomech 38: 159–167, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Burke A, Fitzgerald GA. Oxidative stress and smoking-induced vascular injury. Prog Cardiovasc Dis 46: 79–90, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Burridge KA, Friedman MH. Environment and vascular bed origin influence differences in endothelial transcriptional profiles of coronary and iliac arteries. Am J Physiol Heart Circ Physiol 299: H837–H846, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics 7: 55–63, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A 100: 10623–10628, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292: H1209–H1224, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dolan JM, Meng H, Singh S, Paluch R, Kolega J. High fluid shear stress and spatial shear stress gradients affect endothelial proliferation, survival, and alignment. Ann Biomed Eng 39: 1620–1631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L, Hoi Y, Swartz DD, Kolega J, Siddiqui A, Meng H. Nascent aneurysm formation at the basilar terminus induced by hemodynamics. Stroke 39: 2085–2090, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol 286: H1916–H1922, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Hoi Y, Gao L, Tremmel M, Paluch RA, Siddiqui AH, Meng H, Mocco J. In vivo assessment of rapid cerebrovascular morphological adaptation following acute blood flow increase. J Neurosurg 109: 1141–1147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jou LD, van Tyen R, Berger SA, Saloner D. Calculation of the magnetization distribution for fluid flow in curved vessels. Magn Reson Med 35: 577–584, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol Heart Circ Physiol 239: H14–H21, 1980 [DOI] [PubMed] [Google Scholar]
- 17a.Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics 6: 144, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolega J. Asymmetric distribution of myosin IIB in migrating endothelial cells is regulated by a rho-dependent kinase and contributes to tail retraction. Mol Biol Cell 14: 4745–4757, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolega J, Gao L, Mandelbaum M, Mocco J, Siddiqui AH, Natarajan SK, Meng H. Cellular and molecular responses of the basilar terminus to hemodynamics during intracranial aneurysm initiation in a rabbit model. J Vasc Res 48: 429–442, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HJ, Koh GY. Shear stress activates Tie2 receptor tyrosine kinase in human endothelial cells. Biochem Biophys Res Commun 304: 399–404, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Lehoux S, Tronc F, Tedgui A. Mechanisms of blood flow-induced vascular enlargement. Biorheology 39: 319–324, 2002 [PubMed] [Google Scholar]
- 22.Li X, Kolega J. Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells. J Vasc Res 39: 391–404, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Lindekleiv HM, Valen-Sendstad K, Morgan MK, Mardal KA, Faulder K, Magnus JH, Waterloo K, Romner B, Ingebrigtsen T. Sex differences in intracranial arterial bifurcations. Gend Med 7: 149–155, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Martins GG, Kolega J. Endothelial cell protrusion and migration in three-dimensional collagen matrices. Cell Motil Cytoskeleton 63: 101–115, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Meng H, Metaxa E, Gao L, Liaw N, Natarajan SK, Swartz DD, Siddiqui AH, Kolega J, Mocco J. Progressive aneurysm development following hemodynamic insult. J Neurosurg 114: 1095–1103, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, Kolega J. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 38: 1924–1931, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metaxa E, Meng H, Kaluvala SR, Szymanski MP, Paluch RA, Kolega J. Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am J Physiol Heart Circ Physiol 295: H736–H742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metaxa E, Tremmel M, Natarajan SK, Xiang J, Paluch RA, Mandelbaum M, Siddiqui AH, Kolega J, Mocco J, Meng H. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke 41: 1774–1782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest 78: 1456–1461, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, Shibata M, Nakatsuka T, Harii K, Wada Y, Kohro T, Kodama T, Ando J. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb 10: 304–313, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J 386: 15–27, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem 17: 2059–2069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Tiberio G, Giulini SM, Rossi G, Bernini G, Agabiti-Rosei E. Endothelial dysfunction in hypertension is independent from the etiology and from vascular structure. Hypertension 31: 335–341, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun 293: 501–508, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Schirmer CM, Malek AM. Wall shear stress gradient analysis within an idealized stenosis using non-Newtonian flow. Neurosurgery 61: 853–863; discussion 863–854, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Sho E, Komatsu M, Sho M, Nanjo H, Singh TM, Xu C, Masuda H, Zarins CK. High flow drives vascular endothelial cell proliferation during flow-induced arterial remodeling associated with the expression of vascular endothelial growth factor. Exp Mol Pathol 75: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Sho E, Sho M, Singh TM, Nanjo H, Komatsu M, Xu C, Masuda H, Zarins CK. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp Mol Pathol 73: 142–153, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: article 3, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W, Jr, Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb 12: 120–134, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Szymanski MP, Metaxa E, Meng H, Kolega J. Endothelial cell layer subjected to impinging flow mimicking the apex of an arterial bifurcation. Ann Biomed Eng 36: 1681–1689, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talloen W, Clevert DA, Hochreiter S, Amaratunga D, Bijnens L, Kass S, Gohlmann HW. I/NI-calls for the exclusion of non-informative genes: a highly effective filtering tool for microarray data. Bioinformatics 23: 2897–2902, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Tanweer O, Metaxa E, Liaw N, Sternberg D, Siddiqui A, Kolega J, Meng H. Inhibition of stretch-activated ion channels on endothelial cells disrupts nitric oxide-mediated arterial outward remodeling. J Biorheology 24: 77–83, 2011 [Google Scholar]
- 46.Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol 20: E120–E126, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005 [DOI] [PubMed] [Google Scholar]
- 48.van Everdingen KJ, Klijn CJ, Kappelle LJ, Mali WP, van der Grond J. MRA flow quantification in patients with a symptomatic internal carotid artery occlusion. The Dutch EC-IC Bypass Study Group. Stroke 28: 1595–1600, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Viggers RF, Wechezak AR, Sauvage LR. An apparatus to study the response of cultured endothelium to shear stress. J Biomech Eng 108: 332–337, 1986 [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Kolega J, Hoi Y, Gao L, Swartz DD, Levy EI, Mocco J, Meng H. Molecular alterations associated with aneurysmal remodeling are localized in the high hemodynamic stress region of a created carotid bifurcation. Neurosurgery 65: 169–177; discussion 177–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 53: 502–514, 1983 [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Burridge KA, Friedman MH. In vivo differences between endothelial transcriptional profiles of coronary and iliac arteries revealed by microarray analysis. Am J Physiol Heart Circ Physiol 295: H1556–H1561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.