Abstract
The cytoskeleton participates in many aspects of transporter protein regulation. In this study, by using yeast two-hybrid screening, we identified the cytoskeletal protein actin as a binding partner with the UT-A1 urea transporter. This suggests that actin plays a role in regulating UT-A1 activity. Actin specifically binds to the carboxyl terminus of UT-A1. A serial mutation study shows that actin binding to UT-A1's carboxyl terminus was abolished when serine 918 was mutated to alanine. In polarized UT-A1-MDCK cells, cortical filamentous (F) actin colocalizes with UT-A1 at the apical membrane and the subapical cytoplasm. In the cell surface, both actin and UT-A1 are distributed in the lipid raft microdomains. Disruption of the F-actin cytoskeleton by latrunculin B resulted in UT-A1 accumulation in the cell membrane as measured by biotinylation. This effect was mainly due to inhibition of UT-A1 endocytosis in both clathrin and caveolin-mediated endocytic pathways. In contrast, actin depolymerization facilitated forskolin-stimulated UT-A1 trafficking to the cell surface. Functionally, depolymerization of actin by latrunculin B significantly increased UT-A1 urea transport activity in an oocyte expression system. Our study shows that cortical F-actin not only serves as a structural protein, but directly interacts with UT-A1 and plays an important role in controlling UT-A1 cell surface expression by affecting both endocytosis and trafficking, therefore regulating UT-A1 bioactivity.
Keywords: cytoskeletal protein, latrunculin, membrane protein, urea transport
urea plays a critical role in the urine concentration mechanism in the kidney's inner medulla. The major mechanism for delivering urea to the inner medullary interstitium is urea reabsorption mediated by the UT-A1 urea transporter from the terminal inner medullary collecting duct (IMCD) (26, 34). A UT-A1/A3 knockout mouse has impaired urea clearance and urinary concentrating ability (10). In vivo, UT-A1 transport activity is mainly regulated by vasopressin. Vasopressin increases kidney urea transport activity by increasing UT-A1 in the apical plasma membrane (19) and UT-A1 phosphorylation (43).
Successful trafficking to and residing on the cell surface are the prerequisites for transport proteins to execute their proper functions. The regulatory mechanism for membrane protein trafficking to cell surface, as well as maintaining its membrane expression, is complex. It is under strict spatial and temporal regulation and is particularly controlled by some important accessory proteins. Direct protein-protein interactions by accessory proteins have been shown to determine the specificity of membrane protein in sorting, membrane trafficking, and retrieval (14, 27). During the past decade, much attention has been paid to those proteins. Several proteins, including SPA-1 (27), syntaxin-3 (24), SNAP23 (38), Rho GTPase (16, 25), dynein and dynactin (30), and actin (37, 42), are involved in regulating aquaporin 2 (AQP2) water channel trafficking and membrane expression. UT-A1 membrane trafficking, endocytosis, and degradation has been shown to be regulated by the SNARE-associated proteins snapin (25), dynamin (15), caveolin (9), and MDM2 (5).
The actin cytoskeleton is the basic component for the cell's structure, with actin being one of the most abundant cytosolic proteins. It is a highly conserved protein with three isotypes (α, β, γ > 90% aa homology). Actin is vital to the function of all eukaryotic cells. Not only a structural protein or a simple scaffold protein, actin is also required for mitosis, cytokinesis, cell motility, muscle contract, maintenance of cell shape, endocytosis, and secretion (1, 16). Actin exists in two forms, monomeric actin (G-actin) and filamentous actin (F-actin). F-actin is in dynamic equilibrium with soluble G-actin. The extent of F-actin polymerization/depolymerization changes according to the cell's physiological needs and its specialization.
A number of cytoskeletal proteins like ERM (ezrin/radixin/moesin), many isoforms of actin, cofilin, dynein, and tubulin have been identified in exosomes containing AQP2 (29). The epithelial sodium channel (ENaC) complex from bovine epithelia contains ankyrin, spectrin, and actin (36). These findings suggested that actin cytoskeleton and actin-associated proteins could directly interact with and actively participate in the regulation of these channels. In addition to AQP2 (28) and ENaC (21), the cystic fibrosis transmembrane conductance regulator CFTR (31), the Na-K-2Cl cotransporter NKCC1 (20), cardiac L-type Ca channels (33), the Na+/H+ exchanger NHE1 (41), and the glucose transporter GLUT4 (18) are also reported to associate with actin. Some of their interactions with actin could occur indirectly by actin-associated proteins, like NHE3 indirectly binding to the cytoskeleton via actin binding adaptor protein ezrin (41).
In this study, using a yeast two-hybrid assay, we identified actin as a UT-A1-binding protein. We further found that, in polarized epithelial cells, the cortical actin colocalizes with UT-A1 in the apical membrane and subapical cytoplasm and plays an important role in the regulation of UT-A1 cell surface expression and urea transport activity.
MATERIALS AND METHODS
Constructs.
To generate the bait construct for the yeast two-hybrid assay, a fragment of 144 nt corresponding to the 48 aa COOH terminus of human urea transporter UT-A1 was amplified by PCR from pcDNA3-UT-A1 and subcloned in frame into the multiple cloning sites of Nde I and Sal I digested pGBKT7 vector (Clontech). The insert sequence and reading frame were confirmed by DNA sequencing.
The UT-A1 COOH-terminal amino acids in pGBK-C-UT-A1 were mutated to alanine with the QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions.
Yeast two-hybrid assay.
The bait of C-UT-A1 in pGBKT7 was transformed into the yeast strain AH109 (BD Biosciences) using the lithium acetate method. A single positive AH109 clone (bait) was selected from SD/-Trp plate and mated with the human kidney cDNA pretransformed library in Y187 (BD Biosciences). The mating mixtures were plated onto quadruple minus plates lacking Trp, Leu, Ade, and His. After 7–10 days, positive colonies were collected and replated in quadruple minus plates containing X-α-Gal for the second screening. All positive colonies were then processed for plasmid DNA purification and sequence analysis.
In vitro translation and binding assay.
In vitro translation was carried out using the TNT T7-coupled rabbit reticulocyte lysate system (Promega). The bait gene in pGBKT7 was tagged with c-myc and contains a T7 promoter. The targeted gene actin (clones C30, C35) in pACT2 isolated from cDNA library screening was tagged with hemagglutinin (HA). A 50-μl reaction mixture was prepared by using 25 μl of TNT reticulocyte lysate, 2 μl of reaction buffer, 1 μl of 1 mM amino acid mixture (minus methionine), 2 μl of 10 mCi/ml [35S]methionine (Amersham), 1 μg of DNA template, and 1 μl of T7 RNA polymerase. After incubation for 30 min at 30°C, 10 μl of bait and 10 μl of target reaction were mixed and preincubated for 1 h, then 10 μl of myc antibody (BD Biosciences) was added. For monitoring the quality of the protein synthesis, 10 μl of bait or target reaction was incubated with c-myc or HA antibody. The combined mixtures were precipitated by protein-A beads. After washing, the beads were eluted by boiling in Laemmli sample buffer and the proteins were resolved by SDS-PAGE. The gel was dried and analyzed by autoradiography.
Cell culture and cell treatment.
A stably transfected MDCK cell expressing UT-A1 (12) was used in this study. The cells were grown in DMEM supplemented with 10% FCS, 25 mM HEPES, and antibiotics in a 5% CO2 incubator at 37°C. After becoming confluent, the cells were treated with the following compounds alone or in different combinations: 0.1 μM jasplakinolide (Molecular Probes), 1 μM latrunculin B (Molecular Probes), 5 μM methyl-β-cyclodextrin (MβCD, Sigma), or 50 μg/ml concanavalin A (Sigma), for different times as described in detail in the relevant experiments. Cells were then processed for cell surface biotinylation, lipid raft isolation, Western blot analysis, or immunofluorescence microscopy.
Immunocytochemistry.
UT-A1-MDCK cells were grown on a Transwell insert filter (Corning) for 5–6 days to allow the cells to develop polarity. The cells were fixed with 4% paraformaldehyde for 10 min at room temperature, permeabilized with 0.1% Triton X-100 for 5 min, and then were exposed to primary antibody against UT-A1 (1:400) for 1 h, followed by staining with fluorescein isothiocyanate (FITC)-conjugated secondary goat anti-rabbit antibody (Sigma) for another 1 h. To stain the F-actin, the cells were further incubated with Texas Red-X phalloidin (Molecular Probes) (1:500) for 20 min. After PBS washing, the cells were mounted with Vectashield (Vector Laboratories) and analyzed by confocal laser scanning microscopy.
Lipid raft fractionation.
Lipid raft isolation was performed with a 5–40% sucrose discontinuous gradient as described previously (4, 15). UT-A1-MDCK cells were grown on 100-mm plates and lysed in ice-cold 0.5% Brij96-TNEV buffer (10 mM Tris·HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 2 mM Na vanadate, and protease inhibitor cocktail) for 30 min on ice. Lysates were centrifuged at 3,000 rpm for 5 min. Five hundred microliters of postnuclear supernatant were mixed with an equal volume of 80% sucrose in TNEV and transferred to 13 × 51-mm Beckman centrifuge tubes. Three milliliters of 35% sucrose in TNEV were layered carefully on top of the mixture followed by another 1 ml layer of 5% sucrose. The sucrose gradient was centrifuged in a SW 50.1 rotor (Beckman) at 34,000 rpm (∼110,000 g) for 18 h at 4°C. Equal sizes of fractions (∼400 μl) were collected from the top to bottom of the tube and analyzed by Western blotting with respective antibodies.
Biotinylation and Western blot analysis.
Cell surface biotinylation was performed as described before (3). After treatment, the cells were washed with ice-cold PBS and labeled twice with freshly prepared 1 mg/ml EZ-Link Sulfo-NHS-SS-Biotin (Pierce) in borate buffer for 20 min in a cold room with gentle shaking. The excess biotin was quenched with 0.1 M l-lysine (Sigma). After washing, the cells were solubilized in 700 μl of RIPA buffer (150 mM NaCl, 10 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and protease inhibitors). Fifty microliters of the supernatant was saved for Western blotting as pre-bead total protein, whereas 600 μl of the supernatant was incubated with 25 μl of Immunopure immobilized streptavidin agarose beads (Pierce) overnight at 4°C with gentle shaking. The biotin-labeled proteins were eluted in 35 μl of Laemmli sample buffer and run on SDS-PAGE for Western blot analysis. The following antibodies were used for Western blotting: UT-A1 (4), β-actin (Sigma), tubulin (Sigma), and caveolin (BD Bioscience).
Oocyte experiments.
Xenopus laevis oocytes were prepared and maintained in OR3 medium as described previously (30). The capped cRNA of UT-A1 was transcribed in vitro from linearized cDNAs with T7 polymerase using the mMESSAGE mMACHINE T7 Ultra Kit (Ambion). Two nanograms of UT-A1 cRNA in a total volume of 23 nl water were injected into each oocyte. Three days later, healthy oocytes were selected for functional assays by measuring [14C]urea uptake for each individual cell. For the cell actin depolymerization tests, oocytes were incubated with 0.2 μM latrunculin in OR3 medium for 24 h.
Statistical analysis.
All values are expressed as means ± SD. Statistical analysis of the data was performed by one-way analysis of variance (ANOVA) followed by Tukey honestly significant difference (HSD) tests. Differences were considered significant at P < 0.05.
RESULTS
Identification of actin as a UT-A1 interaction partner.
The urea transporter UT-A1 is a large molecule with 12 transmembrane segments and cytoplasmic NH2 and COOH termini. To identify the UT-A1 binding proteins, we first used a 48-aa COOH-terminal fragment of UT-A1 (C-UT) as bait and screened a 5 × 107 colony-forming units of kidney cDNA library using a yeast two-hybrid assay. After the second round selection, we obtained 149 colonies. DNA sequence analysis revealed four of them are the actin genes.
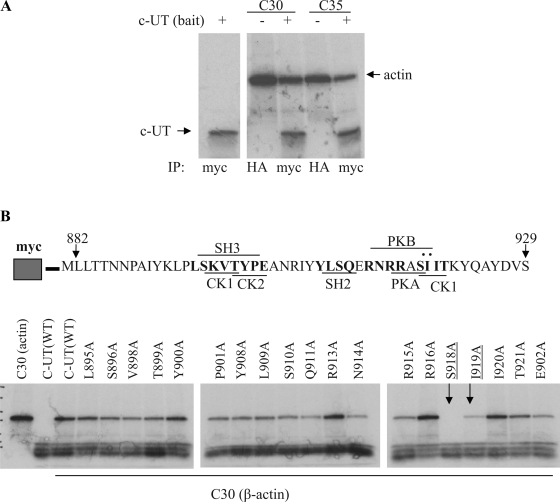
The association of actin and UT-A1 was confirmed by an in vitro binding assay. We used a rabbit reticulocyte lysate translation system and labeled the newly synthesized proteins with [35S]methionine. The bait (C-UT) with c-myc tag and the gene products of interest (C30, C35) conjugated with HA were mixed and immunoprecipitated with c-myc antibody. Figure 1A shows that the UT-A1 COOH terminus binds to actin (C30 and C35).
Fig. 1.
Urea transporter UT-A1 COOH-terminal actin binding site. A: in vitro binding assay. C-UT (COOH-terminal fragment of 48 aa) and the isolated actin clones (C30, C35) were in vitro translated in rabbit reticulocyte lysates and labeled with [35S]methionine. The newly synthesized actin clones (C30, C35) tagged by hemagglutinin (HA) and C-UT bait tagged by myc were processed for immunoprecipitation (IP) with HA or c-myc antibody and analyzed by autoradiography. B: mutation analysis. A series of potentially important amino acids in the UT-A1 COOH terminus were mutated to Ala. In each case, binding to actin clone C30 was assessed as in A. SH3, Src homology 3.
Potential actin binding sites in the UT-A1 COOH terminus.
To determine the actin-binding site on the COOH terminus of UT-A1, we subjected this segment to scanning mutagenesis, converting some key amino acids to alanine. We evaluated the ability to bind actin by an in vitro binding assay. Figure 1B shows that the mutant S918 lost its ability to bind actin.
Cortical actin colocalizes with UT-A1 in polarized UT-A1-MDCK cells.
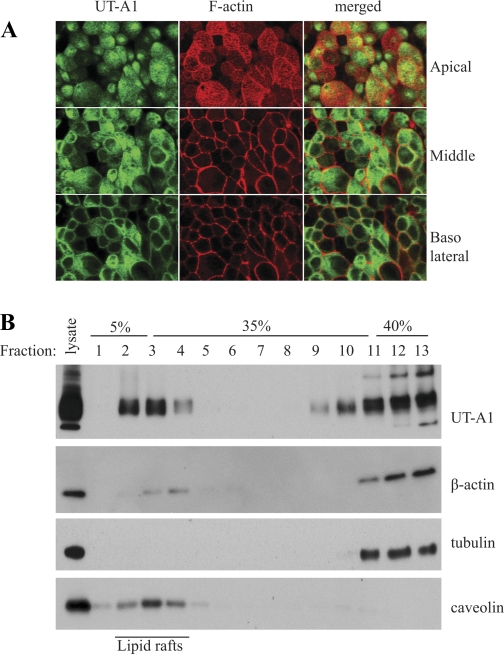
The possible interaction of UT-A1 in vivo with actin was assessed in UT-A1-MDCK cells. Since actin could interact nonspecifically with IgG (8, 21) and immunoprecipitation may yield a false positive result, we performed immunostaining to visualize the colocalization of UT-A1 and actin in MDCK cells. The UT-A1 MDCK cells were grown on the filter for 4–5 days. The UT-A1 was detected by UT-A1 antibody and visualized by FITC-conjugated secondary antibody. The F-actin was stained with Texas Red-X phalloidin. The UT-A1 (green color) is mainly localized in apical membrane and within the subapical cytoplasm of the MDCK cells. Actin is distributed in the cell periphery along the cell membrane. Figure 2A shows the representative scanning from apical, middle, and basolateral sections. Cortical actin in apical sections is observed to colocalize with UT-A1.
Fig. 2.
Colocalization of UT-A1 and the cortical F-actin cytoskeleton. A: confocal microscopy. UT-A1-MDCK cells were grown on Transwell filters for 5–6 days. The cells were then fixed and permeabilized. UT-A1 was immunostained with anti-UT-A1 antibody and subsequently visualized with FITC-conjugated goat anti-rabbit IgG antibody. The filamentous (F) actin was stained by Texas Red-X phalloidin. The image was obtained by confocal microscopy. Whole cells were scanned from the top (apical side) to the bottom (basolateral side) as a cross section through the cell (x-y section). B: lipid raft study. UT-A1-MDCK cells were lysed in 0.5% Brij 96V-TNEV buffer, then applied to 5–40% discontinuous sucrose gradient ultracentrifugation. Fractions were collected from the top to bottom and analyzed by immunoblotting for UT-A1, actin, tubulin, and caveolin.
Both UT-A1 and actin are identified in cell membrane lipid raft fractions.
The cell membrane contains specialized microdomains referred to as lipid rafts, which are enriched in cholesterol and sphingolipids. UT-A1 is mainly associated with lipid rafts in IMCD (4, 9) and HEK293 cells (9, 15), as well as in UT-A1-MDCK cells (4). We examined whether actin is also present in lipid rafts. Lipid raft fractions were isolated from UT-A1-MDCK cells and blotted with UT-A1 and actin antibodies. Figure 2B shows the representative blots of more than three independent experiments. Fractions 2–4 are denoted as the lipid raft fractions as indicated by caveolin, a marker of membrane lipid rafts. Both UT-A1 and actin are identified in cell membrane lipid raft domains. As a control for actin, tubulin, the cytosolic protein, is not found in lipid rafts.
Depolymerization of actin cytoskeleton increases UT-A1 surface accumulation.
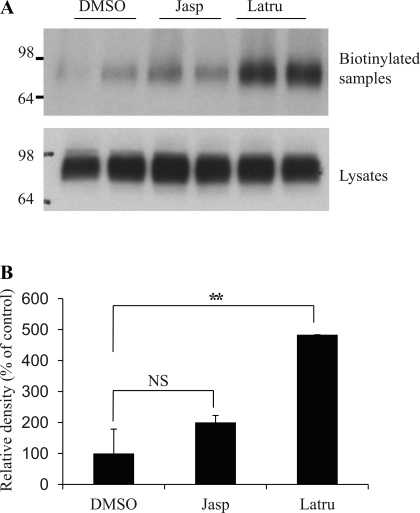
Jasplakinolide binds to and stabilizes actin filaments and promotes actin polymerization (41). In contrast, latrunculin B binds to G-actin monomers and prevents polymerization to F-actin (16). We manipulated the actin polymerization status using these two compounds and examined whether this affects UT-A1 expression in the cell membrane. We treated UT-A1-MDCK cells with 0.1 μM jasplakinolide or 1 μM latrunculin B for 1 h and then processed them for cell surface biotinylation. As seen in Fig. 3, latrunculin B treatment significantly increased the amount of membrane UT-A1. In contrast, jasplakinolide treatment did not affect UT-A1 cell surface expression.
Fig. 3.
Effect of actin remodeling on UT-A1 membrane expression. A: cell biotinylation experiment. UT-A1-MDCK cells were grown in six-well plates to confluence. They were then treated with jasplakinolide (Jasp; 0.1 μM) or latrunculin B (Latru; 1 μM) for 1 h at 37°C. After treatment, cells were processed for cell surface biotinylation. Total cell lysates or biotinylated proteins were subjected to immunoblotting with UT-A1 antibody. B: quantitative densitometric analysis. The intensity of UT-A1 from biotinylated samples was assessed by ImageJ software (National Institutes of Health, Bethesda, MD) from 3 independent experiments (means ± SD; NS, no significance; **P < 0.01).
Actin depolymerization promotes forskolin-stimulated UT-A1 membrane trafficking.
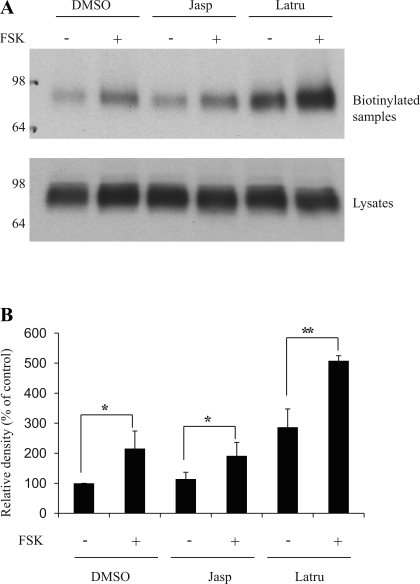
UT-A1 molecules are quickly trafficked to the cell surface upon exposure to vasopressin or the cAMP stimulator forskolin (FSK) (3, 19). We next investigated whether disruption of F-actin will affect FSK-induced UT-A1 membrane trafficking. UT-A1-MDCK cells were pretreated with latrunculin B or jasplakinolide for 40 min followed by addition of 10 μM FSK for another 20 min. Cell surface biotinylation experiments showed that FSK stimulated UT-A1 membrane trafficking, which is consistent with an earlier report (3). Actin depolymerization by latrunculin did not block FSK's effect (Fig. 4). On the contrary, cotreatment with latrunculin and FSK had a synergistic effect on UT-A1 membrane expression. Actin depolymerization facilitates FSK stimulated-UT-A1 trafficking to the surface.
Fig. 4.
Effect of actin depolymerization on forskolin (FSK)-induced UT-A1 membrane trafficking. A: cell biotinylation experiment. UT-A1-MDCK cells were treated with jasplakinolide (0.1 μM) or latrunculin B (1 μM) for 40 min and with or without forskolin (1 μM) for another 20 min. Cells were then processed for biotinylation. Total cell lysates or biotinylated proteins were analyzed for UT-A1 expression. B: quantification of cell surface UT-A1 from 3 independent experiments (means ± SD; *P < 0.05, **P < 0.01).
Actin influences both caveolin- and clathrin- mediated endocytosis of UT-A1.
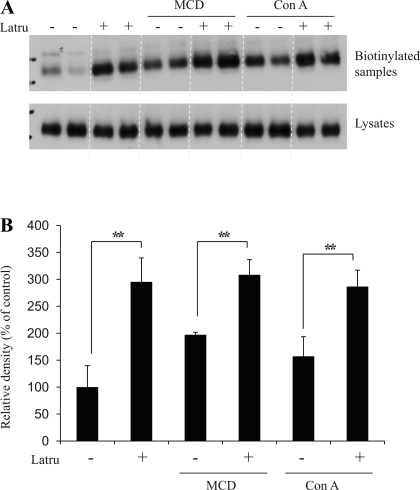
The caveolin- and clathrin-mediated endocytotic pathways are the two major routes for endocytosis in eukaryotic cells. To examine whether actin participates in one or both pathways, UT-A1-MDCK cells were treated with MβCD, which inhibits caveolin-dependent endocytosis, or concanavalin A, which inhibits clathrin-coated pit-mediated endocytosis, and together with or without latrunculin for 1 h. Cell plasma membrane UT-A1 accumulation was measured by biotinylation. In agreement with our previous study (15), inhibition of either the caveolin or clathrin-mediated pathway increased UT-A1 membrane accumulation. Latrunculin treatment caused more UT-A1 accumulation in the cell membrane when either pathway was blocked (Fig. 5). This suggests that disruption of actin has broad inhibitory effects and reduces both the caveolin- and clathrin-mediated UT-A1 endocytosis.
Fig. 5.
Actin depolymerization abrogates UT-A1 endocytosis. A: cell biotinylation experiment. UT-A1-MDCK cells were treated with 5 mM methyl-β-cyclodextrin (MCD) or 50 μg/ml concanavalin A (Con A) together with or without 1 μM latrunculin B for 1 h. Cells were then processed for biotinylation. Total cell lysates or biotinylated proteins were analyzed for UT-A1 expression. B: quantification of cell surface UT-A1 from 3 independent experiments (means ± SD; **P < 0.01).
Actin regulates UT-A1 urea transport activity.
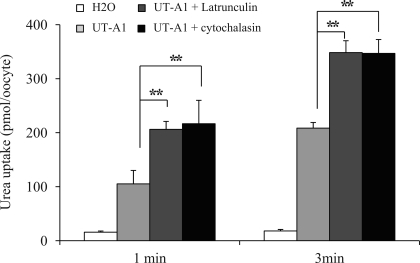
Finally, we examined whether modulation of actin could directly regulate UT-A1 urea transport activity. Unfortunately, neither measuring urea transport activity by tubular perfusion of rat IMCDs (40) nor transepithelial flux in UT-A1-MDCK cells (12) was successful. Latrunculin treatment caused significant tubular leakage and damaged MDCK cell barrier integrity. We then sought to use the oocyte expression system. Incubation of UT-A1-injected oocytes with 0.2 μM latrunculin for 24 h significantly increased UT-A1 urea transport activity. Cytochalasin D, another inhibitor of actin polymerization (16), also increased UT-A1 urea flux activity (Fig. 6).
Fig. 6.
Effect of actin-disrupting agent on UT-A1 activity in Xenopus oocytes. UT-A1 cRNA (2 ng/23 nl per cell) or water (as a control) were microinjected into oocytes and incubated at 18°C for 3 days. For actin depolymerization tests, cells were incubated with 0.2 μM latrunculin B or cytochalasin D for 24 h. Urea transport activity was measured by [14C]urea flux (n = 5–6 oocytes) as described in materials and methods (means ± SD; **P < 0.01).
DISCUSSION
The importance of actin in regulating transporter proteins has been appreciated in many studies. The well-organized actin network, particularly cortical filamentous actin beneath the apical membrane, plays a critical role in transporter protein membrane expression in polarized epithelial cells (16). The major finding of this study is that actin directly interacts with the UT-A1 carboxyl terminus and that the cortical actin plays an important role in the regulation of UT-A1 cell membrane expression and bioactivity.
Intact actin filaments are required for the vesicular trafficking of some ion channels and transporter proteins to the membrane (6, 17, 21, 22) and/or structurally anchoring the protein within the plasma membrane (21). Inhibition of actin polymerization blocks sodium-glucose transporter 1 (SGLT1) apical surface localization stimulated by 8-bromo-cAMP and significantly inhibits SGLT1 activity (17). This indicates that intact actin filaments are necessary to translocate SGLT1 from cytosolic compartments to the plasma membrane (17). cAMP/protein kinase A (PKA) induces CFTR-associated ion channel activity only in the presence of an organized actin cytoskeleton (31). The vasopressin-stimulated Na+ channel (ENaC) activity is inhibited by disrupting the organization of actin filaments (30).
However, other studies show that actin polymerization functions as a barrier for transporter protein trafficking between the apical membrane and the intracellular compartments (21, 28). Actin and actin-binding proteins may cross-link into three-dimensional networks. The vesicles or the transporter molecules are held in place by the cortical actin cytoskeleton (28) and are therefore prevented from contacting the cell membrane and inserting into the cell surface. Thus, depolymerization of cortical F-actin is considered an important prerequisite for transporter protein membrane trafficking and proper activity. AQP2 is another important transporter expressed in the IMCD. Vasopressin-stimulated apical sorting of AQP2 was associated with depolymerization of the actin cytoskeleton (35) in the mammalian collecting duct and in the amphibian bladder (7), indicating that actin filaments might retard the fusion processes. In rat collecting duct epithelium, the total F-actin content decreases by 20–30% after stimulation with vasopressin (35). Depolymerization of actin by latrunculin A has also been found to transiently promote neurotransmitter release in synapses (25). Similar to the regulation of AQP2 by cAMP, UT-A1 is activated by stimulation of the cAMP pathway and activation of PKA (43). Activation of PKA leads to a change in the F-actin cytoskeleton (35). This change could cause the increase in UT-A1 membrane abundance and urea transport activity that is seen in the current study (Figs. 4 and 6).
The amount of transporter protein in the cell plasma membrane is determined by both protein membrane trafficking (exocytosis) and retrieval (endocytosis). Clathrin-mediated endocytosis is the major endocytic pathway of many mammalian cells. Evidence from a variety of cell types demonstrates that the actin cytoskeleton plays an essential and active role in many key endocytic stages (13, 16, 32, 42). Within 5 min, latrunculin causes the actin patch to disappear and endocytosis to be completely abrogated in yeast (32). In the very early stage of clathrin-mediated endocytosis, actin accumulates at sites of endocytosis. Then, endocytic proteins and adaptors, like clathrin, Sla1, Sla2, and End3, are recruited to the actin patch, followed by regulators of the Arp2/3 complex (11, 32). Actin is also required for the invagination during the late stages of endocytic vesicle movement (13). Although cell membrane UT-A1 mainly resides in lipid raft subdomains, its endocytosis occurs through both caveolin- and clathrin-mediated endocytic pathways (15). The current study shows that inhibition of either pathway by concanavalin A or MβCD only partially inhibits UT-A1 endocytosis, which causes UT-A1 membrane accumulation. Latrunculin inhibits both clathrin- and caveolin-mediated UT-A1 endocytosis. The role of actin in the clathrin-mediated pathway has been well studied (11, 16, 42), but the function of actin in the caveolin pathway is less explored. Our results show that actin may also be involved in UT-A1 endocytosis through the caveolin endocytic pathway. In support of this, both actin and UT-A1 are found localized in lipid raft domains in the cell membrane (Fig. 2B).
In our study, treatment with jasplakinolide, which binds to and stabilizes actin filaments, does not alter UT-A1 membrane abundance. One of the possible explanations could be that the normal amount of F-actin on the apical side is enough to fulfill the cell's function. An increase in actin filaments would have no additional effect. An elegant experiment by Apodaca et al. (1) showed that jasplakinolide treatment selectively stimulated basolateral endocytosis of the fluid-phase marker FITC-dextran and horseradish peroxidase, whereas apical endocytosis of these markers was unaffected (1). The reason is that most filamentous actin is on the apical side of polarized epithelial cells.
Although much effort has been made, the consensus actin binding motif is still not certain. A potential motif for actin binding has been suggested as L/I-X-D/E-X-X-L/I (where X = any residue) (2, 39). However, some actin binding proteins do not have this sequence. The mutation L1020A in Slo1 calcium-activated potassium channels caused a reduction in Slo1 binding to β-actin, but the D1022A and L1025A mutations exhibited only slightly reduced binding to actin (44). The AQP2 COOH terminus does not contain typical actin binding motifs (28). A conserved 14-amino acid region (E631–F644) in the carboxyl terminus of αENaC has been shown to contribute specifically to actin's modulation of ENaC (21). In our study, we found that the amino acid residue S918, probably together with I919, in the UT-A1 COOH terminus mediates the actin binding. It is interesting to note that in most studies F-actin binds to the carboxyl terminus of the target proteins, such as α-subunit of the ENaC (21), AQP2 (28), Slo1 channel (44), as well as UT-A1 in the current study.
In summary, actin concentrated at the apical cortex provides many functions in the apical domain of epithelial cells (16). Here we found that by directly binding to UT-A1, cortical actin regulates UT-A1 expression in the cell membrane by regulating both endocytosis and membrane trafficking. This may have important physiological significance. Alteration of the actin cytoskeleton modulates UT-A1 activity. It has been shown that actin is also phosphorylated by PKA and that the phosphorylation reduces the rate of actin polymerization (30). Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct and increases water channel-carrying vesicle fusion with the apical membrane (35). Taken together, we propose that the actin cytoskeleton, by changing its polymerization state, may play an important role in vasopressin-mediated UT-A1 activity in vivo by regulating UT-A1 endocytosis and trafficking.
GRANTS
This work was supported by National Institutes of Health Grants R01-DK087838 and R21-DK080431 (to G. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.X., H.S., and C.B.C. performed the experiments; G.X., H.S., O.F., and G.C. analyzed the data; G.X., H.S., O.F., and G.C. interpreted the results of the experiments; O.F. and G.C. drafted the manuscript; G.C. conception and design of the research; G.C. prepared the figures; G.C. edited and revised the manuscript; G.C. approved the final version of the manuscript.
REFERENCES
- 1. Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Bresnick AR, Warren V, Condeelis J. Identification of a short sequence essential for actin binding by Dictyostelium ABP-120. J Biol Chem 265: 9236–9240, 1990 [PubMed] [Google Scholar]
- 3. Chen G, Froehlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem 281: 27436–27442, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Chen G, Howe AG, Xu G, Fröhlich O, Klein JD, Sands JM. Mature N-linked glycans facilitate UT-A1 urea transporter lipid raft compartmentalization. FASEB J 25: 4531–4539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen G, Huang H, Fröhlich O, Yang Y, Klein JD, Price SR, Sands JM. MDM2 E3 ubiquitin ligase mediates UT-A1 urea transporter ubiquitination and degradation. Am J Physiol Renal Physiol 295: F1528–F1534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Condrescu M, Reeves JP. Actin-dependent regulation of the cardiac Na+/Ca2+ exchanger. Am J Physiol Cell Physiol 290: C691–C701, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Ding GH, Franki N, Condeelis J, Hays RM. Vasopressin depolymerizes F-actin in toad bladder epithelial cells. Am J Physiol Cell Physiol 260: C9–C16, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Fechheimer M, Daiss JL, Cebra JJ. Interaction of immunoglobulin with actin. Mol Immunol 16: 881–888, 1979 [DOI] [PubMed] [Google Scholar]
- 9. Feng X, Huang H, Yang Y, Fröhlich O, Klein JD, Sands JM, Chen G. Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane. Am J Physiol Renal Physiol 296: F1514–F1520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona O, O' Toole E, De Camilli P. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 17: 811–822, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Urea transport in MDCK cells that are stably transfected with UT-A1. Am J Physiol Cell Physiol 286: C1264–C1270, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Galletta BJ, Mooren OL, Cooper JA. Actin dynamics and endocytosis in yeast and mammals. Curr Opin Biotechnol 21: 604–610, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gormley K, Dong Y, Sagnella GA. Regulation of the epithelial sodium channel by accessory proteins. Biochem J 371: 1–14, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang H, Feng X, Zhuang J, Fröhlich O, Klein JD, Cai H, Sands JM, Chen G. Internalization of UT-A1 urea transporter is dynamin dependent and mediated by both caveolae- and clathrin-coated pit pathways. Am J Physiol Renal Physiol 299: F1389–F1395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hyman T, Shmuel M, Altschuler Y. Actin is required for endocytosis at the apical surface of Madin-Darby canine kidney cells where ARF6 and clathrin regulate the actin cytoskeleton. Mol Biol Cell 17: 427–437, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ikari A, Harada H, Takagi K. Role of actin in the cAMP-dependent activation of sodium/glucose cotransporter in renal epithelial cells. Biochim Biophys Acta 1711: 20–24, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kanzaki M, Pessin JE. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem 276: 42436–42444, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Klein JD, Fröhlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol 17: 2680–2686, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Liedtke CM, Hubbard M, Wang X. Stability of actin cytoskeleton and PKC-delta binding to actin regulate NKCC1 function in airway epithelial cells. Am J Physiol Cell Physiol 284: C487–C496, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Mazzochi C, Bubien JK, Smith PR, Benos DJ. The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J Biol Chem 281: 6528–6538, 2006 [DOI] [PubMed] [Google Scholar]
- 22. McCarthy AM, Spisak KO, Brozinick JT, Elmendorf JS. Loss of cortical actin filaments in insulin-resistant skeletal muscle cells impairs GLUT4 vesicle trafficking and glucose transport. Am J Physiol Cell Physiol 291: C860–C868, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mistry AC, Mallick R, Fröhlich O, Klein JD, Rehm A, Chen G, Sands JM. The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J Biol Chem 282: 30097–30106, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Mistry AC, Mallick R, Klein JD, Weimbs T, Sands JM, Fröhlich O. Syntaxin specificity of aquaporins in the inner medullary collecting duct. Am J Physiol Renal Physiol 297: F292–F300, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron 27: 539–550, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci USA 93: 5495–5500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noda Y, Sasaki S. Regulation of aquaporin-2 trafficking and its binding protein complex. Biochim Biophys Acta 1758: 1117–1125, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Noda Y, Horikawa S, Katayama Y, Sasaki S. Water channel aquaporin-2 directly binds to actin. Biochem Biophys Res Commun 322: 740–745, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prat AG, Bertorello AM, Ausiello DA, Cantiello HF. Activation of epithelial Na+ channels by protein kinase A requires actin filaments. Am J Physiol Cell Physiol 265: C224–C233, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Prat AG, Cunningham CC, Jackson GR, Jr, Borkan SC, Wang Y, Ausiello DA, Cantiello HF. Actin filament organization is required for proper cAMP-dependent activation of CFTR. Am J Physiol Cell Physiol 277: C1160–C1169, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Robertson AS, Smythe E, Ayscough KR. Functions of actin in endocytosis. Cell Mol Life Sci 66: 2049–2065, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sadeghi A, Doyle AD, Johnson BD. Regulation of the cardiac L-type Ca2+ channel by the actin-binding proteins α-actinin and dystrophin. Am J Physiol Cell Physiol 282: C1502–C1511, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Sands JM. Molecular mechanisms of urea transport. J Membr Biol 191: 149–163, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Simon H, Gao Y, Franki N, Hays RM. Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct. Am J Physiol Cell Physiol 265: C757–C762, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Smith PR, Saccomani G, Joe EH, Angelides KJ, Benos DJ. Amiloride-sensitive sodium channel is linked to the cytoskeleton in renal epithelial cells. Proc Natl Acad Sci USA 88: 6971–6975, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281: F1092–F1101, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology 146: 5063–5070, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Vandekerckhove JS, Kaiser DA, Pollard TD. Acanthamoeba actin and profilin can be cross-linked between glutamic acid 364 of actin and lysine 115 of profilin. J Cell Biol 109: 619–626, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y, Klein JD, Blount MA, Martin CF, Kent KJ, Pech V, Wall SM, Sands JM. Epac regulates UT-A1 to increase urea transport in inner medullary collecting ducts. J Am Soc Nephrol 20: 2018–2024, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watts3rd BA, George T, Good DW. The basolateral NHE1 Na+/H+ exchanger regulates transepithelial HCO3− absorption through actin cytoskeleton remodeling in renal thick ascending limb. J Biol Chem 280: 11439–11447, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell 16: 964–975, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Zou S, Jha S, Kim EY, Dryer SE. A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane. Mol Pharmacol 73: 359–368, 2008 [DOI] [PubMed] [Google Scholar]