Abstract
The gap junction protein, connexin43 (Cx43), plays an important role in skeletal biology. Previously, we have shown that Cx43 can enhance the signaling and transcriptional response to fibroblast growth factor 2 (FGF2) in osteoblasts by increasing protein kinase C-δ (PKCδ) activation to affect Runx2 activity. In the present study, we show by luciferase reporter assays that the ERK signaling cascade acts in parallel to PKCδ to modulate Runx2 activity downstream of the Cx43-dependent amplification of FGF2 signaling. The PKCδ-independent activation of ERK by FGF2 was confirmed by Western blotting, as was the Cx43-dependent enhancement of ERK activation. Consistent with our prior observations for PKCδ, flow cytometry analyses show that Cx43 overexpression enhances the percentage of phospho-ERK-positive cells in response to FGF2, supporting the notion that shared signals among gap junction-coupled cells result in the enhanced response to FGF2. Western blots and luciferase reporter assays performed on osteoblasts cultured under low-density and high-density conditions revealed that cell-cell contacts are required for Cx43 to amplify ERK activation and gene transcription. Similarly, inhibition of gap junctional communication with the channel blocker 18β-glycyrrhetinic acid attenuates the Cx43-dependent enhancement of Runx2-transcriptional activity. In total, these data underscore the importance of cell-cell communication and activation of the ERK and PKCδ pathways in the coordination of the osteoblast response to FGF2 among populations of osteoblasts.
Keywords: gap junctions, osteoblast, signal transduction, fibroblast growth factor, protein kinase C
the gap junction protein connexin43 (Cx43) is abundantly expressed in osteoblasts and osteocytes and has been shown to be fundamentally important to skeletal function (5, 22, 41). Mutations in Gja1, the gene encoding Cx43, cause the pleiotropic disorder oculodentodigital dysplasia (ODDD) (35, 36), which has several skeletal manifestations. While the bone mass phenotype of patients with ODDD has not been reported, two mouse models of ODDD have markedly reduced bone mass (7, 9). Similarly, genetic ablation of Cx43 in mouse models leads to delayed ossification, markedly reduced peak bone mass, insensitivity to osteoanabolic interventions, such as intermittent parathyroid hormone administration and mechanical load, and a generalized reduction in osteoblast differentiation and mineralizing capacity (4, 15, 27, 48). Conversely, overexpression of Cx43 in cultured osteoblasts enhances osteogenic capacity and responsiveness to extracellular cues (14, 26, 28, 39), suggesting that the degree of Cx43 expression regulates the full elaboration of the osteoblast phenotype. In addition, Cx43 has been implicated in the mechanosensing and signaling by osteocytes in vitro (3, 12, 19, 37, 40, 45, 49). Despite the relevance of Cx43 to bone, the complex molecular mechanisms by which Cx43 regulates skeletal function and osteoblast/osteocyte biology are only beginning to emerge.
Previously, we have shown that modulation of Cx43 could impact growth factor responses and signal transduction cascades, leading to altered gene expression and osteoblast function (28, 42, 43). To understand how Cx43 regulates osteoblast function, we are examining how osteoblasts respond to fibroblast growth factor 2 (FGF2) and how Cx43 affects that response. FGF2 plays an important but complex role in osteoblast proliferation, differentiation, and bone mass accrual (29, 30). Recently, we reported that modulation of Cx43 affects signal transduction in response to FGF2 treatment in osteoblasts in a PKCδ-dependent manner (28). We demonstrated that overexpression of Cx43 could enhance the transcriptional activity of Runx2, a master regulator of osteoblast differentiation, in response to FGF2, an effect that could be partially blocked by inhibition of PKCδ (28).
In the present study, we show that ERK activity is also required for the full effect of Cx43 on osteoblast responsiveness to FGF2. Indeed, by blocking both the ERK and PKCδ pathways, we can cooperatively inhibit the Cx43-dependent amplification of FGF2 signaling and Runx2 transcriptional activity. In addition, we expand upon the underlying molecular details of how Cx43 amplifies the osteoblast response to FGF2 and demonstrate that Cx43 channel function is required for the communication of shared signals. An understanding of Cx43 function in osteoblasts provides insights into how these cells respond to cues (such as hormones, growth factors, and mechanical load), into the identity of the biologically relevant second messengers/signals being propagated by gap junctions, and, ultimately, into how skeletal tissue coordinates function to regulate bone mass.
MATERIALS AND METHODS
Cell culture and FGF2 treatments.
MC3T3-E1 (clone 4) osteoblasts, obtained from American Type Culture Collection (Manassas, VA), were grown in α minimum essential medium (αMEM) containing 10% fetal bovine serum (HyClone, Logan, UT), penicillin (50 IU/ml), and streptomycin (50 μg/ml) as described previously (16). Cultures were kept at 37°C in a humidified atmosphere of 95% air and 5% CO2. Culture media were renewed every 3 days and cells were used at passage numbers <15. For experiments with FGF2 treatment, cells were serum starved in αMEM containing 0.1% fetal bovine serum and 0.3% bovine serum albumin for 24 h. FGF2 (Millipore, Billerica, MA) was added to the starvation media at 10 ng/ml. The vehicle diluent for FGF2 (phosphate-buffered saline, 0.1% bovine serum albumin, 1 mM dithiothreitol) was used as a negative control for FGF2 treatments. Cell viability was routinely monitored using a CCK-8 assay (Dojindo Molecular Technologies, Rockville, MD). For experiments with low-density cells, the day before treatment with FGF2, cells were split using the nonenzymatic Cellstripper reagent (Cellgro, Manassas, VA) from a 12-well plate into a p100 plate to reduce the number of cell-cell contacts between cells. The cells were maintained overnight in starvation media, as above, before treatment with FGF2. High-density (∼125,000 cells/cm2) and low-density (∼8,000 cells/cm2) cultures were scraped from the dish and collected into a cell pellet by centrifugation, before lysis in an equal volume of lysis buffer. Of note, each preparation was made from the same number of cells (500,000 cells/vessel). In low-density cultures, cell-cell contacts were minimal but not wholly eliminated.
Transient transfections and luciferase assays.
MC3T3 (clone 4) cells were seeded at 100,000 cells/well in 24-well plates. Twenty-four hours later, cells were transiently transfected with the appropriate plasmid using FuGene 6 (Roche, Indianapolis, IN). The reporter plasmid (p6xOSE2Luc) was used at 0.4 μg/well and the expression vector (pSFFV-Cx43) was used at 0.8 μg/well. Total DNA content was kept constant by the addition of pSFFV-neo empty vector. The p6xOSE2 luciferase reporter construct, which contains six repeats of the Runx2-binding OSE2 element of the mouse OG2 promoter (8), was provided by Gerard Karsenty (Columbia University, New York, NY). The pSFFV-Cx43 construct (1), which contains the full-length rat Cx43 cDNA cloned into the EcoR1 site of the pSFFV-neo plasmid, was provided by Thomas Steinberg (Washington University, St. Louis, MO). The pSFFV-neo backbone vector (10) was supplied by Gabriel Nunez (University of Michigan, Ann Arbor, MI). Forty-eight hours posttransfection, the cells were serum starved overnight and then treated with FGF2 (10 ng/ml) for 4 h. The cells were rinsed in Hanks' balanced salt solution before lysis in 1× Passive Lysis Buffer (Promega, Madison, WI). Luciferase activity was monitored using a Berthold Centro LB 960 luminometer (Oak Ridge, TN). Transfection efficiency was assessed by cotransfection with an SV-β-gal reporter (Promega). β-Galactosidase activity was monitored by a colorimetric assay. All experiments were performed in triplicate wells and repeated a minimum of three times.
Western blotting.
For inhibitor treatments, cells were pretreated with 10 μM U0126 (Cell Signaling Technology) or 5 μM rottlerin (Sigma, St. Louis, MO) for 30 min, as well as during exposure to FGF2 (10 ng/ml) before protein extraction. Whole cell extracts were prepared from confluent cultures of MC3T3 cells following treatments, as described previously (33). Equal amounts of proteins were electrophoresed on 10% SDS-PAGE gels, blotted to polyvinylidene difluoride membranes, and probed with the indicated antibodies as described previously (32). Membranes were stripped and reprobed with anti-GAPDH antibodies (Millipore) to ensure equal loading of proteins among lanes. Blots were quantitated with ImageJ image analysis software (National Institutes of Health, Bethesda, MD), and the density of phospho-ERK1/2 bands were normalized to GAPDH. Anti-phospho-ERK1/2, anti-phospho-PKCδ (Thr505), and anti-phospho-PKD (Ser744/748) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence detection reagents were purchased from GE Healthcare Biosciences (Piscataway, NJ). Blots were acquired and analyzed using an UVP EpiChem gel documentation system (UVP Bioimaging Systems, Upland, CA).
Flow cytometry.
For phospho-ERK staining, serum-starved, transiently transfected cells were stimulated with FGF2 (10 ng/ml, 10 min) or vehicle and stained with a LIVE/DEAD Fixable Far Red Dead Cell Stain (Invitrogen, Carlsbad, CA) before formaldehyde fixation and permeabilization with ice-cold methanol. Cells were labeled with anti-phospho ERK1/2 primary antibodies and Alexa488-conjugated chicken anti-rabbit IgG secondary antibodies (Invitrogen). Substitution of nonimmune IgG for the primary antibodies was used as a negative control. For enumeration of phospho-ERK positive cells, cells were resuspended in phosphate-buffered saline containing 0.1% bovine serum albumin and analyzed on a FACSCalibur instrument (BD Immunocytochemistry Systems, San Jose, CA).
Real-time PCR.
Total RNA was isolated from cultured cells using TRIzol reagent, according to the manufacturer's directions. Reverse transcription and real-time PCR were carried out as previously described (33). The primer sets used for PCR were as follows: FGFR1, CGG GCT GGA TAA GGA CAA AC (forward), TGC GTC GGA CTT CAA CAT CTT (reverse); FGFR2, CGA GTC CAG CTC CTC CAT GA (forward), TTG AGG ACA GAC GCG TTG TTA (reverse); FGFR3, TTC TGG CCA ATG TTT CTG AAC TT (forward), GCC GGG TCC TGG ATA GCT (reverse); FGFR4, CCT GCC GGG AAT ACT GTC A (forward), TCC TTG AGC CAG TGG ATG GT (reverse); 18SrRNA CAT TAA ATC AGT TAT GGT TCC TTT GG (forward), TCG GCA TGT ATT AGC TCT AGA ATT ACC (reverse). The data are shown relative to the expression of 18SrRNA, as determined by the ΔCT method as described previously (42). All experiments were carried out in triplicate wells and repeated a minimum of three times.
Statistical analysis.
Results are expressed as means ± SD. Data were analyzed by one-way analysis of variance followed by a Tukey's post hoc test or Dunnett's post hoc, as appropriate, using SigmaStat software (Aspire Software, Ashburn, VA). P ≤ 0.05 was used as a threshold for indication of statistical difference among compared groups.
RESULTS
ERK and PKCδ are both required for the Cx43-dependent amplification of Runx2 activity by FGF2.
Upon binding to its cognate receptor, FGF2 activates numerous signaling cascades, including ERK1/2, protein kinase C family members, phosphatidylinositol 3-kinase (PI3K) and Stat1 (6, 46). Previously, we have reported that the potentiation of FGF2 signaling by Cx43 required the activity of PKCδ (28). However, we found that PKCδ inhibition alone was not sufficient to fully abrogate the effects of Cx43 on FGF2-mediated signaling and transcription. Accordingly, we examined the contribution of other signaling pathways to the Cx43 effect on FGF2 signaling and transcription.
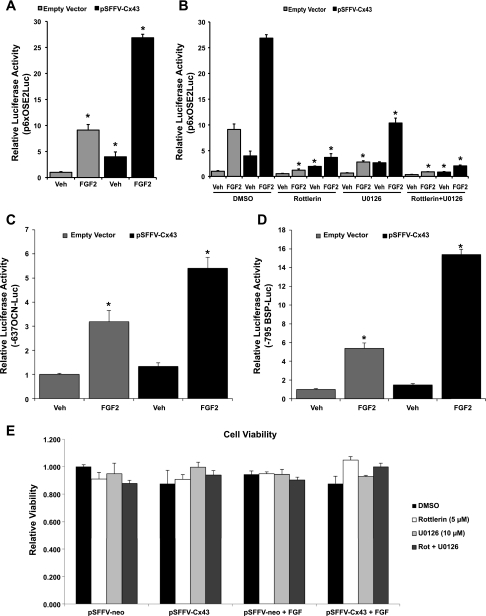
Consistent with our previous findings (28), FGF2 stimulation of MC3T3 cells resulted in an increase in transcriptional activity from the Runx2-binding p6xOSE2-luciferase reporter construct (Fig. 1A). Furthermore, the overexpression of Cx43 resulted in a robust amplification of this response to FGF2. The Cx43-dependent potentiation of transcription activity in response to FGF2 occurs not only in this heterologous Runx2 reporter construct but also in the context of Runx2-regulated osteocalcin and bone sialoprotein gene promoters (Fig. 1, C and D).
Fig. 1.
PKCδ and ERK contribute to the synergistic induction of transcription from a Runx2-binding element by connexin43 (Cx43) and fibroblast growth factor 2 (FGF2). A: luciferase reporter assay of vehicle (Veh) or FGF2 (10 ng/ml, 4 h)-treated MC3T3 cells cotransfected with a Runx2-binding OSE2-luciferase reporter (p6xOSE2Luc) and a Cx43 overexpression vector (pSFFV-Cx43) or an empty vector control (pSFFV-neo). *Significantly increased (P < 0.05) relative to empty vector/vehicle-treated controls. B: luciferase reporter assay as in A but in cells treated with 0.1% DMSO (control), 10 μM U0126, 5 μM rottlerin, or a combination of both U0126 and rottlerin. *Significantly decreased (P < 0.05) relative to its matched DMSO-treated controls. C and D: luciferase reporter assays of vehicle or FGF2 (10 ng/ml, 4 h)-treated MC3T3 cells cotransfected with −637/+32 rat osteocalcin (OCN)-luciferase reporter (C) or −795/+32 bone sialoprotein (BSP)-luciferase reporter (D) and a Cx43 overexpression vector (pSFFV-Cx43) or an empty vector control (pSFFV-neo). *Significantly increased (P < 0.05) relative to empty vector/vehicle-treated controls. E: MC3T3 cells, treated identically to those used for luciferase assays in B, were examined for viability by the colorimetric CCK-8 cell viability assay. Rot, rottlerin. Data were normalized to the empty vector-transfected, vehicle-treated control. All graphs depict means ± SD from representative experiments done in triplicate wells.
As expected, treatment of cells with the PKCδ inhibitor rottlerin (5 μM) markedly reduced both the response to FGF2 and the Cx43 potentiation of this response from the p6xOSE2-luciferase reporter construct (Fig. 1B). Likewise, the MEK inhibitor U0126 (10 μM) reduced the response to FGF2 and the Cx43 potentiation of this response. When administered together, U0126 and rottlerin nearly fully abrogated the Cx43-dependent amplification of the FGF2 response. A viability assay demonstrated that the added effect of combined inhibitor treatments was not due to a synergistic enhancement of cell toxicity (Fig. 1E). Inhibition of the PI3K (LY294002), p38 (SB203580), or JNK (SP600125) pathways did not affect transcription from the p6xOSE2Luc reporter in response to FGF2 and/or Cx43 (data not shown). These data indicate that both the ERK and PKCδ pathways converge on Runx2 to regulate transcription in response to FGF2, and that the activation of both pathways is required for the full potentiation of signaling by Cx43.
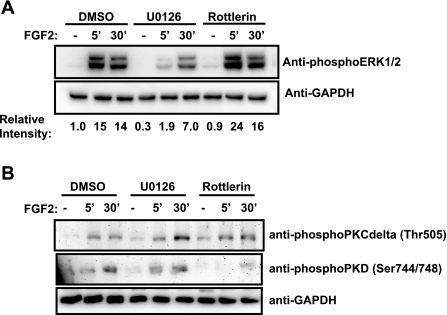
FGF2-stimulated phosphorylation of ERK is independent of PKCδ activity.
In some contexts, it has been shown that PKCδ can stimulate ERK signaling upstream of ERK1/2 (20, 44, 47). To determine whether ERK phosphorylation is dependent on PKCδ activity, we examined by Western blotting the levels of phospho-ERK1/2 in the presence of the PKCδ inhibitor rottlerin or the MEK inhibitor U0126 (Fig. 2A). Stimulation of osteoblasts with FGF2 resulted in an increase in phospho-ERK1/2 levels at both 5 and 30 min. Inhibition of the ERK pathway with U0126 (10 μM) inhibited the FGF2-induced increase in phospho-ERK1/2 levels. In contrast, inhibition of PKCδ with rottlerin (5 μM) failed to impact ERK phosphorylation, indicating that ERK activation by FGF2 is independent of PKCδ activity. Thus, the activation of the Cx43/FGF2-mediated signaling and transcriptional response is due to the independent, parallel action of PKCδ and ERK rather than downstream activation of ERK by PKCδ. Despite the fact that ERK has not been shown to regulate PKCδ, we performed the converse experiment as well. In contrast to the phospho-ERK1/2 response, phospho-PKCδ levels were unaffected by treatment with U0126 or rottlerin (Fig. 2B). The downstream target of PKCδ activity, PKD, whose phosphorylation was stimulated by FGF2 treatment, was not affected by the inhibition of the ERK pathway and yet was sensitive to the PKCδ inhibitor, demonstrating the effectiveness of the rottlerin in the inhibition of PKCδ activity.
Fig. 2.
ERK activation by FGF2 is independent of the activation of PKCδ. Shown are Western blots of whole cell extracts from serum-starved MC3T3 cells treated with vehicle (−) or 10 ng/ml FGF2 (5 min, 30 min) in the presence of 0.1% DMSO (control), 10 μM U0126, or 5 μM rottlerin. Blots were probed with anti-phospho-ERK1/2 antibodies (A) and anti-phospho-PKCδ (Thr505) and anti-phospho-PKD (Ser744/748) antibodies (B). GAPDH was used as a protein load control. Relative expression was determined by densitometric comparison of the band intensities for the ERK1/2 relative to GAPDH using ImageJ software. Data shown are from a representative experiment.
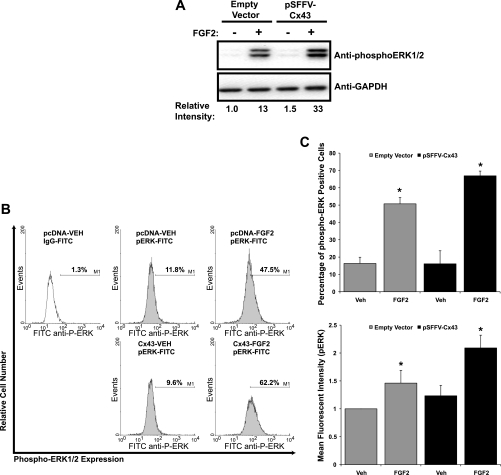
Cx43 enhances ERK activity by increasing the number of cells responding to FGF2.
To expand on the molecular mechanisms by which gap junctions affect downstream signaling, we examined the impact of Cx43 overexpression on phospho-ERK1/2 levels (Fig. 3A). Compared with empty vector transfected cells, Cx43 overexpression enhanced the amount of phospho-ERK1/2 detected in whole cell lysates of FGF2-stimulated cells. These results are analogous to those determined previously for PKCδ (28). Next, we investigated the cause of the increase in ERK activation in response to FGF2 and Cx43. There are two possible explanations for an increase in a detected output in population-based assays like luciferase assays and Western blotting. Either more cells are responding to the signal, resulting in an increase in activation/transcription, or the responding cells are now responding more robustly, generating the same detected increase in activation/transcription. Classically, Cx43 gap junctions are associated with cell-to-cell communication via small-molecule exchange (<1.2 kDa). Based on this function it is likely that the potentiation of signaling is due to increased numbers of cells responding. However, numerous studies have shown that gap junctions function in nonclassic ways, including channel-independent activities (23, 31). Indeed, the COOH-terminal tail of Cx43 has been shown to be a docking platform for numerous signaling molecules (13, 18) and thus may influence signaling and cell function dependent or independent of cell-to-cell communication. Accordingly, to determine the contribution of these two alternatives, we performed flow cytometry to analyze the percentage of phospho-ERK1/2 positive cells following FGF2 treatment in the presence or absence of overexpressed Cx43. The results of a representative experiment are shown in Fig. 3B. The averaged results from triplicate experiments (Fig. 3C) demonstrate that, at basal conditions, 16.3% of empty vector (pcDNA)-transfected, vehicle-treated MC3T3 cells were phospho-ERK1/2 positive. Similarly, in the absence of FGF2, 16.1% of Cx43-overexpressing cells were phospho-ERK1/2 positive. Upon FGF2 stimulation, 50.1% of pcDNA -transfected cells became phospho-ERK positive. Overexpression of Cx43 markedly increased the percentage of phospho-ERK1/2-positive cells (66.9%), suggesting that sharing of signals among cells by Cx43 permitted a larger number of cells to respond to the stimuli. This increase in the percentage of cells responding to FGF2 resulted in a unimodal population shift of cells to a higher mean fluorescence intensity in response to FGF2, which was potentiated by the Cx43 overexpression (Fig. 3B).
Fig. 3.
Cx43-dependent amplification of ERK signaling in response to FGF2. A: Western blots of whole cell extracts from MC3T3 cells that had been transfected with empty vector or pSFFV-Cx43 and subsequently treated with vehicle (−) or 10 ng/ml FGF2 (+) for 15 min. Blots were probed with anti-phospho-ERK1/2 antibodies. GAPDH was used as a protein load control. Relative expression was determined by densitometric comparison of the band intensities for the ERK1/2 relative to GAPDH using ImageJ software. Results from a representative experiment are shown. B: flow cytometry analysis of phospho-ERK1/2-positive cells. MC3T3 cells were transiently transfected with empty vector or pSFFV-Cx43 and treated with vehicle or 10 ng/ml FGF2 for 10 min. Nonimmune IgG antibodies (white) were used as a control to set the marker. Histograms from a representative experiment are shown. C, top: percentage of phospho-ERK1/2 positive cells. C, bottom: mean fluorescence intensity data for each treatment population. Data shown are means ± SD from triplicate flow cytometry experiments. *Significantly increased (P < 0.05) relative to the empty vector/vehicle-treated controls.
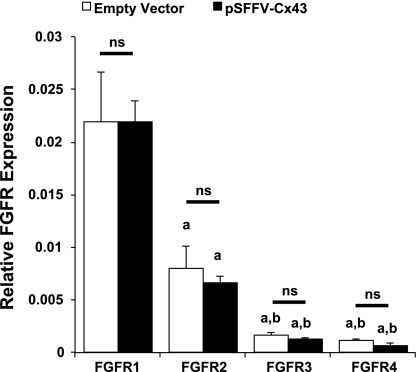
At least two possibilities could support this result. One, overexpression of Cx43 directly regulates FGF receptor (FGFR) expression, permitting a larger percentage of cells to respond to the FGF2 stimulation. Two, Cx43 increases intercellular communication among cells downstream of FGFR activation, leading to an enhanced number of cells responding to FGF2. Real-time PCR analysis of MC3T3 cDNA revealed that FGFR1 is abundantly expressed in these cells, with expression levels more than fivefold higher than that of FGFR2, which is in turn more than three times more abundantly expressed than FGFR3 or FGFR4 (Fig. 4). Notably, the expression levels of all four FGFRs were unaffected by overexpression of Cx43. Accordingly, we examined the role of direct intercellular communication among cells in the amplification of the cellular response.
Fig. 4.
Cx43 overexpression does not regulate FGF receptor (FGFR) expression. A: real-time PCR of FGFR1–4 expression in MC3T3 cells transfected with pSFFV-neo or pSFFV-Cx43. Lowercase letters “a” or “b” indicate a reduction in expression relative to FGFR1 (“a”) and FGFR2 (“b”) with a P < 0.05; ns, no significant difference in expression. Graph depicts means ± SD from a representative experiment done in triplicate wells.
Cell-cell contact and Cx43 function are required for the enhancement of ERK signaling and Runx2 activity by Cx43.
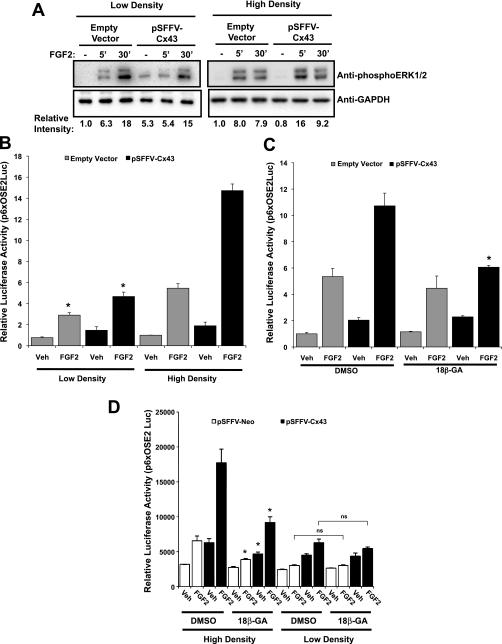
To establish that communication of signals is by direct cell-to-cell exchange of information, we examined the ability of Cx43 to enhance the response of MC3T3 to FGF2 in cells cultured at high and low densities, as well as in Cx43-overexpressing cells treated with a gap junction inhibitor, 18β-glycyrrhetinic acid. The activation of the ERK signaling cascade was examined using Western blotting for phospho-ERK1/2 in cells cultured at ∼100% confluence (high density; ∼125,000 cells/cm2) or at ∼10% confluence (low density; ∼8,000 cells/cm2). In contrast to the high-density cultures, overexpression of Cx43 did not enhance the detected amounts of phospho-ERK1/2 relative to empty vector-transfected cells in cells cultured at low density with few cell-cell contacts (Fig. 5A). Interestingly, there is a change in the dynamics of ERK phosphorylation in low-density versus high-density cultures, with maximal phospho-ERK1/2 levels occurring later in low-density cultures. We are unsure why this occurs, but perhaps it is due to the loss of even basal gap junctional communication at low density.
Fig. 5.
Amplification of ERK phosphorylation and subsequent Runx2-mediated transcription require cell-cell contacts. A: Western blots of whole cell extracts from MC3T3 cells that had been transfected with empty vector or pSFFV-Cx43, plated at high cell density (∼125,000 cells/cm2) or low cell density (∼8,000 cells/cm2) and subsequently treated with 10 ng/ml FGF2 for 0, 5, or 30 min. Blots were probed with anti-phospho-ERK1/2 antibodies. GAPDH was used as a protein load control. Relative expression was determined by densitometric comparison of the band intensities for the ERK1/2 relative to GAPDH using ImageJ software. Data shown are from a representative experiment. B: luciferase reporter assay of vehicle or FGF2 (10 ng/ml, 4 h)-treated MC3T3 cells cotransfected with a Runx2-binding OSE2-luciferase reporter (p6xOSE2Luc) and a Cx43 overexpression vector (pSFFV-Cx43) or an empty vector control (pSFFV-neo). Cells were cultured at low or high density, as described above, before treatment with FGF2. Graph depicts means ± SD from a representative experiment done in triplicate wells. *Significantly reduced (P < 0.05) relative to the corresponding high cell density culture. C: luciferase reporter assay of vehicle or FGF2 (10 ng/ml, 4 h)-treated MC3T3 cells cotransfected with a Runx2-binding OSE2-luciferase reporter (p6xOSE2Luc) and a Cx43 overexpression vector (pSFFV-Cx43) or an empty vector control (pSFFV-neo). Cells were treated with DMSO or 18β-glycyrrhetinic acid (20 μM) before and during FGF2 treatment. Graph depicts means ± SD from a representative experiment done in triplicate wells. *Significantly reduced (P < 0.05) relative to the corresponding DMSO-treated sample. D: luciferase reporter assay of vehicle or FGF2 (10 ng/ml, 4 h)-treated MC3T3 cells cotransfected with a Runx2-binding OSE2-luciferase reporter (p6xOSE2Luc) and a Cx43 overexpression vector (pSFFV-Cx43) or an empty vector control (pSFFV-neo). Cells were cultured at low or high density, as described in materials and methods. Before and during treatment with vehicle or FGF2, cells were treated with DMSO or 18β-glycyrrhetinic acid (20 μM). Graph depicts means ± SD from a representative experiment done in triplicate wells; ns, no significant difference detected between the low-density cultures treated with DMSO or 18β-glycyrrhetinic acid. *Significantly reduced (P < 0.05) relative to the corresponding DMSO-treated sample in the high density cultures.
Similarly, overexpression of Cx43 in the low-density cell cultures failed to potentiate the transcriptional activity from a Runx2 reporter (p6xOSE2 Luc) in response to FGF2 (Fig. 5B). On the other hand, in the high-density cell cultures with abundant cell-cell contacts, Cx43 overexpression potentiated the transcriptional response of the cells to FGF2 treatment (Fig. 5B).
To further confirm that these effects were due to traditional Cx43 functions (i.e., gap junctional communication and/or hemichannel function) and not due to novel nonjunctional mechanisms or an artifact of overexpression, we overexpressed Cx43 and used the gap junction inhibitor 18β-glycyrrhetinic acid (20 μM) to block the effects of Cx43 on the transcriptional response of the cells to FGF2 treatment (Fig. 5C). 18β-Glycyrrhetinic acid prevented the potentiation of transcription caused by Cx43 overexpression with minimal effect on the basal response of the cells in mock-transfected cells. To establish that the effects of 18β-glycyrrhetinic acid were related to cell-to-cell communication, we treated cells seeded at low density with 18β-glycyrrhetinic acid, which failed to further inhibit the already suppressed FGF2 response (Fig. 5D).
DISCUSSION
Previous studies have shown that both the ERK pathway (17, 34, 50) and the PKCδ pathway (24, 25, 28) contribute to Runx2 activation following FGF2 treatment in osteoblastic cells; however, the interdependence of these pathways was previously unknown. Indeed, in some cell contexts, PKCδ is known to directly activate the ERK MAPK cascade at the level of Ras or MEK (20, 44, 47). We present here clear evidence that ERK and PKCδ act in parallel to regulate Runx2 transcriptional activity following FGF2 treatment of MC3T3 osteoblast-like cells.
Importantly, the data presented here demonstrate that these two pathways mediate the amplification of the osteoblast response to FGF2 by Cx43 expression independently. These data imply that Cx43 communicates a signal (or signals) that activates both the ERK and PKCδ pathways. While the identity of the second messenger mediators of the Cx43-dependent amplification of Runx2 activity in osteoblasts are still unclear, an understanding of the pathways downstream of these messengers provides insights into their identity. Undoubtedly there are multiple second messengers that can be communicated by gap junctions to initiate downstream signaling. The identity of these second messengers will be dictated by the extracellular cue to which the cell is responding. Notably, a common theme of downstream pathways that carry out the action of Cx43 is emerging in osteoblasts. ERK (37, 38, 42, 45), protein kinase C (11, 28, 32), and cAMP/protein kinase A (2) pathways have all been shown to contribute to the changes to osteoblast responses mediated by Cx43. Despite the likelihood that Cx43 transmits several signal molecules that converge upon a finite number of signaling cascades, overall the role of Cx43 in bone seems to be osteoanabolic as disruption of Cx43 function in vivo results in decreased bone mass, an attendant defect in osteoblast function and diminished ability to respond to osteogenic cues (4, 7, 9, 15, 27, 48).
Cx43 can function as traditional gap junction channels, hemichannels, and via nontraditional, channel-independent means (21). With respect to FGF2 responsiveness, our flow cytometry data indicate that the effects of Cx43 on ERK phosphorylation in response to FGF2 is mediated by an increase in the number of cells responding to the FGF2 treatment, rather than simply an increase in the robustness of the response by a few cells. Indeed, we observed a unimodal population shift in phospho-ERK- labeled cells rather than a bimodal shift, which is indicative of Cx43-transfected cells [∼30–40% of the total cell population (28)] communicating the response to the nontransfected cell population, rather than just an increase in the responsiveness of the subpopulation of Cx43 transfected cells. In agreement with our Western blot and luciferase reporter data, the observed increase in the percentage of cells responding to FGF2 results in a potentiation of the mean ERK response by the population of cells. In total, these data are consistent with a model of traditional gap junctional communication or hemichannel activity. Furthermore, our data show that cell-cell contacts are required for the action of Cx43 on ERK and the transcriptional responsiveness of osteoblast-like cells to FGF2, as the potentiation of phospho-ERK1/2 and Runx2-mediated transcription only occurred in high-density cell culture. This underscores that the effects of Cx43 on signaling and function in these cells is likely due to the classic cell-cell signaling function of Cx43 rather than on nontraditional, channel independent means. While these data suggest that signaling occurs via classic cell-cell contact-mediated communication through gap junction channels, we cannot fully rule out with these methodologies a contribution of hemichannel activity, as cell density may influence the diffusion of messengers passed via hemichannels. Effective tools to dissect hemichannel and gap junction channels activities have still not been adequately developed.
We envision a model in which cells that sense the extracellular cue (i.e., FGF2) respond by generating a signaling response that includes second messengers. As a result of coupling of cells via gap junctions, these second messengers are propagated to adjacent cells permitting those cells to also respond to the extracellular cue. This results in an amplification of the cell population's ability to respond to the cue by direct sharing of information. Indeed, these results are analogous to those that we have reported for PKCδ and FGF2 (28). This model is also consistent with our previous data on the impact of disrupted Cx43 function on the signaling and transcription in ROS17/2.8 osteosarcoma cells (42, 43).
In conclusion, our data support the concept that Cx43 permits a more homogenous and robust response of cells to FGF2 treatment by sharing signals among cells, resulting in the parallel activation of the ERK and PKCδ pathways which converge on Runx2 activity. These data provide important insights into the phenotypes of Cx43 knockout models, which are likely caused, at least in part, by diminished responsiveness and coordination of osteoblast activity as a result of osteogenic cues. Indeed, defects in osteoblast gene expression and responsiveness to anabolic cues have been reported in Cx43-deficient mouse models (4, 27, 48). By understanding the osteoanabolic pathways regulated by intercellular signaling through Cx43, we gain insight into the molecular mechanisms that bone cells use to coordinate function.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.N., M.S.W., and J.P.S. conception and design of the research; C.N., A.M.B., C.H., and B.T.D. performed the experiments; C.N., A.M.B., and J.P.S. analyzed the data; C.N., A.M.B., and M.S.W. interpreted the results of the experiments; C.N., A.M.B., and J.P.S. prepared the figures; C.N., M.S.W., and J.P.S. edited and revised the manuscript; J.P.S. drafted the manuscript; J.P.S. approved the final version of the manuscript.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health/National Institute for Arthritis, Musculoskeletal and Skin Diseases Grant R01 AR052719 (to J. P. Stains).
REFERENCES
- 1. Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol 105: 2621–2629, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bivi N, Lezcano V, Romanello M, Bellido T, Plotkin LI. Connexin43 interacts with betaarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem 112: 2920–2930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell 16: 3100–3106, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci 119: 4187–4198, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys 473: 188–192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 16: 233–247, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, Bukauskas FF, Civitelli R, Lewalter T, Fleischmann BK, Willecke K. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet 17: 539–554, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol 15: 1858–1869, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, Nieman BJ, Peterson K, Qu D, Roscoe W, Shao Q, Tong D, Veitch GI, Voronina I, Vukobradovic I, Wood GA, Zhu Y, Zirngibl RA, Aubin JE, Bai D, Bruneau BG, Grynpas M, Henderson JE, Henkelman RM, McKerlie C, Sled JG, Stanford WL, Laird DW, Kidder GM, Adamson SL, Rossant J. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 132: 4375–4386, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Fuhlbrigge RC, Fine SM, Unanue ER, Chaplin DD. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci USA 85: 5649–5653, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geneau G, Defamie N, Mesnil M, Cronier L. Endothelin1-induced Ca(2+) mobilization is altered in calvarial osteoblastic cells of Cx43(+/−) mice. J Membr Biol 217: 71–81, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol 212: 207–214, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res 62: 233–245, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Gramsch B, Gabriel HD, Wiemann M, Grummer R, Winterhager E, Bingmann D, Schirrmacher K. Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast-like cell line. Exp Cell Res 264: 397–407, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1). J Bone Miner Res 23: 879–886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta RR, Yoo DJ, Hebert C, Niger C, Stains JP. Induction of an osteocyte-like phenotype by fibroblast growth factor-2. Biochem Biophys Res Commun 402: 258–264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hatch NE, Li Y, Franceschi RT. FGF2 stimulation of the pyrophosphate-generating enzyme, PC-1, in pre-osteoblast cells is mediated by RUNX2. J Bone Miner Res 24: 652–662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol 94: 29–65, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Huo B, Lu XL, Costa KD, Xu Q, Guo XE. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium 47: 234–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson DN, Foster DA. The enigmatic protein kinase Cdelta: complex roles in cell proliferation and survival. FASEB J 18: 627–636, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Jiang JX, Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim Biophys Acta 1711: 208–214, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci 12: 1450–1462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kardami E, Dang X, Iacobas DA, Nickel BE, Jeyaraman M, Srisakuldee W, Makazan J, Tanguy S, Spray DC. The role of connexins in controlling cell growth and gene expression. Prog Biophys Mol Biol 94: 245–264, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kim BG, Kim HJ, Park HJ, Kim YJ, Yoon WJ, Lee SJ, Ryoo HM, Cho JY. Runx2 phosphorylation induced by fibroblast growth factor-2/protein kinase C pathways. Proteomics 6: 1166–1174, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Kim HJ, Kim JH, Bae SC, Choi JY, Kim HJ, Ryoo HM. The protein kinase C pathway plays a central role in the fibroblast growth factor-stimulated expression and transactivation activity of Runx2. J Biol Chem 278: 319–326, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Lecanda F, Towler DA, Ziambaras K, Cheng SL, Koval M, Steinberg TH, Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell 9: 2249–2258, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol 151: 931–944, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lima F, Niger C, Hebert C, Stains JP. Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism. Mol Biol Cell 20: 2697–2708, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marie PJ. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene 316: 23–32, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Marie PJ, Coffin JD, Hurley MM. FGF and FGFR signaling in chondrodysplasias and craniosynostosis. J Cell Biochem 96: 888–896, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Mroue RM, El-Sabban ME, Talhouk RS. Connexins and the gap in context. Integr Biol (Camb) 3: 255–266, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Niger C, Hebert C, Stains JP. Interaction of connexin43 and protein kinase C-delta during FGF2 signaling. BMC Biochem 11: 14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niger C, Howell FD, Stains JP. Interleukin-1beta increases gap junctional communication among synovial fibroblasts via the extracellular-signal-regulated kinase pathway. Biol Cell 102: 37–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park OJ, Kim HJ, Woo KM, Baek JH, Ryoo HM. FGF2-activated ERK mitogen-activated protein kinase enhances Runx2 acetylation and stabilization. J Biol Chem 285: 3568–3574, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet 72: 408–418, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat 30: 724–733, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem 280: 7317–7325, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem 277: 8648–8657, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Rossello RA, Wang Z, Kizana E, Krebsbach PH, Kohn DH. Connexin 43 as a signaling platform for increasing the volume and spatial distribution of regenerated tissue. Proc Natl Acad Sci USA 106: 13219–13224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, Jiang JX. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem 283: 26374–26382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stains JP, Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta 1719: 69–81, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Stains JP, Civitelli R. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol Biol Cell 16: 64–72, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem 278: 24377–24387, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J 384: 449–459, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. Am J Physiol Cell Physiol 292: C545–C552, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10: 116–129, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem 271: 23512–23519, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E, Civitelli R. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell 22: 1240–1251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/beta-catenin signaling. Mol Cell Biol 30: 206–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem 277: 36181–36187, 2002 [DOI] [PubMed] [Google Scholar]