Abstract
Abl is a nonreceptor tyrosine kinase that has a role in regulating migration and adhesion of nonmuscle cells as well as smooth muscle contraction. The role of Abl in smooth muscle cell proliferation has not been investigated. In this study, treatment with endothelin-1 (ET-1) and platelet-derived growth factor (PDGF) increased Abl phosphorylation at Tyr412 (an indication of Abl activation) in vascular smooth muscle cells. To assess the role of Abl in smooth muscle cell proliferation, we generated stable Abl knockdown cells by using lentivirus-mediated RNA interference. ET-1- and PDGF-induced cell proliferation was attenuated in Abl knockdown cells compared with cells expressing control shRNA and uninfected cells. Abl silencing also arrested cell cycle progression from G0/G1 to S phase. Furthermore, activation of smooth muscle cells with ET-1 and PDGF induced phosphorylation of ERK1/2 and Akt. Abl knockdown attenuated ERK1/2 phosphorylation in smooth muscle cells stimulated with ET-1 and PDGF. However, Akt phosphorylation upon stimulation with ET-1 and PDGF was not reduced. Because Abl is known to regulate actin polymerization in smooth muscle, we also evaluated the effects of inhibition of actin polymerization on phosphorylation of ERK1/2. Pretreatment with the actin polymerization inhibitor latrunculin-A also blocked ERK1/2 phosphorylation during activation with ET-1 and PDGF. The results suggest that Abl may regulate smooth muscle cell proliferation by modulating actin dynamics and ERK1/2 phosphorylation during mitogenic activation.
Keywords: Abelson tyrosine kinase, extracellular signal-regulated kinase 1/2
proliferation of vascular smooth muscle cells plays a critical role in the development and homeostasis of blood vessels and contributes to the pathogenesis of vascular diseases such as hypertension and restenosis (3, 15). However, the mechanisms that regulate vascular smooth muscle cell proliferation are not fully understood.
Abl (Abelson tyrosine kinase; C-Abl) is a nonreceptor tyrosine kinase that has been implicated to function in a range of cellular functions, including migration and adhesion of nonmuscle cells (8, 27). Recent studies have suggested that Abl is an important component of the cellular processes that regulate vascular smooth muscle contraction. Abl undergoes phosphorylation at Tyr412 (an indication of Abl activation) in smooth muscle during agonist stimulation (1, 5, 9, 26). Abl knockdown by RNA interference (RNAi) significantly attenuates force development in vascular smooth muscle (1, 5, 9). Nevertheless, the functional role of Abl in vascular smooth muscle cell proliferation has not been investigated.
Abl tyrosine kinase is able to regulate actin polymerization in vascular smooth muscle (1, 5, 9). Actin polymerization transpires in smooth muscle in response to external stimulation. Silencing of Abl in smooth muscle diminishes actin polymerization and force development in smooth muscle (1, 5, 9). There is a wealth of evidence to suggest that actin polymerization is involved in the regulation of nonmuscle cell adhesion and movement, and smooth muscle contraction (1, 5, 8, 9, 27).
Proliferation of smooth muscle cells is in part mediated by ERK1/2 (extracellular signal-regulated kinases), an important member of the mitogen-activated protein kinase (MAPK) family. ERK1/2 is phosphorylated and activated in smooth muscle cells in response to treatment with various stimuli, such as growth factors and vasoactive peptides (3, 16, 28). Inhibition of ERK1/2 by pharmacological tools or knockdown of ERK1/2 by molecular approaches attenuates proliferation of various cell types, including smooth muscle cells (4, 16). It has been proposed that activated ERK1/2 translocates from the cytoplasm to the nucleus, by which ERK1/2 phosphorylates several protein kinases, nuclear transcription factors, and other proteins to promote cell proliferation (3, 28).
The objective of this study was to evaluate whether Abl has a role in regulating vascular smooth muscle cell proliferation. We established stable Abl knockdown cells to assess the role of Abl in cell proliferation. Furthermore, we determined whether Abl kinase and actin polymerization participate in the regulation of ERK1/2 phosphorylation in smooth muscle cells during mitogenic activation.
MATERIALS AND METHODS
Cell culture.
All animal protocols were approved by the Institutional Animal Care and Use Committee at Albany Medical College. Primary vascular smooth muscle cells were isolated from rat aorta. Briefly, two aortic segments (10-mm long) were incubated for 10 min with 5 ml of dissociation solution [130 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES (pH 7.0), 0.25 mM EDTA, 10 mM d-glucose, 10 mM taurine, 4.5 mg of collagenase (type I), 10 mg of papain (type IV), 2 mg of elastase, 1 mg of tryosin inhibitor, 1 mg/ml BSA, and 1 mM dithiothreitol]. All enzymes were obtained from Sigma. The tissue was then washed three times with a HEPES-buffered saline solution (composition in mM: 10 HEPES, 130 NaCl, 5 KCl, 10 glucose, 1 CaCl2, 1 MgCl2, 0.25 EDTA, and 10 taurine, pH 7) and triturated with a pipette to liberate individual smooth muscle cells from the tissue. The cell suspension was mixed with DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (vol/vol) fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin). Cells were cultured in 100-mm dishes at 37°C in the presence of 5% CO2 in the same DMEM. The medium was changed every 2–3 days until the cells reached confluence, and confluent cells were passaged by using trypsin-EDTA solution.
Assessment of cell proliferation.
Cells (1 ×104) were plated in 24-well plates with DMEM supplemented with 5% FBS for ≥18 h. Cells were then serum starved for 24 h. They were subsequently treated with endothelin-1 (ET-1; 200 nM) or platelet-derived growth factor (PDGF)-BB (20 ng/ml) in DMEM containing 0.25% FBS. Additional cells were cultured in DMEM with 0.25% FBS as control. Numbers of viable cells were counted using the trypan blue exclusion test. Briefly, cell suspension was incubated with 0.4% trypan blue for 5 min at room temperature. Unstained cells (viable cells) were counted using a hemocytometer. Duplicated samples were averaged for each independent experiment.
Immunoblot analysis.
Cells were lysed in 40 μl of SDS sample buffer composed of 1.5% dithiothreitol, 2% SDS, 80 mM Tris·HCl (pH 6.8), 10% glycerol, and 0.01% bromophenol blue. The lysates were boiled in the buffer for 5 min and separated by SDS-PAGE. Proteins were transferred to a nitrocellulose membrane. The membrane was blocked with bovine serum albumin or milk for 1 h and probed with primary antibody followed by horseradish peroxidase-conjugated secondary antibody (ICN Biomedicals, Irvine, CA). Proteins were visualized by enhanced chemiluminescence (SuperSignal; Pierce) using the LAS-4000 Fuji Image System. Phospho-Abl (Tyr412) antibody, total-Abl antibody, phospho-ERK1/2 (Thr202/Tyr204) antibody, total ERK1/2 antibody, phospho-Akt (Ser473) antibody, and total Akt antibody were purchased from Cell Signaling Technology. GAPDH antibody was purchased from Fitzgerald.
The levels of phosphoprotein and total protein were quantified by scanning densitometry of immunoblots (Fuji Multigauge Software). Changes in protein phosphorylation were expressed as a magnitude increase over levels of phosphorylation in unstimulated cells. The luminescent signals from all immunoblots were within the linear range (11–13).
Construction of lentivirus encoding short hairpin RNA and virus production.
To construct lentivirus encoding Abl short hairpin RNA (shRNA), oligonucleotides were synthesized by Invitrogen (Carlsbad, CA). The sense target sequence of Abl shRNA was 5′-AAGCAGCTCGATGGAACTCCAAG-3′ (NCBI accession no. DQ294402). Oligonucleotides with scramble sequence (5′-ATTGCTCATATTGGCTAT-3′) were used as control. Oligonucleotides encoding Abl shRNA or control shRNA were subcloned into pFUGW lentiviral vector (14), followed by transformation into Stbl3-competent cells (Invitrogen). These constructs also carry the gene of green fluorescent protein (GFP) to monitor the infection efficiency of cells. The plasmid DNA was harvested and purified using the plasmid maxiprep kits (Invitrogen). To produce viruses, 293FT cells were transfected with pFUGW encoding Abl shRNA or scramble control shRNA plus packaging vector pCMV and envelop vector pVSV-G. Viruses were collected 48 h after transfection. To infect cells, smooth muscle cells were incubated with viruses for 6 h. The cells were then incubated in the growth medium for 2–3 days. GFP fluorescence of infected cells was monitored by a digital fluorescent microscope.
Analysis of F/G-actin ratios.
The content of F-actin and G-actin in smooth muscle was measured using a previously described method (1, 24, 25, 30). Briefly, smooth muscle cells were homogenized in F-actin stabilization buffer (50 mM PIPES, pH 6.9, 50 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 5% glycerol, 0.1% Triton X-100, 0.1% Nonidet P-40, 0.1% Tween 20, 0.1% β-mercaptoethanol, 1 mM ATP, 1 μg/ml pepstatin, 1 μg/ml leupeptin, and 10 μg/ml benzamidine). The supernatants of protein extracts were collected after centrifugation at 151,000 g for 60 min at 37°C. The pellets were resuspended in ice-cold H2O plus 1 μM cytochalasin D and then incubated on ice for 1 h to dissociate F-actin. The resuspended pellets were gently mixed every 15 min. The supernatant of the resuspended pellets was collected after centrifugation at 16,100 g for 2 min at 4°C. Equal volume of the first (G-actin) or second supernatant (F-actin) was subjected to immunoblot analysis using α-actin antibody. The amount of F-actin and G-actin was determined by scanning densitometry.
Fluorescence microscopy.
Cells were fixed for 15 min in 4% paraformaldehyde and then washed three times in Tris-buffered saline containing 50 mM Tris, 150 mM NaCl, and 0.1% NaN3, followed by permeabilization with 0.2% Triton X-100 dissolved in Tris-buffered saline for 5 min. The samples were fluorescently stained for F-actin using rhodamine-labeled phalloidin (Invitrogen-Molecular Probes). Images of labeled F-actin were viewed under a high-resolution fluorescent microscope (Leica). The time of image capturing, intensity gaining, and image black levels in channels were optimally adjusted and kept constant for all experiments to standardize the fluorescence intensity measurements among the experiments. For each independent experiment, three regions were randomly selected for observation. Fluorescence intensities were measured using National Institutes of Health ImageJ software. Measurements from three areas were averaged for a single data point.
Cell cycle analysis by flow cytometry.
Cells were cultured in serum-free medium for 24 h. They were then treated with mitogenic factors for 24 h. Cells were washed with phosphate-buffered saline (PBS) and collected by exposure to trypsin and centrifugation at 600 g for 5 min. Cells were resuspended in PBS and fixed in 70% ethanol at 4°C overnight. The fixed cells were washed with PBS, stained with propidium iodide (50 μg/ml), and incubated with RNase A (100 μg/ml) overnight at 4°C and analyzed using a FACSCanto flow cytometer (BD Biosciences).
Statistical analysis.
All statistical analysis was performed using Prism 4 software (GraphPad Software, San Diego, CA). Comparison among multiple groups was performed by one-way analysis of variance followed by Tukey's multiple comparison test. Differences between pairs of groups were analyzed by Student-Newman-Keuls test or Dunn's method. Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
RESULTS
Treatment with ET-1 and PDGF induces proliferation of vascular smooth muscle cells.
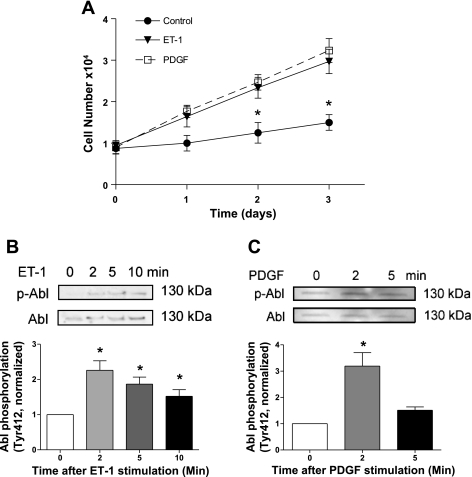
We evaluated the effects of ET-1 and PDGF stimulation on cell proliferation. Both ET-1 (a small vasoactive peptide) and PDGF have been implicated in cell growth (3, 17). Rat aortic smooth muscle cells were stimulated with ET-1 (200 nM) and PDGF (20 ng/ml) or left unstimulated. Numbers of viable cells were determined using the trypan blue exclusion test. As shown in Fig. 1A, the numbers of cells treated with ET-1 and PDGF were increased compared with control cells, which is time dependent. These results demonstrate the positive roles of ET-1 and PDGF in vascular smooth muscle cell proliferation.
Fig. 1.
Stimulation with endothelin-1 (ET-1) and platelet-derived growth factor (PDGF) induces cell proliferation and Abelson tyrosine kinase (Abl) phosphorylation. A: rat aortic smooth muscle cells were stimulated with 200 nM ET-1 and 20 ng/ml PDGF for 1–3 days. The numbers of viable cells were determined using the trypan blue exclusion test. Data are means ± SE of 4 independent experiments. *Significantly different between unstimulated cells and stimulated cells on given days (P < 0.05). B and C: cells were stimulated with 200 nM ET-1 (B) or 20 ng/ml PDGF (C) for different time points or left unstimulated. Abl phosphorylation in these cells was evaluated by immunoblotting using phospho-Abl (Tyr412) antibody and total Abl antibody. The phosphorylation values of protein in stimulated cells were normalized to the phosphorylation level in unstimulated smooth muscle cells. *Significantly higher Abl phosphorylation in stimulated cells compared with unstimulated cells (P < 0.05). Values represent means ± SE (n = 4–6).
Stimulation with ET-1 and PDGF leads to an increase in phosphorylation of Abl in smooth muscle cells.
Next, we determined whether Abl is activated in smooth muscle cells upon mitogenic stimulation. Vascular smooth muscle cells were treated with ET-1 (200 nM) and PDGF (20 ng/ml) for different time points. Abl phosphorylation at Tyr412 in these cells, an indication of Abl activation (1), was determined by immunoblot analysis.
Stimulation with ET-1 induced Abl phosphorylation in a time-dependent manner. Phosphorylation levels of Abl were increased approximately twofold 2–5 min after stimulation with ET-1. Abl phosphorylation levels declined slightly 10 min after ET-1 stimulation (Fig. 1B). Exposure to PDGF also enhanced Abl phosphorylation in a time-dependent manner (Fig. 1C). These results indicate that ET-1 and PDGF are able to activate Abl in smooth muscle cells.
Generation of stable Abl knockdown cells.
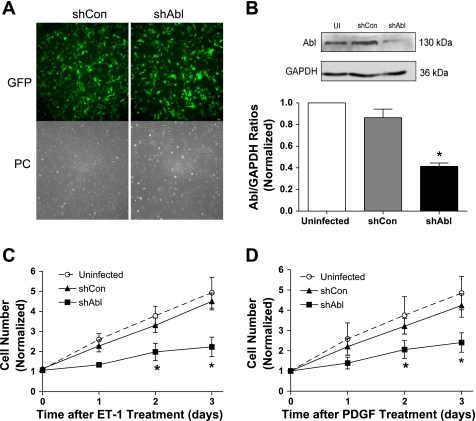
To reveal the functional role of Abl in cell proliferation, we constructed recombinant lentiviruses encoding control or Abl shRNA. These constructs also carry the gene of GFP to monitor the infection efficiency of cells. Cells were infected with lentiviruses encoding control or Abl shRNA for 3 days. The infection efficiency was nearly 100% as evaluated by fluorescent microscopy (Fig. 2A). Immunoblot analysis was used to determine the expression levels of Abl in these cells. The level of Abl protein in cells treated with control shRNA was comparable with uninfected cells. However, the protein level of Abl in cells expressing Abl shRNA was obviously lower compared with uninfected cells and cells expressing control shRNA. The expression level of GAPDH was similar in these cells. Abl/GAPDH ratios were significantly lower in Abl shRNA-treated cells than in uninfected cells and in cells generating control shRNA (Fig. 2B). In addition, these cells expressing shRNAs are stable for at least five passages after initial infection.
Fig. 2.
Knockdown of Abl attenuates cell proliferation induced by ET-1 and PDGF. A: cells were infected with lentiviruses encoding control short hairpin (sh)RNA (shCon) or Abl shRNA (shAbl) for 3 days. These constructs also carry the gene of green fluorescence protein (GFP) to monitor the infection efficiency of cells. Almost every cell displayed GFP fluorescence, suggesting nearly 100% infection efficiency. PC, phase contrast images. B: blots of uninfected (UI) cells, cells infected with viruses encoding shCon, and cells infected with viruses producing shAbl were probed with use of antibodies against Abl and GAPDH. Ratios of Abl/GAPDH protein in cells producing shCon or shAbl were normalized to ratios obtained in cells not treated with lentiviruses. Values are means ± SE (n = 4). *Significantly lower Abl/GAPDH ratios in cells producing Abl shRNA compared with UI cells and cells producing control shRNA (P < 0.05). C and D: UI cells, cells expressing shCon, and stable Abl knockdown cells were treated with 200 nM ET-1 (C) or 20 ng/ml PDGF (D). The numbers of viable cells were determined using the trypan blue exclusion test. Data are means ± SE (n = 4). *Significantly lower numbers of Abl knockdown cells compared with UI cells and cells expressing control shRNA on the same day (P < 0.05).
Knockdown of Abl attenuates cell growth induced by ET-1 and PDGF.
We determined the effects of Abl knockdown on cell proliferation in response to mitogenic stimulation. Uninfected cells, cells stably expressing control shRNA, and stable Abl knockdown cells were stimulated with ET-1 and PDGF or left unstimulated. The numbers of viable cells were then determined 1–3 days after the treatment. Stimulation with ET-1 and PDGF led to the increase in cell numbers in uninfected cells and cells producing control shRNA. However, the increase in cell numbers induced by ET-1 and PDGF was dramatically reduced in Abl-deficient cells (Fig. 2, C and D).
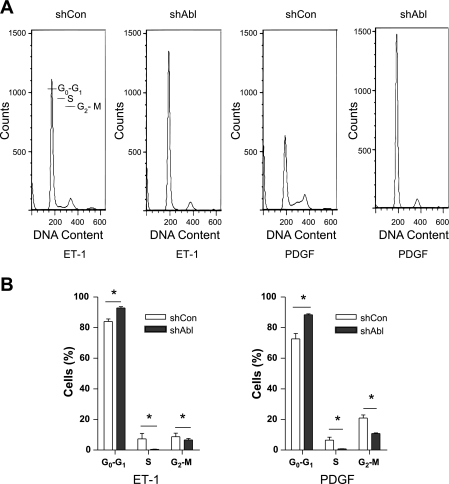
We next evaluated cell cycle progression in Abl knockdown cells by flow cytometric analysis. Cells were synchronized in G0/G1 by serum deprivation for 24 h and stimulated to reenter the cell cycle by exposure to ET-1 and PDGF. Abl knockdown blocked cell cycle at the G0/G1 phase. The percentage of Abl-deficient cells at the G0/G1 phase was higher compared with control cells. In contrast, the percentage of Abl knockdown cells in the S and G2/M phases was lower than that of control cells (Fig. 3).
Fig. 3.
Effects of Abl knockdown on cell cycle progression. A: cells were synchronized in G0/G1 by serum deprivation for 24 h and harvested for flow cytometry 24 h after mitogenic exposure. Plots represent cell numbers. vs. DNA content. B: histograms derived from experiments similar to that in A showing the proportion of cells in G0/G1, S, and G2/M phases of the cell cycle. *P < 0.05. Data are means ± SE (n = 3).
Activation with ET-1 and PDGF induces ERK1/2 phosphorylation.
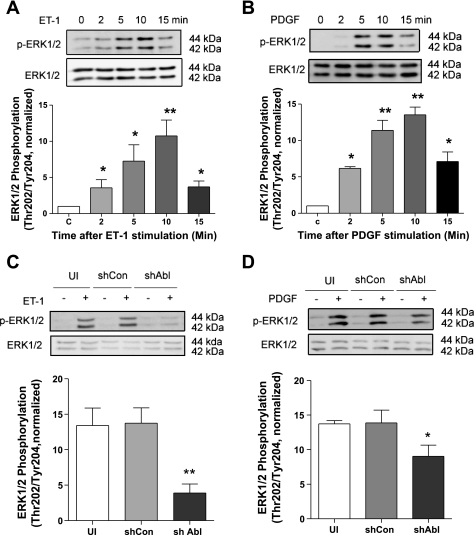
We evaluated the effects of activation with ET-1 and PDGF on phosphorylation of ERK1/2, a well-documented protein kinase regulating cell proliferation (28). Smooth muscle cells were stimulated with ET-1 and PDGF. Phosphorylation of ERK1/2 was evaluated by immunoblot analysis. Activation with ET-1 and PDGF induced time-dependent ERK1/2 phosphorylation that reached the maximal level 10 min after stimulation (Fig. 4, A and B). Phosphorylation levels and time course of ERK1/2 were similar in cells stimulated with ET-1 and in cells treated with PDGF.
Fig. 4.
Effects of Abl silencing on ERK1/2 phosphorylation. A and B: cells were stimulated with 200 nM ET-1 (A) and 20 ng/ml PDGF (B) for 2–15 min or left unstimulated. ERK1/2 phosphorylation (Thr202/Tyr204) was evaluated by immunoblot analysis. Stimulation with ET-1 (n = 5) and PDGF (n = 5) significantly induces ERK1/2 phosphorylation in a time-dependent manner. C, control. C and D: UI cells, cells expressing shCon, and cells producing shAbl were treated with or without 200 nM ET-1 (C) or 20 ng/ml PDGF (D) for 10 min. Protein phosphorylation was then evaluated by immunoblotting. ERK1/2 phosphorylation (Thr202/Tyr204) induced by ET-1 (n = 5) and PDGF (n = 4–5) was reduced significantly in Abl knockdown cells compared with UI cells and cells treated with control shRNA. Protein phosphorylation in cells induced by ET-1 and PDGF was normalized to the levels in unstimulated cells. Values are means ± SE. *P < 0.05; **P < 0.01.
ERK1/2 phosphorylation induced by ET-1 and PDGF is attenuated in Abl-deficient cells.
Since stimulation with ET-1 and PGDF activates both Abl and ERK1/2, we questioned whether Abl has a role in regulating ERK1/2 in cells. Uninfected cells, cells expressing control shRNA, and cells producing Abl shRNA were stimulated with ET-1 and PDGF or left unstimulated. ERK1/2 phosphorylation was assessed for these cells. Basal ERK1/2 phosphorylation was similar in uninfected cells and in cells producing control or Abl shRNA. However, ET-1-induced ERK1/2 phosphorylation was reduced by ∼70% in Abl-deficient cells compared with uninfected cells or control shRNA-treated cells (Fig. 4C). Furthermore, ERK1/2 phosphorylation induced by PDGF is reduced by ∼30% in Abl knockdown cells compared with control cells (Fig. 4D). These results suggest that Abl has a role in regulating ERK1/2 activation upon stimulation with ET-1 and PDGF. However, ET-1-regulated ERK1/2 phosphorylation is largely dependent on Abl kinase, whereas PDGF-induced ERK1/2 activation is less dependent on the tyrosine kinase.
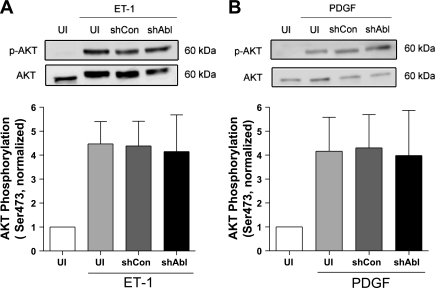
Abl silencing does not inhibit Akt phosphorylation during stimulation with ET-1 and PDGF.
In addition to ERK1/2 activation, exposure to growth-associated factors also activates Akt/PKB in smooth muscle cells (20). To determine whether Akt activation is regulated by Abl kinase, we assessed the effects of Abl knockdown on Akt phosphorylation during stimulation with ET-1 and PDGF. Stimulation with ET-1 and PDGF induced a significant increase in Akt phosphorylation in control cells, cells expressing control shRNA, and Abl-deficient cells (Fig. 5). The average increase in Akt phosphorylation in these cells was not significantly different 10 min after ET-1 and PDGF stimulation.
Fig. 5.
Akt phosphorylation stimulated by ET-1 and PDGF is not reduced in Abl knockdown cells. UI cells, cells expressing control shRNA, and stable Abl knockdown cells were treated with 200 nM ET-1 (A) or 20 ng/ml PDGF (B) for 10 min. Akt phosphorylation (Ser473) induced by ET-1 and PDGF was similar in UI cells, cells treated with control shRNA, and Abl knockdown cells (P > 0.05). Stimulated Akt phosphorylation (p-Akt) was normalized to the basal level of uninfected cells. Values are means ± SE (n = 5–6).
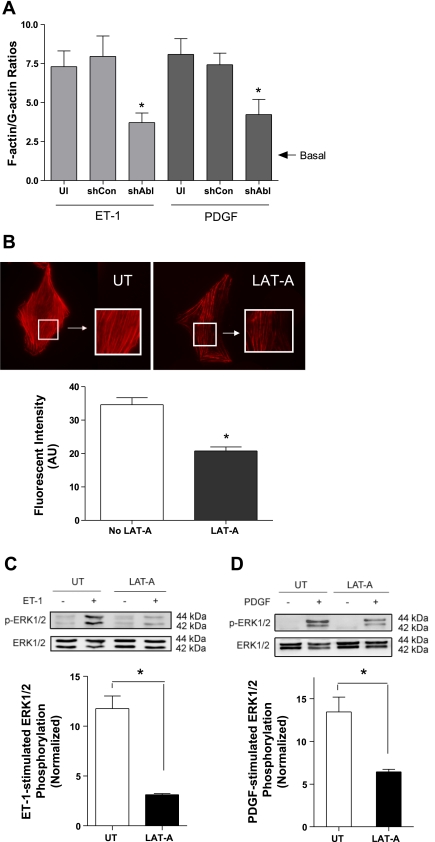
Blockade of actin polymerization inhibits ET-1- and PDGF-induced ERK1/2 activation.
Abl has been shown to regulate actin filament polymerization in smooth muscle during contractile activation (1, 5, 9). To determine whether Abl affects actin polymerization in smooth muscle during proliferative response, we assessed the effects of Abl silencing on F/G-actin ratios in response to stimulation with ET-1 and PDGF. F/G-actin ratios induced by the mitogenic factors were enhanced in uninfected cells and control shRNA-treated cells. However, F/G-actin ratios induced by ET-1 and PDGF were reduced in Abl knockdown cells (Fig. 6A). Basal F/G-actin ratios were similar in the three groups. Furthermore, treatment with the actin polymerization inhibitor latrunculin-A (LAT-A; 1 μM, 30 min) diminished actin polymerization, as evidenced by fluorsescent microscopy. However, treatment with LAT-A did not disrupt the actin cytoskeleton in cells (Fig. 6B).
Fig. 6.
Blockage of actin polymerization by latrunculin-A (LAT-A) diminishes ERK1/2 phosphorylation induced by ET-1 and PDGF. A: UI cells, cells expressing control shRNA, and stable Abl knockdown cells were stimulated with ET-1 and PDGF for 5 min. F/G-actin ratios were then assessed using the method described in materials and methods. *Significantly lower F/G-actin ratios upon ET-1 or PDGF stimulation in Abl knockdown cells than in UI cells and shCon-treated cells (P < 0.05). Values are means ± SE (n = 3). B: treatment with LAT-A (1 μM, 30 min) inhibited actin polymerization without disrupting the actin cytoskeleton, as evidenced by phalloidin staining. Images are representative of 3 identical experiments. The bar graph shows statistical analysis (*P < 0.05; n = 3–4). UT, untreated cells; AU, arbitrary units. C and D: UT cells and LAT-A-pretreated cells were stimulated with ET-1 (200 nM, 10 min) and PDGF (20 ng/ml, 10 min) or left unstimulated. ERK1/2 phosphorylation was evaluated by immunoblot analysis (*P < 0.05; n = 4–5).
We then evaluated the effects of actin polymerization inhibition by LAT-A on ERK1/2 phosphorylation. Untreated cells and LAT-A-pretreated cells were stimulated with ET-1 and PDGF. Stimulation with ET-1 and PDGF induced ERK1/2 phosphorylation in untreated cells. On the contrary, ERK1/2 phosphorylation induced by ET-1 and PDGF was suppressed in LAT-A-pretreated cells (Fig. 6, C and D). Treatment with LAT-A did not affect basal ERK1/2 phosphorylation. These results suggest that ERK1/2 activation is regulated by actin polymerization during activation with mitogenic factors.
DISCUSSION
Abl is a nonreceptor tyrosine kinase that has a role in the regulation of nonmuscle cell migration and adhesion (8, 27) and smooth muscle contraction (1, 5, 9). The role of Abl in smooth muscle cell proliferation is largely unknown. Our present studies suggest that Abl is an essential molecule that regulates smooth muscle cell proliferation. Furthermore, Abl may regulate cell growth by modulating actin polymerization and the activation of ERK1/2 during proliferative responses.
Although the role of vascular smooth muscle cell proliferation in tissue/organ homeostasis and various chronic diseases is well recognized (3, 15), the mechanisms that regulate smooth muscle cell proliferation are not fully understood. In the present study, Abl underwent phosphorylation at Tyr412 in smooth muscle cells during activation with the vasoactive peptide ET-1 and the growth factor PDGF. The results suggest that Abl is activated in smooth muscle cells during mitogenic stimulation. Tyr412 is located at the activation loop of Abl kinase domain. When unstimulated, the activation loop of the Abl kinase domain folds into the active site, thereby preventing binding of both the substrate and ATP. Phosphorylation at Tyr412 induces conformation changes; the activation loop no longer blocks the active site, which leads to the increase in kinase activity (27).
Abl kinase may be activated by Src in smooth muscle cells. ET-1 receptor is a member of G protein-coupled receptor (GPCR) family, whereas PDGF receptor belongs to the family of tyrosine kinase-containing receptors. Both GPCR and tyrosine kinase receptors play an important role in the regulation of smooth muscle cell functions. In vascular smooth muscle cells, activation of GPCR by agonists induces phosphorylation of Src at Tyr416, which in turn triggers the activation of Abl (9, 26). Similarly, cellular challenge with PDGF is able to activate Src, which subsequently mediates phosphorylation of Abl in mammalian cells (6, 7, 21, 22).
To unveil the functional role of Abl in smooth muscle cell proliferation, we generated stable Abl knockdown cells by using lentivirus-mediated RNAi. Abl knockdown by RNAi attenuated the ET-1- and PDGF-induced smooth muscle cell proliferation. Furthermore, Abl silencing also arrested cell cycle progression from the G0/G1 to the S phase. To the best of our knowledge, this is the first evidence to suggest that Abl plays a critical role in the regulation of smooth muscle cell proliferation and cell cycle progression.
Upon the binding of ligands such as vasoactive peptides and growth factors to corresponding receptors, ERK1/2 is phosphorylated and activated in smooth muscle cells, which may orchestrate cell proliferation by triggering activation of many genes necessary for cell growth (3, 28). As described above, Abl knockdown arrested smooth muscle cells in the G0/G1 phase, suggesting that the effects of Abl on cell proliferation may occur in the early stage of cellular responses to activation with mitogenic factors. Since phosphorylation of ERK1/2 is the major cellular event during the early phase of mitogenic response, we assessed the effects of Abl silencing on phosphorylation of ERK1/2. Phosphorylation of ERK1/2 upon stimulation with ET-1 and PDGF was reduced by Abl knockdown. However, Akt phosphorylation activated by the two agents was not affected by Abl silencing. Akt phosphorylation has also been implicated in signaling pathways in smooth muscle cells in response to mitogenic activation (3, 19, 20). The results suggest that Abl differentially regulates the activation of ERK1/2 and Akt in smooth muscle cells during mitogenic responses.
Abl regulates cell functions by affecting actin polymerization and dynamics in various cell types (1, 9, 27). In response to external stimulation, Abl tyrosine kinase in smooth muscle cells may regulate phosphorylation of actin-regulatory proteins such as p130 Crk-associated substrate (CAS). Phosphorylated CAS may enhance its binding to the adapter protein CrkII, which may facilitate the formation of the multiprotein compound that includes CrkII, neuronal Wiskott-Aldrich syndrome protein, and the actin-related protein 2/3 complex, initiating actin polymerization and branching mediated by the actin-related protein 2/3 complex (18, 24, 25, 30).
In the present study, actin polymerization transpired in smooth muscle cells upon stimulation with mitogenic factors. More importantly, the inhibition of actin polymerization attenuated ERK1/2 phosphorylation in response to activation with mitogenic factors. These findings suggest that actin polymerization is an essential cellular process that regulates the activation of ERK1/2 in smooth muscle cells during proliferative responses.
Abl may regulate the activation of ERK1/2 by controlling actin polymerization because there is evidence that Abl controls actin dynamics via the CAS-mediated pathway in smooth muscle (see above). Moreover, Raf-1 (a critical upstream regulator of ERK1/2) has been shown to be activated and recruited to the cellular membrane from the cytoplasm in response to external stimulation (10, 29), which is a critical step to activate ERK1/2 (10, 28, 29). Pretreatment with the actin polymerization inhibitor cytochalasin D prevented Raf-1 activation/membrane recruitment and ERK1/2 phosphorylation (10, 29). Thus, actin polymerization may regulate the spatial translocation and activation of Raf-1, which may in turn modulate the activation of ERK1/2 in cells in response to external stimulation.
Although Abl knockdown attenuated ERK1/2 phosphorylation induced by both ET-1 and PDGF, ET-1-mediated ERK1/2 phosphorylation is mediated largely by Abl kinase, whereas ERK1/2 activation induced by PDGF is less dependent upon the tyrosine kinase. It is known that ERK1/2 activation by ET-1 is mediated mainly by the Raf/MEK (MAPK kinase) pathway in smooth muscle cells (3). However, PDGF-induced ERK1/2 phosphorylation is orchestrated by multiple signaling pathways, including the Raf/MEK pathway, the protein tyrosine phosphatase SHP2 and RPTP (receptor protein tyrosine phosphatase) pathway, and PYK2 (proline-rich tyrosine kinase 2) (2, 17). It is possible that Abl kinase may just modulate the activation of the Raf/MEK pathway without affecting the functions of SHP2/RPTP and PYK2. Thus, PDGF-induced ERK1/2 phosphorylation is slightly affected by Abl knockdown.
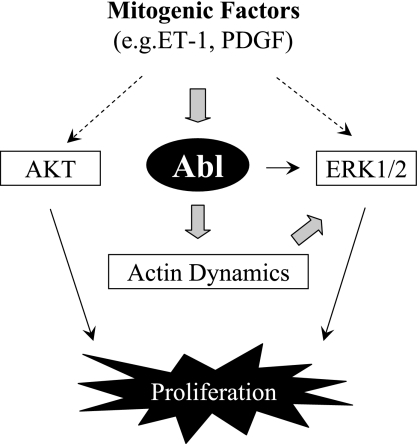
In summary, we demonstrate that Abl is a critical component of cellular mechanisms that regulate smooth muscle cell proliferation. In response to stimulation with mitogenic factors such as ET-1 and PDGF, Abl gets phosphorylated and activated. Activated Abl may regulate actin dynamics, which subsequently mediates the activation of ERK1/2 and cell proliferation (Fig. 7).
Fig. 7.
Proposed mechanism. Abl gets phosphorylated and activated in response to stimulation with mitogenic factors such as ET-1 and PDGF. Activated Abl may control actin polymerization, which in turn regulates the activation of ERK1/2 and cell proliferation.
GRANTS
This work was supported by Grant No. R01-HL-110951 from the National Heart, Lung, and Blood Institute and by grants from Albany Medical College.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.J. and R.W. performed the experiments; L.J., R.W., and D.D.T. analyzed the data; L.J. and D.D.T. interpreted the results of the experiments; L.J. and R.W. prepared the figures; L.J. drafted the manuscript; L.J. and D.D.T. edited and revised the manuscript; L.J., R.W., and D.D.T. approved the final version of the manuscript; D.D.T. did the conception and design of the research.
ACKNOWLEDGMENTS
We thank Qingfen Li for technical assistance.
REFERENCES
- 1.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res 101: 420–428, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki T, Nawa H, Neel BG. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J Biol Chem 278: 41677–41684, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bouallegue A, Daou GB, Srivastava AK. Endothelin-1-induced signaling pathways in vascular smooth muscle cells. Curr Vasc Pharmacol 5: 45–52, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37–40, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Wang R, Li QF, Tang DD. Abl knockout differentially affects p130 Crk-associated substrate, vinculin, and paxillin in blood vessels of mice. Am J Physiol Heart Circ Physiol 297: H533–H539, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorey K, Engen JR, Kretzschmar J, Wilm M, Neubauer G, Schindler T, Superti-Furga G. Phosphorylation and structure-based functional studies reveal a positive and a negative role for the activation loop of the c-Abl tyrosine kinase. Oncogene 20: 8075–8084, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol 5: 33–44, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Hu H, Bliss JM, Wang Y, Colicelli J. RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr Biol 15: 815–823, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Jia L, Tang DD. Abl activation regulates the dissociation of CAS from cytoskeletal vimentin by modulating CAS phosphorylation in smooth muscle. Am J Physiol Cell Physiol 299: C630–C637, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krepinsky JC, Li Y, Tang D, Liu L, Scholey J, Ingram AJ. Stretch-induced Raf-1 activation in mesangial cells requires actin cytoskeletal integrity. Cell Signal 17: 311–320, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Li QF, Spinelli AM, Tang DD. Cdc42GAP, reactive oxygen species, and the vimentin network. Am J Physiol Cell Physiol 297: C299–C309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem 281: 34716–34724, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li QF, Tang DD. Role of p47(phox) in regulating Cdc42GAP, vimentin, and contraction in smooth muscle cells. Am J Physiol Cell Physiol 297: C1424–C1433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295: 868–872, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv 4: 104–111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orsini MJ, Krymskaya VP, Eszterhas AJ, Benovic JL, Panettieri RA, Jr, Penn RB. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol Lung Cell Mol Physiol 277: L479–L488, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Perez J, Torres RA, Rocic P, Cismowski MJ, Weber DS, Darley-Usmar VM, Lucchesi PA. PYK2 signaling is required for PDGF-dependent vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol 301: C242–C251, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 36: 451–477, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res 90: 1243–1250, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Sirvent A, Benistant C, Roche S. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol Cell 100: 617–631, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Sirvent A, Boureux A, Simon V, Leroy C, Roche S. The tyrosine kinase Abl is required for Src-transforming activity in mouse fibroblasts and human breast cancer cells. Oncogene 26: 7313–7323, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J 388: 773–783, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem 279: 51722–51728, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Tang DD, Zhang W, Gunst SJ. The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J Biol Chem 280: 23380–23389, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res 97: 829–836, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Wang JY. Controlling Abl: auto-inhibition and co-inhibition? Nat Cell Biol 6: 3–7, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 79: 143–180, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Bewick M, Lafrenie RM. EGFR and ErbB2 differentially regulate Raf-1 translocation and activation. Lab Invest 82: 71–78, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol 288: C1145–C1160, 2005 [DOI] [PubMed] [Google Scholar]