Abstract
High interstitial K+ concentration ([K+]) has been reported to impede normal propagation of electrical impulses along the muscle cell membrane (sarcolemma) and then also into the transverse tubule system; this is one considered underlying mechanism associated with the development of muscle fatigue. Interestingly, the extracellular buildup of lactic acid, once considered an additional cause for muscle fatigue, was recently shown to have force-restoring effects in such conditions. Specifically, it was proposed that elevated lactic acid (and intracellular acidosis) may lead to inhibition of voltage-gated chloride channels, thereby reestablishing better excitability of the muscle cell sarcolemma. In the present study, using an in vitro muscle contractile experimental setup to study functionally viable rectus abdominis muscle preparations obtained from normal swine, we examined the effects of 20 mM lactic acid and 512 μM 9-anthracenecarboxylic acid (9-AC; a voltage-gated chloride channel blocker) on the force recovery of K+-depressed (10 mM K+) twitch forces. We observed a similar muscle contractile restoration after both treatments. Interestingly, at elevated [K+], myotonia (i.e., hyperexcitability or afterdepolarizations), usually present in skeletal muscle with inherent or induced chloride channel dysfunctions, was not observed in the presence of either lactic acid or 9-AC. In part, these data confirm previous studies showing a force-restoring effect of lactic acid in high-[K+] conditions. In addition, we observed similar restorative effects of lactic acid and 9-AC, implicating a beneficial mechanism via voltage-gated chloride channel modulation.
Keywords: skeletal muscle fatigue, chloride channel
lactic acidosis and intracellular acidification have long been considered as potential causes of skeletal muscle fatigue (9, 10, 27). In experiments, however, only minor inhibitory effects of lactate on normal excitation-contraction coupling could be found (7), and there is a host of research showing beneficial effects (e.g., lactate preferentially utilized by the heart as a readily diffusible fuel) (4). Furthermore, although acidosis of the myoplasm was observed to affect the Ca2+ sensitivity of the myofilaments and/or the Ca2+ release from ryanodine receptors (2), the role of intracellular acidosis as a main cause of muscle fatigue has been challenged recently, because several studies found low pH to have only little or no effect on contractions within mammalian skeletal muscles at physiological temperatures (5, 24, 28, 32, 34). Another proposed cause of muscle fatigue is the accumulation of K+ in the interstitial fluid. The increased K+ concentration ([K+]) can inhibit the propagation of the action potentials along the cell membrane (sarcolemma) and subsequently into the transverse (t)-tubule system, thereby modifying excitation-contraction coupling (15, 33). Interestingly, in such conditions of increased interstitial [K+], newer findings suggest a force-restoring effect of increasing levels of lactic acid; i.e., acidosis of the myoplasm may protect from skeletal muscle fatigue. Specifically, such studies showed skeletal muscle force recovery during high interstitial [K+] with treatment using lactic acid (16, 21, 25). In their subsequent experiments, Pedersen et al. (26) then determined that intracellular acidosis decreased the Cl− permeability within the t-tubule system and thereby restored membrane excitability.
The aims of the present study were to test the earlier reported beneficial findings of lactic acid on skeletal muscle force recovery in hyperkalemic conditions in swine tissue, to determine whether this force restoration could be mimicked by Cl− channel inhibition, and if so, whether this was associated with myotonic-like behavior of the treated muscle bundles.
METHODS
Approval for these animal experiments was granted from the Institutional Animal Care and Use Committee of the University of Minnesota (Minneapolis, MN). Nine castrated male Yorkshire crossbred swine were pretreated with an intramuscular injection of a mixture of 250 mg of tiletamine and 250 mg of zolazepam (Telazol; Fort Dodge Animal Health, Fort Dodge, IA). After induction of surgical anesthesia with 500 mg of methohexital (Brevital sodium; JHP Pharmaceuticals, Rochester, MI), intubation was performed and mechanical ventilation started. Rectus abdominis muscle biopsies were obtained (within 15 min), and samples were placed in preoxygenated Krebs-Ringer solution (gassed with carbogen, 95% O2 and 5% CO2) and then immediately transferred to a dissecting dish equipped with continuous gassing. The muscle biopsies were dissected into small muscle bundles of intact fibers (from tendon to tendon) (19, 20). After transfer to 1 of 24 in vitro, temperature-controlled (37 ± 0.1°C) muscle experimental chambers (containing Krebs-Ringer solution bubbled continuously with carbogen), each bundle was independently stimulated with individually determined supramaximal electrical field stimulation pulses of 1-ms duration at a frequency of 0.1 Hz (14, 17). The muscle bundles were stretched to their optimal muscle length, as previously described in more detail (11, 13, 23). In one experimental setting, a tetanic stimulus protocol was followed; these bundles were stimulated every 10 s with a 1.5-s 33-Hz train of pulses with a 0.2-ms pulse width. All other studies maintained the pulse protocol used during setup. On average, 20 ± 2 muscle bundles were obtained from each pig for each experiment. After a stable baseline was maintained for ∼10–15 min, muscle bundles were treated according to the study protocol. In one additional experimental setting, surplus human tissue obtained from contracture testing for malignant hyperthermia was used. During regional anesthesia, an open muscle biopsy of vastus lateralis muscle was taken. The muscle sample was immediately placed in a preoxygenated Krebs-Ringer solution (gassed with carbogen, 95% O2 and 5% CO2) in the operating room and then immediately transferred to the laboratory for in vitro diagnostic contracture testing (to determine susceptibility to malignant hyperthermia). The surplus muscle bundles not needed for testing (waste tissue) were used for the experiments. Dissection, attachment to the force transducer, and stimulation were conducted according to the description given above. The tissue was obtained from a healthy 31-yr-old male subject and was deemed not susceptible to malignant hyperthermia. Consent was obtained in the context of the testing for malignant hyperthermia. The lengths of the various muscle bundles were determined at their optimal lengths (measured as the distance between the suture ties holding the muscle in the tissue bath). Muscle bundle mass was determined as the wet weight of each specimen. Cross-sectional areas were then calculated as follows (30): area (mm2) = muscle mass (mg)/[fiber length (mm) × muscle density (mg/mm3)], using 1.06 mg/mm3 as the density of skeletal muscle (6).
Pharmacological Agents
The standard incubation medium was normal Krebs-Ringer solution, consisting of 118.1 mM NaCl, 3.4 mM KCl, 1.2 mM KH2PO4, 1.0 mM MgSO4·7H2O, 11.0 mM d-glucose, 25.0 mM NaHCO3, and 2.5 mM CaCl2·2H2O. The Krebs solutions with high K+ levels were constituted similarly, except that the KCl concentration was adjusted to obtain 6, 8, 10, and 12 mM total [K+]. The lactic acid used was purchased from Fluka Analytical (Sigma-Aldrich, Buchs, Switzerland/Sigma-Aldrich, Steinheim, Germany). The investigated drugs were epinephrine (Hospira, Lake Forest, IL), ouabain octahydrate (Sigma-Aldrich, Steinheim, Germany), and 9-anthracenecarboxylic acid (9-AC; Sigma-Aldrich, Steinheim, Germany) (8).
Investigation Protocols
Lactic acid and voltage-gated Cl− channel inhibition in high-[K+] conditions.
After a control period (15–20 min), the buffer was exchanged with a modified Krebs solution containing a high [K+] (10 mM). After incubation in this high [K+] for 45 min, either 20 mM lactic acid (n = 14) or 512 μM 9-AC (n = 15) was added to the baths, and data were collected for another 120 min. For control groups, we used muscle bundles incubated in conventional Krebs (n = 13) as well as in 10 mM K+ Krebs solution (n = 14), respectively. Additional preliminary experiments were performed as described below.
TISSUE BATH TEMPERATURE AT 30°C.
Protocol was followed as described above, except tissue bath temperature was set at 30°C throughout the experimental period: time control with conventional Krebs (n = 5), 10 mM K+ Krebs (n = 5), 512 μM 9-AC (n = 6), and 20 mM lactic acid (n = 6).
HUMAN TISSUE.
Protocol was followed as described above at 37°C, except with the use of human tissue: time control with conventional Krebs (n = 1), 10 mM K+ Krebs (n = 1), 512 μM 9-AC (n = 1), and 20 mM lactic acid (n = 2).
TETANIC STIMULATION.
After a control period of 20 min, the buffer was exchanged with a modified Krebs solution containing a high [K+] (10 mM). After incubation in this high [K+] for 60 min, either 20 mM lactic acid (n = 5) or 512 μM 9-AC (n = 5) was added to the baths, and data were collected for another 120 min. As control groups, muscle bundles incubated in conventional Krebs (n = 5) or in 10 mM K+ Krebs solution (n = 5) were used.
Influence of rising [K+] on myotonia induced by Cl− channel inhibition.
After a control period (30 min), 512 μM 9-AC was added to the baths to induce myotonia (8, 12, 18). Thirty minutes after addition of the 9-AC, the normal Krebs solution within the tissue baths was exchanged with buffers containing [K+] of 4.6 (n = 4), 6 (n = 4), 8 (n = 4), 10 (n = 4), or 12 mM (n = 4), and data were collected for another 120 min.
Effect of epinephrine, ouabain, and the combination of ouabain/epinephrine or ouabain/lactic acid in high-[K+] conditions.
After control periods (15–20 min), the buffer was exchanged for a Krebs solution with 10 mM K+. In 15 baths, after incubation in high [K+] for 25 min, 100 μM ouabain was added, and after another 20 min, either 1 μM epinephrine (n = 6) or 20 mM lactic acid (n = 5) was added, while four baths were maintained with ouabain treatment alone. In 6 baths, 1 μM epinephrine alone was given after incubation in high [K+] for 45 min. Data in all cases were then collected for another 120 min.
Data Collection and Data Analysis
Peak forces were recorded and analyzed as an indicator of the skeletal muscle contractile function. The forces produced by each muscle bundle were normalized to its relative baseline forces (at the beginning of each study) and subsequently expressed as percent change from this value. As an indicator of the relative degree of elicited myotonia, the areas under the force curves (AUCs) were divided by peak forces (AUC/PF) to normalize any changes in AUC that may have been due solely to changes in peak force. AUC was measured from the stimulus until the force fell to 10% of its peak force. We waited 8 min after each administered treatment to allow for maximal effects of a given agent; we then collected the data during the subsequent 60 s (i.e., 6 twitch responses per collection period). These data were used for later analyses. All data collection was automated, employing a customized LabView software program (ADEPT; Adept Scientific, Letchworth Garden City, UK). Tissue bath pH was measured using an Accumet AR25 meter with an Accumet 13-620-290 probe (Fisher Scientific, Pittsburgh, PA) 5 min after addition of lactic acid to observe the pH difference between groups.
Statistical Analyses
For measurements with respect to time, data were analyzed using two-way ANOVA with repeated measures, and one-way ANOVA was used for determining differences between drug groups at given time points. To identify intergroup differences, Bonferroni post hoc tests were employed. All data are means ± SE, except the muscle characteristics data, which are given as means ± SD; P < 0.05 was considered statistically significant. The significance of the relative pH changes in the tissue baths, after addition of lactic acid to the baths, was analyzed using Student's t-test. All statistical analyses were performed using a Prism software package (GraphPad Software, La Jolla, CA).
RESULTS
Lactic acid and Cl− Channel Modulation in High-[K+] Conditions
In the experiments initiated at high K+ levels, the parameters of length, mass, or cross-sectional area of the muscle bundles used in the various treatment groups did not differ (Table 1, treatment group A). The mean values of the muscle bundles' characteristics were 33.6 ± 4.1 mm, 116.3 ± 48.4 mg, and 3.27 ± 1.3 mm2, respectively.
Table 1.
Muscle bundle characteristics
| Treatment Group | Length, mm | Mass, mg | Area, mm2 | n |
|---|---|---|---|---|
| A | ||||
| Time control | 35.1 ± 4.5 | 125.0 ± 33.1 | 3.43 ± 1.0 | 13 |
| High K+ only | 33.5 ± 4.3 | 111.5 ± 41.5 | 3.13 ± 1.1 | 14 |
| High K+ and lactic acid | 32.4 ± 4.1 | 103.2 ± 47.3 | 2.99 ± 1.3 | 14 |
| High K+ and 9-AC | 33.2 ± 3.8 | 121.5 ± 67.7 | 3.40 ± 1.7 | 15 |
| B (tissue bath 30°C) | ||||
| Time control | 33.3 ± 2.7 | 153.6 ± 31.3 | 4.36 ± 0.7 | 5 |
| High K+ only | 34.0 ± 2.1 | 142.1 ± 24.1 | 3.98 ± 0.8 | 5 |
| High K+ and lactic acid | 34.6 ± 2.9 | 140.4 ± 24.5 | 3.83 ± 0.6 | 6 |
| High K+ and 9-AC | 33.8 ± 2.7 | 162.4 ± 10.3 | 4.59 ± 0.6 | 6 |
| C (human tissue) | ||||
| Time control | 53.4 | 328.4 | 5.82 | 1 |
| High K+ only | 60.5 | 324.9 | 5.09 | 1 |
| High K+ and lactic acid | 53.7 ± 4.7 | 404.3 ± 3.3 | 7.17 ± 0.7 | 2 |
| High K+ and 9-AC | 42.0 | 122.0 | 2.75 | 1 |
| D (tetanic stimulation) | ||||
| Time control | 30.3 ± 3.4 | 136.7 ± 31.8 | 3.98 ± 1.0 | 5 |
| High K+ only | 31.7 ± 1.9 | 134.2 ± 10.7 | 4.02 ± 0.5 | 5 |
| High K+ and lactic acid | 30.3 ± 3.4 | 128.3 ± 32.0 | 3.99 ± 0.8 | 5 |
| High K+ and 9-AC | 30.6 ± 2.2 | 134.3 ± 15.1 | 4.17 ± 0.5 | 5 |
| E | ||||
| 9-AC and 4.6 mM K+ | 32.4 ± 4.2 | 128.9 ± 32.3 | 3.73 ± 0.6 | 4 |
| 9-AC and 6 mM K+ | 29.9 ± 3.8 | 96.0 ± 22.6 | 3.06 ± 0.6 | 4 |
| 9-AC and 8 mM K+ | 36.2 ± 2.7 | 106.7 ± 35.4 | 2.76 ± 0.7 | 4 |
| 9-AC and 10 mM K+ | 29.2 ± 3.5 | 96.1 ± 22.3 | 3.13 ± 0.6 | 4 |
| 9-AC and 12 mM K+ | 30.4 ± 4.3 | 93.4 ± 29.0 | 2.86 ± 0.6 | 4 |
| F | ||||
| High K+ and epinephrine | 27.4 ± 5.9 | 101.6 ± 41.0 | 3.55 ± 1.3 | 6 |
| High K+ and ouabain | 30.8 ± 2.2 | 109.0 ± 20.6 | 3.39 ± 0.8 | 4 |
| High K+ and ouabain/lactic acid | 28.0 ± 2.3 | 94.5 ± 17.0 | 3.20 ± 0.6 | 5 |
| High K+ and ouabain/epinephrine | 31.8 ± 2.3 | 106.3 ± 28.5 | 3.14 ± 0.7 | 6 |
Values are means ± SD. 9-AC, 9-anthracenecarboxylic acid.
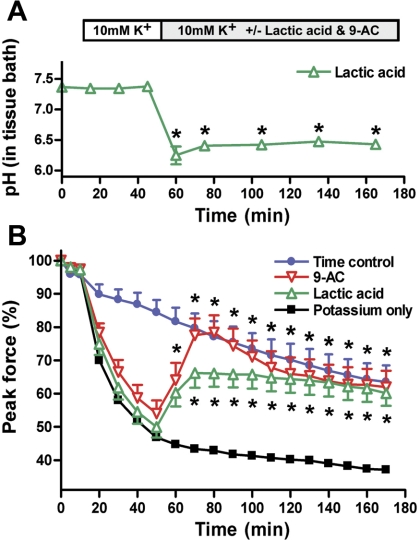
The pH of the buffers in the tissue baths decreased significantly after addition of lactic acid to the baths; e.g., adding 20 mM lactic acid to the tissue baths decreased the pH of the buffer solution from 7.37 ± 0.11 to 6.40 ± 0.23 (P < 0.05). Measurements were recorded every 15–30 min in 8 tissue baths throughout an entire experimental period (Fig. 1A). At the end of the high-[K+] experimental period and with exchange of the tissue buffer, the pH normalized again to 7.21 ± 0.15; note that in the control group where no lactic acid was added, the pH remained at 7.30 ± 0.03.
Fig. 1.
Lactic acid or the Cl− channel blocker 9-anthracenecarboxylic acid (9-AC) lead to peak force recovery in K+-depressed skeletal muscle. A: pH change after addition of 20 mM lactic acid to the tissue baths (n = 8). Data are means ± SE. *P < 0.05, significantly different from normal Krebs solution (Student's t-test). B: evolution of peak force in muscle bundles incubated in 10 mM K+ after addition of 20 mM lactic acid (n = 14) or 512 μM 9-AC (n = 15) to the baths. Muscle bundles incubated in either normal Krebs-Ringer solution (n = 13) or in 10 mM K+ Krebs-Ringer solution (n = 14) were used as controls. Both treatments (lactic acid and 9-AC) led to increased peak force compared with the K+-only group (*P < 0.05 by 2-way ANOVA for repeated measures). Data are means ± SE.
Peak force of all the muscle bundles decreased significantly after 20 min of incubation in 10 mM K+ compared with the muscle bundles in conventional Krebs solution (P < 0.001) (Fig. 1B). Osmolarity in the control tissue baths was 293 ± 1.30 mosM (n = 5), whereas osmolarity in the 10 mM K+ solution was 302 ± 0.58 mosM (n = 3). The subsequent treatment with 9-AC or lactic acid significantly increased the peak forces of the treated muscle bundles compared with the untreated muscle bundles in the 10 mM K+ solution after 10 or 20 min, respectively (Fig. 1B). Osmolarity of the solution was not further affected by adding either 9-AC (n = 1) or lactic acid to the tissue baths (n = 1). Compared with time control, the muscle bundles treated with lactic acid or 9-AC regained normal function 20 min after addition of lactic acid or 9-AC to the tissue baths. It is important to note that we did not observe any myotonic activity elicited by any of the muscle bundles tested. Only after the buffer was changed to normal Krebs solution with 4.6 mM K+ (with 9-AC) did myotonic activity of the muscle bundles become observable (Fig. 2). In additional preliminary experiments on temperature, testing protocols were employed to determine whether similar muscle force recovery due to lactic acid administration would also occur with 9-AC administrations to skeletal muscle bundles in hyperkalemic conditions. More specifically, in one set of experiments, 30°C tissue bath conditions were utilized (Table 1, treatment group B; Fig. 3); at this temperature, the peak force of the muscle bundles increased as soon as lactic acid was added to the hyperkalemic tissue baths. In other studies, adding 9-AC to the hyperkalemic solution had the same force-restoring effects as lactic acid. However, this effect took some time to be elicited, i.e., after ∼20 min. Importantly, in pilot studies employing human skeletal muscle bundles (Table 1, treatment group C; Fig. 4), we again observed the force-restoring effects of either lactic acid or 9-AC treatment, yet the 9-AC administration resulted in a more pronounced force recovery compared with lactic acid (especially in the first 30 min after treatment). In the studies in which we utilized a tetanic stimulation protocol (Table 1, treatment group D; Fig. 5), we observed a pattern similar to that noted above, i.e., the force-restoring effects of either lactic acid or 9-AC administration. Yet, we observed more pronounced benefit of 9-AC compared with lactic acid, especially in the early phase posttreatment.
Fig. 2.
Myotonic activity change after normalization of [K+] (the same experiments as in Fig. 1). In the experiments with force decline due to elevated [K+] (10 mM) in the tissue baths (see Fig. 1), myotonic activity was absent irrespective of the treatment group. On exchange to normal Krebs solution, however, the muscle bundles treated with 512 μM 9-AC showed an abrupt and significant increase in the area under the force curve (AUC) divided by peak force compared with the other groups (induced myotonia) (*P < 0.05 by 2-way ANOVA for repeated measures). Data are means ± SE.
Fig. 3.
Effects of lactic acid or the Cl− channel blocker 9-AC on peak force recovery in K+-depressed skeletal muscle: tissue bath temperature set at 30°C. Evolution of peak force in muscle bundles incubated in 10 mM K+ after addition of 20 mM lactic acid (n = 6) or 512 μM 9-AC (n = 6) to the baths is shown. Muscle bundles incubated in either normal Krebs-Ringer solution (n = 5) or in 10 mM K+ Krebs-Ringer solution (n = 5) were used as controls. Data are means ± SE.
Fig. 4.
Effects of lactic acid or the Cl− channel blocker 9-AC on peak force recovery in K+-depressed skeletal muscle: human tissue. Evolution of peak force in muscle bundles incubated in 10 mM K+ after addition of 20 mM lactic acid (n = 2) or 512 μM 9-AC (n = 1) to the baths is shown. Muscle bundles incubated in either normal Krebs-Ringer solution (n = 1) or in 10 mM K+ Krebs-Ringer solution (n = 1) were used as controls. Data are means ± SE.
Fig. 5.
Effects of lactic acid or the Cl− channel blocker 9-AC on peak force recovery in K+-depressed skeletal muscle: tetanic stimulation protocol. Evolution of peak force in muscle bundles incubated in 10 mM K+ after addition of 20 mM lactic acid (n = 5) or 512 μM 9-AC (n = 5) to the baths is shown. Muscle bundles incubated in either normal Krebs-Ringer solution (n = 5) or in 10 mM K+ Krebs-Ringer solution (n = 5) were used as controls. Data are means ± SE.
Influence of Elevated [K+] on Myotonia Induced by Cl− Channel Inhibition
Again in the experiments with the 9-AC-induced myotonia, length, mass, or cross-sectional area parameters of the muscle bundles used in the various treatment groups were not statistically different (Table 1, treatment group E). The mean values of the muscle bundles' characteristics were 31.7 ± 4.3 mm, 103.3 ± 29.9 mg, and 3.07 ± 0.7 mm2, respectively. The Cl− channel blocker 9-AC (512 μM) led to significantly increased AUCs. The AUCs were then divided by peak force values to normalize any changes in AUC that may have been due solely to changes in peak forces (this was then considered as a relative indicator of the degree of induced myotonia). A decrease in myotonia was observed on exposure to elevated [K+] (Fig. 6).
Fig. 6.
Myotonic activity after pharmacological blockade of the voltage-gated Cl− channels depends on [K+] in the tissue baths. AUC/peak force was increasing in the muscle bundles treated with 9-AC (512 μM). After 30 min, the muscle bundles were exposed to various [K+]: 4.6 (n = 4), 6 (n = 4), 8 (n = 4), 10 (n = 4), and 12 mM (n = 4). At a concentration of 10 mM K+ in the tissue baths, myotonic activity of the muscle bundles ceased despite continued blockade of the voltage-gated Cl− channels by 9-AC. Data are means ± SE.
Effect of Epinephrine, Ouabain, and the Combination of Ouabain/Epinephrine or Ouabain/Lactic Acid in High-[K+] Conditions
In the experiments with the combined administration of epinephrine/ouabain/lactic acid, the mean values of the muscle bundles' characteristics were 29.4 ± 4.0 mm, 102.2 ± 28.4 mg, and 3.31 ± 0.9 mm2 (Table 1, treatment group F); there were no statistical differences in the muscle bundles' characteristics in the various treatment groups. Initially, muscle forces declined in all the muscle bundles after exposure to 10 mM K+ (Fig. 7). On exposure to 100 μM ouabain, muscle forces decreased further; this led to significant force losses in the ouabain-treated muscle bundles after 20 min compared with the muscle bundles exposed to 10 mM K+ only. Neither 1 μM epinephrine nor 20 mM lactic acid were able to reestablish increased muscle forces in the ouabain-treated muscle bundles. In contrast, the muscle bundles exposed to 10 mM K+ only elicited significant force recoveries after exposure to 1 μM epinephrine.
Fig. 7.
Effects of epinephrine, ouabain, and the combination of ouabain with either epinephrine or lactic acid on peak force recovery in K+-depressed skeletal muscle. Peak forces in the muscle bundles declined roughly 40% after incubation in 10 mM K+ in all groups. In groups where 100 μM ouabain was added, peak forces declined steeper over the next 20 min. Epinephrine (1 μM) was then given to baths free of ouabain (n = 6) or in combination with 100 μM ouabain (n = 6). In addition, 20 mM lactic acid was added to ouabain-pretreated baths (n = 5) or ouabain was the sole treatment (n = 4). The peak force in the epinephrine-only group was significantly higher compared with all the groups where ouabain was added to the tissue baths (P < 0.05 by 2-way ANOVA for repeated measures). Data are means ± SE.
DISCUSSION
It was previously reported that the administration of lactic acid improves contractile forces in muscle in which its function is depressed by K+ exposure (a proposed model for induced fatigue) (16, 21, 25). In the in vitro studies described here, in which we utilized rectus abdominis muscle bundles from normal swine, we observed a similar recovery of twitch forces associated with the treatment of lactic acid (20 mM) in bundles in which forces were decreased by incubation in buffers with high [K+]. It had been suggested that this force-recovering effect of lactic acid might be linked to reduced conductance of the voltage-gated Cl− channels (26). Therefore, we specifically employed a known Cl− channel inhibitor (9-AC) to verify whether this observed action of lactic acid on K+-depressed skeletal muscle could be mimicked by decreasing Cl− channel conductance. Indeed, we observed that decreasing Cl− channel conductance yielded results, relative to muscle force production, similar to those seen in the presence of lactic acid.
In resting skeletal muscle, the sarcolemmal (and t-tubule) permeabilities for K+ and Cl− are relatively high, whereas the permeability of Na+ is low (22). Hence, during such a resting state, one can consider that the chemical distribution of both K+ and Cl− is in equilibrium with the membrane potential; i.e., thus the net currents both ions carry across the membrane are minimal. In situations where there may be an induced change in resting membrane potentials, these two ions would tend to support the reestablishment of more normal resting membrane potentials. In other words, both ion currents can be considered to have important stabilizing effects on the overall resting membrane potentials. More specifically, in the case of the Cl−, it is known that 70–80% of the resting membrane conductance in skeletal muscle is associated with voltage-gated Cl− channels.
Hyperpolarizing currents counter the excitability (depolarization) of the muscle membrane, e.g., in the presence of the functional resting K+ and Cl− currents, spontaneously opening Na+ channels will not be able to lead to a full-blown (propagating) depolarization of the cell membrane; in other words, despite the spontaneous opening of a small number of Na+ channels, the muscle membrane will stay in a resting state. In case of normal initiation of the action potential, e.g., at the neuromuscular junction, a strong cation current is generated through acetylcholine-activated receptors, which in turn activates the opening of nearby voltage-gated Na+ channels. The abundance of voltage-gated Na+ channels within the sarcolemma then ensures safe propagation of the action potential along the total length of the muscle fiber and into the t-tubules, where excitation-contraction coupling then occurs. So, in nonfatigued skeletal muscle, this large potential Na+ current easily overcomes the inhibitory and repolarizing currents carried by K+ and Cl− channels. It is considered that during prolonged exercise, however, interstitial [K+] rises, namely, in the t-tubules (1), and this rise in [K+] has several consequences: 1) the electrochemical gradient decreases, and this thereby leads to a relative depolarization of the resting muscle membrane; and 2) depending on the extent of this depolarization, more and more voltage-gated Na+ channels enter a state of slow inactivation (29, 31). As more Na+ channels become inactivated, the muscle membrane becomes unexcitable to a greater extent, and it is considered that muscle fatigue has ensued. The t-tubular system, in particular, due to a reduced volume-to-surface area ratio, is prone to the accumulating K+ and thus the associated consequences of conduction (action potential) failure.
In our experiments, according to the mechanisms depicted above, we observed a force decline of 30–40% in the muscle bundles exposed to 10 mM K+ (in the tissue baths) compared with controls. Interestingly, the administration of 20 mM lactic acid was able to reestablish normal peak force values within 20 min; this is in accordance with previously reported studies (16, 21, 25). It was observed that adding 20 mM lactic acid to the tissue baths reduced buffer pH by 0.97 (from 7.34 ± 0.11 to 6.40 ± 0.23, n = 8). It should be noted that Nielsen et al. (21) reported that adding 20 mM lactic acid to such tissue baths not only affected the pH within the baths but also reduced the intracellular pH of the muscles, i.e., in their study from 7.28 ± 0.05 to 6.89 ± 0.06. These same authors also described that decreasing the intracellular pH by other means (i.e., propionic acid or 23% CO2) could also lead to the same beneficial force recovery of the K+-depressed muscle function as seen when exposed to lactic acid (21). This was interpreted to signify that the actual mechanism of muscle force recovery was due to the decrease in pH rather than to the metabolic effects of lactic acid. Furthermore, in 2004, Pedersen et al. (26) then noted that intracellular acidosis of skeletal muscle was associated with decreased currents through the voltage-gated Cl− channels. This decrease in Cl− channel function was considered to normalize the excitability of the muscle membrane, since a smaller Na+ current is then needed to overcome the stabilizing effects of K+ and Cl− currents. Such a balance between stimulation and inhibition would be reestablished, because decreased stimulatory Na+ channel gating is countered by a decreased inhibitory Cl− channel gating. Therefore, in the case of accumulating lactic acid during exercise, the resultant decrease in intracellular pH could protect the muscle membrane from becoming inexcitable due to increasing interstitial [K+] or, as perhaps was the case in our experiment, render the membrane excitable again after the prior exposure to high extracellular [K+].
In normal conditions, i.e., with normal interstitial K+ levels, decreased Cl− channel function has been associated with severe muscular dysfunction, as illustrated by several Cl− channel myotonic disorders (e.g., myotonic dystrophy, Thomsen's disease, and Becker-type myotonia). As stated previously, Cl− channel function is considered paramount for the resting membrane potential of skeletal muscle, and especially its relative stability. In cases of disturbed Cl− channel function, due to either genetic factors or, as in our experiments, pharmacological treatment (9-AC), the membrane becomes hyperexcitable. In this scenario, an already small amount of spontaneously opening Na+ channels can be sufficient to overcome the counteracting currents (K+ and the diminished Cl− current), leading to repetitive stimulatory Na+ currents and the elicitation of myotonia. This has been shown by our group in several studies (3, 12), where pharmacological treatment of skeletal muscle bundles with 9-AC in normal Krebs-Ringer solution led to myotonic characteristics of the treated muscle bundles. However, in the current experimental setting (elevated [K+]), we did not observe any myotonic activity induced in the 9-AC-treated muscle bundles, despite high dosing (512 μM) of the Cl− channel blocker. Nevertheless, as soon as the buffer solution was exchanged with normal Krebs solution, the muscle bundles exhibited myotonic behaviors. In subsequent dose-response studies, we then could show that increasing [K+] to 6 mM nearly abolished myotonic behavior in 9-AC-treated muscle bundles.
In other experiments we observed that ouabain (a Na+-K+ pump inhibitor)-treated muscle bundles elicited an additional force decline, beyond the K+-induced depression. At this relatively high dose of ouabain (100 μM), we also observed that neither adrenaline nor lactic acid had effects on force recovery. Perhaps the rising intracellular [Na+], and thereby a decreased electrochemical gradient, may have averted any potentially beneficial effects of lactic acid on excitability in such experiments, since large doses of ouabain were used. Hence, we acknowledge that further dose-response studies may be needed.
A further limitation to our studies may be that the tissue bath solution was not adjusted for changes in ionic strength and osmolality after addition of K+, lactic acid, or the other pharmacological agents. However, these changes might have significantly influenced our study results.
In conclusion, after treatment with 20 mM lactic acid, muscle bundles with prior K+-depressed muscle forces regained normal contractile properties within 20 min. In addition, the administration of the pharmacological Cl− channel blocker 9-AC led to a similar force recovery. Interestingly, despite this induced diminished Cl− channel conductance, there was no elicitation of myotonia within these respective muscle bundles; this was true for both lactic acid- and 9-AC-treated muscle bundles. It is considered that the presence of a high interstitial [K+] was associated with an antimyotonic effect, likely due to the increasing proportion of slow-inactivated Na+ channels, thereby reestablishing the balance between stimulatory and inhibitory currents.
GRANTS
This research was funded in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant P30 AR057220 (Muscular Dystrophy Research Center Core Laboratories), the Departments of Anesthesia and Intensive Care of the University of Basel, and the University of Minnesota's Malignant Hyperthermia Diagnostic Test Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.B., C.L.S., and P.A.I. conception and design of research; O.B. and C.L.S. performed experiments; O.B., C.L.S., and P.A.I. analyzed data; O.B., C.L.S., and P.A.I. interpreted results of experiments; O.B. prepared figures; O.B. drafted manuscript; O.B., C.L.S., and P.A.I. approved final version of manuscript; C.L.S. and P.A.I. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Monica Mahre for help with proofing the text and manuscript submission.
REFERENCES
- 1. Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Allen DG, Lannergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol 80: 497–527, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Bandschapp O, Ginz HF, Soule CL, Girard T, Urwyler A, Iaizzo PA. In vitro effects of propofol and volatile agents on pharmacologically induced chloride channel myotonia. Anesthesiology 111: 584–590, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol 587: 5591–5600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruton JD, Lannergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28°C. J Appl Physiol 85: 478–483, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Cooper TE, Trezek GJ. Correlation of thermal properties of some human tissue with water content. Aerospace Med 42: 24–27, 1971 [PubMed] [Google Scholar]
- 7. Dutka TL, Lamb GD. Effect of lactate on depolarization-induced Ca2+ release in mechanically skinned skeletal muscle fibers. Am J Physiol Cell Physiol 278: C517–C525, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Dutka TL, Murphy RM, Stephenson DG, Lamb GD. Chloride conductance in the transverse tubular system of rat skeletal muscle fibres: importance in excitation-contraction coupling and fatigue. J Physiol 586: 875–887, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Fletcher WM. Lactic acid in amphibian muscle. J Physiol 35: 247–309, 1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hong J, Sigg DC, Coles JA, Jr, Oeltgen PR, Harlow HJ, Soule CL, Iaizzo PA. Hibernation induction trigger reduces hypoxic damage of swine skeletal muscle. Muscle Nerve 32: 200–207, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Iaizzo PA, Lehmann-Horn F. The correlation between electrical after-activity and slowed relaxation in myotonia. Muscle Nerve 13: 240–246, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Iaizzo PA, Quasthoff S, Lehmann-Horn F. Differential diagnosis of periodic paralysis aided by in vitro myography. Neuromuscul Disord 5: 115–124, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Iaizzo PA, Wedel DJ, Gallagher WJ. In vitro contracture testing for determination of susceptibility to malignant hyperthermia: a methodologic update. Mayo Clin Proc 66: 998–1004, 1991 [DOI] [PubMed] [Google Scholar]
- 15. Jones DA. High-and low-frequency fatigue revisited. Acta Physiol Scand 156: 265–270, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Kristensen M, Albertsen J, Rentsch M, Juel C. Lactate and force production in skeletal muscle. J Physiol 562: 521–526, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larach MG. Standardization of the caffeine halothane muscle contracture test. North American Malignant Hyperthermia Group. Anesth Analg 69: 511–515, 1989 [PubMed] [Google Scholar]
- 18. Liu SH, Fu WM, Lin-Shiau SY. Studies on the inhibition by chlorpromazine of myotonia induced by ion channel modulators in mouse skeletal muscle. Eur J Pharmacol 231: 23–30, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Moulds RF, Denborough MA. Procaine in malignant hyperpyrexia. Br Med J 4: 526–528, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson TE, Austin KL, Denborough MA. Screening for malignant hyperpyrexia. Br J Anaesth 49: 169–172, 1977 [DOI] [PubMed] [Google Scholar]
- 21. Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol 536: 161–166, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nielsen OB, de Paoli FV. Regulation of Na+-K+ homeostasis and excitability in contracting muscles: implications for fatigue. Appl Physiol Nutr Metab 32: 974–984, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Ording H, Brancadoro V, Cozzolino S, Ellis FR, Glauber V, Gonano EF, Halsall PJ, Hartung E, Heffron JJ, Heytens L, Kozak-Ribbens G, Kress H, Krivosic-Horber R, Lehmann-Horn F, Mortier W, Nivoche Y, Ranklev-Twetman E, Sigurdsson S, Snoeck M, Stieglitz P, Tegazzin V, Urwyler A, Wappler F. In vitro contracture test for diagnosis of malignant hyperthermia following the protocol of the European MH Group: results of testing patients surviving fulminant MH and unrelated low-risk subjects. The European Malignant Hyperthermia Group. Acta Anaesthesiol Scand 41: 955–966, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol 486: 689–694, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pedersen TH, Clausen T, Nielsen OB. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and beta2-agonist. J Physiol 551: 277–286, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science 305: 1144–1147, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Philp A, Macdonald AL, Watt PW. Lactate—a signal coordinating cell and systemic function. J Exp Biol 208: 4561–4575, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Posterino GS, Fryer MW. Effects of high myoplasmic l-lactate concentration on E-C coupling in mammalian skeletal muscle. J Appl Physiol 89: 517–528, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Ruff RL. Sodium channel slow inactivation and the distribution of sodium channels on skeletal muscle fibres enable the performance properties of different skeletal muscle fibre types. Acta Physiol Scand 156: 159–168, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol Cell Physiol 248: C265–C270, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Sjogaard G. Exercise-induced muscle fatigue: the significance of potassium. Acta Physiol Scand Suppl 593: 1–63, 1990 [PubMed] [Google Scholar]
- 32. Westerblad H, Bruton JD, Lannergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol 500: 193–204, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerblad H, Lee JA, Lannergren J, Allen DG. Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol Cell Physiol 261: C195–C209, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Wiseman RW, Beck TW, Chase PB. Effect of intracellular pH on force development depends on temperature in intact skeletal muscle from mouse. Am J Physiol Cell Physiol 271: C878–C886, 1996 [DOI] [PubMed] [Google Scholar]