Abstract
Background
In this prospective cohort study, we have undertaken a comprehensive evaluation of clinical parameters along with variation in 29 genes (including CYP2C9 and VKORC1) to identify factors determining interindividual variability in warfarin response.
Methods
Consecutive patients (n = 311) were followed up prospectively for 26 weeks. Several outcomes chosen to capture both warfarin efficacy and toxicity were assessed. Univariate and multiple regression analyses were undertaken to assess the combined effect of clinical and genetic factors.
Results
CYP2C9 was the most important gene determining initial anticoagulant control, whereas VKORC1 was more important for stable anticoagulation. Novel associations with some clinical outcomes were found with single nucleotide polymorphisms in the cytochrome 450 genes CYP2C18 and CYP2C19, which were independent of the associations observed with CYP2C9 and in genes encoding CYP3A5, protein S and clotting factor V, although the variability explained by these genes was small. On the basis of the results of microcosting, adverse events were shown to be a significant predictor of total cost.
Conclusion
Accurate prediction of warfarin dose requirement needs to take into account multiple genetic and environmental factors, the contributions of which vary in the induction and maintenance phases of treatment.
Keywords: dosing algorithms, haemorrhage, pharmacogenetics, variability, warfarin
Introduction
Warfarin is a highly effective anticoagulant [1], but its use is complicated by the unpredictability of dose requirements, and difficulty in achieving and maintaining anticoagulation within a defined therapeutic window [typically an international normalized ratio (INR) of between 2 and 3]. This predisposes patients to either rethrombosis from underanticoagulation or bleeding from overanticoagulation. Major bleeding events occur in the first 3 months of therapy, particularly when the INR is greater than 3 [2]. This emphasizes the need to stabilize patients as soon as possible after the initiation of warfarin therapy.
The stability of anticoagulation with warfarin can be affected by various environmental factors including age, body weight, diet, alcohol intake and interacting medications [3]. More recently, genetic determinants of warfarin dose requirements have been identified with a consistent association shown with polymorphisms in CYP2C9, the main P450 isoform responsible for the metabolism of S-warfarin, and VKORC1, the drug’s main pharmacological target [3]. The association between warfarin dose and CYP2C9 and VKORC1 is highly robust – it has been replicated by many different studies including those that have been retrospective in nature, those that have recruited only patients who have already achieved stable dosage and those that have excluded patients on interacting medications [4–18]. This has led to a change in the drug label for warfarin by the FDA [19]. However, despite this, there is scepticism whether there is adequate evidence to introduce preprescription genetic testing for warfarin therapy [20,21]. Indeed, the recent guidelines from the American College of Chest Physicians recommended against pharmacogenetic-based dosing until data from randomized trials are available [22]. An additional area of concern is the cost-effectiveness of preprescription genotyping. Widely varying estimates of cost-effectiveness have been published which may partly be because of the lack of methodological rigour [23] and the absence of accurate cost data.
Although the majority of studies are retrospective in nature (see above), more prospective studies have been published recently [24–26]. Prospective cohort studies are important as they reduce bias and may help in the design of randomized studies, as they allow information on variable patient response to be captured right from initiation onto warfarin. Furthermore, they allow inclusion of an important sector of the warfarin patient population who struggle to achieve stable dose and who are typically excluded from retrospective studies which usually recruit only those patients already on a stable dose, and are thus at risk of selection bias. In this study, we present the findings of the first UK prospective study. Our aims were to determine whether the impact of the CYP2C9 and VKORC1 genes in our prospective cohort accords with that seen in retrospective studies, and more recently in prospective studies; and to systematically evaluate the effect of genetic and clinical factors, not only on stable dose, but also on other outcome measures that capture treatment response. In addition to CYP2C9 and VKORC1, the effect of a further 27 genes on response to warfarin was also explored. We have also undertaken a comprehensive microcosting exercise, which was possible in our study because of its prospective nature.
Methods
Patient recruitment and follow-up
Patients initiated onto warfarin irrespective of indication were recruited from the Royal Liverpool and Broadgreen University Hospitals Trust and University Hospital Aintree between November 2004 and March 2006. The only exclusion criterion was inability or refusal to give informed consent. The study was approved by the Birmingham South Research Ethics Committee, and each patient provided informed consent to participate in the study.
The study design was observational and pragmatic. Patients received usual clinical care where the warfarin loading dose and subsequent maintenance dose were determined according to in-hospital guidelines. There were four fixed study visits for each patient, the first at the time of initiation of warfarin (index visit), then at 1 week, 8 weeks and 26 weeks of warfarin therapy. Seventy-four percent of patients had their index visit within 2 days of commencing warfarin, 14% on the third day of warfarin therapy and 2% between the fourth and ninth days of therapy. The remaining 10% of patients had their index visit between 1 and 4 days before the start of warfarin therapy. At the index visit, patient demographics were recorded and baseline INR, clotting factor activity and protein levels were measured (Table 1). At the remaining follow-up visits INR was again measured, and dose changes since the previous visit were recorded. In addition to the four fixed study visits, patients also attended anticoagulant clinic according to their clinical needs. This meant that, at the end of follow-up, data on warfarin dose changes and INR levels were available longitudinally for each patient, which together provided a complete picture of treatment progress from warfarin initiation onwards. For patients who missed one or more fixed follow-up visits, INR measurements and dose changes missing as a consequence were obtained from clinical records. The number of patients attending each visit is shown in Supplementary Figure A (see Supplemental Digital Content 1, http://links.lww.com/FPC/A54), whereas the numbers of patients contributing to each analysis are summarized in the results section.
Table 1.
Patient demographics
| Characteristics | Mean (range)/n (%) |
|---|---|
| Sex | |
| Male | 184 (59%) |
| Age (years) | 66 (19–95) |
| Weight (kg) | 81 (36–172)a |
| Height (cm) | 169 (125–195)b |
| Indication for warfarin Rx | |
| Atrial fibrillation | 165 (53) |
| Pulmonary embolism | 75 (24) |
| Deep vein thrombosis | 43 (14) |
| Cerebrovascular accident and transient ischaemic attacks |
10 (3) |
| Myocardial infarction | 1 (0.3) |
| Mechanical heart valve | 1 (0.3) |
| Otherd | 16 (5) |
| Loading dose first 2 daysc | |
| 10; 10 | 194 (63) |
| 10; 5 | 52 (17) |
| 3; 3 | 23 (7) |
| 5; 5 | 15 (5) |
| 7; 7 | 11 (4) |
| 8; 8 | 3 (1) |
| 1; 1 | 2 (1) |
| Othere | 9 (3) |
| Ethnicity | |
| White | 304 (98) |
| Black African | 1 (0.3) |
| Black Caribbean | 1 (0.3) |
| Black other | 3 (0.9) |
| Otherf | 2 (0.6) |
| Medical/surgical history | |
| Cardiovascular disease | 208 (67) |
| Musculoskeletal problems | 142 (46) |
| Respiratory disease | 102 (33) |
| Gastrointestinal disease | 84 (27) |
| Endocrinological disorder | 75 (24) |
| Neurological disease | 73 (23) |
| Urological condition | 45 (14) |
| History of falls | 27 (9) |
| Renal disease | 23 (7) |
| Hepatic disease | 9 (3) |
| Clotting factor activity levelsg | |
| Factor II | 71.78 (8.37–140.69) |
| Factor V | 143.43 (11.11–249.66) |
| Factor VII | 57.80 (0.52–210.62) |
| Factor IX | 86.91 (4.63–231.49) |
| Factor X | 73.50 (17.74–151.89) |
| Protein Cg | 60.46 (0.14–199.42) |
| Protein Sg | 123.85 (26.73–254.28) |
Weight is missing for five patients.
Height is missing for three patients.
Loading dose regime is missing for two patients.
Other indications for warfarin treatment include the following: prevention of clotting in arm for dialysis; axillary vein thrombosis; short saphenous vein thrombosis; valvular heart disease; saggital sinus thrombosis; dilated left ventrical; occluded graft in leg; aortic and mitral regurgitation; poor liver function and pseudoaneurysm; ischaemic leg; dilated left ventrical; brachial artery thrombosis; previous pulmonary embolism and abdo clot; mitral stenosis and postsurgery (n=2).
Other loading dose regimes for the first 2 days include: (10, 6); (10, 8); (8, 7); (7, 10); (6, 7); (5, 10); (5, 0); (4, 4); (2, 2).
Other self-reported ethnicities include Hungarian, Black British.
These measurements are missing for one patient.
Outcome measures of warfarin efficacy and toxicity
To capture both efficacy and toxicity of warfarin treatment, we chose two primary and several secondary outcome measures. The two primary outcomes were as follows:
INR greater than 4 during the first week; and
Warfarin sensitivity (a dose of ≤ 1.5 mg/day on three successive clinic visits).
The secondary outcome measures were as follows:
(iii) Warfarin resistance (a dose of > 10 mg/day on three successive clinic visits).
(iv) Time to achievement of stable warfarin dosing from initiation. Stable warfarin dosing was defined as the mean daily dose required to achieve three consecutive INR measurements within the individual’s target range, at the same daily dose.
(v) Stable warfarin dose as defined above.
(vi) Time to therapeutic INR. This was defined as the time of first achieving INR measurement within the individual’s target range, providing that INR was also within the target range at the subsequent clinic visit.
(vii) Haemorrhagic complications. All adverse events reported by the patients were assessed for causality by M.P. (blinded to genotype) and the events categorized as definitely, probably, possibly or unlikely to be related to warfarin. Haemorrhagic complications were defined as major or minor according to the classification provided by Fihn et al. [27]. Only those events considered to be possibly, probably or definitely associated with warfarin were included in the analyses.
The cut-off of 4 for INR during the first week was chosen on the basis that the large majority (99%) of patients had target INR in the range 2–3. It is therefore an important outcome in terms of investigating factors that elevate INR to a level that exposes the patient to an increased risk of bleeding, before INR tracking can be used effectively to inform dose adjustments. It is true that not all patients with INR above 4 will suffer bleeding events; however, identification of those most likely to achieve elevated INR early on will allow suitable dose adjustments to reduce this risk. The cut-off values for defining warfarin sensitivity and resistance of 1.5 and 10 mg/day, respectively, were chosen based on the estimated 5th and 95th percentiles of the distribution of stable dose within our patient population. These outcomes were considered appropriate surrogates for investigating the factors influencing the extent of drug (in)tolerability at both ends of the spectrum.
Genotyping
DNA from blood samples was extracted at the Sanger Institute using the phenol–chloroform method. Genotyping was performed either by matrix assisted laser desorption /ionization-time of flight mass spectrometry or by real-time PCR as described by Wadelius et al. [28], in which the quality control measures adopted are also summarized. Any single nucleotide polymorphism (SNP) with an initial call rate less than 90% was regenotyped to minimize the amount of missing genotype data. Genotype frequencies at each SNP were also compared with previously published frequencies for the same population and any SNPs for which frequencies differed by more than 20% in absolute terms were regenotyped to safeguard further against genotyping errors. Twenty-nine candidate genes were genotyped (see Supplementary Table A, Supplemental Digital Content 2, http://links.lww.com/FPC/A55), all derived from the review undertaken by Wadelius and Pirmohamed [3]. A combination of tagging SNPs and functional variants, 196 in total, identified in a study undertaken in a Swedish cohort [29] and explaining at least 95% of the genetic diversity in each candidate gene were genotyped. Details of genotype frequencies at each SNP together with the number of genotypes missing are summarized in Supplementary Table B (see Supplemental Digital Content 3, http://links.lww.com/FPC/A56). Genotypes were classed as missing if they failed quality control procedures.
Analysis of clotting factors
Analysis of clotting factors (II, V, VII, IX, X, proteins C and S) was carried out at the Royal Liverpool University Hospital using an automated analyzer [Multi-Channel Discrete Analyzer (MDA)-180; bioMérieux, Inc., Durham, North Carolina, USA]. Factors II, VII and X activity levels were determined by a one-stage prothrombin time-based clotting assay using specific factor-depleted plasmas (Precision BioLogic Inc., Dartmouth, Canada) and simplastin human thromboplastin factor as the thromboplastin reagent (bioMérieux) [coefficient of variation (CV): 5.5%]. Activity levels of factors V and IX were quantified by a one-stage activated partial thromboplastin time-based clotting assay using factor-deficient plasmas (Precision BioLogic) and MDA Platelin LS test kit – APTT reagent (bioMérieux) (CV: 4.5%). Factor II activity levels were measured with a chromogenic method using commercial kits (bioMérieux) (CV: 7%). A functional clotting assay based on the prolongation of APTT was used to measure protein C activity using protein C-deficient plasma and Protac (Technoclone GmbH, Vienna, Austria) (CV: approximately 7%), whereas free protein S activity was measured with an immuneturbidimetric assay using commercial kits of STA, Diagnostica Stago, Asnieres-sur-Seine, France (CV: 8%). All kits were used according to the manufacturer’s recommendations.
Statistical analysis of association with outcomes of efficacy and toxicity
Assessing conformity with Hardy–Weinberg equilibrium
Statistical analysis was undertaken for each SNP using the Hardy–Weinberg equilibrium (HWE) test function in the genetics package of R (http://cran.r-project.org/web/packages/genetics/index.html). A P value of less than 0.001 was assumed to indicate deviation from HWE.
Assessing association with individual single nucleotide polymorphisms
Two tests of association were undertaken for each SNP–outcome combination, and the maximum test statistic referred to in each case. The first made no assumptions regarding the underlying mode of inheritance (binary outcomes: Pearson’s χ2 test or Fisher’s Exact test; time to event outcomes: log-rank test; continuous outcomes: analysis of variance), whereas the second assumed an additive mode of inheritance (binary outcomes: Cochrane–Armitage test for trend; time to event outcomes: log-rank test for trend; continuous outcomes: univariate linear regression).
Assessing association with clinical factors
The following baseline clinical factors were identified in advance as being of potential interest in terms of influencing response to warfarin: age; sex; ethnic origin; BMI; levels of clotting factors II, V, VII, IX and X; proteins C and S; indication for warfarin treatment; warfarin loading dose; medications that interact with warfarin and pre-existing medical conditions. Each factor was univariately assessed for association with each outcome. All statistically significant factors (P < 0.05) were included in the multiple regression models described below. To confirm that timing of initiation of warfarin relative to index visit had no effect on baseline clotting factor and proteins C and S activity, patients were stratified according to this relative timing and mean levels of activity compared between strata. No significant differences were found (data available on request). For investigating interacting medications, a record was made of all medications taken by each patient during follow-up, and all those known to interact with warfarin (according to the British National Formulary [30]) were highlighted. Analyses were limited to the eight drugs that were being commonly taken (by at least 10% of the participants), namely simvastatin, amiodarone, omeprazole, unfractionated heparin, aspirin, dalteparin, enoxaparin and clopidogrel.
Multiple regression models
To mirror previous attempts at developing models to predict warfarin maintenance dose based on a combination of genetic and environmental factors [4,6–8,11, 16–18,28,31,32], multiple regression models were fitted for each outcome. These included covariates to represent all clinical factors significant univariately (P < 0.05) as well as covariates representing the SNPs significant univariately [false discovery rate (FDR) [33] < 0.05]. To avoid colinearity, correlation between each pair of factors, as well as linkage disequilibrium (LD) between each pair of SNPs, was assessed with only one from each highly correlated pair represented in the model. This was particularly important when considering the clotting factors as they are in significant correlation with each other. For each model, Nagelkerke’s r2 value was calculated.
Correction for multiple testing
For each test, the FDR [33] was calculated in addition to the raw P value. When calculating the FDR, all analyses undertaken across the 29 candidate genes were accounted for.
Methodology used for the identification, measurement and valuation of healthcare resource use and costs
An analysis adopting a UK National Health Service perspective was performed to quantify the overall healthcare resource use and direct costs attributable to warfarin therapy. The time horizon of analysis was 6 months, and all costs are reported in UK£ for the year 2006–2007. Patients’ use of resources was categorized according to two general headings (i) anticoagulation services; (ii) management of thrombotic and haemorrhagic and other adverse events. Adverse events were assessed for causality, and the procedures, products and services associated with their management were assessed retrospectively from patients’ notes. All other data were collected prospectively in the clinical forms. Unit costs were obtained from National Health Service (NHS) reference costs for 2006 (Department of Health Reference Costs. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_082571), Curtis and Netten [34], and National Blood Transfusion Service (National Blood Transfusion Service: http://hospital.blood.co.uk/library/pdf/ncg_letter_Dec06_NBS__component_prices_2007_08.pdf) (see Supplementary Table C, Supplemental Digital Content 4, http://links.lww.com/FPC/A57). Double counting was avoided by acknowledging that NHS reference costs include low-cost drugs but exclude some blood products (NHS costing manual).
Mann–Whitney U tests and Kruskal–Wallis tests were used to assess association between resource use and baseline clinical and genetic factors (defined by age; sex; CYP2C9 genotype; VKORC1 genotype; adverse events; comedication; comorbidities; and smoking status). Categorical variables were tested for significance by Fisher’s Exact test for independence. P values less than 0.05 were considered statistically significant. Mean patient costs per 6 months were reported with 95% bias corrected and accelerated confidence intervals estimated using nonparametric bootstrap sampling. Two thousand samples were taken for the calculation of each confidence interval. A multiple generalized linear regression, including baseline clinical factors and genetic factors, with an inverse Gaussian distribution and log link was used to model direct costs. The appropriateness of the link and error distribution was tested using the Box-Cox and Park tests, respectively [35]. Nonparametric bootstrapping and generalized linear modelling were carried out using STATA Version 8.2 (StataCorp LP, College Station, Texas, USA); other analyses were conducted using SPSS Version 16.0.2 (SPSS Inc., Chicago, Illinois, USA).
Results
Patient demographics
The baseline demographic factors including underlying comorbidities are summarized in Table 1. The majority of patients (n=309), 60% of whom were inpatients, had a target INR range of 2–3, with the remaining two patients having a target range of 3–4. The majority of the patients were White with atrial fibrillation being the most common indication for warfarin therapy. There was significant variation in the loading doses prescribed. Sixty-three percent were given 10 mg on the first 2 days, 17% were given 10 mg on day 1 followed by 5 mg on day 2, 7% were given 3 mg on both days whereas 5% were given 5 mg on both days. The median number of INRs recorded for each patient was 16 (range: 1–57).
The maximum daily dose prescribed to a patient ranged from 1 to 18 mg (mean: 5.75 mg). Mean daily dose ranged from 0.29 to 12.19 mg (mean: 4.25 mg). Number of dose changes during follow-up also varied between patients, ranging from 1 to 34 (median: 7) for patients with complete 26-week follow-up, reflecting the significant variation in stability of INR between patients. Of the patients for which sufficient dose data was available (n=273), 13% (n=35) were sensitive to warfarin whereas 4% (n=10) were resistant. Sixty-six percent of patients (n=204) achieved stable dose during their follow-up and median time to stable dose was 58.6 days. Median stable dose was 4 mg/day (range: 1–12 mg). As its distribution was skewed, all analyses of stable dose were undertaken on its square-root transformation. Two hundred and seventy-four patients achieved therapeutic INR during follow-up, and median time to therapeutic dose was 10.6 days. Eighteen percent of patients (n=57) experienced an INR greater than 4 during the first week of treatment and 22% (n=68) experienced at least one bleeding event during follow-up, with 5% (n=16) experiencing a major bleed.
Univariate analysis of associations between single nucleotide polymorphisms and outcomes
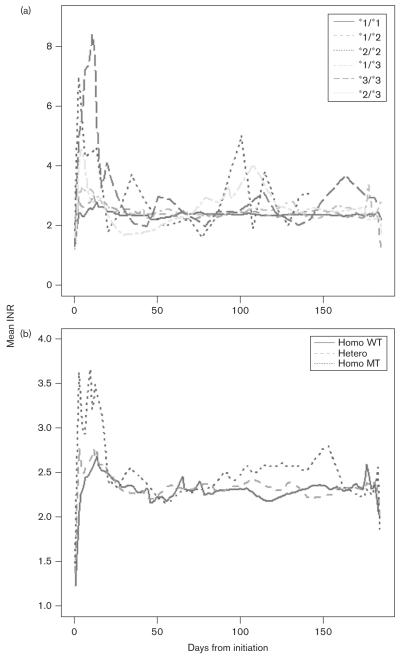
All SNPs investigated had less than 5% genotypes missing (See Supplementary Table B, Supplemental Digital Content 3, http://links.lww.com/FPC/A56) and less than 10% of genotypes were missing for the majority of individuals. As the amount of missing genotype data was small, no attempt was made at imputation. None were found to deviate from HWE. Results from univariate analyses for association between each SNP in CYP2C9 and VKORC1 and outcome are summarized in Table 2. In accordance with previous studies, SNPs in both the VKORC1 and CYP2C9 genes were significantly associated with the square root of stable warfarin dose. However, SNPs in CYP2C9, but not VKORC1, were statistically significant at the FDR level for the outcomes INR greater than 4 within the first week, time to stable warfarin dose and time to therapeutic INR. For the outcome of warfarin sensitivity, although SNPs in both genes were statistically significant, those in CYP2C9 were generally more so. This is further illustrated by plots of mean INR profile during study follow-up, stratified by genotype (Fig. 1). Individuals with variant genotypes were more likely to be unstable in terms of INR control than those with wild-type genotypes, particularly during the initiation phase of therapy.
Table 2.
Results of univariate analyses of association between each SNP in CYP2C9 and VKORC1 and clinical outcomes
| INR>4d |
Warfarin sensitivee |
Warfarin resistantf |
Time to stable warfarin doseb,g |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | SNP | n c | P value | n | Pvalue | n | Pvalue | n | Pvalue |
| CYP2C9 | rs1799853 | 301 | 7.30E-02 | 265 | 2.37E-02 | 265 | 8.49E-02 | 301 | 4.98E-02 |
| rs2860905 | 309 | 3.00E-03 | 271 | 3.08E-05a | 271 | 9.01E-02 | 308 | 3.94E-01 | |
| rs2475376 | 307 | 2.09E-01 | 269 | 3.94E-02 | 269 | 7.79E-01 | 306 | 9.59E-04 | |
| rs1856908 | 307 | 1.29E-01 | 269 | 7.09E-05a | 269 | 1.29E-01 | 306 | 1.85E-01 | |
| rs9332197 | 311 | 7.16E-01 | 273 | 8.04E-01 | 273 | 2.17E-01 | 310 | 3.45E-04a | |
| rs1057910 | 306 | 2.00E-05a | 268 | 2.18E-08a | 268 | 2.59E-01 | 309 | 4.52E-02 | |
| VKORC1k | rs7294 | 309 | 8088E-02 | 272 | 2.74E-01 | 272 | 5.74E-02 | 308 | 1.32E-01 |
| rs2359612 | 305 | 5.66E-04 | 267 | 5.17E-05a | 267 | 3.93E-02 | 304 | 1.36E-01 | |
| rs99232331 | 307 | 2.10E-03 | 269 | 7.07E-05a | 269 | 2.51E-02 | 306 | 1.14E-01 | |
| Time to therapeutic INRh | Square root of stable daily warfarin dose |
Any haemorrhagic eventi | Major haemorrhagic eventj | ||||||
| CYP2C9 | rs1799853 | 301 | 1.49E-04a | 198 | 5.57E-01 | 301 | 7.01E-01 | 301 | 9.65E-01 |
| rs2860905 | 308 | 7.08E-01 | 203 | 1.19E-06a | 309 | 7.88E-01 | 309 | 8.11E-02 | |
| rs2475376 | 306 | 2.53E-01 | 201 | 2.10E-01 | 307 | 6.04E-01 | 307 | 4.53E-01 | |
| rs1856908 | 306 | 1.98E-01 | 201 | 1.98E-01 | 307 | 3.52E-01 | 307 | 6.47E-03 | |
| rs9332197 | 310 | 1.44E-01 | 204 | 8.38E-01 | 311 | 6.08E-01 | 311 | 4.71E-01 | |
| rs1057910 | 309 | 3.53E-02 | 198 | 2.55E-05a | 306 | 4.35E-01 | 306 | 1.09E-03 | |
| VKORC1k | rs7294 | 308 | 2.77E-01 | 202 | 4.30E-04a | 309 | 5.31E-01 | 309 | 6.84E-01 |
| rs2359612 | 304 | 1.74E-01 | 198 | 1.17E-09a | 305 | 1.73E-01 | 305 | 4.56E-01 | |
| rs99232331 | 306 | 1.22E-01 | 202 | 8.30E-10a | 307 | 6.50E-02 | 307 | 3.63E-01 | |
INR, international normalized ratio; SNP, single nucleotide polymorphism.
False discovery rate< 0.05.
Only 204 patients achieved stable warfarin dose during the course of the study.
n= number participants contributing to analysis.
57 out of 311 (18%) patients experienced INR>4 during first week.
Complete dosage information unavailable for 38 patients. Of remaining patients, 33 (12%) were warfarin sensitive.
Complete dosage information unavailable for 38 patients. Of remaining patients, 10 (4%) were warfarin resistant.
204 (66%) of patients achieved stable dose during follow-up period. Median time to achieving stable dose was 33 days (range: 2–189 days).
274 (88%) of patients achieved therapeutic INR during follow-up period. Median time to achieving this was 8 days (range: 1–155 days).
A haemorrhagic complication occurred in 68 (22%) patients.
A major haemorrhagic complication occurred in 16 (5%) patients. One patient experienced three major events; two patients experienced two events. Major events were: haematemesis (2), malaena (5), muscle haematoma (5), haematuria (3), haemoptysis (2), epistaxis (2), bleeding into joints (1).
One SNP in the VKORC1 gene, rs11150606 is excluded as it is monomorphic in this study population.
Fig. 1.
The mean international normalized ratio (INR) profiles in the patient cohort stratified by CYP2C9 genotypes (a) and VKORC1 (rs9923231) genotypes (b). WT, wild type; MT, mutant type.
Univariate analysis for the other 27 genes showed associations of a number of the SNPs with the different outcomes, even after correction for multiple testing (Table 3). These can be summarized as follows:
SNP rs3814637 in CYP2C19 was significantly associated with elevated INR during the first week, stable dose and warfarin sensitivity.
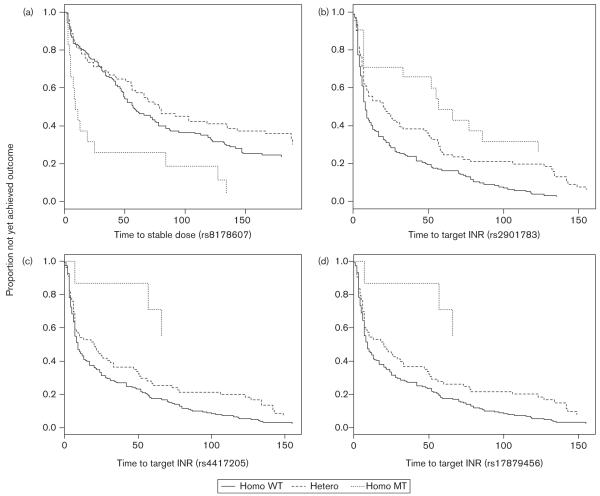
SNP rs2901783 in gene CYP2C18 was associated with warfarin sensitivity; patients who were homozygous wild-type for this SNP achieved therapeutic INR the fastest, the homozygous variant genotype group took the longest, with heterozygotes occupying an intermediate position (Fig. 2b).
A further two SNPs in CYP2C19, rs4417205 and rs17879456 (Fig. 2c and d, respectively) also had an effect on time to therapeutic INR with a recessive mode of inheritance, although for these SNPs, homozygous mutants are relatively rare (n=7) and the results should be treated with caution. A significant P value was also obtained for association between SNP rs3817939 in the clotting factor IX gene and this outcome, but again the relevance of this finding is limited by the small number of patients with the homozygous variant genotype (≤ 2).
A further SNP, rs6976017 in gene CYP3A5 was also associated with warfarin sensitivity.
For SNP rs8178607, which is in the protein S gene, the time to stable dose for the wild-type and heterozygous genotype groups were similar, but the homozygous mutant group seemed to achieve stability faster, suggesting a possible recessive mode of inheritance for the variant allele at this SNP (Fig. 2a).
One SNP, rs6018 in the clotting factor V gene, was associated with major bleeding events.
Table 3.
Univariate analyses of association between SNPs in 27 candidate genes (excluding CYP2C9 and VKORC1) and clinical outcomes (only SNPs giving false discovery rate<0.05 are shown)
| Outcome | n | SNP | Gene | P value |
|---|---|---|---|---|
| INR> 4 in week 1 | 270 | rs3814637a | CYP2C19 | 3.85E-04 |
| Warfarin sensitive | 271 | rs2901783a | CYP2C18 | 4.57E-06 |
| 235 | rs3814637a | CYP2C19 | 4.59E-05 | |
| 233 | rs6976017 | CYP3A5 | 2.14E-04 | |
| Time to stable dose | 307 | rs8178607 | PROS1 | 5.00E-05 |
| 306 | GS30681 | CYP3A5 | 6.00E-04 | |
| Time to therapeutic INR | 300 | rs3817939 | F9 | 6.40E-09 |
| 308 | rs2901783a | CYP2C18 | 1.20E-05 | |
| 309 | rs4417205 | CYP2C19 | 1.50E-04 | |
| 301 | rs17879456 | CYP2C19 | 1.60E-04 | |
| Major bleeds | 300 | rs6018 | F5 | 4.00E-05 |
| Stable dose | 176 | rs3814637a | CYP2C19 | 8.60E-06 |
INR, international normalized ratio; LD, linkage disequilibrium; SNP, single nucleotide polymorphism.
This SNP is in LD with CYP2C9 SNP rs1057910 (r2=0.51).
Fig. 2.
Kaplan–Meier plots of clinical outcomes associated with various single nucleotide polymorphism (SNPs): (a) For SNP rs8178607, in the protein S gene, the association with time to stable dose is shown; (b) for SNP rs2901783 in CYP2C18, (c) SNP rs4417205 in CYP2C19 and (d) SNP rs17879456 in CYP2C19, the association with time to therapeutic international normalized ratio (INR) is shown. WT, wild type; MT, mutant type.
CYP2C18 and CYP2C19 both exist within the CYP2C cluster on chromosome 10, together with CYP2C9, and as such many SNPs within CYP2C18 and CYP2C19 are in reasonably high linkage disequilibrium (r2 > 0.50) with SNPs in CYP2C9. Consequently, we hypothesized that the significant associations found with SNPs in both CYP2C18 and CYP2C19 could be because of this LD with CYP2C9. To investigate this, for each outcome with which SNPs in CYP2C18 or CYP2C19 were significantly associated, a regression model including CYP2C9 SNPs only was compared, using the likelihood ratio test, with a model that also included the CYP2C18 and/or CYP2C19 SNPs as appropriate. For the outcome of INR greater than four during the first week, addition of the CYP2C19 SNP had a significant effect (P =4.5 × 10−10). For the outcome warfarin sensitivity, addition of the CYP2C18 SNP was not significant (P = 0.48), whereas the addition of CYP2C19 SNP was significant (P = 1.47 ×10 −8). For the outcome of time to therapeutic INR, addition of both the CYP2C18 SNPs and the CYP2C19 SNP had a significant effect on the model (P =3 × 10 −7 and 6.14 × 10 −24 respectively). Furthermore, for the outcome of stable dose, the addition of the CYP2C19 SNP had a significant effect (P = 2.09 × 10 −13).
Clinical and biochemical determinants of outcomes
A number of clinical factors, and clotting factor activity levels, were shown to be significantly associated with the outcomes univariately (Table 4) (P < 0.05). Taking all significant clinical and clotting factors together, the total proportion of variability in outcome explained varied between the different clinical outcomes with r2 ranging from 0 to 0.33. Incorporation of SNPs found significant univariately (FDR < 0.05) into the multiple regression models improved the proportion of variability explained for the outcomes of INR greater than 4, warfarin sensitivity and the square root of stable warfarin dose (Table 4).
Table 4.
Environmental and clinical factors included in multiple regressions for each outcome
| Outcome | Environmental and clinical factors included in multiple regression models after eliminating one from each pair of highly correlated factorsd |
Total r2 explained by all environmental and clinical factors togetherm |
SNPs (gene) found significant univariately at the FDR level |
Nagelkerke’s r2 for model including environmental and clinical factors and SNPsm |
|---|---|---|---|---|
| INR>4a,e | Sex; BMI; clotting factor VII; warfarin loading dose |
0.17 | rs1057910 (CYP2C9); rs3814637 (CYP2C19) |
0.27 |
| Warfarin sensitivitya,f | None | NA | rs1057910 (CYP2C9); rs2901783 (CYP2C18); rs2860905 (CYP2C9); rs3814637 (CYP2C19); rs2359612 (VKORC1); rs9923231 (VKORC1); rs1856908 (CYP2C9); rs6976017 (CYP3A5) |
0.54 |
| Warfarin resistancea,g | Age; warfarin loading dose | 0.33 | None | NA |
| Time to stable doseb,h | Clotting factor IX and indication for warfarin |
0.03 | rs8178607 (PROS1); rs9332197 (CYP2C9); gs30681 (CYP3A5) |
0.09 |
| Time to therapeutic INRb,i | BMI; clotting factor VII; warfarin loading dose and ethnic origin |
0.06 | rs3817939 (F9); rs2901783 (CYP2C18); rs4417205 (CYP2C19); rs1799853 (CYP2C9); rs17879456 (CYP2C19) |
0.13 |
| Square-root stable warfarin dosec,j | Age, BMI, clotting factor II, warfarin loading dose, and suffering from a neurological condition |
0.19 | rs9923231 (VKORC1); rs2359612 (VKORC1); rs2860905 (CYP2C9); rs3814637 (CYP2C19); rs1057910 (CYP2C9); rs7294 (VKORC1) |
0.53 |
| All haemorrhagic eventsa,k | None | NA | None | NA |
| Major haemorrhagic eventsa,l | Clotting factor IX, warfarin loading dose and concurrent use of omeprazole |
0.14 | rs6018 (F5) | 0.15 |
FDR, false discovery rate; INR, international normalized ratio.
For these binary outcomes Student’s t-test was used to test for association when the factor was continuous whereas Pearson’s χ2 test or Fisher’s Exact test were used when the factor was categorical.
For these time to event outcomes the univariate Cox proportional hazards regression model was used to test for association when the factor was continuous and a log-rank test was used when the factor was categorical.
For this continuous outcome univariate linear regression was used to test for association when the factor was continuous, analysis of variance was used when the factor was categorical whereas the Student’s t-test was used when the factor was binary.
For continuous variables, pairwise correlation was assessed by calculating Pearson’s correlation coefficient and r2> 0.70 taken as being significant. To assess correlation between a binary and continuous variable a two-sample t-test was undertaken whilst Pearson’s χ2 test was used to assess correlation between two binary or categorical variables. A P value less than 0.01 was assumed to indicate significant correlation for these two tests.
57 out of 311 (18%) patients experienced INR>4 during first week.
Complete dosage information unavailable for 38 patients. Of remaining patients, 33 (12%) were warfarin sensitive.
Complete dosage information unavailable for 38 patients. Of remaining patients, 10 (4%) were warfarin resistant.
204 (66%) of patients achieved stable dose during follow-up period. Median time to achieving stable dose was 33 days (range: 2–189 days).
274 (88%) of patients achieved therapeutic INR during follow-up period. Median time to achieving this was 8 days (range: 1–155 days).
204 (66%) of patients achieved stable dose during follow-up period.
A haemorrhagic complication occurred in 68 (22%) patients.
A major haemorrhagic complication occurred in 16 (5%) patients. One patient experienced three major events; two patients experienced two events. Major events were haematemesis (2), malaena (5), muscle haematoma (5), haematuria (3), haemoptysis (2), epistaxis (2), bleeding into joints (1).
Clotting factor levels were important determinants for several outcomes but as clotting factor levels are not routinely measured, Nagelkerke’s r2 value was also calculated excluding any clotting factor levels to assess how much additional variability was being explained by them. This resulted in a decrease in Nagelkerke’s r2 value for the outcome of INR> 4 (0.17–0.11), time to stable dose (0.03–0.01), time to therapeutic INR (0.06–0.05) and square root of stable dose (0.19–0.16), but did not change the r2 value for major bleeding events.
Microcosting exercise
Complete 6-months data were available for 254 patients (inclusive of 10 patients who had died). During the study period, a total of 930 anticoagulation visits (median 3 per patient, IQR 1, 5) and 4059 INR measurements (median 15 per patient, IQR 10, 20) were recorded. Univariate analyses showed no significant association between any of the baseline clinical or genetic factors and the number of appointments with the anticoagulation clinics or the number of INR measurements (results not shown). Of the 70 (27.6%) patients who had experienced a thrombotic or haemorrhagic adverse event, 16 (6.3%) required hospitalization. VKORC1 status was associated significantly with hospitalization. Logistic regression analysis controlling for age, sex and comorbidities in patients who experienced an adverse event showed that the odds ratio for hospitalization was 8.35 [95% confidence interval (CI): 1.44, 48.35] for patients with the VKORC1 TT genotype compared with other genotypes. The mean duration of hospital stay for any one admission was 7.5 days (median 5.5; range 1–26 days). However, no significant association was found between length of stay and any of the clinical or genetic factors (results not shown).
The total cost of care for the evaluable cohort over the 6-months period was £98 480. The management of warfarin-related adverse events contributed to 53% of this cost (£46 453), with anticoagulation services accounting for the remainder; whereas the mean costs for the 179 patients who did not experience an adverse event was £178 (95% CI: 164, 192), those who experienced an adverse event incurred a mean cost of £884 (95% CI: 554, 1837) (Table 5). Patients experiencing thrombotic events incurred a mean cost of care of £1865 (95% CI: 1204, 2527), compared with £579 (95% CI: 396, 889) in those who had complications secondary to overanticoagulation. The multiple generalized linear regression was used to assess the importance of age, sex, adverse events, concomitant interacting medications, comorbidities, smoking status, CYP2C9 genotype and VKORC1 genotype in predicting overall costs (Table 6). The model indicated that the presence of adverse events was a significant predictor of total cost, after adjusting for the baseline clinical and genetic factors. Total costs were estimated to be 4.50 (95% CI: 2.61, 7.77) times higher in patients who had experienced adverse events versus those who had not (Table 6). Other variables were not significant independent predictors of cost.
Table 5.
Six-month total costs for patients initiated on warfarin therapy
| Subgroup | Bootstrap mean cost (£) |
95% confidence intervald |
|---|---|---|
| All patients | 391.66 | 290.19, 742.74 |
| Age | ||
| < 65 years (n =87) | 466.02 | 222.93, 1600.03 |
| ≥65 years (n= 167) | 352.92 | 270.22, 480.31 |
| Sex | ||
| Male (n= 151) | 404.56 | 246.97, 938.73 |
| Female (n =103) | 372.74 | 280.81, 533.75 |
| Smoking status | ||
| Yes (n =211) | 381.29 | 264.66, 735.79 |
| No (n =43) | 442.53 | 260.11, 864.79 |
| CYP2C9*2 (rs1799853) genotypea | ||
| No mutant allele (n =191) | 438.51 | 299.56, 883.34 |
| One mutant allele (n= 52)e | 223.60 | 172.40, 355.00 |
| CYP2C9*3 (rs1057910) genotypea | ||
| No mutant allele (n =214) | 403.25 | 284.53, 806.33 |
| One mutant allele (n= 27) | 296.22 | 171.82, 730.22 |
| Two mutant alleles (n =2) | 545.00 | 545.00,827.00 |
| VKORC1 rs9923231 Genotypeb | ||
| VKORC1 CC (n=99) | 260.14 | 215.59, 355.79 |
| VKORC1 CT (n =123) | 323.57 | 237.90, 483.21 |
| VKORC1 TT (n= 28) | 1185.77 | 370.32, 4039.96 |
| VKORC1 rs7294 genotypec | ||
| VKORC1 GG (n= 93) | 508.60 | 262.03, 1311.55 |
| VKORC1 AG (n= 124) | 337.40 | 256.11, 484.28 |
| VKORC1 AA (n= 36) | 383.67 | 208.11, 511.33 |
| Warfarin-related adverse events | ||
| No (n =177) | 177.63 | 164.33, 191.54 |
| Yes (n =77) | 883.64 | 554.14, 1837.17 |
| Thrombotic (n= 4) | 1865.5 | 1204.00, 2527.00 |
| Overanticoagulation (n= 66) | 579.48 | 395.75, 889.46 |
| Others (n= 7) | 178.20 | 107.40, 237.00 |
| Concomitant medication | ||
| No (n =44) | 260.11 | 193.05, 485.89 |
| Yes (n =210) | 419.22 | 297.24, 799.52 |
| CYP450 enzyme inhibitors (n= 38) | 390.50 | 222.55, 936.50 |
| Synergistic (n= 93) | 534.64 | 294.40, 1588.58 |
| Mixed (n =77) | 296.34 | 210.90, 493.36 |
| Preexisting medical conditions | ||
| No (n =236) | 384.33 | 167.61, 1224.67 |
| Yes (n =18) | 392.21 | 283.48, 738.23 |
n= 243.
n=250.
n=253.
Bias-corrected and accelerated.
Including one patient with two copies of the mutant allele.
Table 6.
Multivariate generalized linear model of predictors of total cost related to warfarin therapy
| Variables | Coefficient (SE) | Relative change in total cost associated with variable (baseline =1) |
|---|---|---|
| Age | 0.0026 (0.0057) | 1.0026 (0.9914, 1.0139) |
| Femalea | 0.0952 (0.1455) | 1.0999 (0.8270, 1.4627) |
| CYP2C9 rs1799853b | − 0.2075 (0.1550) | 0.8126 (0.5998, 1.1009) |
| CYP2C9 rs1057910c | ||
| One mutant allele | − 0.3539 (0.1965) | 0.7020 (0.4776, 1.0318) |
| Two mutant alleles | 0.2418 (0.9024) | 1.2736 (0.2172, 7.4676) |
| VKORC1 rs9923231 genotyped |
||
| VKORC1 C/T | − 0.0017 (0.1599) | 0.9983 (0.7297, 1.3657) |
| VKORC1 T/T | 0.1044 (0.3273) | 1.1100 (0.5844, 2.1083) |
| VKORC1 rs7294 genotypee | ||
| VKORC1 A/G | 0.0625 (0.1543) | 1.0645 (0.7867, 1.4405) |
| VKORC1 A/A | 0.0267 (0.2431) | 1.0271 (0.6377, 1.6542) |
| Warfarin-related adverse eventsf | 1.5042 (0.2789)* | 4.5005 (2.6052, 7.7747) |
| Smokingg | 0.1454 (0.1832) | 1.1566 (0.8076, 1.6562) |
| Concomitant medicationh | ||
| Synergistic | 0.0503 (0.1870) | 1.0516 (0.7289, 1.5172) |
| CYP450 enzyme inhibitor | 0.0806 (0.2163) | 1.0839 (0.7094, 1.6561) |
| Mixed | − 0.1082 (0.1835) | 0.8975 (0.6264, 1.2858) |
| Preexisting medical conditionsi | − 0.1062 (0.3702) | 0.8993 (0.4353, 1.8578) |
| Intercept | 5.0916 (0.5042)* |
Reference category male.
Reference category ‘no mutant allele at rs1799853’.
Reference category ‘no mutant allele at rs1057910’.
Reference category VKORC1 rs9923231 C/C (wild-type genotype).
Reference category VKORC1 rs7294 G/G (wild-type genotype).
Reference category ‘no adverse event’.
Reference category ‘nonsmokers’.
Reference category ‘no concomitant medications’.
Reference category ‘no comorbidity’.
Statistically significant at P value <0.001.
Discussion
The response to warfarin therapy is associated with a number of genetic and environmental determinants [3]. An ideal dosing algorithm should allow the patient to reach therapeutic INR as soon as possible, without overshooting (or being underanticoagulated), with quick and effective prediction of the stable maintenance dose, such that the patient does not have wild swings in their INR. Although some bleeding events occur when a patient is within therapeutic range, there is a significantly higher risk when the INR is elevated [36]. Thus, an effective dosing algorithm would reduce bleeding complications, and would in theory, reduce the frequency of INR monitoring visits. This is going to be a complex and difficult task, and one that should not be underestimated. Even before the advent of pharmacogenetic dosing, a number of dosing strategies have been described [37–40], none of which seem to have been universally accepted.
To assess the difficulty, we have undertaken the most comprehensive assessment to date of different outcomes that can be associated with warfarin anticoagulation to dissect the clinical and genetic factors acting as determinants of response during the initiation as well as maintenance phases of treatment. Our data show the complexity of the clinical factors affecting anticoagulation response, and the variable contribution of polymorphisms in CYP2C9 and VKORC1 (Table 2), in particular, and other candidate genes. The amount of variability explained by individual SNPs and clinical factors (including age and BMI) combined varied between 9 and 54%, depending on outcome. It is of note that the additional variability in stable dose explained by genetic variants was 33%, and this is in line with the prospective study published recently by Limdi et al. [41]. It is important to stress that the aim of our analysis was not to develop a dosing algorithm. The numerous multiple regression models built do not have the same clinical utility as a dosing algorithm and should not be misinterpreted as such. However, these models were essential for identifying the genetic factors that significantly contributed to warfarin response, after adjusting for clinical factors, as well as for assessing the total influence of all significant clinical and genetic factors in combination. The knowledge gained from these models should be used to guide the development of future dosing algorithms (both loading and maintenance, as appropriate), which will subsequently need to be validated independently and tested within a randomized controlled trial setting.
We have also measured the clotting factor activity levels in the vitamin-K-dependent pathway to assess their contribution to anticoagulation response to warfarin. Our aim here was not to suggest measurement of coagulation factors in practice, but more to provide mechanistic insight. Our data show that overall contribution of clotting factor activity levels was relatively small (2–9% depending on outcome, results not shown).
In terms of the genetic determinants of response, we have analyzed 29 genes in the warfarin response pathway. Our data show that CYP2C9 and VKORC1 are the most important genetic factors, consistent with previous studies. However, it seems that CYP2C9 is more important in determining the outcomes that reflect initial anticoagulation control (as shown by the outcome measures INR greater than 4 in the first week, time to stable warfarin dose and time to therapeutic INR), whereas VKORC1 is more important for determining what the eventual stable dose will be (Table 2 and Fig. 1). These findings are consistent with two recent studies [26,42], but are at odds with the findings of Schwarz et al. [25]. This may reflect the different loading dose regimens used between the two populations, with the higher loading doses in our population being reflected in the effect of CYP2C9 variation initially. Furthermore, although Schwarz et al. [25] also investigated time to therapeutic INR, this was defined as time to achieve first INR within therapeutic range, whereas our definition was more stringent and required two consecutive INR measurements.
Of the other genes analyzed, significant associations were found with a further six genes: CYP2C18, CYP2C19, CYP3A5, PROS1, factor IX and factor V. The associations with CYP2C18 and CYP2C19 may have been because of LD with CYP2C9, but our analysis suggests that variants within these genes seemed to have an independent effect on warfarin response, over and above that imposed by CYP2C9. The associations with the other genes are unlikely to have any clinical relevance in relation to stable maintenance dose, consistent with the recent studies in a Swedish cohort [26,43]. However, it is important to note that many of the associations were with other clinically relevant outcome measures in our study rather than stable dose; whether these parameters and their clinical and genetic determinants can be used in any clinically significant manner, for example in loading dose algorithms, is unclear at present.
The most important adverse clinical outcome associated with warfarin therapy is haemorrhage. Clearly, stability of anticoagulation is an important surrogate in preventing haemorrhage [36], but the relationship with INR is not perfect, with some bleeds occurring at therapeutic INRs. In our patients, the rate of major haemorrhage was approximately 15.8/100 patient years consistent with previous studies [44]. An association between major bleeding and CYP2C9 variants was present, but did not retain significance after correction for multiple testing. This is likely to have been because of lack of power as the total number of major bleeding events was small (n=16). An association with major haemorrhage was also seen with the factor V gene; although this association is biologically plausible, the effect size was small. Bleeding with warfarin has also previously been related to factor IX polymorphisms [45,46]; although there was no association with bleeding in our patients, there was a significant association with time to therapeutic INR (Table 3).
The majority of studies to date have concentrated on stable dose as an outcome measure [47]. However, in our study, a third of patients did not achieve stable dose during their 26-week follow-up period, supporting our suggestion that retrospective studies recruiting only patients who have already achieved stable dose exclude a substantial patient subgroup. Furthermore, it can be argued that stable dose can be achieved through regular INR monitoring. As our data show, the majority of patients who have the wild-type genotypes, even with the current dosing strategies, are more readily stabilized (Fig. 1), whereas instability is particularly seen in those with variant genotypes who tend to be the most sensitive in terms of dose requirements. Another group to consider is the patients who are resistant to warfarin, who represented 4% of our cohort, where we cannot currently predict stable dose requirements [48]. There is a great deal of effort ongoing internationally to develop dosing algorithms. This has been led by the International Warfarin Pharmacogenetics Consortium which recently showed that the greatest benefit of a maintenance dose algorithm is seen in those patients on less than 3 mg/day or more than 7 mg/day of warfarin [49]. However, to date, no loading dose algorithm has been developed although we are currently exploring this possibility, making use of the variability in loading doses observed within our cohort. The acceptability of any dosing algorithms will ultimately be dependent on demonstration of their clinical validity and utility through randomized studies. Indeed the lack of randomized data has led the American College of Chest Physicians to advise against the use of pharmacogenetic-based warfarin dosing [22]. There have been two randomized trials, one of which only took account of CYP2C9 variants [50], while another trial [51] was relatively small. There are two larger trials of pharmacogenetic-based warfarin dosing planned, one in the US and the other in the European Union which will hopefully provide some answers.
Another issue that will influence the implementation of pharmacogenetic-based dosing is treatment cost [23]. We present some of the most detailed data on costs associated with the use of warfarin. Our analysis indicates that the bootstrapped mean 6-month cost of healthcare that is directly attributable to warfarin therapy is £392, with the majority (53%) resulting from the management of adverse events. This is comparable with previous estimates from cost and economic analyses related to warfarin [52,53]. Our analysis showed that patients who had experienced an adverse event and who were carriers of VKORC1 TT (rs9923231) were eight times more likely to be hospitalized, but this was not accompanied by an independent effect on total cost. However, this analysis should be regarded as exploratory in nature, and needs to be replicated by other studies. Published economic analyses of pharmacogenetic testing relating to warfarin therapy have assumed, rather than directly measured, the level of healthcare resource use related to genotype in order to calculate costs [23]. This gross-costing (top–down) approach is inferior to the microcosting (bottom-up) approach adopted in this study. A microcosting analysis, which requires that each component of resource use is measured and a unit cost derived for each, results in more precise estimates of healthcare costs [54]. By adopting a 6-month analytic horizon, our analysis may underestimate the true cost of adverse events, as costs related to the rehabilitation and management of disability related to thrombotic events were not valued. The analysis also excluded direct nonmedical costs, such as those borne by patients, which have been reported as €12.20 (2003 figures) per patient, per anticoagulation clinic visit [55]. Despite the advantages of the microcosting exercise, it is nevertheless a limitation when compared with cost-effectiveness analysis, which we are currently developing using the comprehensive dataset presented in this study.
In conclusion, our prospective study highlights the complexity of warfarin pharmacogenetics by showing that different genetic and environmental factors influence different outcomes. Genetic variation in CYP2C9 is more influential during the initial treatment period, whereas VKORC1 is more important in determining stable dose. This complexity needs to be factored in to the future development of pharmacogenetic-based dosing algorithms both for maintenance and initiation, particularly for patients at the extremes of the warfarin daily dose requirements. Although recent genome-wide association studies have shown large effects with only CYP2C9 and VKORC1, they have concentrated on stable maintenance dose [43,56]. We are currently undertaking a genome-wide association study in a larger patient population that will be analysed not only in relation to stable dose, but also with reference to the other clinical outcome measures which have been described in this study.
Supplementary Material
Acknowledgements
The authors acknowledge the UK Department of Health for funding the study. The support of the Wellcome Trust is also acknowledged. The authors thank all the clinicians, nurses and pharmacists who helped us in recruiting patients, and the patients for taking part in the study, and the Roald Dahl Anticoagulant clinic in the Royal Liverpool Hospital. The authors also acknowledge the contribution of Dr Vanessa Martlew (Royal Liverpool Hospital) and Dr Dasgupta (University Hospital Aintree) for patient recruitment. The authors thank Susanna Dodd and Fabio Miyajima, University of Liverpool, for their assistance with the analysis of the cost data.
The conception and design of the study was undertaken by M.P., D.A.H., A.C., F.K., A.D., D.F., P.R.W., B.K.P. and P.D. A.L.J. was responsible for monitoring of data (also done by P.R.W.), statistical analyses of associations with response to warfarin, and wrote the first draft of the manuscript. J.E.Z. undertook all laboratory analyses of clotting factors and preparation of laboratory samples, while A.K. undertook genotyping, supervised by P.D. A.H., D.E., K.H. and L.S. recruited the patients, designed the C.R.F. and validated the patient data, while C.H.T. facilitated patient identification and quality assurance of the coagulation based laboratory tests. A.C. supervised the DNA extraction. S.A.Z. and D.A.H. undertook the cost analysis. M.P. is the principal investigator, conceived the design of the study, coordinated the study, was involved in data monitoring and assessment of patients, and co-wrote first draft. All authors were involved in reviewing the manuscript and approved the final version. M.P. acts as guarantor for the study.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pharmacogeneticsandgenomics.com)
References
- 1.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD006186.pub2. CD006186. [DOI] [PubMed] [Google Scholar]
- 2.Fanikos J, Grasso-Correnti N, Shah R, Kucher N, Goldhaber SZ. Major bleeding complications in a specialized anticoagulation service. Am J Cardiol. 2005;96:595–598. doi: 10.1016/j.amjcard.2005.03.104. [DOI] [PubMed] [Google Scholar]
- 3.Wadelius M, Pirmohamed M. Pharmacogenetics of warfarin: current status and future challenges. Pharmacogenomics J. 2007;7:99–111. doi: 10.1038/sj.tpj.6500417. [DOI] [PubMed] [Google Scholar]
- 4.D’Andrea G, D’Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–649. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 5.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Shennan M, Reynolds KK, Johnson NA, Herrnberger MR, Valdes R, Jr, Linder MW. Estimation of warfarin maintenance dose based on VKORC1 (–1639 G > A) and CYP2C9 genotypes. Clin Chem. 2007;53:1199–1205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 7.Millican EA, Lenzini PA, Milligan PE, Grosso L, Eby C, Deych E, et al. Genetic-based dosing in orthopedic patients beginning warfarin therapy. Blood. 2007;110:1511–1515. doi: 10.1182/blood-2007-01-069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gage B, Eby C, Johnson J, Deych E, Rieder M, Ridker P, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther. 2008;84:326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet. 2005;14:1745–1751. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 10.Vecsler M, Loebstein R, Almog S, Kurnik D, Goldman B, Halkin H, Gak E. Combined genetic profiles of components and regulators of the vitamin K-dependent gamma-carboxylation system affect individual sensitivity to warfarin. Thromb Haemost. 2006;95:205–211. doi: 10.1160/TH05-06-0446. [DOI] [PubMed] [Google Scholar]
- 11.Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, Wood P, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 12.Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 13.Loebstein R, Yonath H, Peleg D, Almog S, Rotenberg M, Lubetsky A, et al. Interindividual variability in sensitivity to warfarin–Nature or nurture? Clin Pharmacol Ther. 2001;70:159–164. doi: 10.1067/mcp.2001.117444. [DOI] [PubMed] [Google Scholar]
- 14.Lee SC, Ng SS, Oldenburg J, Chong PY, Rost S, Guo JY, et al. Interethnic variability of warfarin maintenance requirement is explained by VKORC1 genotype in an Asian population. Clin Pharmacol Ther. 2006;79:197–205. doi: 10.1016/j.clpt.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Wadelius M, Sorlin K, Wallerman O, Karlsson J, Yue QY, Magnusson PK, et al. Warfarin sensitivity related to CYP2C9, CYP3A5, ABCB1 (MDR1) and other factors. Pharmacogenomics J. 2004;4:40–48. doi: 10.1038/sj.tpj.6500220. [DOI] [PubMed] [Google Scholar]
- 16.Wu AHB, Wang P, Smith A, Haller C, Drake K, Linder M, Valdes R. Dosing algorithm for warfarin using CYP2C9 and VKORC1 genotyping from a multi-ethnic population: comparison with other equations. Pharmacogenomics. 2008;9:169–178. doi: 10.2217/14622416.9.2.169. [DOI] [PubMed] [Google Scholar]
- 17.Herman D, Peternel P, Stegnar M, Breskvar K, Dolzan V. The influence of sequence variations in factor VII, gamma-glutamyl carboxylase and vitamin K epoxide reductase complex genes on warfarin dose requirement. Thromb Haemost. 2006;95:782–787. [PubMed] [Google Scholar]
- 18.Caldwell MD, Berg RL, Zhang KQ, Glurich I, Schmelzer JR, Yale SH, et al. Evaluation of genetic factors for warfarin dose prediction. Clin Med Res. 2007;5:8–16. doi: 10.3121/cmr.2007.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuehn BM. Warfarin label update. JAMA. 2007;298:1389. [Google Scholar]
- 20.Bussey HI, Wittkowsky AK, Hylek EM, Walker MB. Genetic testing for warfarin dosing? Not yet ready for prime time. Pharmacotherapy. 2008;28:141–143. doi: 10.1592/phco.28.2.141. [DOI] [PubMed] [Google Scholar]
- 21.Garcia DA. Warfarin and pharmacogenomic testing: the case for restraint. Clin Pharmacol Ther. 2008;84:303–305. doi: 10.1038/clpt.2008.131. [DOI] [PubMed] [Google Scholar]
- 22.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 23.Hughes DA, Pirmohamed M. Warfarin pharmacogenetics: economic considerations. Pharmacoeconomics. 2007;25:899–902. doi: 10.2165/00019053-200725110-00001. [DOI] [PubMed] [Google Scholar]
- 24.Limdi NA, McGwin G, Goldstein JA, Beasley TM, Arnett DK, Adler BK, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther. 2008;83:312–321. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, et al. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadelius M, Chen LY, Lindh JD, Eriksson N, Ghori MJ, Bumpstead S, et al. The largest prospective warfarin-treated cohort supports genetic forecasting. Blood. 2008;113:784–792. doi: 10.1182/blood-2008-04-149070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fihn SD, Callahan CM, Martin DC, McDonell MB, Henikoff JG, White RH. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics. Ann Intern Med. 1996;124:970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5:262–270. doi: 10.1038/sj.tpj.6500313. [DOI] [PubMed] [Google Scholar]
- 29.Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.British National Formulary. (Edition 54) 2007 Sep; www.bnf.org.
- 31.Carlquist JF, Horne BD, Muhlestein JB, Lappe DL, Whiting BM, Kolek MJ, et al. Genotypes of the cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. J Thromb Thrombol. 2006;22:191–197. doi: 10.1007/s11239-006-9030-7. [DOI] [PubMed] [Google Scholar]
- 32.Aquilante CL, Langaee TY, Lopez LM, Yarandi HN, Tromberg JS, Mohuczy D, et al. Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin Pharmacol Ther. 2006;79:291–302. doi: 10.1016/j.clpt.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodological) 1995;57:289–300. [Google Scholar]
- 34.Curtis L, Netten A. Unit costs of health and social care 2006. Personal Social Services Research Unit, University of Kent; Canterbury: 2006. [Google Scholar]
- 35.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 36.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 37.Fennerty A, Dolben J, Thomas P, Backhouse G, Bentley DP, Campbell IA, Routledge PA. Flexible induction dose regimen for warfarin and prediction of maintenance dose. BMJ (Clinical Research Ed) 1984;288:1268–1270. doi: 10.1136/bmj.288.6426.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fergusson RJ, Eade OE, Logie AW, Gaddie J. A flexible loading dose schedule for warfarin therapy. Scott Med J. 1987;32:169–171. doi: 10.1177/003693308703200604. [DOI] [PubMed] [Google Scholar]
- 39.Cosh DG, Moritz CK, Ashman KJ, Dally RJ, Gallus AS. Prospective evaluation of a flexible protocol for starting treatment with warfarin and predicting its maintenance dose. Aust N Z J Med. 1989;19:191–197. doi: 10.1111/j.1445-5994.1989.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 40.Sawyer WT, Poe TE, Canaday BR, Weiner JS, Williams DM, Webb CE, Ellison MJ. Multicenter evaluation of six methods for predicting warfarin maintenance-dose requirements from initial response. Clin Pharmacy. 1985;4:440–446. [PubMed] [Google Scholar]
- 41.Limdi NA, Beasley TM, Crowley MR, Goldstein JA, Rieder MJ, Flockhart DA, et al. VKORC1 polymorphisms, haplotypes and haplotype groups on warfarin dose among African-Americans and European-Americans. Pharmacogenomics. 2008;9:1445–1458. doi: 10.2217/14622416.9.10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meckley LM, Wittkowsky AK, Rieder MJ, Rettie AE, Veenstra DL. An analysis of the relative effects of VKORC1 and CYP2C9 variants on anticoagulation related outcomes in warfarin-treated patients. Thromb Haemost. 2008;100:229–239. [PubMed] [Google Scholar]
- 43.Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5:e1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 45.Kristensen SR. Warfarin treatment of a patient with coagulation factor IX propeptide mutation causing warfarin hypersensitivity. Blood. 2002;100:2676–2677. doi: 10.1182/blood-2002-06-1753. [DOI] [PubMed] [Google Scholar]
- 46.Van der Heijden JF, Rekke B, Hutten BA, van der Meer FJ, Remkes MG, Vermeulen M, et al. Non-fatal major bleeding during treatment with vitamin K antagonists: influence of soluble thrombomodulin and mutations in the propeptide of coagulation factor IX. J Thromb Haemost. 2004;2:1104–1109. doi: 10.1111/j.1538-7836.2004.00768.x. [DOI] [PubMed] [Google Scholar]
- 47.Au N, Rettie AE. Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab Rev. 2008;40:355–375. doi: 10.1080/03602530801952187. [DOI] [PubMed] [Google Scholar]
- 48.Gak E, Halkin H. Shifting paradigms in the pharmacogenetics of warfarin. Pharmacogenomics. 2008;9:1373–1375. doi: 10.2217/14622416.9.10.1373. [DOI] [PubMed] [Google Scholar]
- 49.Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caraco Y, Blotnick S, Muszkat M. CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clin Pharmacol Ther. 2008;83:460–470. doi: 10.1038/sj.clpt.6100316. [DOI] [PubMed] [Google Scholar]
- 51.Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 52.Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, Song F. Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anticoagulation therapy: a systematic review and economic modelling. Health Technol Assess. 2007;11:iii–iv. ix–66. doi: 10.3310/hta11380. [DOI] [PubMed] [Google Scholar]
- 53.Hallinen T, Martikainen JA, Soini EJ, Suominen L, Aronkyto T. Direct costs of warfarin treatment among patients with atrial fibrillation in a Finnish health care setting. Curr Med Res Opin. 2006;22:683–692. doi: 10.1185/030079906X100014. [DOI] [PubMed] [Google Scholar]
- 54.Raftery J. Costing in economic evaluation. BMJ. 2000;320:1597. doi: 10.1136/bmj.320.7249.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jowett S, Bryan S, Mahe I, Brieger D, Carlsson J, Kartman B, Nevinson M. A multinational investigation of time and traveling costs in attending anticoagulation clinics. Value Health. 2008;11:207–212. doi: 10.1111/j.1524-4733.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 56.Cooper GM, Johnson JA, Langaee TY, Feng H, Stanaway IB, Schwarz UI, et al. A genome-wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112:1022–1027. doi: 10.1182/blood-2008-01-134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.