Abstract
Cytidine deaminase, an enzyme that catalyses the deamination of both cytidine and its nucleoside analogues including the antineoplastic agents cytosine arabinoside (ara-C) and 5-azacytidine (5-azaC), has been partially purified from normal and leukemic human granulocytes. The purification procedure included heat precipitation at 70°C, ammonium sulfate precipitation, calcium phosphate gel ion exchange, and Sephadex G-150 gel filtration. The enzyme has mol wt 51,000, isoelectric pH of 4.8, and maximum activity over a broad pH range of 5-9.5. The enzyme is stabilized by the presence of the sulfhydryl reagent, dithiothreitol.
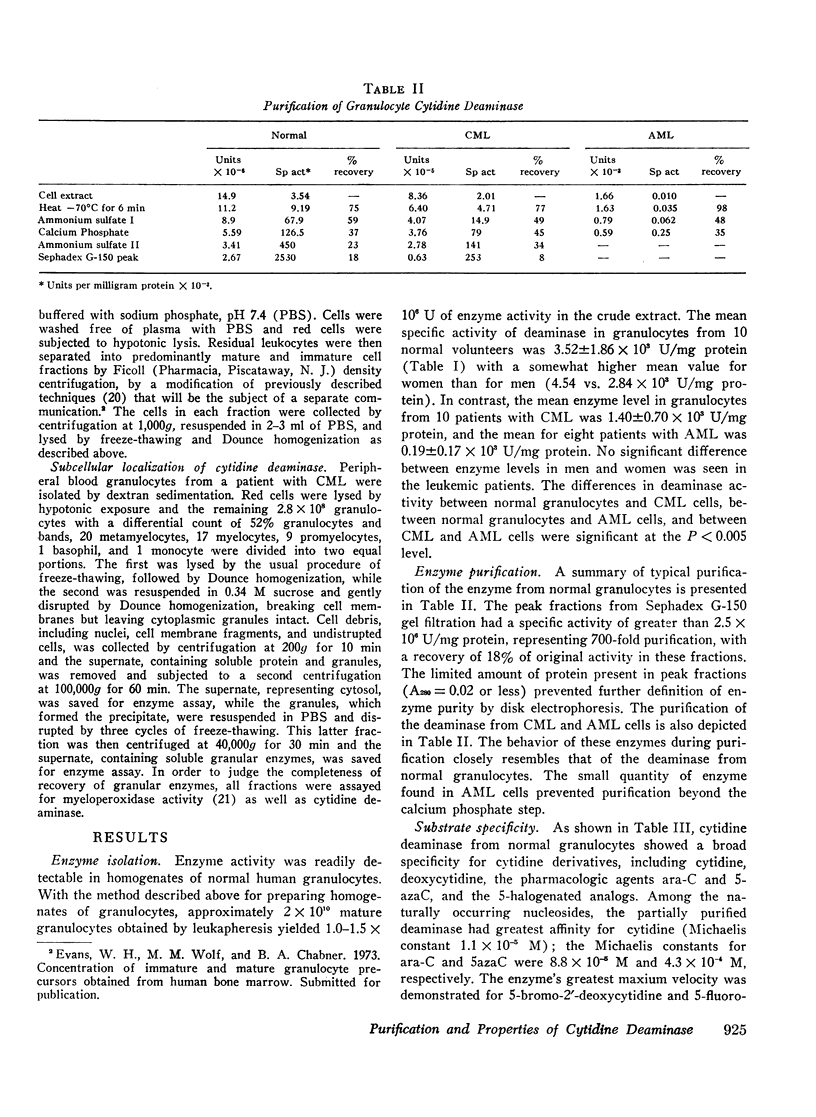
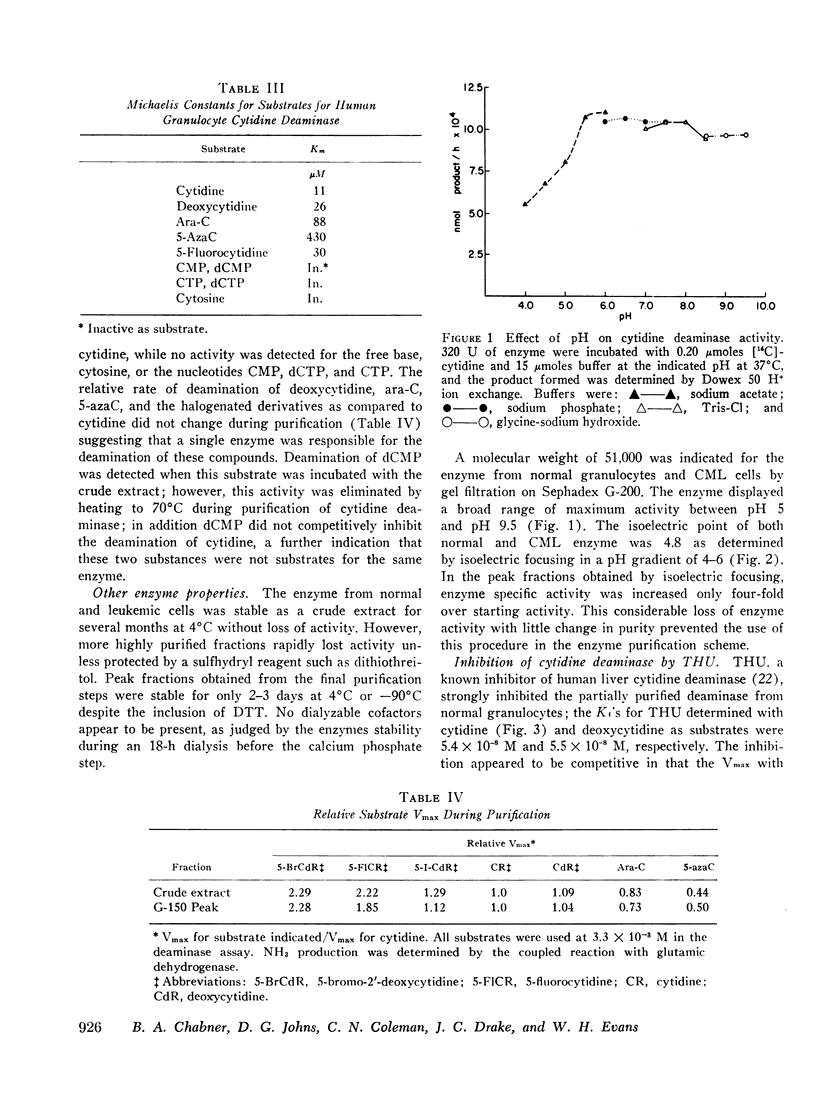
Cytidine deaminase from normal human granulocytes has a greater affinity for its physiologic substrate cytidine (Km = 1.1 × 10−5 M) than for ara-C (8.8 × 10−5 M) or 5-azaC (4.3 × 10−4 M). Halogenated analogues such as 5-fluorocytidine and 5-bromo-2′-deoxycytidine also exhibited substrate activity, with maximum velocities greater than that of the physiologic substrates cytidine and deoxycytidine. No activity was observed with nucleotides or deoxynucleotides. The relative maximum velocity of the enzyme for cytidine and its nucleoside analogues remained constant during purification, indicating that a single enzyme was responsible for deamination of these substrates.
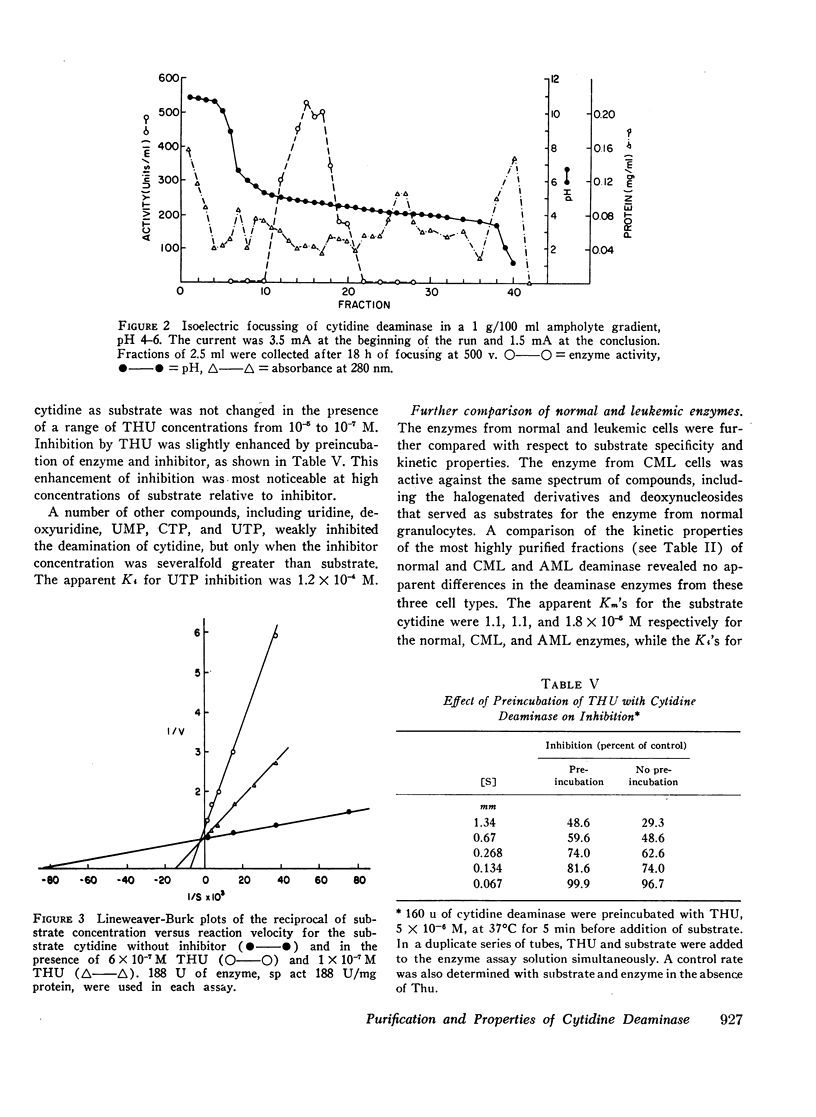
Tetrahydrouridine (THU) was found to be a strong competitive inhibitor of partially purified deaminase with a Ki of 5.4 × 10−8 M.
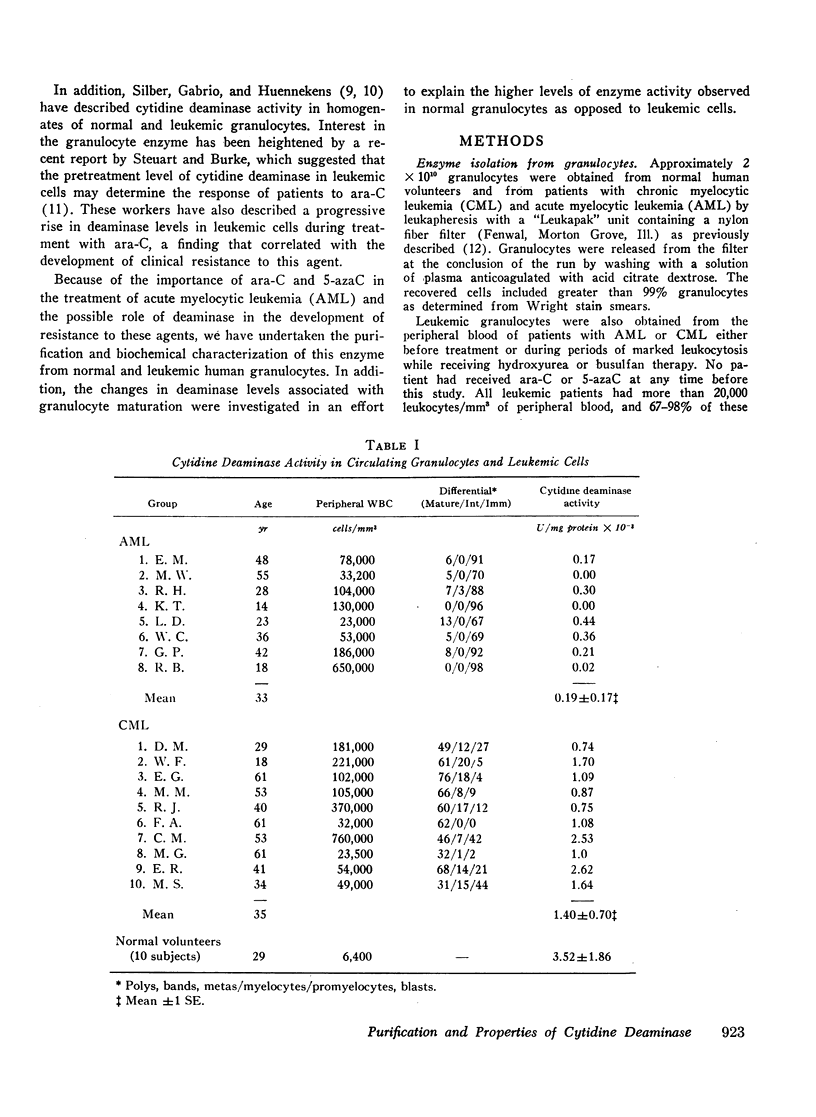
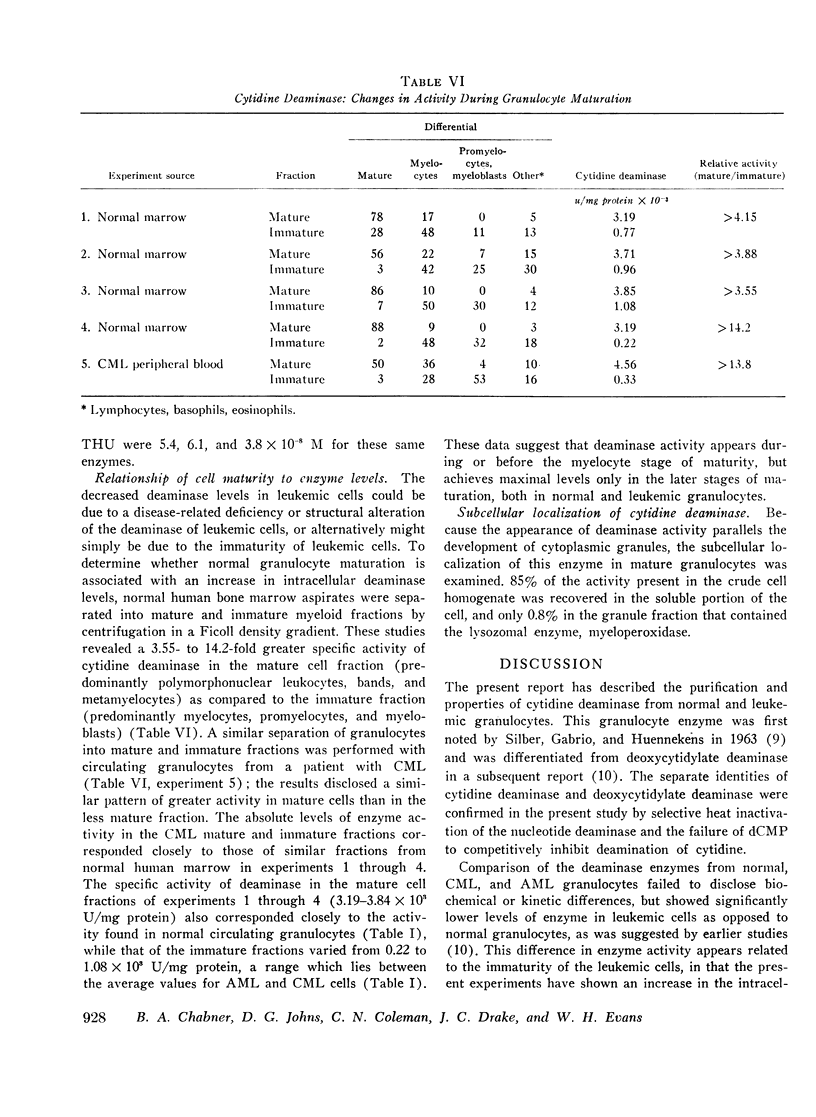
The biochemical properties of partially purified preparations of cytidine deaminase from normal and leukemic cells were compared with respect to isoelectric pH, molecular weight, and substrate and inhibitor kinetic parameters, and no differences were observed. However, normal circulating granulocytes contained a significantly greater concentration of cytidine deaminase (3.52±1.86 × 103/mg protein) than chronic myelocytic leukemia (CML) cells (1.40±0.70 × 103 U/mg protein) or acute myelocytic leukemia (AML) cells (0.19±0.17 × 103 U/mg protein). To explain these differences in enzyme levels in leukemic versus normal cells, the changes in cytidine deaminase levels associated with maturation of normal granulocytes were studied in normal human bone marrow. Myeloid precursors obtained from bone marrow aspirates were separated into mature and immature fractions by Ficoll density centrifugation. Deaminase activity in lysates of mature granulocytes was 3.55-14.2 times greater than the activity found in the lysates of immature cells. Decreased enzyme activity was also found in immature myeloid cells from a patient with CML as compared to mature granulocytes from the same patient. These observations support the conclusion that the greater specific activity of cytidine deaminase in normal mature granulocytes as compared to leukemic cells is related to the process of granulocyte maturation rather than a specific enzymatic defect in leukemic cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTINO J. R., PERKINS J. P., JOHNS D. G. PURIFICATION AND PROPERTIES OF DIHYDROFOLATE REDUCTASE FROM EHRLICH ASCITES CARCINOMA CELLS. Biochemistry. 1965 May;4:839–846. doi: 10.1021/bi00881a007. [DOI] [PubMed] [Google Scholar]
- BERTINO J. R., SILBER R., FREEMAN M., ALENTY A., ALBRECHT M., GABRIO B. W., HUENNEKENS F. M. STUDIES ON NORMAL AND LEUKEMIC LEUKOCYTES. IV. TETRAHYDROFOLATE-DEPENDENT ENZYME SYSTEMS AND DIHYDROFOLIC REDUCTASE. J Clin Invest. 1963 Dec;42:1899–1907. doi: 10.1172/JCI104875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU M. Y., FISCHER G. A. COMPARATIVE STUDIES OF LEUKEMIC CELLS SENSITIVE AND RESISTANT TO CYTOSINE ARABINOSIDE. Biochem Pharmacol. 1965 Mar;14:333–341. doi: 10.1016/0006-2952(65)90198-x. [DOI] [PubMed] [Google Scholar]
- CREASEY W. A. Studies on the metabolism of 5-iodo-2'-deoxycytidine in vitro. Purification of nucleoside deaminase from mouse kidney. J Biol Chem. 1963 May;238:1772–1776. [PubMed] [Google Scholar]
- Camiener G. W., Smith C. G. Studies of the enzymatic deamination of cytosine arabinoside. I. Enzyme distribution and species specificity. Biochem Pharmacol. 1965 Oct;14(10):1405–1416. doi: 10.1016/0006-2952(65)90175-9. [DOI] [PubMed] [Google Scholar]
- Camiener G. W. Studies of the enzymatic deamination of ara-cytidine. V. inhibition in vitro and in vivo by tetrahydrouridine and other reduced pyrimidine nucleosides. Biochem Pharmacol. 1968 Sep;17(9):1981–1991. doi: 10.1016/0006-2952(68)90114-7. [DOI] [PubMed] [Google Scholar]
- Camiener G. W. Studies of the enzymatic deamination of cytosine arabinoside. 3. Substrate requirements and inhibitors of the deaminase of human liver. Biochem Pharmacol. 1967 Sep 9;16(9):1691–1702. doi: 10.1016/0006-2952(67)90244-4. [DOI] [PubMed] [Google Scholar]
- Camiener G. W. Studies of the enzymatic deamination of cytosine arabinoside. II. Properties of the deaminase of human liver. Biochem Pharmacol. 1967 Sep 9;16(9):1681–1689. doi: 10.1016/0006-2952(67)90243-2. [DOI] [PubMed] [Google Scholar]
- Camiener G. W., Tao R. V. Studies of the enzymatic deamination of ara-cytidine. IV. Inhibition by an acridine analogue and organic solvents. Biochem Pharmacol. 1968 Jul;17(7):1411–1423. doi: 10.1016/0006-2952(68)90077-4. [DOI] [PubMed] [Google Scholar]
- Chabner B. A., Drake J. C., Johns D. G. Deamination of 5-azacytidine by a human leukemia cell cytidine deaminase. Biochem Pharmacol. 1973 Nov 1;22(21):2763–2765. doi: 10.1016/0006-2952(73)90137-8. [DOI] [PubMed] [Google Scholar]
- Creasey W. A., Papac R. J., Markiw M. E., Calabresi P., Welch A. D. Biochemical and pharmacological studies with 1-beta-D-arabinofuranosylcytosine in man. Biochem Pharmacol. 1966 Oct;15(10):1417–1428. doi: 10.1016/0006-2952(66)90186-9. [DOI] [PubMed] [Google Scholar]
- Evans B. E., Wolfenden R. V. Catalysis of the covalent hydration of pteridine by adenosine aminohydrolase. Biochemistry. 1973 Jan 30;12(3):392–398. doi: 10.1021/bi00727a005. [DOI] [PubMed] [Google Scholar]
- Evans B., Wolfenden R. [A potential transition state analog for adenosine deaminase]. J Am Chem Soc. 1970 Jul 29;92(15):4751–4752. doi: 10.1021/ja00718a056. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Wilson S., Mage M. G., Grieshaber C. K., Himmelhoch S. R. Biochemical characterization of isolated immature granulocytes from guinea pig bone marrow. J Reticuloendothel Soc. 1971 Mar;9(3):209–224. [PubMed] [Google Scholar]
- Gallo R. C., Perry S. The enzymatic mechanisms for deoxythymidine synthesis in human leukocytes. IV. Comparisons between normal and leukemic leukocytes. J Clin Invest. 1969 Jan;48(1):105–116. doi: 10.1172/JCI105958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J Chromatogr. 1967 May;28(1):69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- HEIDELBERGER C., GRIESBACH L., CRUZ O., SCHNITZER R. J., GRUNBERG E. Fluorinated pyrimidines. VI. Effects of 5-fluorouridine and 5-fluoro-2'-deoxyuridine on transplanted tumors. Proc Soc Exp Biol Med. 1958 Feb;97(2):470–475. doi: 10.3181/00379727-97-23777. [DOI] [PubMed] [Google Scholar]
- Hart J. S., Ho D. H., George S. L., Salem P., Gottlieb J. A., Frei E., 3rd Cytokinetic and molecular pharmacology studies of arabinosylcytosine in metastatic melanoma. Cancer Res. 1972 Dec;32(12):2711–2716. [PubMed] [Google Scholar]
- Herzig G. P., Root R. K., Graw R. G., Jr Granulocyte collection by continuous-flow filtration leukapheresis. Blood. 1972 Apr;39(4):554–567. [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Ho D. H., Frei E., 3rd Clinical pharmacology of 1-beta-d-arabinofuranosyl cytosine. Clin Pharmacol Ther. 1971 Nov-Dec;12(6):944–954. doi: 10.1002/cpt1971126944. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALEY F., MALEY G. F. Nucleotide interconversions. II. Elevation of deoxycytidylate deaminase and thymidylate synthetase in regenerating rat liver. J Biol Chem. 1960 Oct;235:2968–2970. [PubMed] [Google Scholar]
- Malathi V. G., Silber R. Effects of murine viral leukemia on spleen nucleoside deaminase: purification and properties of the enzyme from leukemic spleen. Biochim Biophys Acta. 1971 May 27;238(3):377–387. doi: 10.1016/0005-2787(71)90612-5. [DOI] [PubMed] [Google Scholar]
- McCullough J. L., Chabner B. A., Bertino J. R. Purification and properties of carboxypeptidase G 1 . J Biol Chem. 1971 Dec 10;246(23):7207–7213. [PubMed] [Google Scholar]
- Meyers R., Malathi V. G., Cox R. P., Silber R. Studies on nucleoside deaminase. Increase in activity in HeLa cell cultures caused by cytosine arabinoside. J Biol Chem. 1973 Sep 10;248(17):5909–5913. [PubMed] [Google Scholar]
- Rabinowitz Y. DNA polymerase and carbohydrate metabolizing enzyme content of normal and leukemic glass column separated leukocytes. Blood. 1966 Apr;27(4):470–481. [PubMed] [Google Scholar]
- SILBER R., GABRIO B. W., HUENNEKENS F. M. STUDIES ON NORMAL AND LEUKEMIC LEUKOCYTES. VI. THYMIDYLATE SYNTHETASE AND DEOXYCYTIDYLATE DEAMINASE. J Clin Invest. 1963 Dec;42:1913–1921. doi: 10.1172/JCI104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. H., Jr, BAKER F. A., SULLIVAN M. Pyrimidine metabolism in man. II. Studies of leukemic cells. Blood. 1960 Mar;15:360–369. [PubMed] [Google Scholar]
- Scholar E. M., Calabresi P. Identification of the enzymatic pathways of nucleotide metabolism in human lymphocytes and leukemia cells. Cancer Res. 1973 Jan;33(1):94–103. [PubMed] [Google Scholar]
- Silber R. Regulatory mechanisms in the human leukocyte. I. The feedback control of deoxycytidylate deaminase. Blood. 1967 Jun;29(6):896–905. [PubMed] [Google Scholar]
- Steuart C. D., Burke P. J. Cytidine deaminase and the development of resistance to arabinosyl cytosine. Nat New Biol. 1971 Sep 22;233(38):109–110. doi: 10.1038/newbio233109a0. [DOI] [PubMed] [Google Scholar]
- Tomchick R., Saslaw L. D., Waravdekar V. S. Mouse kidney cytidine deaminase. Purification and properties. J Biol Chem. 1968 May 25;243(10):2534–2537. [PubMed] [Google Scholar]
- Uchida K., Kreis W. Studies on drug resistance. I. Distribution of l-beta-D-arabinofuranosylcytosine, cytidine and deoxycytidine in mice bearing ara-C-sensitive and-resistant P815 neoplasms. Biochem Pharmacol. 1969 May;18(5):1115–1128. doi: 10.1016/0006-2952(69)90115-4. [DOI] [PubMed] [Google Scholar]
- Wisdom G. B., Orsi B. A. The purification and properties of cytidine aminohydrolase from sheep liver. Eur J Biochem. 1969 Jan;7(2):223–230. doi: 10.1111/j.1432-1033.1969.tb19595.x. [DOI] [PubMed] [Google Scholar]
- Wolfenden R. Transition state analogues for enzyme catalysis. Nature. 1969 Aug 16;223(5207):704–705. doi: 10.1038/223704a0. [DOI] [PubMed] [Google Scholar]



