Abstract
Alcohol use disorders (AUDs), including alcohol abuse and dependence, have been linked to the development of acute lung injury (ALI). Prior clinical investigations suggested an association between AUDs and abnormal alveolar epithelial permeability mediated through pulmonary oxidative stress that may partially explain this relationship. We sought to determine if correcting pulmonary oxidative stress in the setting of AUDs would normalize alveolar epithelial permeability in a double-blinded, randomized, placebo-controlled trial of Protandim, a nutraceutical reported to enhance antioxidant activity. We randomized 30 otherwise healthy AUD subjects to receive directly observed inpatient oral therapy with either Protandim (1,350 mg/day) or placebo. Subjects underwent bronchoalveolar lavage (BAL) and blood sampling before study drug administration and after 7 days of therapy; all AUD subjects completed the study protocol without adverse events. BAL total protein was measured at each timepoint as an indicator of alveolar epithelial permeability. In subjects with AUDs, before study drug initiation, BAL total protein values were not significantly higher than in 11 concurrently enrolled controls (P = 0.07). Over the 7-day study period, AUD subjects did not exhibit a significant change in BAL total protein, regardless of their randomization to Protandim {n = 14, −2% [intraquartile range (IQR), −56–146%]} or to placebo [n = 16, 77% (IQR −20–290%); P = 0.19]. Additionally, among those with AUDs, no significant changes in BAL oxidative stress indexes, epithelial growth factor, fibroblast growth factor, interleukin-1β, or interleukin-10 were observed regardless of drug type received. Plasma thiobarbituric acid reactive substances, a marker of lipid peroxidation, decreased significantly over time among AUD subjects randomized to placebo (P < 0.01). These results suggest that Protandim for 7 days in individuals with AUDs who are newly abstinent does not alter alveolar epithelial permeability. However, our work demonstrates the feasibility of safely conducting clinical trials that include serial bronchoscopies in a vulnerable population at risk for acute lung injury.
Keywords: alveolar epithelial permeability, acute lung injury
alcohol consumption contributes to ∼6.8% of the disease burden in North American countries, a factor equivalent or exceeding the contribution of tobacco, hypertension, and unsafe sex (35). The use of alcohol is particularly evident in hospitalized intensive care unit (ICU) patients, where the prevalence of alcohol dependence is ∼10% (31), while the number of admissions related to unhealthy alcohol use alcohol ranges from 9 to 21% (3, 22).
Alcohol use disorders (AUDs), including alcohol abuse and alcohol dependence, in hospitalized ICU patients are associated with significant morbidity, including an increased risk for requiring mechanical ventilation (10), developing septic shock and nosocomial infections (31), and acquiring acute lung injury (ALI; Refs. 25, 27). ALI is characterized by the development of noncardiogenic pulmonary edema that occurs in the setting of common ICU illnesses, such as severe pulmonary infections, aspiration, and trauma, and is associated with a mortality of ∼30–40% (36). The underlying factors that predispose individuals with AUDs to develop ALI remain elusive and are likely multifactorial. One potential contributor is these individuals' propensity for severe pulmonary infections, mediated through abnormal pulmonary innate immunity (2, 5, 8) that may culminate in ALI development. Additionally, an association between intrapulmonary as well as systemic oxidative stress in the setting of AUDs has also been reported (7, 26, 47). Most notably, these include abnormalities in homeostasis of glutathione, a critical pulmonary antioxidant. Excessive oxidative stress in the lung has a myriad of effects on cellular function and may contribute to abnormal permeability of the alveolar epithelial membrane, thereby promoting the development of pulmonary edema. The importance of this association has been highlighted in previous investigations, where the diagnosis of an AUD in the absence of overt, symptomatic lung illness was determined to correlate with an increased transit time of an inhaled radioactive isotope within the lung, suggesting abnormal alveolar epithelial permeability (9). While animal models of AUDs have demonstrated that administration of exogenous antioxidants, such as procysteine, can normalize alveolar epithelial permeability in vitro (17), therapies to decrease intrapulmonary oxidative stress as a means of correcting alveolar epithelial permeability have not been specifically explored in humans.
To determine if normalization of intrapulmonary oxidative stress would be associated with normalized alveolar epithelial permeability in subjects with AUDs, we conducted a study with the agent Protandim, a nutraceutical with a lengthy history of use in homeopathic, Ayurvedic, and traditional Chinese medicine. It comprised of five phytochemical components derived from Bacopa monniera, Silybum marianum (milk thistle), Withania somnifera (Ashwagandha), Camellia sinensis (green tea), and Curcuma longa (turmeric). Combining these individual extracts at low, nontoxic doses is performed with the intention of optimizing each of their unique properties while minimizing toxicity. This product achieves synergistic activation of the Nrf2 transcription factor pathway that is integral to several antioxidant enzymes, including γ-glutamyl cysteine synthase, an enzyme that catalyzes the committed step in glutathione synthesis (43). In cell culture, Protandim has demonstrated the ability to potently induce heme oxygenase-1 and to increase cellular glutathione production (44). Clinical investigations have further suggested that this compound can directly induce other endogenous antioxidant enzymes, such as SOD and catalase, while decreasing lipid peroxidation. In healthy individuals, measurable in vivo effects have been demonstrated after 30 days of oral supplementation, including decreased thiobarbituric acid reactive substances (TBARS), reflecting decreased lipid peroxidation, along with increased erythrocyte antioxidant enzyme activity (30). Moreover, the agent was well tolerated by these subjects. The effects of Protandim on intrapulmonary oxidative stress have not been examined; however, one might infer that the correction of systemic oxidative stress among those with AUDs could have beneficial effects on the redox status in other organ systems, including the lung.
We conducted a randomized, double-blinded, placebo-controlled trial to determine the effects of 7 days of directly observed oral Protandim therapy on measures of intrapulmonary oxidative stress among otherwise healthy subjects with a history of AUDs, hypothesizing that administration of Protandim would be associated with normalization of alveolar epithelial permeability as reflected by decreases in bronchoalveolar lavage (BAL) total protein over time. We further postulated that randomization to active study drug would result in quantitatively decreased glutathione and cystine oxidation, lipid peroxidation, nucleic acid oxidation, and proinflammatory cytokines in BAL and in plasma. Finally, we sought to confirm the relationship between AUDs and increased BAL total protein and intrapulmonary oxidative stress and the effect of abstinence on these parameters.
METHODS
Subject Screening, Recruitment, and Enrollment
Subjects with AUDs were recruited between June 2009 and September 2010 at the Denver Comprehensive Addictions Rehabilitation and Evaluation Services (Denver CARES) center, an inpatient detoxification facility affiliated with Denver Health and Hospital System (Denver, CO). Control subjects without AUDs were recruited from the Denver Veterans Affairs Medical Center's smoking cessation clinic and via approved flyers posted on the University of Colorado Denver's medical campus. The institutional review boards at all participating sites approved this study, and all subjects provided written informed consent before their participation in this protocol. This trial was registered at http://clinicaltrials.gov (identification number NCT00936000).
Subjects with AUDs were eligible to participate if they met all of the following criteria at study entry: 1) an Alcohol Use Disorders Identification Test (AUDIT) score of ≥8 for men or ≥5 for women, 2) alcohol use within the 7 days before enrollment, and 3) age of ≥21. The AUDIT questionnaire is a standardized survey to detect current and previous alcohol abuse that has been validated in a variety of clinical settings (33). Further, to meet eligibility as a control, control subjects' AUDIT values were required to be <8 for men or <5 for women, and they should not have consumed alcohol within the prior month. Screening of potential controls focused on balancing these individuals with AUD subjects in terms of age and smoking history.
In an effort to minimize the effects of comorbidities on our outcome variables, AUD subjects and controls were ineligible to participate in the study if they met any of the following criteria: 1) prior medical history of liver disease (documented history of cirrhosis, total bilirubin ≥2.0 mg/dl, or albumin <3.0), 2) prior medical history of gastrointestinal bleeding (due to the concern for varices), 3) prior medical history of heart disease (documentation of ejection fraction <50%, myocardial infarction, or severe valvular dysfunction), 4) prior medical history of renal disease (end-stage renal disease requiring dialysis or a serum creatinine ≥2 mg/dl), 5) prior medical history of lung disease defined as an abnormal chest radiograph or spirometry (first second of exhalation and forced vital capacity <75%), 6) concurrent illicit drug use defined as a positive toxicology screen, 7) prior history of diabetes mellitus, 8) prior history of HIV infection, 9) failure of the patient to provide informed consent, 10) pregnancy, and 11) abnormal nutritional status. The nutritional status was assessed using the nutritional risk index (NRI) with the subject's albumin, current weight, and usual weight values in the following equation (1): NRI = 1.519 (albumin in g/l) + (current weight/usual weight) * 100 + 0.417. Patients were considered to have a normal nutritional status if the NRI was ≥90, and an abnormal NRI if it was <90. Potential subjects >55 yr of age were also excluded to minimize the presence of concomitant but asymptomatic comorbidities.
Study Protocol
Eligible subjects with AUDs were admitted to the University of Colorado Hospital Clinical and Translational Research Center (CTRC) for bronchoscopy and study drug administration. All bronchoscopy procedures were performed utilizing telemetry monitoring and standard conscious sedation protocols as previously described (18). The bronchoscope was wedged into a subsegment of either the right middle lobe or the lingula. Three to four 50-ml aliquots of sterile, room temperature 0.9% saline were sequentially instilled and recovered with gentle aspiration. The first aspirated aliquot was not utilized in experiments for this investigation. The second and subsequent aliquots were combined and used in subsequent experiments as representative of the distal airspaces. Subjects with AUDs had their first bronchoscopy performed before the receipt of any study drug and within 24 h of hospital admission; their second bronchscopy was performed within 24 h after completion of the 7-day study drug protocol. Control subjects had a single bronchoscopy performed in a similar fashion. BAL samples were transported to the laboratory in sterile 50-ml conical tubes. Plasma and serum samples were also collected at the time of bronchoscopy.
After initial bronchoscopy, subjects with AUDs were randomized to receive Protandim or placebo in a double-blind fashion, blocked by smoking history. Randomization was performed by the research pharmacist at the University of Colorado Hospital; none of the investigators, the CTRC nursing staff, nor the subject were aware of the drug type the patient received. Subjects received their study medication or identical-appearing placebo twice daily at 10:00 AM and 10:00 PM. Active Protandim was administered at a dose of 675 mg twice daily, producing a dosage near 20 mg·kg−1·day−1. Both Protandim and placebo capsules were provided to the study investigators at no cost by the makers of Protandim (LifeVantage, South Jordan, UT). All subjects received a standard diet (3 meals daily plus morning and afternoon snacks) consisting of ∼30 kcal·kg−1·day−1 comprised of equal percentages of 30% fat, 15% protein, and 55% carbohydrates, prepared in the metabolic kitchen of the CTRC. They also received an oral multivitamin, 100 mg thiamine, and 1 mg folate daily. Additionally, subjects were visited daily by trained study personnel and were monitored for alcohol withdrawal with the Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) protocol (41), receiving intermittent benzodiazepines for alcohol withdrawal symptoms. Laboratory values were assessed after 4 and 7 days of therapy to monitor for adverse events. After 7 days of directly observed study drug treatment (14 total doses of study medication), subjects with AUDs underwent a second BAL using identical methods. AUD subjects were discharged ∼24 h after their second bronchoscopy.
Laboratory Processing
Whole blood was immediately centrifuged (900 g, 5 min) to separate red blood cells from plasma or serum. BAL fluid was immediately centrifuged (900 g, 5 min) after collection to separate cellular and acellular components. Specimens that were not assayed immediately were aliquotted and stored at −80°C. Urea values were measured from both plasma and acellular BAL with an adapted Berthelot method (Pointe Scientific, Canton, MI) that has a sensitivity range of 0.05 to 150 mg/dl. The urea dilution factor of BAL for each subject was calculated as: [urea]plasma/[urea]BALF, where brackets denote concentration and BALF is BAL fluid. Each subject's BAL measurements (described below) were multiplied by his or her unique dilution factor to correct for differences in BAL yield (34).
Protein assays.
Total protein in acellular BAL was measured in duplicate using the Coomassie (Bradford) protein assay kit (Thermo Scientific, Rockford, IL) from 25-ul aliquots for each subject and control.
Thiol/disulfide measurements and calculation of redox potential.
To assess the effect of alcohol consumption on endogenous antioxidants, thiols and disulfides were measured in acellular BAL and plasma samples (47). Briefly, acellular BAL and plasma samples were preserved immediately after collection in a 5% perchloric acid solution containing iodoacetic acid (6.7 μM) and boric acid (0.1 M) and 5 μM of the internal standard γ-glutamyl-glutamate (γ-Glu-Glu). After protein removal, samples were derivatized with dansyl chloride and separated on a 10-μm Ultrasil amino column by HPLC (Waters 2690; Waters, Milford, MA). Fluorescence detection was recorded by two detectors (Waters 474 and Gilson model 121; Gilson, Middleton, WI). The micromolar concentrations of reduced glutathione (GSH), oxidized glutathione (GSSG), cysteine (Cys), and cystine (CySS) were calculated by quantitating the integrated areas relative to that of γ-Glu-Glu. The micromoler values of these thiols were corrected for urea dilution when measured in BAL. Additionally, to assess the relative quantity of oxidized thiol species (disulfides) to total thiol present, the percentage of GSSG present in BAL or plasma relative to total glutathione (e.g., GSH + GSSG) was calculated as [GSSG uM/(GSSG uM + GSH uM)] × 100. The percentage of CySS was calculated similarly.
To quantitate the oxidant/antioxidant balance present in lung and systemically (19), the redox potential (Eh) of each thiol/disulfide pair, namely GSH/GSSG and Cys/CySS, in the plasma and the BALF were calculated with the Nernst equation, Eh = Eo + RT/nF ln [disulfide]/([thiol 1] [thiol 2]). The Eo is the standard potential for the redox couple, R is the gas constant, T is the absolute temperature, n is 2 for the number of electrons transferred, F is Faraday's constant, and RT is room temperature. The standard potential Eo at pH 7.4 for the 2 GSH/GSSG couple was −264 mV, while the standard potential Eo for the 2Cys/CySS couple was −250 mV (16). Thiol and disulfide values from BAL used in the calculation for redox potential were corrected for urea dilution.
8-Hydroxydeoxyguanosine assay.
Acellular BAL was used with the OxiSelect Oxidative DNA damage ELISA kit (Cell Biolabs, San Diego, CA) to quantify 8-hydroxydeoxyguanosine (8-OHdG) as an assessment of nucleic acid oxidation. Where the concentration of 8-OhdG was below detection in unconcentrated (neat) BAL, samples were vacuum concentrated at room temperature from 500 ul starting volume to 250 ul in a SpeedVac (Thermo Scientific, Pittsburg, PA) before the assay.
Cytokine/growth factor assays.
To assess differences in cytokine/chemokine/growth factors within the alveolar space, acellular BAL fluid was also used in assays to measure cytokines/chemokines including epidermal growth factor (EGF) and fibroblast growth factor (FGF). Differences in tumor necrosis factor (TNF)-α and IL-10 were also assessed as pro- and anti-inflammatory chemokines, respectively. All were measured using a custom MILLIPLEX MAP multiplex magnetic bead based immunoassay with Luminex xMAP technology (Millipore, Billerica, MA). All measurements were performed in duplicate.
TBAR and paroxonase assays.
To assess for products of lipid peroxidation in plasma, assays for TBARS and paroxonase (PON)-1 activity were performed. TBARS were quantified using 1,1,3,3-tetramethoxypropane (Sigma, St. Louis, MO) as a standard (32) and are reported in micromolar malondialdehyde equivalents that are formed when highly reactive lipid hydroperoxides degrade. PON-1 (arylesterase) activity was measured in heparinized plasma spectrophotometrically using phenylacetate (Sigma) as substrate (11). The reaction mixture contained 1 mM phenylacetate, 9 mM of Tris·HCl, and 0.9 mM of CaCl2 at pH 8.0. The increase in absorbance at 270 nm was read using a molar extinction coefficient of 1,310 M−1·cm−1. Arylesterase activity is expressed in units per milliliters plasma.
Statistical Analyses
Data was classified as parametrically or nonparametrically distributed based on testing of the variances. Parametrically distributed data were analyzed with a Student's t-test or paired t-test for continuous outcome variables. Nonparametrically distributed data were analyzed with a Kruskal-Wallace or Wilcoxon signed rank test. Categorical data were compared using Fisher's exact test. Univariate correlations between continuous variables were also assessed.
The primary outcome variable for these investigations was the effect of Protandim on the percent change in BAL protein concentrations between the first and second bronchoscopy. We determined the sample size needed to detect significant BAL protein differences based on our previous work examining the change in BAL total protein concentration over 7 days (7). We assumed that subjects with a history of AUDs randomized to placebo would have a mean increase in BAL protein of 50 ug/ml with a SD of 50 μg/ul over the 7-day study period. To perceive a significant decrease in BAL total protein concentration toward normal levels (130 μg/ul) in the group randomized to Protandim, with an α of 0.05 and a power of 80%, using a 2-tailed, t-test analysis, a total of 34 patients, or 17 in each arm, would need to be enrolled in the study. Secondary outcome variables included the quantification of thiols/disulfides in BAL and plasma; calculation of GSSG and CySS redox potentials in BAL and plasma; 8-OHdG in BAL; quantification of selected cytokines/growth factors in BAL; and quantification of TBARS and PON-1 activity in plasma.
RESULTS
We enrolled 30 subjects with AUDs during the study period, slightly below our predicted sample size due to issues with financial limitations related to the study. Demographic features of all subjects are presented in Table 1. Subjects with AUDs scored above the cut point for unhealthy alcohol use and drank a majority of days of the week. Despite this heavy alcohol consumption, total bilirubin values were within the normal range for these individuals, while AST values were on average 1.5 times normal. Spirometric values (forced expiratory volume in the first second of exhalation and forced vital capacity) were within the range considered to be normal (≥80% predicted). None of the 30 subjects was found to be nutritionally compromised by NRI.
Table 1.
Demographic features of subjects with auds and controls
| AUD Subjects (n = 30) | Control Subjects (n = 11) | P Value | |
|---|---|---|---|
| Mean age, yr | 45 ± 6 | 41 ± 5 | 0.06 |
| Men/women, % (number) | 90% (27)/10% (3) | 55% (6)/45% (5) | 0.02 |
| Smokers, % (number) | 67% (20) | 36% (4) | 0.15 |
| Mean AUDIT score | 27 ± 6 | 3 ± 2 | <0.0001 |
| Days/week drinking | 5 ± 1 | <1 | <0.0001 |
| Racial background | 40% (12/30) White | 82% (9/11) White | 0.14 |
| 7% (2/30) African-American | 9% (1/11) African-American | ||
| 23% (7/30) Hispanic | 9% (1/11) Hispanic | ||
| 27% (8/30) Native American | |||
| 3% (1/30) Asian | |||
| FEV1, %predicted | 92.5 ± 8.8 | 97.7 ± 14.0 | 0.16 |
| FVC, %predicted | 95.1 ± 10.6 | 96.0 ± 15.0 | 0.83 |
| Creatinine, mg/dl | 0.82 ± 0.11 | 0.89 ± 0.20 | 0.17 |
| Albumin, g/dl | 4.0 ± 0.4 | 4.0 ± 0.3 | 0.94 |
| Aspartate transaminase, U/l | 63.6 ± 54.7 | 24.8 ± 8.1 | 0.03 |
| Total bilirubin, mg/dl | 0.93 ± 0.45 | 0.83 ± 0.30 | 0.47 |
| Nutritional risk index | 105 ± 4 | 107 ± 3 | 0.17 |
Values are means ± SE. AUDIT, Alcohol Use Disorders (AUD) Identification Test; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity. Normal range for creatinine 0.4–1.2 mg/dl; for albumin 3.4–5.0 g/dl; for aspartate transaminase 0–47 U/l; and for total bilirubin 0–1.3 mg/dl. A normal nutritional risk index was considered to be ≥90.
Fourteen subjects with AUDs were randomized to Protandim, while 16 with AUDs were randomized to placebo. The mean age of the group randomized to Protandim was slightly younger than the placebo group (43 ± 6 vs. 47 ± 4 yr; P < 0.04), and the NRI between the two groups indicated somewhat better nutritional status among the Protandim-randomized group (107 ± 3 vs. 104 ± 4; P < 0.02). There were no significant differences between the groups in terms of gender (P = 0.59), smoking (P = 1.0), racial distribution (P = 0.50), or AUDIT scores (P = 0.50).
All 30 subjects with AUDs completed the study drug protocol as described, and no drug doses were missed. No adverse events were observed among this group related to the study drug administration or the bronchoscopy protocol. All blood chemistry values assessed after 4 and 7 days of study drug administration, including liver function tests and creatinine, either did not change significantly or decreased to a normal range during the study period in these subjects. None of the subjects developed complicated alcohol withdrawal while in the CTRC.
The characteristics and composition of the two BAL samples obtained from AUD subjects are presented in Table 2. The percent yield from the procedure (amount of BAL returned as a percentage of the total amount of saline instilled) was consistent between the two procedures. Moreover, the total cell count did not change significantly over time in AUD subjects (P = 0.68). Interestingly, an increase in the percentage of BAL lymphocytes (P < 0.02) and neutrophils (P < 0.05) was observed between the first and second procedures, with a concomitant decrease in the percentage of monocytes/macrophages present (P < 0.008).
Table 2.
Characteristics of bronchoalveolar lavage fluid from subjects with AUDs and controls
| AUD Subjects, Prestudy Drug (day 1; n = 30) | AUD Subjects, Poststudy Drug (day 8; n = 30) | P Value (Comparison Between AUD Subjects) | Control Subjects (n = 11) | |
|---|---|---|---|---|
| %Fluid instilled that was returned, mean | 43.4 ± 14.4 | 42.3 ± 11.0 | 0.65 | 46.6 ± 7.4 |
| Millions of cells per mm in BAL fluid, median (intraquartile range) | 11.8 (8.6, 17.1) | 12.6 (9.0, 16.7) | 0.68 | 11.0 (7.3, 29.3) |
| %Monocytes/macrophages | 89 ± 5 | 84 ± 9 | 0.008 | 90 ± 8 |
| %Neutrophils | 2 ± 2 | 3 ± 3 | 0.05 | 2 ± 4 |
| %Lymphocytes | 9 ± 5 | 14 ± 9 | 0.02 | 8 ± 5 |
| Total protein, μg/ml | 106 (80, 142) | 109 (83, 187) | 0.16 | 120 (112, 157)* |
| Total protein, μg/ml (corrected for urea dilution) | 299 (135, 1015) | 326 (189, 865) | 0.30 | 153 (108, 225)† |
Values are means ± SE. BAL, bronchoalveolar lavage;
P < 0.05, when compared with day-1 AUD subjects;
P = 0.07, when compared with day-1 AUD subjects.
Administration of Protandim did not Significantly Alter BAL Total Protein
Four subjects with AUDs did not have measurable urea in BAL (2 randomized to placebo and 2 to Protandim) and therefore could not have BAL values corrected for dilution. Accordingly, BAL total protein changes over time both with and without urea dilution are presented (Table 2). With respect to study drug administration, we found that while AUD subjects randomized to placebo tended to display increased BAL total protein (corrected for dilution) over time (median increase of 77%), the BAL total protein in subjects randomized to Protandim tended to remain stable (median decrease of −2%); however, this was not statistically significant (P = 0.19; Fig. 1A). Nonsignificant changes in BAL total protein values were also observed when these values were not corrected for dilution, with a median increase of 19% in the placebo group and median decrease of 5% in the Protandim group (P = 0.66; Fig. 1B). In the entire cohort of 30 AUD subjects, BAL total protein data between the first and second bronchoscopy were compared. BAL total protein values did not change significantly over time despite 7 days of abstinence from alcohol {BAL total protein corrected for urea dilution of 299 ug/ml [intraquartile range (IQR) 135, 1,015] in first BAL vs. BAL total protein 327 ug/ml (IQR 189, 864) in second BAL; P = 0.67}. Moreover, BAL protein concentrations uncorrected for dilution did also not vary significantly between the first [106 ug/ml (IQR 80–142)] and second [109 ug/ml (IQR 83–187)] BAL procedure (P = 0.14). For comparison, in our previously published cohort of 18 subjects with AUDs (7), BAL total protein concentrations, uncorrected for dilution, from the first BAL were 413 ug/ml (IQR 307–540), whereas at the second BAL after 1 wk of abstinence were 445 ug/ml (IQR 397–762) (P = 0.98).
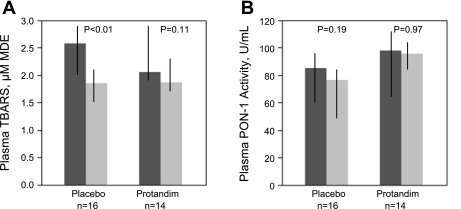
Fig. 1.
Bronchoalveolar lavage (BAL) total protein measurements were performed after centrifugation of fluid to remove cellular elements. A: %change in total protein, corrected for urea dilution. Amount of total protein increased in the group of alcohol use disorders (AUDs) subjects randomized to placebo but remained relatively stable among those randomized to Protandim, although this was not statistically significant. B: %change in total protein, uncorrected for dilution. A nonsignificant increase was observed in the total protein concentration among those randomized to placebo. Thick bars represent median values, while black crossbars represent the intraquartile range of the data. Statistical comparisons made with Wilcoxon signed rank test.
Given the random differences in age and NRI between subjects who received Protandim or placebo, multivariable modeling including these potential confounders was performed to assess their impact on the relationship between type of study drug received (placebo vs. Protandim) and the change in BAL total protein. Ultimately, the study drug type was not found to be associated with the change in BAL total protein [either corrected (P = 0.15) or uncorrected (P = 0.64) for dilution] accounting for age and NRI in these multivariable models.
Thiol/Disulfide Alterations in BAL
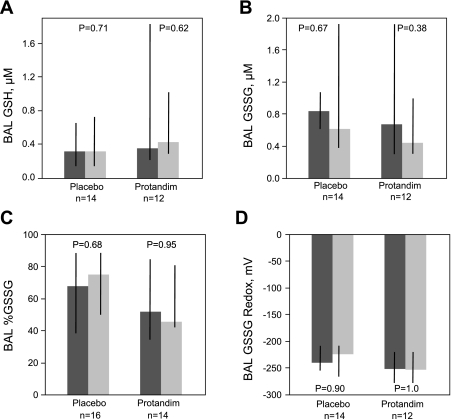
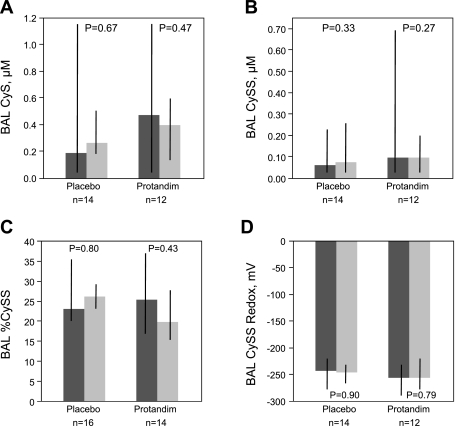
BAL concentrations for the thiol/disulfide pairs, GSH/GSSG and Cys/CySS, were measured in samples from all 30 subjects. %GSSG, %CySS, GSSG redox potential, and CySS redox potential were calculated from these values (Figs. 2 and 3). Administration of Protandim was not associated with alterations in thiols (GSH, CyS) or disulfide (GSSG, CySS) species in BAL. Similarly, the percentage of oxidized species (%GSSG and %CySS) was not altered over the week of therapy with Protandim or placebo, nor did GSSG or CySS redox potentials change significantly. Comparing values for the entire AUD cohort (n = 30) between the first and second bronchoscopy revealed no significant changes over time for either % GSSG (increase of 4%; P = 0.52) or GSSG redox (increased oxidation by 3 mV; P = 0.67).
Fig. 2.
BAL thiol/disulfide glutathione measurements were performed via HPLC. A: reduced glutathione (GSH), corrected for urea dilution. B: oxidized glutathione (GSSG), corrected for urea dilution. C: %GSSG relative to total GSSG plus GSH in BAL was calculated with uM values of GSH and GSSG. D: GSSG redox potential in BAL was calculated using the Nernst equation with GSH and GSSG values corrected for urea dilution. No significant changes over time for any of these parameters were observed for subjects randomized to either placebo or Protandim. Thick bars represent median values, where dark grey is pretreatment (day 1) and light grey is posttreatment (day 8) time points, while black crossbars represent the intraquartile range of the data. Statistical comparisons made with Wilcoxon signed rank test.
Fig. 3.
BAL thiol/disulfide cysteine (CyS) and cystine (CySS) measurements were performed via HPLC. A: CyS, corrected for urea dilution. B: CySS, corrected for urea dilution. C: %cystine relative to total cystine plus cysteine in BAL was calculated with the uM values of CyS and CySS. D: CySS redox potential in BAL was calculated using the Nernst equation with CyS and CySS values corrected for urea dilution. No significant differences over time for any measured parameters were observed for subjects randomized to either placebo or Protandim. Thick bars represent median values, where dark grey is pretreatment (day 1) and light grey is posttreatment (day 8) time points, while black crossbars represent the intraquartile range of the data. Statistical comparisons made with Wilcoxon signed rank test.
Nucleic Acid Oxidation in BAL
In a subset of subjects with AUDs randomized to active Protandim (n = 10) or placebo (n = 9), 8-OHdG was measured. There were no significant changes in the 8-OHDG values in BAL associated with the drug type received (P = 0.49 for placebo; P = 1.0 for Protandim groups with Wilcoxon's signed rank test).
Cytokine/Growth Factor Measurements in BAL from Subjects with AUDs
In a subset of 13 AUD subjects (Protandim, n = 6; placebo, n = 7), BAL cytokines/growth factors were measured before and after completion of the study protocol. EGF, FGF, IL-1β, and IL-10 were determined to be low (<1 pg/ml) in BAL. Median EGF levels were 0.01 (0–0.19) pg/ml at the time of the first BAL, and 0 (0–0.27) pg/ml after 7 days of abstinence (P = 0.45). Similarly, FGF at first BAL was 0 (0–0.53) pg/ml, and after 7 days of abstinence was 0 (0–1.65) pg/ml (P = 0.88). For the proinflammatory cytokine TNF-α, levels tended to increase over 7 days of abstinence among subjects with AUDs. At the first BAL, levels were determined to be 0.01 (0–0.03) pg/ml, while at the second BAL they were 0.03 (0.01–0.18) (P = 0.12). Finally, levels of the anti-inflammatory cytokine IL-10 were also not influenced by abstinence, with first BAL values measured at 0.44 (0.28–0.61) pg/ml, and second BAL values measured at 0.55 (0.44–0.73) (P = 0.77). Reception of Protandim did not significantly influence the change any measured cytokine/growth factor within subjects.
Correlation Among Oxidative Stress, Pulmonary Cytokine Milieu, and Alveolar Epithelial Permeability
To determine the strength of the relationship between intrapulmonary oxidative stress and alveolar epithelial permeability among those with AUDs, we assessed the correlation between GSH/GSSG or CyS/CySS homeostasis and BAL total protein using data from the first bronchoscopy. No correlation between BAL total protein values and GSSG redox potential, %GSSG, CySS redox potential, or %CySS was observed (all P values >0.05). Similarly, no significant relationship in BAL total protein with the growth factors/cytokines EGF, FGF, TNF-α, or IL-10 was observed (all P values >0.05).
Thiol/Disulfide Alterations in Plasma
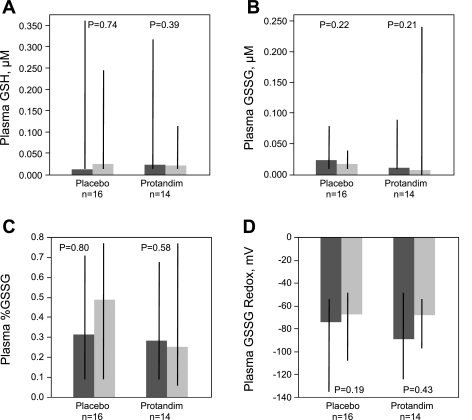
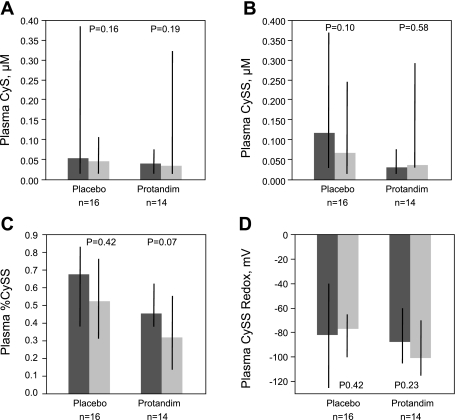
The thiol/disulfide pairs GSH/GSSG and Cys/CySS were measured in plasma from all 30 AUD subjects (Figs. 4 and 5). Similar to what was observed in BAL, treatment with Protandim was not associated with significant alterations in GSH, GSSG, CyS, or CySS in plasma over the 7-day study period. Moreover, there were no significant changes in the %GSSG, %CySS, GSSG redox potential, or CySS redox potential between the first and last day of the study, regardless of treatment type.
Fig. 4.
Plasma thiol/disulfide glutathione measurements were performed via HPLC. A: GSH. B: GSSG. C: %GSSG present in plasma was calculated with uM values of GSH and GSSG. D: redox potential of oxidized glutathione in plasma was calculated using the Nernst equation with GSH and GSSG values. No significant differences over time were observed subjects randomized to either placebo or Protandim. Thick bars represent median values, where dark grey is pretreatment (day 1) and light grey is posttreatment (day 8) time points, while black crossbars represent the intraquartile range of the data. Statistical comparisons made with Wilcoxon signed rank test.
Fig. 5.
Plasma cystine/cysteine measurements were performed via HPLC. A: CyS. B: CySS. C: %CySS present in plasma was calculated with uM values of CyS and CySS. D: CySS redox potential in plasma was calculated using the Nernst equation with CyS and CySS values. A trend for decreased %CySS was observed in the Protandim group; however, no statistically significant differences in parameters over time were observed in either the placebo or Protandim group for any parameter assessed. Thick bars represent median values, where dark grey is pretreatment (day 1) and light grey is posttreatment (day 8) time points, while black crossbars represent the intraquartile range of the data. Statistical comparisons made with Wilcoxon signed rank test.
Plasma Markers of Lipid Peroxidation
Plasma TBARS (uM malondialdehyde equivalents) and PON-1 activity were measured from all 30 subjects (Fig. 6). A decrease in plasma TBARS over time was observed in both the Protandim and placebo groups that was statistically significant in the placebo group (P < 0.01). Similarly, there was a nonsignificant decrease in plasma PON-1 activity between the first and last day of the study for both the Protandim and placebo groups.
Fig. 6.
Plasma samples were assessed for the change in thiobarbituric acid reactive substances (TBARS; A) over time in association with Protandim or placebo administration, reported as uM malondialdehyde equivalents (MDE) and the change in paroxanase-1 (PON-1; B) activity over time in association with Protandim or placebo administration. Placebo group was observed to have a significant decrease in plasma TBARS over the 7 days of observation, while a trend was observed for decreased TBARS in the Protandim group. Neither the placebo nor Protandim groups' PON-1 activity changed significantly over the duration of the study. Thick bars represent median values, with dark grey is pretreatment (day 1) and light grey is posttreatment (day 8) time points, while black crossbars represent the intraquartile range of the data. Statistical comparisons made with Wilcoxon signed rank test.
Validation of Prior Comparisons Between AUD Subjects and Healthy Controls
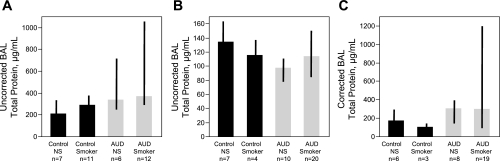
During the same time frame for enrollment of AUD subjects, we enrolled 11 age-matched controls; demographic features are listed in Table 1 and BAL data are listed in Table 2. Compared with the AUD subjects, controls were slightly younger, more likely to be women, and less likely to be smokers than our AUD subjects. Previous investigations by our group (6) demonstrated that otherwise healthy subjects with AUDs had an associated higher BAL total protein than did a group of age- and smoking-matched controls (Fig. 7A; P < 0.01). Therefore, we sought to confirm this original observation in the present investigation. Additionally, we wanted to confirm earlier findings of increased intrapulmonary oxidative stress (26) among those with AUDs and also to determine if measureable differences in selected cytokines and growth factors in BAL would be detectable between those with AUDs and controls.
Fig. 7.
Total protein in BAL measured in subjects with AUDs and controls. A: in our previously published cohort (7), the diagnosis of an AUD was associated with higher BAL total protein when the analysis was performed using BAL total protein values uncorrected for dilution (P < 0.01 for comparison between 18 AUD subjects vs. 18 controls group matched for age, smoking, and gender). NS, nonsmoker. B: in the present cohort, AUDs were associated with small but significantly lower BAL total protein when the analysis was performed without correcting total protein values for dilution (P < 0.05 between 30 AUD and 11 control subjects). However, when BAL total protein values were corrected for dilution (urea techniques; C), subjects with AUDs (n = 27) tended to have increased total protein compared with controls (n = 9; P = 0.07). Compared with the previously published cohort, there were an increased number of smokers in our AUD subjects and fewer smokers among controls in the current cohort.
When we compared BAL total protein values uncorrected for urea dilution between our current cohort of 30 AUD subjects (smokers, n = 20; nonsmokers, n = 10) obtained at the time of the first BAL, before abstinence, with 11 control subjects' values (smokers, n = 4; nonsmokers, n = 7), a small but significantly higher BAL total protein was detected in controls (Fig. 7B; P < 0.05). However, when BAL total protein values were corrected for urea dilution, compared with BAL total protein from healthy control subjects, BAL total protein from subjects with AUDs tended to be higher [total protein in controls, 153 ug/ml (IQR 108, 225) vs. AUD subjects, 299 ug/ml (IQR 135, 1015); Fig. 7C; P = 0.07], similar to what we have previously reported (7).
To validate prior observations of increased glutathione oxidation in the setting of AUDs and to determine alcohol's effect on cysteine and nucleic acid oxidation, data from the AUD group's first BAL was compared with control subject BAL (BAL available from control nonsmokers; n = 6; control smokers; n = 3). We observed that the %GSSG was significantly higher in the subjects with AUDs [66 (IQR 35–84)% vs. 25 (IQR 18–69)%; P < 0.05], while GSSG redox potential tended to be more highly oxidized [−248 mV (IQR −216 to −269) vs. −279 mV (IQR-233 to −293); P = 0.06]. No significant differences were observed in control cystine, cysteine, %CySS, or CySS redox potential between AUD subjects and controls. Limiting our analyses to nonsmoking AUD subjects (n = 10) and controls (n = 6) to minimize the potential confounding effect of smoking (13, 25), we observed an even more pronounced association between alcohol use and glutathione oxidation. Specifically, the %GSSG was threefold higher in AUD subjects compared with controls [61 (IQR 40–88)% in those with AUDs vs. 18 (IQR 16–25)% in controls; P < 0.01], while the GSSG redox potential was ∼13% more oxidized [−250 mV (IQR −203 to −255) in those with AUDs vs. −287 mV (IQR −278 to −296); P < 0.002] in this group. Similarly, values for 8-OHdG were approximately twofold higher among those with AUDs compared with the values measured in controls [0.16 (0.09–0.33) ng/ml in AUDs vs. 0.07 (0.05–0.10) ng/ml in controls; P < 0.02].
Cytokines/growth factors were compared between BAL samples from AUD subjects' first BAL and control subjects' BAL. A tendency for lower EGF was observed in subjects with AUDs, where levels were 0 (0–0.11) pg/ml compared with 0.03 (0–0.57) pg/ml in controls (P = 0.13). Values for TNF-α were 0.03 (0.01–0.04) pg/ml among those with AUDs, a small but statistically significant increase from controls whose values were 0 (0–0.02) pg/ml. Differences in IL-10 were most robust between these two types of subjects; BAL values for those with AUDs were 0.59 (0.44–0.69) pg/ml, while those for controls were 0.3 (0.23–0.44) pg/ml (P < 0.02). No significant differences in BAL FGF were observed between groups (P = 0.17).
Finally, systemic oxidative stress measured in plasma from AUD subjects obtained at the time of the first bronchoscopy was compared with measurements in plasma from controls. Both GSSG (P < 0.004) and CySS (P < 0.05) redox potential measured at the time of the first bronchoscopy were significantly more oxidized in the group with AUDs. Examining plasma values from nonsmokers only (available plasma from AUD subjects, n = 6; controls, n = 4), GSH and Cys were quantitatively greater in the plasma from controls (P < 0.04 and P < 0.01, respectively), while GSSG redox potential was significantly more oxidized among those with AUDs (P < 0.03). CySS redox potential was not significantly different between AUD subjects and controls who were nonsmokers (P = 0.33). At the beginning of the study period, plasma TBARS from subjects with AUDs were significantly higher than those measured in healthy controls [2.3 uM MDE (IQR 1.9–2.9) vs. 1.8 uM MDE (IQR 1.7–2.2); P < 0.02]. At the end of the 7-day study period, however, TBARS values for those with AUDs approximated what was observed in controls [1.9 uM MDE (IQR 1.6, 2.1) in those with AUDs vs. 1.8 uMDE (IQR 1.7, 2.2) in controls; P = 0.80]. Plasma PON-1 activity in the setting of AUDs did not significantly differ from that in controls at either time point (AUD subjects vs. controls; P = 0.68 at study day 1; P = 0.96 at study day 7).
DISCUSSION AND CONCLUSIONS
In these investigations, we sought to determine the efficacy of the nutraceutical Protandim in normalizing alveolar epithelial permeability in individuals with AUDs via correction of underlying intrapulmonary oxidative stress. We determined that neither receiving active drug nor placebo was associated with significant alterations in BAL total protein levels over a 7-day period of abstinence among individuals with AUDs. One possible explanation for the apparent lack of Protandim's influence on BAL total protein values was that it failed to have an appreciable effect on intrapulmonary oxidative stress as assessed by thiol homeostasis and lipid peroxidation measurements. Alternatively, given our relatively small sample size of 30 subjects, we may have had insufficient power to detect significant differences in BAL total protein between the Protandim and placebo groups. Despite these neutral results for our primary outcome variable, conducting this randomized, controlled trial in subjects with AUDs allowed our group to establish new information regarding the effect of AUDs on pulmonary oxidative stress and demonstrated the feasibility of conducting complex clinical trials in this vulnerable population.
In subjects with AUDs, BAL total protein did not change significantly over the 7 days of directly observed abstinence, similar to what we observed in our prior investigation (7), where total protein concentrations did not change significantly over 1 wk of abstinence, regardless of whether raw or corrected protein values were examined. Our current and prior observations suggest relative stability in BAL total protein among asymptomatic subjects with AUDs over time, despite no alcohol consumption. It should be noted that although BAL total protein is fairly straightforward to measure, data obtained from BAL may be influenced by differences in technique (or patient tolerance of the procedure) that can ultimately affect the yield of the procedure leading to wide intersubject variability in data; accordingly, we included BAL data corrected for these potential dilutional effects. Moreover, since our initial publication, assessments of alveolar epithelial permeability have been performed in individuals with AUDs using different but complementary techniques to further substantiate the validity of our hypothesis that alveolar epithelial permeability is abnormal in the setting of AUDs. First, a shortened half-life of an inhaled radiolabeled isotope was reported in 33 asymptomatic, otherwise healthy subjects with AUDs, compared with 13 age- and smoking-matched controls, suggesting enhanced permeability of the alveolar epithelial membrane among those with AUDs (9). In a second study, PiCCO transpulmonary thermodilution catheter placement was performed in 35 patients with ALI to measure extravascular lung water (EVLW), a surrogate for pulmonary edema, over a 7-day time period (4). Thirteen of these patients met criteria for an AUD. These investigators determined that mean EVLW was significantly higher among patients with ALI who had a history of an AUD and that EVLW remained persistently and significantly greater among AUD patients during the study period. Collectively, this literature suggests that permeability of the alveolar epithelium is abnormal in individuals with AUDs in the absence of overt pulmonary illness and that it does not resolve rapidly during abstinence from alcohol. Therefore, enhanced alveolar epithelial permeability in the setting of AUDs could specifically contribute to the development of noncardiogenic pulmonary edema and ALI or hamper its resolution.
Based on our prior work in humans (7) and in animal models (17), correction of intrapulmonary oxidative stress was a logical strategy to correct alveolar epithelial permeability among those with AUDs. However, administration of oral Protandim for the 7-day study period was not associated with any appreciable normalization of BAL glutathione or cystine homeostasis nor did it appear to influence the redox potential in the alveolar space. Notably, compared with control subjects' values, both %GSSG and 8-OHdG values in BAL were significantly higher among those with AUDs before study drug administration. After 7 days of abstinence, neither of these parameters changed significantly in those with AUDs, regardless of whether subjects received active study drug or placebo. Therefore, persistently abnormal intrapulmonary oxidative stress is one consistent observation that may influence the pulmonary health of those with AUDs. However, its relationship to alveolar epithelial permeability is uncertain given the fact that in univariate analyses, unlike our prior work (7), correlations between oxidative stress parameters and BAL total protein values were not observed.
Recently, other effectors important in maintaining alveolar epithelial permeability have been reported. Catecholamines, inflammatory factors, and the presence of specific pathogens (i.e., influenza) have been reported to influence alveolar fluid clearance (reviewed in Ref. 15). Given our inability to consistently detect an association between AUD-associated oxidative stress and alveolar epithelial permeability, exploration of these other mechanisms is warranted. Finigan et al. (13) recently reported that the ADAM17-neuregulin 1-human EGF receptor 2 axis may represent an important pathway in the regulation of alveolar epithelial permeability. Perturbations of this pathway in human airway epithelial cell monolayers enhanced permeability; moreover, elevated levels of neuregulin were measured in epithelial lining fluid from patients with ALI, suggesting the clinical importance of this protein in regulating alveolar epithelial permeability. Moreover, polymorphisms in the EGF gene, particularly in men, have been associated with the development in acute respiratory distress syndrome (38). EGF is one factor previously demonstrated to influence alveolar epithelial permeability in vitro (6) and in vivo (42) through potential effects on claudin expression (14). Claudin family tight junction proteins, found on both alveolar epithelial type I as well as type II cells, have a role in regulating paracellular permeability and may influence the development of pulmonary edema (24, 46). Although EGF was slightly lower in BAL from subjects with AUDs, no definitive conclusions regarding its relevance to maintenance of alveolar epithelial permeability can be drawn from these investigations. However, differences we observed between AUD subjects and controls in BAL quantity of IL-10 and TNF-α suggest a potential influence of alcohol on the intrapulmonary inflammatory milieu. The deleterious anti-inflammatory effects of chronic alcohol consumption have been previously reported by Spies and colleagues (37, 39–40) in clinical studies of patients with AUDs, where they are believed to contribute to the higher incidence of postoperative pneumonias among those with AUDs. A more complete understanding of alcohol's effect on intrapulmonary inflammation may help to determine its relevance to alveolar epithelial permeability.
Information derived from these investigations also provided new insights to the duration of systemic oxidative stress among those with AUDs with respect to not only thiol/disulfide homeostasis but also indexes of lipid peroxidation. In contrast to the relative stability of plasma thiol/disulfide antioxidant quantity and redox potential, TBARS concentrations were increased in those with AUDs before randomization but decreased to values found in healthy subjects after 7 days of abstinence, regardless of the study drug administered. Plasma thiol/disulfide levels have been noted to be influenced by dietary composition, particularly sulfur amino acid containing compounds (20, 21). The influence of diet on TBARS quantity is not well established. However, the fact that TBARS values decreased to a normal range after detoxification, as we observed, contrasts with what others (45) have recently reported in a similar cohort of subjects with AUDs. In this earlier investigation, although TBARS values did decrease over time, they did not return to normal values in a 7-day period. Decreased PON-1 activity has been reported in the presence of chronic liver diseases, including those related to alcohol abuse (12); moreover, it is believed to participate in mechanisms related to the development of hepatic fibrosis as a hepatically synthesized enzyme with lactonase and esterase activities important in the metabolism of lipid peroxides. In contrast to our results, a previously described cohort of 328 patients with AUDs and varying degrees of liver dysfunction reported significantly decreased serum PON-1 activity in those with AUDs compared with healthy controls, including 110 AUD subjects with no evidence of liver disease (23). In our small cohort of subjects and controls, it is not possible to clearly establish the effects of the nutrition provided to the subjects, or the effect of vitamin, folic acid, thiamine supplementation, and any unmeasured effects of Protandim administered to those with AUDs. Therefore, these dietary effects and other unassessed confounders might have influenced plasma measurements of these substances.
Although these investigations provided new insight regarding longitudinal alterations in alveolar epithelial barrier function and oxidative stress in both the lung and systemically in the setting of AUDs, they are not without limitations. First, our relatively small sample size might have precluded us from observing significant differences in our primary outcome variable as well as some of the secondary outcome variables measured. A larger sample size of AUD and control subjects, particularly one that included more women and had greater racial diversity would enhance the strength and generalizability of our observations. Moreover, differences between our AUD subjects and controls subjects in terms of age, gender, racial background, and smoking could have confounded the comparison of BAL total protein values between these groups. Additionally, since AUDs have been associated with impaired intestinal absorption, the use of an oral agent, instead of an intravenous product, could have limited absorption of active drug (29). Further, compared with prior investigations using Protandim (30), the shorter duration of study drug administration could have influenced our results. However, the strength of the methods that we employed to safely conduct an inpatient study on vulnerable subjects who were at risk for alcohol withdrawal, including the performance of two bronchoscopies, a randomization strategy to ensure equal numbers of active smokers in each group, and tightly controlled diet/vitamin supplement protocols will allow us to conduct studies of similar or greater complexity with larger cohorts in the future. Further, we know of no other investigations where BAL samples have been obtained sequentially during a period of directly observed abstinence from alcohol; this has provided us with new directions for subsequent research.
In conclusion, we observed that 7 days of administration of an oral agent reported to enhance endogenous antioxidants in subjects with AUDs did not significantly alter alveolar epithelial permeability nor did it appreciably affect intrapulmonary or systemic oxidative stress. These observations are tempered by the fact that BAL total protein values between those with AUDs in our cohort and a smaller cohort of healthy controls did not differ at baseline. However, in longitudinal sampling of the AUD cohort, directly observed to be abstinent from alcohol, the persistence of thiol/disulfide homeostatic abnormalities and DNA oxidation abnormalities was observed. Collectively, our findings suggest that these persistent abnormalities could be contributors to the predisposition of those with AUDs to develop noncardiogenic pulmonary edema when affected by an ALI risk factor. Novel strategies to target these abnormalities can potentially limit the development of this devastating disorder with its attendant poor outcomes among individuals with AUDs.
GRANTS
This work was supported by National Institutes of Health Grants R01-AA-014435, UL1-RR-025780, K24-HL-089223, and R24-AA-019661.
DISCLOSURES
J. McCord is an employee of LifeVantage, the manufacturer of Protandim, and has a financial interest in the company exceeding $10,000.
AUTHOR CONTRIBUTIONS
Author contributions: E.L.B., S.B., L.A.B., R.H., M.M., and J.G. conception and design of research; E.L.B., J.M.M., S.B., and J.G. performed experiments; E.L.B., L.A.B., M.M., and J.G. analyzed data; E.L.B., J.M.M., L.A.B., and M.M. interpreted results of experiments; E.L.B. prepared figures; E.L.B. and J.G. drafted manuscript; E.L.B., J.M.M., S.B., L.A.B., and M.M. edited and revised manuscript; E.L.B., J.M.M., L.A.B., R.H., M.M., and J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the support provided by clients and staff of Denver CARES, the kind assistance of the University of Colorado Hospital CTRC, and provision of the study medication by LifeVantage.
REFERENCES
- 1. Anonymous Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med 325: 525– 532, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Bagasra O, Howeedy A, Kajdacsy-Balla A. Macrophage function in chronic experimental alcoholism. I. Modulation of surface receptors and phagocytosis. Immunology 65: 405– 409, 1988 [PMC free article] [PubMed] [Google Scholar]
- 3. Baldwin WA, Rosenfeld BA, Breslow MJ, Buchman TG, Deutschman CS, Moore RD. Substance abuse-related admissions to adult intensive care. Chest 103: 21– 25, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Berkowitz DM, Danai PA, Eaton S, Moss M, Martin GS. Alcohol abuse enhances pulmonary edema in acute respiratory distress syndrome. Alcohol Clin Exp Res 33: 1690– 1696, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boe DM, Richens TR, Horstmann SA, Burnham EL, Janssen WJ, Henson PM, Moss M, Vandivier RW. Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response. Alcohol Clin Exp Res 34: 1723– 1732, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borok Z, Hami A, Danto SI, Lubman RL, Kim KJ, Crandall ED. Effects of EGF on alveolar epithelial junctional permeability and active sodium transport. Am J Physiol Lung Cell Mol Physiol 270: L559– L565, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Burnham EL, Brown LA, Halls L, Moss M. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcohol Clin Exp Res 27: 1167– 1172, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Burnham EL, Gaydos J, Hess E, House R, Cooper J. Alcohol use disorders affect antimicrobial proteins and anti-pneumococcal activity in epithelial lining fluid obtained via bronchoalveolar lavage. Alcohol Alcohol 45: 414– 421, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burnham EL, Halkar R, Burks M, Moss M. The effects of alcohol abuse on pulmonary alveolar-capillary barrier function in humans. Alcohol Alcohol 44: 8– 12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeWit M, Best AM, Gennings C, Burnham EL, Moss M. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res 31: 1224– 1230, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Eckerson HW, Oseroff A, Lockridge O, La Du BN. Immunological comparison of the usual and atypical human serum cholinesterase phenotypes. Biochem Genet 21: 93– 108, 1983 [PubMed] [Google Scholar]
- 12. Ferre N, Marsillach J, Camps J, Mackness B, Mackness M, Riu F, Coll B, Tous M, Joven J. Paraoxonase-1 is associated with oxidative stress, fibrosis and FAS expression in chronic liver diseases. J Hepatol 45: 51– 59, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Finigan JH, Faress JA, Wilkinson E, Mishra RS, Nethery DE, Wyler D, Shatat M, Ware LB, Matthay MA, Mason R, Silver RF, Kern JA. Neuregulin-1-human epidermal receptor-2 signaling is a central regulator of pulmonary epithelial permeability and acute lung injury. J Biol Chem 286: 10660– 10670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flores-Benitez D, Ruiz-Cabrera A, Flores-Maldonado C, Shoshani L, Cereijido M, Contreras RG. Control of tight junctional sealing: role of epidermal growth factor. Am J Physiol Renal Physiol 292: F828– F836, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol 35: 10– 19, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gould NS, Min E, Gauthier S, Chu HW, Martin R, Day BJ. Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am J Respir Crit Care Med 182: 1114– 1122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol 279: L127– L135, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Hunninghake GW, Gadek JE, Kawanami O, Ferrans VJ, Crystal RG. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol 97: 149– 206, 1979 [PMC free article] [PubMed] [Google Scholar]
- 19. Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med 28: 625– 635, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Jones DP, Park Y, Gletsu-Miller N, Liang Y, Yu T, Accardi CJ, Ziegler TR. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition 27: 199– 205, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mannery YO, Ziegler TR, Park Y, Jones DP. Oxidation of plasma cysteine/cystine and GSH/GSSG redox potentials by acetaminophen and sulfur amino acid insufficiency in humans. J Pharmacol Exp Ther 333: 939– 947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marik P, Mohedin B. Alcohol-related admissions to an inner city hospital intensive care unit. Alcohol Alcohol 31: 393– 396, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Marsillach J, Ferre N, Vila MC, Lligona A, Mackness B, Mackness M, Deulofeu R, Sola R, Pares A, Pedro-Botet J, Joven J, Caballeria J, Camps J. Serum paraoxonase-1 in chronic alcoholics: relationship with liver disease. Clin Biochem 40: 645– 650, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol 301: L40– L49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 275: 50– 54, 1996 [PubMed] [Google Scholar]
- 26. Moss M, Guidot DM, Wong-Lambertina M, Ten HT, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 161: 414– 419, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 31: 869– 877, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Nagai K, Betsuyaku T, Kondo T, Nasuhara Y, Nishimura M. Long term smoking with age builds up excessive oxidative stress in bronchoalveolar lavage fluid. Thorax 61: 496– 502, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nazer H, Wright RA. The effect of alcohol on the human alimentary tract: a review. J Clin Gastroenterol 5: 361– 365, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radic Biol Med 40: 341– 347, 2006 [DOI] [PubMed] [Google Scholar]
- 31. O'Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med 35: 345– 350, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351– 358, 1979 [DOI] [PubMed] [Google Scholar]
- 33. Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res 26: 272– 279, 2002 [PubMed] [Google Scholar]
- 34. Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60: 532– 538, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Room R, Babor T, Rehm J. Alcohol and public health. Lancet 365: 519– 530, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest 131: 554– 562, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Sander M, Irwin M, Sinha P, Naumann E, Kox WJ, Spies CD. Suppression of interleukin-6 to interleukin-10 ratio in chronic alcoholics: association with postoperative infections. Intensive Care Med 28: 285– 292, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Sheu CC, Zhai R, Su L, Tejera P, Gong MN, Thompson BT, Chen F, Christiani DC. Sex-specific association of epidermal growth factor gene polymorphisms with acute respiratory distress syndrome. Eur Respir J 33: 543– 550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spies C, Eggers V, Szabo G, Lau A, von Dossow V, Schoenfeld H, Althoff H, Hegenscheid K, Bohm B, Schroeder T, Pfeiffer S, Ziemer S, Paschen C, Klein M, Marks C, Miller P, Sander M, Wernecke KD, Achterberg E, Kaisers U, Volk HD. Intervention at the level of the neuroendocrine-immune axis and postoperative pneumonia rate in long-term alcoholics. Am J Respir Crit Care Med 174: 408– 414, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Spies CD, von DV, Eggers V, Jetschmann G, El-Hilali R, Egert J, Fischer M, Schroder T, Hoflich C, Sinha P, Paschen C, Mirsalim P, Brunsch R, Hopf J, Marks C, Wernecke KD, Pragst F, Ehrenreich H, Müller C, Tonnesen H, Oelkers W, Rohde W, Stein C, Kox WJ. Altered cell-mediated immunity and increased postoperative infection rate in long-term alcoholic patients. Anesthesiology 100: 1088– 1100, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 84: 1353– 1357, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Sznajder JI, Ridge KM, Yeates DB, Ilekis J, Olivera W. 1998. Epidermal growth factor increases lung liquid clearance in rat lungs. J Appl Physiol 85: 1004– 1010 [DOI] [PubMed] [Google Scholar]
- 43. Tan XL, Spivack SD. Dietary chemoprevention strategies for induction of phase II xenobiotic-metabolizing enzymes in lung carcinogenesis: a review. Lung Cancer 65: 129– 137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Velmurugan K, Alam J, McCord JM, Pugazhenthi S. Synergistic induction of heme oxygenase-1 by the components of the antioxidant supplement Protandim. Free Radic Biol Med 46: 430– 440, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Wozniak B, Musialkiewicz D, Wozniak A, Drewa G, Drewa T, Drewa S, Mila-Kierzenkowska C, Porzych M, Musialkiewicz M. Lack of changes in the concentration of thiobarbituric acid-reactive substances (TBARS) and in the activities of erythrocyte antioxidant enzymes in alcohol-dependent patients after detoxification. Med Sci Monit 14: CR32– CR36, 2008 [PubMed] [Google Scholar]
- 46. Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol 297: L219– L227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med 176: 270– 276, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]