Abstract
Efficient clearance of apoptotic cells from the lung by alveolar macrophages is important for the maintenance of tissue structure and function. Lung tissue from humans with emphysema contains increased numbers of apoptotic cells and decreased levels of vascular endothelial growth factor (VEGF). Mice treated with VEGF receptor inhibitors have increased numbers of apoptotic cells and develop emphysema. We hypothesized that VEGF regulates apoptotic cell clearance by alveolar macrophages (AM) via its interaction with VEGF receptor 1 (VEGF R1). Our data show that the uptake of apoptotic cells by murine AMs and human monocyte-derived macrophages is inhibited by depletion of VEGF and that VEGF activates Rac1. Antibody blockade or pharmacological inhibition of VEGF R1 activity also decreased apoptotic cell uptake ex vivo. Conversely, overexpression of VEGF significantly enhanced apoptotic cell uptake by AMs in vivo. These results indicate that VEGF serves a positive regulatory role via its interaction with VEGF R1 to activate Rac1 and enhance AM apoptotic cell clearance.
Keywords: alveolar, apoptosis, efferocytosis
apoptosis and the resultant clearance of apoptotic cells are essential for the maintenance of normal tissue structure and function. Clearance of apoptotic cells, also known as efferocytosis, prevents release of toxic contents from dying cells, promotes the resolution of inflammation, and produces growth factors that support tissue structure including vascular endothelial growth factor (VEGF) and hepatocyte growth factor (39). In the absence of disease, apoptotic cells are rarely observed in the lung. Similarly, in acute inflammatory states, such as community acquired pneumonia and adult respiratory distress syndrome (ARDS), few apoptotic cells are present, suggesting that even in times of high cellular turnover, efferocytosis is highly efficient (8, 25). In comparison, increased numbers of apoptotic cells have been documented in a variety of chronic pulmonary diseases including cystic fibrosis and chronic obstructive pulmonary disease (COPD) (39).
Lung tissue sections from subjects with emphysema contain elevated numbers of apoptotic pneumocytes and endothelial cells. Importantly these tissue sections also contain decreased tissue VEGF levels and have reduced VEGF receptor 2 (VEGF R2 or KDR/Flk-1) expression (21). In a similar fashion, increased numbers of apoptotic cells and airspace enlargement are detectable in rats and mice treated with SU5416, a receptor tyrosine kinase inhibitor that is specific for VEGF receptors (22, 30, 38). Alveolar macrophages (AMs) from patients with COPD are defective in their ability to clear apoptotic cells (16). Since VEGF levels are reduced in lung sections from subjects with emphysema, these findings raise the intriguing possibility that VEGF influences the ability of AMs to clear apoptotic cells.
Macrophage function is highly dependent on the environment in which the macrophage resides (12, 19). AMs are bathed in alveolar epithelial lining fluid that contains a 500-fold higher concentration of VEGF relative to plasma or other organs (2, 20). Importantly, AMs express high levels of VEGF receptor 1 (VEGF R1/Flt-1) in contrast to the more well-described VEGF R2, which is absent (7). The effect of VEGF and VEGF R1 on AM efferocytosis has not previously been determined.
Using murine AMs and human monocyte-derived macrophages (HMDMs), we illustrate that deficient VEGF levels, VEGF R1 blockade, and inhibition of VEGF receptor signaling decreased apoptotic cell uptake. Utilizing transgenic animals, we also demonstrate that targeted overexpression of VEGF in the lungs leads to increased uptake of apoptotic cells by AMs.
METHODS
Human and animal experimentation.
This study was approved by and performed in accordance with the ethical standards of the National Jewish Health Institutional Review Board and the Institutional Animal Care and Use Committees at the University of Colorado Health Sciences Center and National Jewish Health.
Animals.
Transgenic mice with doxycycline-inducible expression of human VEGF165 via a CC10 promoter were kindly provided by Drs. Chun G. Lee and Jack A. Elias (Yale University School of Medicine) (24). Doxycycline-containing diet (200 mg/kg Bio-serv, Frenchtown, NJ) was fed to the mice to induce lung-specific VEGF165 expression. Age- and sex-matched littermate controls were used in experiments when appropriate. C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME) and used in selected experiments.
Isolation of primary cells.
Murine AMs were obtained by bronchoalveolar lavage (BAL). After euthanasia, an intratracheal catheter was placed and the lungs were lavaged with 10 1-ml aliquots of PBS with 5 mM EDTA. Cells were isolated by centrifugation at 1,000 rpm, resuspended in DMEM (Mediatech, Manassas, VA) with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), and 1× penicillin-streptomycin glutamine (PSG) (American Type Culture Collection, Manassas, VA) and plated at a concentration of 100,000–150,000 cells per well in 96-well, tissue culture-treated plates (BD Falcon, Franklin Lakes, NJ). AMs were then cultured for 24 h at 37°C in 10% CO2.
HMDMs were obtained from healthy donors and isolated by Percoll gradient centrifugation, as previously described (32). Monocytes were plated in 24-well tissue culture plates (BD Falcon) and matured to macrophages by culturing in X-vivo medium (BioWhittaker, Walkersville, MD) containing 10% human serum pooled from five donors, at 37°C in 10% CO2 for 6–8 days. Cells were cultured at a concentration of ∼5 × 105 per well.
Human T lymphocyte Jurkat cells were obtained from the American Type Culture Collection (ATCC). Jurkat cells were cultured in RPMI (MediaTech) with 10% heat-inactivated FBS (Gemini BioProducts, Calabasas, CA) and supplemented with 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma Chemical, St. Louis, MO).
Thymocytes were harvested from 4- to 6-wk-old C57BL/6 mice. After isolation by dissection, the thymus was placed in a 40 μM cell strainer and washed with PBS. The tissue was disrupted with the plunger from a 1-ml syringe. Cells were isolated by centrifugation of this suspension at 1,000 rpm at 25°C for 5 min. The cell pellet was resuspended at concentration of 3 × 106 cells/ml in RPMI, 10% FBS, and PSG in a 75-cm2 cell culture flask (Corning, Corning, NY).
Apoptosis was induced via exposure to UV irradiation with a wavelength of 312 nm for 10 min. Cells were then incubated at 37°C in 5% CO2 for 2.5 h before utilization in apoptotic cell uptake assays. Apoptosis was confirmed by annexin V/propidium iodide staining and analysis by flow cytometry.
Red blood cells (RBC) expressing phosphatidylserine (PS) on the cell surface were also utilized in apoptotic cell uptake assays. Human RBCs were washed in PBS three times. Washed RBCs were then treated with CaCl2 and ionomycin. RBC were incubated for 15 min at 37°C and then washed in PBS before use in experiments.
Ex vivo and in vitro phagocytosis experiments.
Clearance experiments were performed ex vivo with AMs and in vitro with HMDMs. AMs or HMDMs were treated with anti-mouse VEGF antibody (R & D Systems, Minneapolis, MN), anti-mouse VEGF R1 antibody (R & D Systems), or isotype control antibodies. In some experiments, cells were treated with SU5416, a receptor tyrosine kinase inhibitor specific for VEGF receptors (kindly provided by Dr. Laimute Taraseviciene-Stewart), or DMSO control. AMs were pretreated for 2 h and then cocultured with apoptotic thymocytes at a 10:1 ratio (thymocyte:AM). For supplementation experiments, recombinant mouse (rm) VEGF120, rmVEGF164, and placenta growth factor-2 (PlGF-2) (R & D systems) were added to cells in culture. For experiments involving HMDMs, 6- to 8-day-old HMDMs were cocultured with apoptotic Jurkat cells suspended in 500 μl of X-vivo at 37°C in 10% CO2 in the absence of human serum for 1 h at a ratio of 5:1 (apoptotic cell:HMDM). Cells were then gently washed to remove uningested cells and then fixed and stained with a modified Wright-Giemsa stain (Fisher Scientific, Waltham, MA).
Uptake experiments were also performed with latex beads. Nonmodified or carboxylate-modified 5-μm beads (Bangs Laboratories, Fishers, IN) were coincubated with AMs for 30 min in X-vivo serum-free media at 37°C in 10% CO2 for 30 min. Cells were then washed to remove uningested beads before fixation and staining.
Phagocytosis was determined by visual inspection of samples and expressed as a phagocytic index. The phagocytic index was calculated by counting total apoptotic cell ingestions divided by 200 HMDMs or 400 AMs multiplied by 100.
In vivo phagocytosis experiments.
To assess effect of VEGF receptor blockade, mice were treated with SU5416 by subcutaneous injection at doses of 20 or 200 mg/kg. Control mice were treated with subcutaneous carboxymethylcellulose, which is the vehicle for SU5416. Mice were anesthetized with isoflurane, and 10 × 106 apoptotic thymocytes in 50 μl PBS were instilled intratracheally via a gavage needle at day 7 after SU5416 treatment, as previously described (27, 31, 37, 40). One hour after instillation, mice were euthanized and AMs were harvested by BAL. Mice were lavaged with three 1-ml aliquots of cold PBS with 5 mM EDTA. Cytospin was performed with 150 μl of lavage fluid. Cells were fixed and stained with modified Wright-Giemsa stain, and phagocytosis was assessed by visual inspection.
C57BL/6 mice were also treated with anti-VEGF R1 (MF-1), anti-VEGF R2 (DC101), rat IgG, or PBS control (ImClone Systems, Bridgewater, NJ). Mice were treated with an 800 μg intraperitoneal dose of antibody or isotype on days 0, 3, and 5. One group of mice was also treated with anti-VEGF R1 and anti-VEGF R2 concurrently (total ab dose 1,600 μg). On day 6, mice were exposed to apoptotic cells intratracheally and harvested as described above.
For VEGF supplementation experiments, wild-type and VEGF overexpressor mice were fed either regular chow or doxycycline-containing chow for either 3 or 7 days before experimentation. Mice were subsequently handled in a manner identical to prior in vivo experiments.
Rac1 activation.
Rac1 activation was assessed using the G-LISA Rac1 Activation Assay Biochem Kit (Cytoskeleton, Denver, CO) (36). RAW 264.7 cells (ATCC) were plated and cultured in ATCC DMEM with 10% ATCC FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin for 24 h. RAW 264.7 cells were serum starved in X-vivo for 18 h to minimize background Rac1 activation before treatment with rmVEGF164. After treatment, RAW 264.7 cells were lysed and snap frozen. Samples were then processed as described in the manufacturer's instructions. Briefly, samples were thawed and added to a plate coated with Rac-GTP binding protein. After a wash step, an antigen-presenting buffer was applied followed by primary and secondary antibody binding steps. The result was detected by addition of horseradish peroxidase detection agent followed by measurement of the absorbance at 490 nm on a microplate spectrophotometer (Biotek, Winooski, VT).
Statistics.
Data are presented as means ± SE of at least three independent experiments. Statistical analysis was performed with JMP software (SAS, Cary, NC). In vitro and ex vivo studies were analyzed using paired t-tests. The Tukey-Kramer method was utilized for multiple group comparison of in vivo studies using the VEGF overexpressor mice.
RESULTS
VEGF blockade inhibits uptake of apoptotic cells by murine AMs.
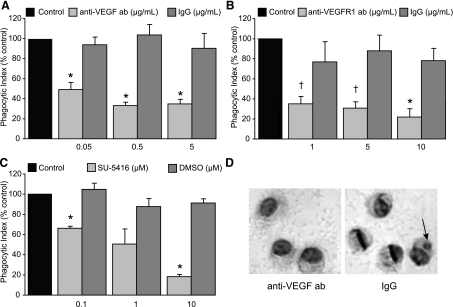
To evaluate the effect of VEGF on uptake of apoptotic cells, murine AMs were treated with reagents that depleted VEGF or disrupted VEGF signaling. AMs obtained by lavage were cultured for 24 h in the presence of complete media. Anti-VEGF neutralizing antibody or isotype control were added to the AMs in complete media for 2 h before the addition of apoptotic thymocytes. After coincubation for 2 h, AMs were fixed and stained, and phagocytic uptake was assessed by microscopy. Treatment with anti-VEGF neutralizing antibody decreased phagocytic uptake by murine AMs (Fig. 1A).
Fig. 1.
Inhibition of vascular endothelial growth factor (VEGF) signaling decreases uptake of apoptotic cells by alveolar macrophages (AMs). A–C: phagocytic uptake of apoptotic thymocytes after 2 h of coculture with murine AMs. AMs in complete media were pretreated for 2 h before addition of apoptotic thymocytes. A: AMs cultured in the presence of VEGF-depleting antibody or IgG isotype control. N = 4. B: AMs treated with VEGF R1 blocking antibody or IgG isotype. N = 3. C: AMs treated with SU-5416, a VEGF receptor tyrosine kinase inhibitor, or DMSO. N = 3. D: uptake of apoptotic cells by AMs treated with VEGF-depleting antibody or IgG isotype control. Aarrowhead, apoptotic body. Control phagocytic indices were 3.5 ± 0.4 (A), 3.0 ± 0.5 (B), and 4.1 ± 0.1(C). *P < 0.05 versus isotype control. †P < 0.06 isotype control.
To test the role of VEGF R1 in AM efferocytic function, AMs were treated with a VEGF R1 blocking antibody in a manner identical to the previously described experiment. VEGF R1 blockade also decreased phagocytic uptake in a dose-dependent manner (Fig. 1B). The importance of VEGF receptor signaling on efferocytosis was further assessed by treating AMs with SU5416, a VEGF receptor tyrosine kinase inhibitor, or DMSO control for 2 h before coincubation with apoptotic cells. SU5416-treated AMs had decreased uptake of apoptotic cells (Fig. 1C). These experiments indicate that VEGF and VEGF receptor signaling enhance the uptake of apoptotic cells.
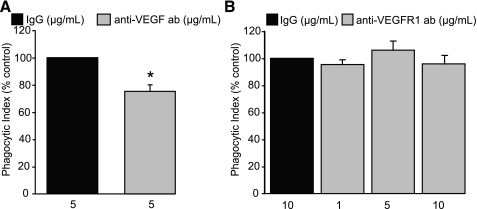
Decreased presence of apoptotic bodies in anti-VEGF-treated AMs could represent diminished uptake or enhanced digestion of apoptotic cells. To determine whether depletion of VEGF or disruption of VEGF signaling altered digestion of apoptotic cells, we performed uptake experiments using carboxylate-modified latex beads. Carboxylate-modified latex beads have a similar surface charge and mimic the behavior of apoptotic cells in uptake assays (9). They also offer the benefit of not being degraded within the phagosome. Anti-VEGF neutralizing antibody or isotype control were added to AMs in complete media for 2 h. After washing was completed, 5 μm carboxylate-modified beads were added to the AMs in a 1:1 ratio in serum-free media. AMs and beads were coincubated for 30 min. Noningested beads were washed off and AMs were then fixed and stained. Consistent with our data using apoptotic cells as targets, anti-VEGF neutralizing antibody inhibited the uptake of carboxylate-modified beads (Fig. 2A). This experiment confirms that the decreased phagocytic index noted following VEGF neutralization is due to inhibited uptake and not enhancement of digestion.
Fig. 2.
Inhibition of VEGF signaling decreases uptake of carboxylate-modified latex beads but not nonmodified latex beads. A and B: phagocytic uptake of 5-μm latex beads after 30 min of coculture with murine AMs. AMs were pretreated for 2 h before addition of beads. A: AMs treated with VEGF-depleting antibody or isotype control and then incubated with 5 μm carboxylate-modified beads. N = 4. B: AMs treated with VEGF R1-blocking antibody and exposed to 5 μm nonmodified latex beads for 30 min. N = 2. *P < 0.05 versus isotype control.
Efferocytosis is a unique method of phagocytosis in which tethering between apoptosis-related recognition molecules on the dying cell and receptor complexes on the phagocyte leads to apoptotic cell engulfment. To determine whether the effect of VEGF on macrophage phagocytosis extended to other forms of phagocytosis, AMs were exposed to nonmodified latex beads. AMs in complete media were pretreated for 2 h with anti-VEGF R1 or IgG isotype control antibodies. Nonmodified latex beads (5 μm) were then added to the AMs and coincubated for 30 min. VEGF R1 blockade did not inhibit nonmodified latex bead uptake by AMs (Fig. 2B), suggesting that the effect of VEGF on AM phagocytosis is not universal and may be specific for apoptotic cells.
VEGF supplementation enhances apoptotic cell uptake after media VEGF depletion.
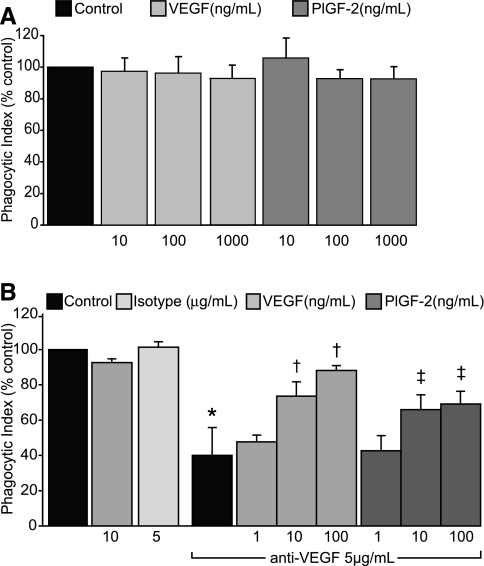
To determine whether VEGF supplementation could enhance ingestion of apoptotic cells, murine AMs were treated with increasing concentrations of rmVEGF or placenta growth factor (PlGF)-2, a member of the VEGF family that exclusively binds and signals through VEGF R1 (29). Contrary to our hypothesis, the addition of rmVEGF120 or PlGF-2 did not enhance apoptotic cell uptake above that of untreated AMs cultured in complete media (Fig. 3A). The addition of rmVEGF164 also failed to augment apoptotic cell uptake (Fig. 3B).
Fig. 3.
VEGF enhances uptake of apoptotic cells by murine alveolar macrophages. A: AMs were cultured in complete media and pretreated with rmVEGF120 or rmPlGF-2 for 2 h before addition of apoptotic thymocytes. Phagocytic uptake was assessed after 2 h of coculture. Control phagocytic index was 3.7 ± 0.8. N = 2. B: AMs were cultured for 24 h in DMEM with 10% FCS and a VEGF neutralizing antibody. Media was removed, and apoptotic thymocytes were added in serum-free media supplemented with rmVEGF164 or PlGF-2. Phagocytosis was assessed 3 h later. Control phagocytic index was 3.5 ± 0.5. N = 3.*P < 0.05 versus IgG isotype control. †P < 0.05 versus anti-VEGF ab-treated AMs. ‡P < 0.07 versus anti-VEGF ab treated AMs.
Since our prior experiments suggested that VEGF neutralization decreased apoptotic cell uptake, we questioned whether bovine VEGF present within cell culture media was maximally stimulating apoptotic cell uptake under basal conditions. To assess this possibility, AMs were pretreated with anti-VEGF antibody at a dose previously shown to decrease apoptotic cell uptake. Complete media was then removed from the AMs. Apoptotic thymocytes were added to the AMs in serum-free media with supplementary doses of VEGF164 or PlGF-2 in concentrations ranging from 0.1 to 100 ng/ml. These doses are comparable to VEGF levels in human respiratory epithelial lining fluid which ranges from 6 to 16 ng/ml (20). AMs pretreated with anti-VEGF antibody had significantly decreased uptake of apoptotic cells relative to AMs maintained in complete media or media treated with IgG isotype (Fig. 3B). AMs supplemented with VEGF164 after VEGF depletion from the media had significantly enhanced apoptotic cell uptake in a dose-dependent manner. Furthermore, there was a trend toward increased apoptotic cell uptake in AMs treated with 100 ng/ml of PlGF-2 (P = 0.066) after VEGF depletion, suggesting that VEGF promotes efferocytosis through engagement of VEGF R1.
The ability to restore apoptotic cell phagocytic activity with VEGF supplementation after VEGF depletion also suggests that inhibition of VEGF or VEGF signaling did not affect AM cellular viability in the time periods utilized in our experiments. This is also supported by our data in which phagocytic uptake of latex beads by AMs was not affected by treatment with anti-VEGF R1 antibody (Fig. 2B).
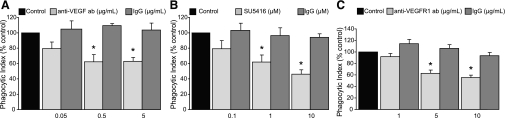
VEGF blockade diminishes uptake of apoptotic cells by HMDMs.
To determine whether the pro-efferocytic effects of VEGF extended to human cells, HMDMs were cocultured with apoptotic cells. VEGF signaling was interrupted by anti-VEGF neutralizing antibody or VEGF R1 tyrosine kinase activity was blocked using SU5416. Control HMDMs were cocultured with apoptotic human Jurkat T-cells in media alone or in the presence of an isotype antibody. Consistent with our data in murine AMs, neutralization of VEGF with the anti-VEGF antibody decreased uptake of apoptotic cells (Fig. 4A). To confirm that VEGF was exerting its effect though VEGF receptors, VEGF receptor tyrosine kinases were inhibited with SU5416. Control HMDMs were cocultured in media with DMSO. VEGF receptor signaling inhibition decreased the efferocytic activity of HMDMs (Fig. 4B).
Fig. 4.
Inhibition of VEGF signaling decreases apoptotic cell uptake by human monocytes-derived macrophages (HMDMs). A: HMDMs were treated with VEGF-depleting antibody or IgG isotype, and cocultured with apoptotic Jurkat cells. Phagocytosis was assessed 1 h later. Control phagocytic index was 20.1 ± 1.1. N = 5. B: HMDMs were treated with SU5416 or DMSO control and cocultured with apoptotic Jurkat cells. Control phagocytic index was 20.9 ± 1.2. N = 5–6. C: HMDMs were treated with VEGF R1 blocking antibody or IgG isotype control and exposed to phosphatidylserine-expressing red blood cells (RBCs). Phagocytosis was assessed 1 h later. Control phagocytic index was 109.7 ± 24.2. N = 4. *P < 0.05 versus isotype control.
Exposure of phosphatidylserine (PS) on the surface of the apoptotic cells is a major recognition signal that promotes efferocytosis (17). To determine whether blockade of VEGF R1 would inhibit phagocytosis of PS-expressing cells, RBCs were treated with ionomycin to induce cell surface PS expression and then added to HMDMs in the presence of VEGF R1 blocking antibody or isotype control. Blockade of VEGF R1 led to a dose-dependent inhibition of efferocytosis by HMDMs, supporting a role for VEGF-VEGF R1 interaction in PS-mediated uptake of apoptotic cells by macrophages (Fig. 4C). Consistent with our data using murine AMs, these studies illustrate that efferocytosis by HMDMs is regulated via a VEGF/VEGF R1 mechanism.
VEGF enhances Rac1 activation.
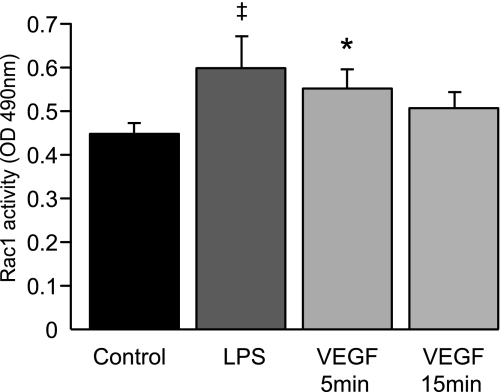
Macrophage membrane ruffling and resultant efferocytosis are enhanced by activation of the Rho-GTPase Rac1 (23). VEGF activates Rac1 in endothelial cells but its effect on macrophages is unknown (11, 34). To determine whether VEGF enhances macrophage Rac1 activation, RAW 264.7 cells were cultured and treated with rmVEGF164. RAW 264.7 cells, which are a murine monocyte macrophage cell line, were utilized due to the high number of macrophages required to obtain adequate protein concentration for the assay. VEGF treatment of RAW 264.7 cells increased Rac1 activation (Fig. 5). This experiment illustrates a possible mechanism for VEGF-mediated enhancement of efferocytosis.
Fig. 5.
VEGF supplementation enhances Rac1 activation in RAW 264.7 cells. After serum starvation in X-vivo, RAW 264.7 cells were treated with rmVEGF164 (100 ng/ml), LPS-positive control (200 ng/ml), or PBS control. Cells were lysed, harvested, and processed using the Rac1 G-Lisa activation assay. N = 4. *P < 0.05 versus control. ‡P < 0.07 versus control.
Inhibition of VEGF receptor activity in the naïve mouse does not alter apoptotic cell clearance.
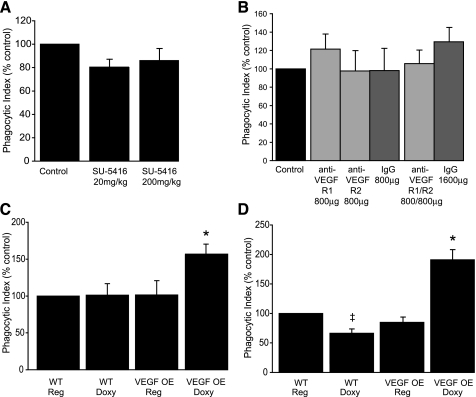
To test whether VEGF receptor inhibition would impair uptake of apoptotic cell in vivo, experimental mice were treated with SU5416 at doses of 20 mg/kg and 200 mg/kg. SU5416 has previously been shown to induce airspace enlargement and alveolar cell apoptosis in mice 4 wk after a single subcutaneous treatment of 20 mg/kg (30). To avoid potential confounding effects of structural lung disease, apoptotic cells were instilled directly into the tracheas of experimental animals 7 days after SU5416 administration. AMs were isolated by BAL 1 h after instillation, and efferocytosis was assessed using light microscopy of Wright-Giemsa-stained cytospins. AMs from SU5416-treated mice did not have a significant decrease in apoptotic cell uptake (Fig. 6A).
Fig. 6.
VEGF augmentation enhances apoptotic cell uptake but VEGF receptor inhibition does not decrease efferocytic activity in the murine lung. A: C57BL/6 mice were treated with a single subcutaneous dose of SU5416 or vehicle control. Apoptotic thymocytes were intratracheally instilled 7 days after SU5416 treatment. AM phagocytosis was assessed 1 h later. Control phagocytic index was 5.0 ± 0.5. N ≥ 19 per group. P = 0.12 for SU5416 20 mg/kg dose. B: C57BL/6 mice were treated with intraperitoneal anti-VEGF R1 (800 μg), anti-VEGF R2 (800 μg), both anti-VEGF R1 and anti-VEGF R2, or rat IgG on days 0, 3, and 5. Apoptotic thymocytes were administered intratracheally on day 6. Phagocytosis was assessed in AMs from bronchoalveolar lavage (BAL) 1 h later. Control phagocytic index was 7.1 ± 1.9. N = 6 per group. C and D: transgenic mice with doxycycline (doxy)-inducible expression of human VEGF165 and C57BL/6 mice were treated with doxy-containing or regular chow. At 3 (C) or 7 (D) days after transgene activation, apoptotic thymocytes were instilled intratracheally. AMs were then harvested by BAL 1 h after instillation and phagocytic uptake was assessed. WT, wild-type mice; OE, overexpressor mice. N = 11–12 per group and 5 per group, respectively. *P < 0.05 versus WT/doxy, VEGF OE/Reg, and WT/Reg. ‡P < 0.05 versus WT/Reg.
To further assess the effect of VEGF receptor blockade on AM efferocytic activity, mice were treated systemically with blocking antibodies to VEGF R1, VEGF R2, or both VEGF R1 and VEGF R2. Monoclonal antibody blockade of VEGF R1 and R2 has previously been shown to induce alveolar cell apoptosis when used in combination at 4 wk (30). Mice were treated with three intraperitoneal injections of antibody (days 0, 3, and 5) and then underwent intratracheal instillation of apoptotic cells on day 6. One hour later, AMs were harvested by BAL, and efferocytosis was assessed. As shown in Fig. 6B, VEGF receptor blocking antibody treatment also did not significantly alter apoptotic cell uptake.
VEGF overexpression augments AM uptake of apoptotic cells in vivo.
To further test the hypothesis that enhanced VEGF expression would increase efferocytosis, transgenic mice that overexpress human VEGF165 in the lungs in a doxycycline-dependent manner were used (24). VEGF overexpressor mice and matched littermate controls were fed normal chow or doxycycline-supplemented chow for 3 or 7 days. Doxycycline-supplemented chow resulted in a four- to fivefold increase in human VEGF in the BAL fluid in the VEGF overexpressor mice but did not alter BAL cell counts (Table 1). After doxycycline induction of VEGF, mice were intratracheally instilled with apoptotic thymocytes. One hour after thymocyte instillation, the mice were euthanized, and BAL was performed. VEGF overexpression increased ingestion of apoptotic cells in vivo at both day 3 and 7 relative to wild-type mice treated with doxycycline and VEGF overexpressor mice without transgene activation (Fig. 4, C and D). VEGF overexpressor mice that did not receive doxycycline had uptake of apoptotic cells similar to wild-type mice. There was a minor decrement in apoptotic cell uptake at day 7 in wild-type mice given a doxycycline diet relative to wild-type mice given a regular diet. Doxycycline has a multitude of effects but to our knowledge its effect on apoptotic cell uptake has not been studied. Regardless, VEGF overexpressing mice had enhanced apoptotic cell uptake on both day 3 and 7 relative to all groups studied.
Table 1.
Transgenic mice with doxycycline-inducible expression of human vascular endothelial growth factor
| VEGF165, pg/ml | Macrophages, Cells × 104/ml | Lymphocytes, Cells × 104/ml | |
|---|---|---|---|
| VEGF OE | |||
| Doxy | 429 ± 31* | 7.03 ± 4.57 | 0.13 ± 0.10 |
| Ctrl | 81 ± 16 | 8.49 ± 3.88 | 0.50 ± 0.32 |
| Wild-type | |||
| Doxy | 45 ± 12 | 6.95 ± 2.32 | 0.21 ± 0.17 |
| Ctrl | 69 ± 27 | 3.86 ± 1.78 | 0.30 ± 0.15 |
Transgenic mice with doxycycline (Doxy)-inducible expression of human vascular endothelial growth factor 165 (VEGF165) and C57BL/6 mice were treated with doxycycline-containing or regular chow. Bronchoalveolar lavage (BAL) was performed on day 7 of transgene activation. VEGF levels were obtained by ELISA of BAL supernatants. Cell counts and differentials were also performed on the BAL fluid. Ctrl, control; OE, overexpressor mice.
P < 0.001 versus C57BL/6 mice given doxycycline or regular diet.
DISCUSSION
We describe a novel regulatory role for VEGF and its cognate receptor VEGF R1 on efferocytosis by macrophages. Depletion of VEGF, VEGF R1 blockade, and inhibition of VEGF receptor signaling were all shown to inhibit apoptotic cell uptake by murine AMs and HMDMs. We demonstrate that this effect is in part related to PS expression and is not generalized to other forms of phagocytic uptake. Our in vivo models illustrate that augmented VEGF enhances macrophage efferocytic function.
Given the complex nature of apoptotic cell clearance, there are many factors that VEGF could influence. For successful efferocytosis to occur, multiple interactions must happen including: 1) expression of “eat-me” signals on apoptotic cells, 2) migration of phagocytic cells to the apoptotic target or random cell-to-cell contact, 3) interaction between cell surface receptors and bridging molecules on the two cells, and 4) engulfment of the apoptotic cell. Engulfment of apoptotic cells occurs through a process known as ruffling in which cytoskeletal and morphological changes occur to create lamellipodia which surround the target cell (17). This process is regulated by the relative balance of members of the Rho-GTPase family, which includes Rac1 and RhoA (28). Rac1 activation enhances membrane ruffling and efferocytosis, whereas RhoA activation inhibits the process (23). Activation of Rac1 in AM by molecules like MCP-1 enhances efferocytosis (36). Our studies indicate that VEGF also activates Rac1 in cells of a monocyte/macrophage lineage.
The implication of VEGF/VEGF R1 interaction on macrophages phagocytic function has not been previously studied. VEGF signals via VEGF R1 (Flt-1) in AMs (7). VEGF R1 mediates anti-apoptotic gene expression in macrophages and is implicated in VEGF-induced migration of monocytes and peritoneal macrophages (1, 13, 33). It is suggested that VEGF R1 serves to trap free VEGF and limit VEGF R2 signaling (29). This stems from VEGF R1's high affinity for VEGF but limited kinase activity relative to VEGF R2 (13). Our studies, therefore, offer a novel role for VEGF/VEGF R1 interaction in macrophages to enhance efferocytosis.
Depletion of VEGF ex vivo led to a decrease in apoptotic cell uptake by macrophages, suggesting a direct role for VEGF in enhancing apoptotic cell clearance. Surprisingly, the addition of VEGF or PlGF-2 to cultured AMs in complete media failed to enhance apoptotic cell clearance above basal levels. VEGF supplementation only enhanced efferocytosis in AMs that were previously cultured in VEGF-depleted media. Treatment of AMs in complete media with a VEGF-depleting antibody decreased basal levels of efferocytosis by ∼50%. AM phagocytic capacity was restored in a dose-dependent manner in these experiments with VEGF164 supplementation. The most likely explanation for these observations is that VEGF present in the fetal bovine serum enhanced the baseline level of efferocytosis of the AMs. In fact, the concentration of VEGF in DMEM with 10% FBS is ∼60 pg/ml (26).
Treatment with the VEGF receptor tyrosine kinase inhibitor SU5416 and VEGF R1/VEGF R2 blocking antibodies have been shown to induce alveolar cell apoptosis in vivo (22, 30). However, these studies did not explore the effect of VEGF receptor inhibition on AMs. Based on our ex vivo studies, we hypothesized that treatment with SU5416 or anti-VEGF receptor antibodies would inhibit uptake of apoptotic cells by AMs. However, in vivo VEGF receptor blockade or VEGF receptor activity inhibition did not influence efferocytic activity of AMs. The failure to suppress AM efferocytic function in our studies may be related to a number of factors. One possibility is that the modes of delivery (i.e., intraperitoneal for blocking antibodies and subcutaneous for SU5416) may have resulted in antibody or drug concentrations in the alveolus that were too low to inhibit macrophage efferocytic function. Alternatively, the ability of VEGF to support efferocytosis in vivo may be more complex than in our in vitro model. For example, the effects of VEGF in vivo may depend on the induction of yet-to-be-identified cofactors, such as one of the many receptors or opsonins that support efferocytosis. These cofactors may persist with very low levels of VEGF receptor activity or may not immediately decrease in response to impaired VEGF signaling.
Our studies utilizing VEGF-overexpressing mice confirm that VEGF is important for the efferocytic activity of AMs. Efferocytosis is dependent on the expression and function of a number of cell surface receptors and soluble bridging molecules. The lung collectins SP-A and SP-D influence efferocytosis, and their levels may be regulated by VEGF. VEGF upregulates SP-A and SP-D in ovine fetal lung and increases SP-A and SP-C mRNA expression in human fetal lung explants (5, 6, 35). The lung collectins, most specifically SP-D, bind calreticulin on apoptotic cell surfaces and direct uptake via CD91 (40). Individuals who smoke or who have emphysema have decreased BAL SP-D levels and deficient AM efferocytic activity (14–16, 41). VEGF may function to upregulate or activate any number of these factors.
The potential for VEGF to upregulate apoptotic cell receptors or influence collectin production could explain the absence of enhancement of efferocytic activity with VEGF supplementation ex vivo. The abbreviated exposure to VEGF supplementation in culture may not allow time for upregulation and expression of cell surface receptors and bridging molecules involved in efferocytosis. VEGF may also affect protein production by lung epithelial cells that enhance efferocytosis.
Prolonged overexpression of lung-specific VEGF leads to a TH2-related asthma phenotype (24). In the initial description of this model, increased leukocytes were present in digested lung tissue as early as 2 days after doxycycline induced VEGF enhancement and in the air spaces by day 14 (3). Since acute inflammation is known to increase phagocytic capacity of AMs, we chose to perform our experiments at days 3 and 7 to minimize the presence of alveolar inflammatory cells (19). In our experiments performed at day 7 after VEGF transgene activation, no increase in leukocyte cell count or differential in BAL fluid was observed. The absence of acute inflammation in our VEGF-overexpressing mice could be multifactorial. In addition to using an earlier time point than Bhandari et al., we utilized doxycycline chow instead of doxycycline water, which resulted in lower BAL VEGF levels than previously described (4, 24). In contrast to the enhancement of AM phagocytic activity in acute inflammation, AMs from humans with severe persistent asthma have deficient uptake of apoptotic cells, relative to subjects with mild-moderate asthma (10, 18). We therefore chose to expose the mice to apoptotic cells at early time points to minimize the influence of the VEGF-induced asthma phenotype on efferocytosis.
In conclusion, our investigations indicate a novel role for VEGF via VEGF R1 signaling on AM apoptotic cell clearance. Our ex vivo studies demonstrate that VEGF depletion and VEGF R1 inhibition decreases macrophage efferocytic activity without clear effect on macrophage viability. Our studies also indicate that VEGF supplementation only enhances macrophage apoptotic cell clearance ex vivo if VEGF has previously been depleted from the media. In contrast, we demonstrate that pulmonary VEGF supplementation enhances AM apoptotic cell clearance in vivo. These studies suggest that in addition to its direct effect on AMs, VEGF may influence other factors in the lung that enhance uptake.
GRANTS
This work was supported by National Institutes of Health Grants HL-88138 (to P. M. Henson and R. W. Vandivier), GM-61031 and HL068864 (to P. M. Henson), and PPG HL-34303 (to D. L. Bratton) and by Flight Attendant Medical Research Institute Clinical Innovator Awards 072001_CIA and 092054_CIA (to R. W. Vandivier).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.T.K., S.D., T.R.R., N.F.V., L.T.-S., W.J.J., C.G.L., J.A.E., D.B., R.M.T., P.M.H., and R.W.V. conception and design of research; M.T.K., S.D., S.A.H., T.R.R., T.T., J.M.D., D.M.B., and R.W.V. performed experiments; M.T.K., S.D., S.A.H., T.R.R., T.T., J.M.D., D.M.B., and R.W.V. analyzed data; M.T.K., S.D., S.A.H., T.R.R., T.T., D.M.B., D.B., P.M.H., and R.W.V. interpreted results of experiments; M.T.K. prepared figures; M.T.K. drafted manuscript; M.T.K., N.F.V., W.J.J., J.A.E., P.M.H., and R.W.V. edited and revised manuscript; M.T.K. and R.W.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank ImClone Systems for the kind contribution of reagents. We also thank Weipeng Xiong for contributions.
REFERENCES
- 1. Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res 62: 2749– 2752, 2002 [PubMed] [Google Scholar]
- 2. Berse B, Brown LF, Van De Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differenetially in normal tissues, macrophages, and tumors. Mol Biol Cell 3: 211– 220, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhandari V. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci USA 103: 11021– 11026, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, Ma B, Baluk P, Lin MI, McDonald DM, Homer RJ, Sessa WC, Elias JA. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci USA 103: 11021– 11026, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown KRS, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol 281: L1001– L1010, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Chen C, Wang L. High-dose vascular endothelial growth factor increases surfactant protein gene expressions in preterm rat lung. Early Hum Dev 83: 581– 584, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Clauss M, Weich H, Breier G, Knies U, Röckl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor flt-1 mediates biological activities. J Biol Chem 271: 17629– 17634, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Droemann D. Decreased apoptosis and increased activation of alveolar neutrophils in bacterial pneumonia. Chest 117: 1679– 1684, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci USA 103: 12825– 12830, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fitzpatrick AM, Holguin F, Teague WG, Brown LAS. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol 121: 1372– 1378, e1373, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res 313: 3285– 3297, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 296: L936– L946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 95: 9349– 9354, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hodge S, Hodge G, Ahern J, Jersmann H, Holmes M, Reynolds PN. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 37: 748– 755, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Hodge S, Hodge G, Jersmann H, Matthews G, Ahern J, Holmes M, Reynolds PN. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. A J Respir Crit Care Med 178: 139– 148, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 81: 289– 296, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol 155: 649– 660, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huynh MLN. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med 172: 972– 979, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Janssen WJ, McPhillips KA, Dickinson MG, Linderman DJ, Morimoto K, Xiao YQ, Oldham KM, Vandivier RW, Henson PM, Gardai SJ. Surfactant proteins A and D suppress alveolar macrophage phagocytosis via interaction with SIRP. Am J Respir Crit Care Med 178: 158– 167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaner R, Crystal R. Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mo Med 7: 240– 246, 2001 [PMC free article] [PubMed] [Google Scholar]
- 21. Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 163: 737– 744, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311– 1319, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, Schnabel R, Hengartner MO. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 434: 93– 99, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, Elias JA. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nature Med 10: 1095– 1103, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matute-Bello G, Liles WC, Radella F, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 1997: 1969– 1977, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Mitra SK, Mikolon D, Molina JE, Hsia DA, Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG, Cheresh DA, Schlaepfer DD. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene 25: 5969– 5984, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, Xiao YQ, Kench JA, Henson PM, Vandivier RW. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol 176: 7657– 7665, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Nakaya M, Kitano M, Matsuda M, Nagata S. Spatiotemporal activation of Rac1 for engulfment of apoptotic cells. Proc Natl Acad Sci USA 105: 9198– 9203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269: 25646– 25654, 1994 [PubMed] [Google Scholar]
- 30. Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, Zhang B, Song S, Hicklin D, Voelkel NF, Flotte T, Tuder RM. A Novel antiapoptotic role for α1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med 173: 1222– 1228, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richens TR, Linderman DJ, Horstmann SA, Lambert C, Xiao YQ, Keith RL, Boe DM, Morimoto K, Bowler RP, Day BJ, Janssen WJ, Henson PM, Vandivier RW. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am J Respir Crit Care Med 179: 1011– 1021, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 83: 865– 875, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sawano A. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97: 785– 791, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Soga N, Connolly JO, Chellaiah M, Kawamura J, Hruska KA. Rac regulates vascular endothelial growth factor stimulated motility. Cell Commun Adhes 8: 1– 13, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Sow FB, Gallup JM, Meyerholz DK, Ackermann MR. Gene profiling studies in the neonatal ovine lung show enhancing effects of VEGF on the immune response. Dev Comp Immunol 33: 761– 771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanaka T, Terada M, Ariyoshi K, Morimoto K. Monocyte chemoattractant protein-1/CC chemokine ligand 2 enhances apoptotic cell removal by macrophages through Rac1 activation. Biochem Biophys Res Commun 399: 677– 682, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Puré E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 296: 155– 158, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Tuder RM. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 29: 88– 97, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Vandivier RW. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129: 1673– 1682, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 169: 3978– 3986, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Winkler C, Atochina-Vasserman E, Holz O, Beers M, Erpenbeck V, Krug N, Roepcke S, Lauer G, Elmlinger M, Hohlfeld J. Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Resp Res 12: 29, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]