Abstract
Autophagy is a process for cells to degrade proteins or entire organelles to maintain a balance in the synthesis, degradation, and subsequent recycling of cellular products. Increased reactive oxygen species formation is known to induce autophagy. We previously reported that increased NADPH oxidase (NOX) activity in pulmonary artery endothelial cells (PAEC) from fetal lambs with persistent pulmonary hypertension (PPHN) contributes to impaired angiogenesis in PPHN-PAEC compared with normal PAEC. We hypothesized that increased NOX activity in PPHN-PAEC is associated with increased autophagy, which, in turn, contributes to impaired angiogenesis in PPHN-PAEC. In the present study, we detected increased autophagy in PPHN-PAEC as shown by increased ratio of the microtubule-associated protein 1 light chain (LC3)-II to LC3-I and increased percentage of green fluorescent protein-LC3 punctate positive cells. Inhibiting autophagy by 3-methyladenine, chloroquine, and beclin-1 knockdown in PPHN-PAEC has led to decreased autophagy and increased in vitro angiogenesis. Inhibition of autophagy also decreased the association between gp91phox and p47phox, NOX activity, and superoxide generation. A nonspecific antioxidant N-acetylcysteine and a NOX inhibitor apocynin decreased autophagy in PPHN-PAEC. In conclusion, autophagy may contribute to impaired angiogenesis in PPHN-PAEC through increasing NOX activity. Our results suggest that, in PPHN-PAEC, a positive feedback relationship between autophagy and NOX activity may regulate angiogenesis.
autophagy, or self-eating, is a regulated cellular process to reutilize intracellular organelles and proteins during starvation or nutrient deficiency. Autophagy also functions as a quality-control mechanism for cells to handle ubiquitinated or misfolded proteins (15). During autophagy, some of the cytoplasmic components are sequestered into double-membrane vesicles (autophagosomes) and degraded upon fusion with lysosomal compartments (27). Autophagy is believed to serve either as a regulator in cellular homeostasis to prolong survival during nutrient deprivation, via regenerating metabolic precursors for macromolecular synthesis and ATP generation, or to serve as a quality-control mechanism (basal autophagy) by selective disposal of protein aggregates and damaged organelles (27, 39). However, elevated autophagy is also observed in dying cells (10, 21, 23). Increased autophagy has been reported in kringle 5- and endostatin-induced angiogenesis inhibition (36) and regression of hyaloid vessels during ocular development (17), and both were reported to associate with increased apoptosis.
There are three forms of autophagy: microautophagy, macroautophagy, and chaperone-mediated autophagy (17, 24). More than 30 autophagy-related proteins are involved in autophagy, and one of them is the microtubule-associated protein 1 light chain (LC3), which is important in the formation of autophagosome (43). During autophagy, phosphatidylethanolamine will conjugate to the cytosolic form of LC3 (LC3-I) to form LC3-II (8), and the LC3-II/LC3-I ratio is commonly used as an indicator for autophagy (19). Autophagy participates in a wide variety of physiological and pathophysiological processes, including embryonic development (2, 34). Its function has been studied extensively in lower eukaryotes, but data in vertebrate development remain limited (2). Basal autophagy represents a reparative, life-sustaining process, but, paradoxically, unrestrained autophagic activity may promote cell death (type II program cell death) (7, 25) or apoptosis (38). The role of autophagy in the pathogenesis of pulmonary vascular disease remains unclear, especially during the early developmental stage (38).
Persistent pulmonary hypertension (PPHN) is a severe lung disease with high mortality that affects neonates during the perinatal transition (48). Impaired vasorelaxation and decreased blood vessel density that leads to increased pulmonary vascular resistance are key components of altered adaptation in PPHN (13). PPHN is associated with endothelial dysfunction and decreased nitric oxide (NO) production (6, 46). Intrauterine ductus arteriosus constriction of fetal lambs during late gestation is the most commonly used animal model to study PPHN (9, 29). Using pulmonary artery endothelial cells (PAEC) from this model, our group has reported that uncoupled endothelial nitric oxide synthase (eNOS) activity contributes to the decreased NO production with increased superoxide (O2−) formation (20). We recently also showed that PAECs from this animal model (PPHN-PAEC) have increased NADPH oxidase (NOX)-mediated O2− formation that contributes to increased apoptosis and impaired in vitro angiogenesis. N-acetylcysteine (NAC) (nonspecific antioxidant) and apocynin (NOX inhibitor) have protective effects that decrease apoptosis and improve angiogenesis in these cells (44). Recent studies have demonstrated that reactive oxygen species (ROS) are important signaling molecules in autophagy (39). Because O2− is known to be the major ROS that regulates autophagy (5), it is logical to consider that increased O2− production can also lead to increased autophagy in PPHN-PAEC. We hypothesize that 1) autophagy is increased in PPHN-PAEC compared with control PAEC, 2) autophagy is proapoptotic in PPHN-PAEC, and 3) increased autophagy contributes to the impaired angiogenesis in PPHN-PAEC.
In this report, we show that a positive feedback exists between NOX activity and autophagy in that inhibition of NOX decreases autophagy, and inhibition of autophagy in turn decreases NOX activity. These findings provide mechanistic explanation of how increased NOX activity contributes to impaired angiogenesis in PPHN-PAEC, which further supports our previous observation of impaired angiogenesis in PPHN-PAEC (44).
MATERIALS AND METHODS
All animal studies were approved by Medical College of Wisconsin Institutional Animal Care and Use Committee (IACUC) and conformed to the current guidelines of NIH for care and use of laboratory animals. PPHN was induced by fetal ductus arteriosus constriction at 128 ± 2 days gestation, term gestation being ∼145 days (20). Control fetal lambs received sham operation without ductal constriction. After 8 days of ductal constriction, the ewe was euthanized and fetal lungs were removed en bloc. PAEC were isolated from pulmonary arteries with the use of 0.25% collagenase type A, and cells were grown in endothelial growth media (20). Identity of the cells was verified by staining for factor VIII antigen (14) and by acetylated LDL uptake (47). PAEC from at least five fetal sheep each with PPHN or control were used for experiments between passages 3 and 6. Passage numbers of the control and PPHN-PAEC were the same for all experiments whenever indicated. Lung tissues from the right upper lobe of both PPHN and control lambs (4 in each group) were frozen in liquid nitrogen and kept in −80°C right after dissection.
Rabbit LC3B XP™ antibody was obtained from Cell Signaling (Boston, MA). Goat anti-p47phox antibody, goat anti-p67phox antibody, rabbit and goat anti-gp91phox antibodies, nonspecific normal goat IgG, beclin-1 siRNA (cow), scramble siRNA, transfection medium, and transfection reagent were from Santa Cruz Biotechnology (Santa Cruz, CA). Annexin V-FITC/ propidium iodide (PI) apoptosis detection kit, mouse anti-Rac1 antibody, and growth-factor-reduced Matrigel were from BD Biosciences (Belford, MA). Dihydroethidium (DHE), 4-amino-5-methylamino-2′,7′-difluorofluoresceine diacetate (DAF-FM-DA), Opti-MEM I reduced serum medium, Lipofectamine LTX and Plus reagent, and Lipofectamine RNAiMAX Transfection Reagent were from Invitrogen (Carlsbad, CA). 3-Methyladenine (3MA), chloroquine (CQ), NAC, hydrogen peroxide (H2O2), and sodium 4, 5-dihydroxybenzene-1, 3-disulfonate (Tiron) were from Sigma-Aldrich (St. Louis, MO). 3MA was dissolved in double-distilled water by heating up to 60°C just before use. Apocynin (Apo) was from Calbiochem/EMD Biosciences (La Jolla, CA) and was dissolved in ethanol.
Cell culture.
PAEC were cultured in high glucose Dulbecco Modified Eagle's Medium (DMEM) supplemented with l-glutamine, 20% FCS and 1% antibiotic/antimycotic in humidified incubator at 37°C, 5% CO2-95% room air. Serum starvation was achieved by using 2% FCS in the culture medium, as we previously found that using 1% or less FCS in the media leads to extensive cell death in 2 h.
Enhanced green fluorescence protein (EGFP)-LC3 plasmid transfection.
PAEC were seeded in Lab-Tek II four-well chamber slides (2 × 104 cells/well), and media were changed into Opti-MEM-I for 1 h before transfection. EGFP-LC3 plasmids (4 μg Addgene) were transfected into PAEC using Lipofectamine LTX and Plus reagent, and 12 h later the transfected cells were changed into medium with different treatments for another 12 h before photography was taken by fluorescence microscopy at ×10 (objective) magnification. Cells were randomly inspected and scored by a coauthor that was blinded to the treatments, and the percentages of cells showing GFP-LC3 punctate were calculated. Fifty GFP-positive cells were counted for each field, and cells having more than two GFP-LC3 punctates were taken as positive. For each culture condition, four fields were counted for analysis.
Autophagy inhibition.
3MA (5 mM), a class III phosphatidylinositol 3-kinase (PI3K) inhibitor (45), and CQ (2 μg/ml), which increases lysosomal pH (12, 49), were used to inhibit autophagy and added into culture media 4 h before harvesting the PAEC for analyzing NOX activity, protein expression of NOX2 subunits, and for determining LC3-I and II expressions. NH4Cl (20 mM) was used to inhibit lysosomal enzymes for studying autophagic flux (4).
Increasing ROS stress to PAEC.
H2O2 was used to increase cellular ROS stress for its ability to enter into cells and to increase intracellular O2− production (50). Control PAEC were treated with 40 μM H2O2 for 4 h before harvesting for immunoblots.
Immunoblotting and immunoprecipitation.
After treatments were applied, PAEC were scraped into RIPA buffer containing protease and phosphatase inhibitor cocktails (Sigma). The cell lysates were directly separated by SDS-PAGE and blotted with anti-LC3B antibody (1:1,000), and after electro-transfer to PVDF membrane, horseradish peroxidase-conjugated anti-rabbit IgG antibody (1:10,000; Bio-Rad, Hercules, CA) was used to detect the signals. Immunoblots were also blotted with rabbit anti-gp91phox (1:500), mouse anti-Rac1 (1:1,000), goat, anti-p47phox (1:1,000), or goat anti-p67phox (1:500) and anti-β-actin antibodies. Signals were generated using SuperSignal West Pico (Pierce, Rockford, IL) and recorded on CL-Xposure films (Pierce). The integrated optical density was processed by Image J. The LC3-II/LC3-I ratio was used as an indicator of autophagy (19), and β-actin was used as loading control.
3MA or CQ was added when PAEC reached ∼80% confluence and incubated for 4 h. PAEC cultures without either 3MA or CQ treatment were used as controls. Cell lysates were immunoprecipitated with goat anti-p47phox antibody or nonspecific goat IgG as negative control before proteins were separated by SDS-PAGE and immunoblotted with rabbit anti-gp91phox (1:500), goat antibody, anti-p47phox (1:1,000), or goat anti-p67phox (1:500) antibodies. Cell lysates were also immunoprecipitated with goat anti-gp91phox antibody followed by immunoblotting with goat anti-p47phox antibody and rabbit anti-gp91phox to study the association between p47phox and gp91phox. Signals were analyzed as described above.
In vitro angiogenic activities.
Tube formation, monolayer scratch recovery, and apoptosis were used to study the in vitro angiogenesis of PAEC according to previously published protocols (11, 44).
Tube formation assay.
Growth-factor-reduced Matrigel (50 μl) was thawed at 4°C overnight and then added to each well of a 96-well plate and was allowed to polymerize at 37°C in a humidified incubator for at least 30 m before 4 × 104 PAEC in 200 μl of culture medium (5% FCS) were plated with/without 3MA or CQ. Capillary-like structures between cell clusters were measured as the tubes by one of the coauthors, who was blinded to the treatment. One picture with representative tube formation per well was taken 6 h after plating at ×10 (objective) magnification. Total tube length per high-power-field was measured for each picture for later analyses. Tube formation assay was also performed, with/without 40 μM H2O2, in control PAEC to evaluate the effect of ROS stress on tube formation.
Monolayer scratch recovery assay.
PAEC were grown to confluence in six-well plates. The cells were serum starved for 90 m. Scratch lines were created by a 1-ml pipette tip, and the scraped cells were gently rinsed away with HBSS. The medium was then changed back to regular medium (20% FCS), with/without 3MA or CQ, for 20 h. Picture of the narrowest distance of the gap between the frontlines of recovery was taken by a coauthor who was blinded to the treatment.
Apoptosis.
Apoptosis was evaluated by in situ TUNEL staining as reported by Sgonc et al. (41). PAEC (3 × 105) were cultured on the Lab-Tek II four-well chamber slides for 20 h with/without 3MA or CQ. The cells were fixed with 4% formalin in HBSS followed by 3% H2O2 in methanol for 10 min at 25°C to quench the internal peroxidase activity. The cells were then permeabilized with 0.1% Triton X-100 on ice and treated with labeling mixture for 60 min at 37°C in the dark. Next the cells were pretreated with peroxidase converter solution for 30 min at 37°C and then stained with 3,3′-diaminobenzidine. Finally, hematoxylin and eosin stain was applied, and the dark brown stained nuclei were counted to obtain the percent of number of cells with apoptosis.
Annexin-V-FITC/PI staining kit from BD Pharmingen™ was used to study early stage apoptosis. PAEC at confluence were treated with 3MA or CQ for 4 h and then trypsinized. After washing twice with PBS, the cells were resuspended in 100 μl 1× binding buffer provided by the kit and stained with 2 μl Annexin-V-FITC and 5 μl PI at room temperature for 15 m in the dark after gentle mixing. The stained cells were then fixed with another 400 μl binding buffer and analyzed on a fluorescence-activated cell sorting Calibur (Becton Dickinson, San Jose, CA) flow cytometer using CellQuest Pro software. A total of 20,000 cells were analyzed for each sample.
Measurements of ROS production by DHE staining.
ROS production was evaluated by DHE fluorescence as we described previously (33, 44). PAEC (1 × 105) were seeded into each well of a Lab-Tek II four-well chamber slide for 2 h before treatment with 3MA or CQ at 37°C for 20 h. The PAEC were then incubated with HBSS containing DHE (5 μM) for 15 min, with/without ATP (10−5 M), at 37°C to detect the intracellular O2− levels. Fluorescence was imaged using a Nikon Eclipse TE200 fluorescence microscope with excitation and emission at 510 and 590 nm, respectively. Fluorescence was quantified using MetaView software and expressed as relative light units (RLU).
Measurement of NOX activity.
PPHN-PAEC were grown in 60-mm plates to 80% confluence and then treated with 3MA, CQ, or double-distilled water (control) for 4 h. Cells were washed twice with ice-cold HBSS and scraped into 120 μl of buffer (20 mM potassium phosphate buffer pH 7.0 and 1 mM EGTA). The cell suspension was freeze-thawed twice in liquid nitrogen and then passed through a 27.5-gauge syringe 10 times. The lysates containing 50 μg of protein were mixed with 150 μl of working solution (50 mM potassium phosphate buffer at pH 7.0, 1 mM EGTA, 150 mM sucrose, 100 μM NADPH, and 5 μM lucigenin) at room temperature. The chemiluminescence generated was measured by GloMax-Multi Detector System (Promega, Madison, WI) every 60 s for 10 min and expressed as RLU. Working solution without NADPH was used for background activity, and NADPH oxidase activity was obtained after subtracting the corresponding background activity. Tiron (50 mM) was used as the O2− scavenger in the assay. Protein contents were assayed by bicinchoninic acid (BCA) method. Data are expressed as RLU/mg protein.
Measurements of NO production.
NO production was quantified using DAF-FM-DA fluorescence as we previously described (44). PAEC were grown to ∼80% confluence, and the medium was replaced with HBSS containing l-arginine (25 μM) and DAF-FM-DA (5 μM) and then incubated at 37°C. Half of the cultures were stimulated with the NOS agonist ATP (10−5 M) for 10 m. The fluorescence was imaged under a fluorescence microscope (Ex 495/Em 515 nm).
Beclin-1 knockdown.
SiRNA against bovine beclin-1 for reverse transfection was used according to the Lipofectamine RNAiMAX Transfection Reagent protocol. SiRNA (1 or 2 μg per well), or scramble siRNA, was mixed with 500 μl Opti-MEM-I reduced serum medium in six-well plates, and then Lipofectamine™ RNAiMAX 1 μl was added and incubated for 20 min at room temperature. PPHN-PAEC (1 × 105) suspended in antibiotic-free culture medium was added into the siRNA mixture for 72 h at 37°C in a humidified CO2 incubator. Beclin-1 expression was assayed by immunoblot, and angiogenesis was studied by tube formation assay and monolayer scratch recovery assay.
Statistical analysis.
Data were expressed as means ± SE. Student's t-test, or Mann-Whitney U-test, was used for comparing two groups wherever appropriate. One-way ANOVA followed by Student-Newman-Keuls post hoc test was used for comparisons among more than two groups. A P value <0.05 was considered statistically significant.
RESULTS
Autophagy is increased in PPHN.
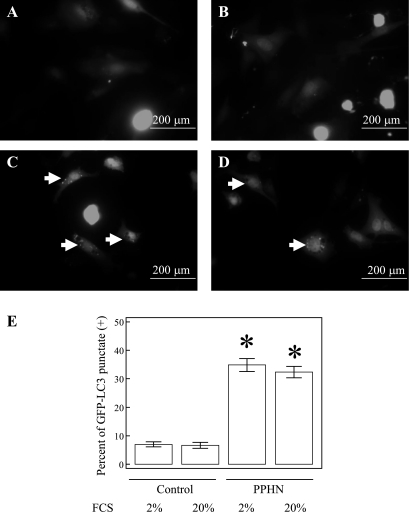
Increased LC3-II/LC3-I ratio (16) and increased GFP-LC3 punctate cells (31) are two commonly used indicators to assess autophagy (28, 49). The LC3-II/LC3-I ratios are increased markedly in PPHN-PAEC compared with control PAEC (n = 3, P < 0.001, Fig. 1A). It is known for eukaryotes that growth factor, or nutritional, deprivation activates autophagy (34). We, therefore, studied the effect of low content of FCS on autophagy in PAEC. The LC3-II/LC3-I ratios were higher in PPHN-PAEC in both 20% FCS (9.3 ± 1.2) and 2% FCS (12.2 ± 0.7) compared with the ratios in control PAEC in both 20% (1.8 ± 0.1) and 2% FCS (2.3 ± 0.3). The LC3-II/LC3-I ratio was also higher in PPHN lung tissue (2.6 ± 0.5) than the control lung tissue (0.6 ± 0.2, n = 4, P = 0.013, Fig. 1B). Serum starvation increased the LC3-II/LC3-I ratio, whereas the presence of NH4Cl further increased the ratio in control PAEC, indicating an increased autophagic flux (n = 3, P = 0.021, Fig. 1C) in nutrient-starved control PAEC. Different from control PAEC, serum starvation with and without NH4Cl did not further increase LC3-II/LC3-I ratio in PPHN-PAEC (n = 3, P = 0.45, Fig. 1D). These data suggest that the increased LC3-II/LC3-I ratio in PPHN-PAEC is mainly attributable to high basal autophagy with defective autophagic degradation (26). The percent of GFP-LC3 punctate-positive cells was also higher in PPHN-PAEC (32.4 ± 2.0% for 20% FCS, 34.8 ± 2.2 for 2% FCS, n = 3, P < 0.001, Fig. 2) than control PAEC (6.7 ± 1.1 for 20% FCS, 7.0 ± 0.9 for 2% FCS) when the cells were transfected with EGFP-LC3 plasmid. This further confirmed that autophagy is increased in PPHN-PAEC.
Fig. 1.
Autophagy is increased in persistent pulmonary hypertension (PPHN) compared with controls. Microtubule-associated protein 1 light chain (LC3)-II/LC3-I ratio by immunoblot is used to study the extent of autophagy and shows an increase in PPHN-pulmonary artery endothelial cells (PAEC) compared with control PAEC. The direction of LC3-II/LC3-I ratio change between PPHN-PAEC and control PAEC remains similar with different fetal calf serum (FCS) concentrations (A). The LC3-II/LC3-I ratio in PPHN lungs are increased compared with control lungs (B). Serum starvation increases LC3-II/LC3-I ratio in control PAEC and increases even more in the presence of NH4Cl, indicating upregulated autophagic flux (C). The LC3-II/LC3-I ratios in PPHN-PAEC do not change under serum starvation even in the presence of NH4Cl, indicating defective autophagic process (D). *P < 0.05 compared with control PAEC in 20% FCS. #P < 0.05 compared with control PAEC in 2% FCS. †P < 0.05 compared with control lungs. ‡P < 0.05 compared with control PAEC without serum starvation.
Fig. 2.
The percent of green fluorescent protein (GFP)-LC3 punctate-positive cells increases in PPHN-PAEC compared with control PAEC. Representative pictures for enhanced GFP (EGFP)-LC3 plasmid transfected control PAEC in 2% FCS (A), control PAEC in 20% FCS (B), PPHN-PAEC in 2% FCS (C), and PPHN-PAEC in 20% FCS (D). A quantitative representation of A–D in the percent of GFP-LC3 punctate-positive cells shows significant difference among groups (E). *P < 0.05 compared with control PAEC in either 20% or 2% FCS.
Autophagy inhibition improved in vitro angiogenesis in PPHN-PAEC.
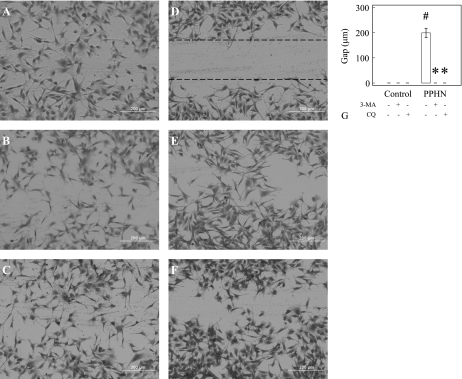
We investigated whether increased autophagy contributes to the impaired angiogenesis in PPHN-PAEC (44) by using autophagy inhibitors. We tested two widely used autophagy inhibitors, 3MA and CQ, in these experiments. Similar to our previous report, PPHN-PAEC (Fig. 3E) had impaired tube formation compared with control PAEC (Fig. 3A). Both 3MA (Fig. 3B) and CQ (Fig. 3C) had no effect on tube formation in control PAEC (Fig. 3D), but both had significantly increased tube formation in PPHN-PAEC (n = 8, P < 0.01, Fig. 3, E–H). The data suggest that increased autophagy is associated with impaired tube formation in PPHN-PAEC. The scratch recovery assay showed that, 20 h after scratch, the gaps almost disappeared in control PAEC (Fig. 4A) with/without 3MA (Fig. 4B) or CQ (Fig. 4C). Untreated PPHN-PAEC showed obvious gaps after 20 h. However, the gaps in PPHN-PAEC (Fig. 4D) almost disappeared after 3MA (Fig. 4E) and CQ (Fig. 4F) treatments (n = 12, P < 0.001, Fig. 4G).
Fig. 3.
Autophagy inhibitors increase tube formation in PPHN-PAEC but not in control PAEC. There is no difference (D) in tube formation for control PAEC (A) after treatment with 3-methyladenine (3MA) (B) or chloroquine (CQ) (C). Tube formation increases (H) in PPHN-PAEC (E) after treatment with both 3MA (F) and CQ (G). *P < 0.05 compared with PPHN-PAEC.
Fig. 4.
Autophagy inhibitors accelerate scratch recovery in PPHN-PAEC. There is no difference in scratch recovery in control PAEC (A) after 3MA (B) and CQ (C) treatments, whereas scratch recovery in PPHN-PAEC (D) is significantly improved (G) after 3MA (E) and CQ (F) autophagy inhibition. *P < 0.001 compared with PPHN-PAEC without treatment. #P < 0.001 compared with control PAEC.
Autophagy inhibition is associated with decreased apoptosis in PPHN-PAEC.
Appropriate amount of apoptosis is known to be an important part of angiogenesis, but exaggerated apoptosis can impair angiogenesis in PPHN-PAEC (44). 3MA and CQ treatment decreased the percentage of apoptotic cells in PPHN-PAEC (2.3 ± 0.5% and 2.2 ± 0.3%, respectively, Fig. 5, B and C) compared with untreated PPHN-PAEC (9.7 ± 1.6%, n = 8, P < 0.05, Fig. 5, A and D) by in situ TUNEL staining. Annexin-V/PI staining showed a high percentage (23.1 ± 0.6%, Fig. 5E) of early-stage apoptosis in PPHN-PAEC, whereas 3MA (10.6 ± 3.0%, Fig. 5F) and CQ (16.0 ± 1.2%, Fig. 5G) drastically decreased apoptosis (n = 5, P = 0.002, Fig. 5H). These results indicate that inhibition of autophagy in PPHN-PAEC also decreases apoptosis.
Fig. 5.
Autophagy inhibitors decrease apoptosis in PPHN-PAEC. Apoptosis in PPHN-PAEC (A) decreases after 3MA (B) and CQ (C) treatments (D) by in situ TUNEL stain. Similar findings are seen by Annexin-V/propidium iodide (PI) stain with the percentage of early apoptosis in PPHN-PAEC (E) decreases significantly after 3MA (F) and CQ (G) treatment (H). *P < 0.05 compared with PPHN-PAEC.
Antioxidants decrease autophagy in PPHN-PAEC.
We previously reported that decreasing ROS, either by NAC or Apo, in PPHN-PAEC increases angiogenesis (44). Here we investigated their effects on autophagy in PPHN-PAEC. Both 3MA and CQ decreased the percent of GFP-LC3 punctate-positive cells in PPHN-PAEC transfected with EGFP-LC3 plasmid (8.0 ± 2.5% for NAC and 8.0 ± 1.4% for Apo vs. 34.3 ± 1.9% for PPHN-PAEC, n = 4, P < 0.001, Fig. 6, A–D). The LC3-II/LC3-I ratio in these cells also decreased after NAC and Apo treatment (n = 3, P < 0.05, Fig. 6E). These findings suggest that ROS production leads to increased autophagy.
Fig. 6.
N-acetylcysteine (NAC) and Apocynin (Apo) decrease autophagy in PPHN-PAEC. The percent of GFP-LC3 punctate-positive cells (A) decreases in the presence of NAC (B) and Apo (C). The difference is significant among groups (D). Both NAC and Apo decrease LC3-II/LC3-I ratio in PPHN-PAEC (E). *P < 0.05 compared with PPHN-PAEC.
Autophagy inhibition decreases ROS production.
To further elucidate the relationship between ROS formation and autophagy in PPHN-PAEC, we monitored ROS formation by DHE fluorescence in these cells. Both 3MA and CQ treatment decreased DHE fluorescence in PPHN-PAEC (n = 10, P < 0.01, Fig. 7, A–D). NOX activity in PPHN-PAEC (7.1 ± 1.0×106 RLU/mg protein) was decreased by both 3MA (2.0 ± 0.8×106 RLU/mg protein) and CQ (2.7 ± 1.4×106 RLU/mg protein). Tiron almost completely (99.6 ± 0.1%) abolished the signals (n = 4, P < 0.001, Fig. 7E). These findings indicate that increased autophagy contributes to increased ROS, especially from NOX, in PPHN-PAEC. Scrambled siRNA did not alter DHE fluorescence signals in PPHN-PAEC (Fig. 7F), whereas beclin-1 knockdown showed a trend for decreased DHE fluorescence (Fig. 7G). However, the PPHN-PAEC activated by ATP (Fig. 7H) increased DHE fluorescence by approximately twofold after scramble siRNA, whereas beclin-1 knockdown significantly decreased the DHE fluorescence (n = 8, P = 0.035, Fig. 7, I and J).
Fig. 7.
Autophagy inhibitors and Beclin-1 knockdown decrease the O2− formation in PPHN-PAEC. Dihydroethidium (DHE) fluorescence after ATP simulation is high in PPHN-PAEC (A) but decreases after both CQ (B) and 3MA (C) treatment. The DHE fluorescence decreases in both cells with and without ATP stimulation (D). Both CQ and 3MA decreases NADPH oxidase (NOX) activity in PPHN-PAEC, and sodium 4, 5-dihydroxybenzene-1, 3-disulfonate (Tiron) almost completely blocked the signals (E). Similar findings are observed in beclin-1 knockdown with high DHE fluorescence in PPHN-PAEC treated with scramble siRNA (F), which increases further with ATP (G). Beclin-1 siRNA shows a trend of decrease in PPHN-PAEC DHE fluorescence (H), and a significant decrease is seen after ATP stimulation (I). The DHE fluorescence decreases mainly in ATP-stimulated beclin-1 knockdown PPHN-PAEC (J). *P < 0.05 compared with nonstimulated PPHN-PAEC. †P < 0.05 compared with ATP-stimulated PPHN-PAEC. ‡P < 0.05 compared with PPHN-PAEC. #P < 0.05 compared with PPHN-PAEC. RLU, relative light units.
Autophagy inhibition does not affect NO production in PPHN-PAEC.
Another possibility for increased tube formation and decreased ROS production in PPHN-PAEC is the recoupling of eNOS. DAF-FM-DA was used to assess NO production. 3MA and CQ treatments did not alter the DAF-FM-DA fluorescence in PPHN-PAEC (data not shown), indicating that eNOS recoupling may not be the reason for the decreased ROS production after autophagy inhibition.
Autophagy inhibition decreases association between p47phox and gp91phox.
NOX2 is one of the major ROS forming enzymes in PAEC, and its activity is increased in PPHN-PAEC (44). Because gp91phox, p22phox, p47phox, p67phox, p40phox, and Rac are believed to form a functional complex for NOX2-dependent ROS formation in endothelial cells (22), we studied the effects of 3MA and CQ on interactions among NOX2 subunits by using immunoprecipitation and immunoblotting. The expression of p47phox was decreased by 3MA and CQ, but the expression of p67phox was increased by CQ. No significant changes in Rac-1 expression were observed by immunoblots in PPHN-PAEC (Fig. 8A) treated with 3MA or CQ. However, both 3MA and CQ decreased gp91phox-p47phox association in PPHN-PAEC detected by immunoprecipitation with p47phox antibody (n = 3, P = 0.008, Fig. 8B). The p47phox-gp91phox association in PPHN-PAEC was also decreased by 3MA and CQ treatment as detected by reverse immunoprecipitation using gp91phox antibody (n = 3, P = 0.01, Fig. 8C). The p67phox-p47phox association was not altered by either 3MA or CQ. These findings indicate that 3MA and CQ decrease NOX2 activity in PPHN-PAEC by decreasing p47phox expression and the association between gp91 phox and p47phox.
Fig. 8.
Autophagy inhibitors decrease the p47phox expression and association between p47phox and gp91phox in PPHN-PAEC. 3MA (hatched bar) and CQ (black bar) treatment decreased p47phox expression, whereas CQ also increased p67phox expression in PPHN-PAEC (A). Immunoprecipitation using p47phox antibody and immunoblot with gp91phox (empty bar) or p67phox (black bar) antibody showed decreased association between gp91phox and p47phox in both 3MA- and CQ-treated PPHN-PAEC. Minus sign, negative control using nonspecific goat immunoglobulin for immunoprecipitation (B). Immunoprecipitation using gp91phox antibody and immunoblot with p47phox antibody showed decreased p47phox association with gp91phox in both 3MA- and CQ-treated PPHN-PAEC (C). *P < 0.05 compared with nontreated PPHN-PAEC.
Beclin-1 knockdown improves angiogenesis in PPHN-PAEC.
Beclin-1 knockdown in PPHN-PAEC was able to decrease DHE fluorescence in PPHN-PAEC under ATP stimulation (Fig. 7, I and J). Immunoblots showed ∼30% decrease in beclin-1 expression after knockdown experiments (n = 4, P = 0.023, Fig. 9A). This beclin-1 knockdown improved both tube formation (Fig. 9, B–D) and scratch recovery (Fig. 9, E–G) in PPHN-PAEC. These results indicate that increased autophagy contributes to the impaired angiogenesis and increased NOX activity in PPHN-PAEC.
Fig. 9.
Beclin-1 knockdown improves PPHN-PAEC angiogenesis. Beclin-1 siRNA decreases beclin-1 expression in PPHN-PAEC (A). Representative pictures of tube formation (B and C) and scratch test (E and F). The impaired tube formation in PPHN-PAEC (B) is improved after beclin-1 knockdown (C and D). Similarly the scratch test of PPHN-PAEC (E) is significantly improved after beclin-1 knockdown (F and G). KD-1μg: knockdown with 1 μg of beclin-1 siRNA; KD-2μg: knockdown with 2 μg of beclin-1 siRNA. *P < 0.05 compared with scramble siRNA-transfected PPHN-PAEC. IOD, integrated optical density.
ROS stress increases autophagy and impairs tube formation in control PAEC.
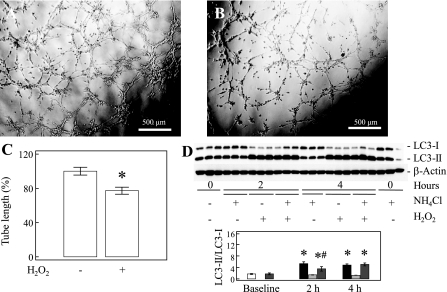
Control PAEC formed appropriate tubular structures (Fig. 10A), whereas H2O2 (Fig. 10B) decreased the tube formation by ∼20% (n = 10, P = 0.001, Fig. 10C). H2O2 also increased the LC3-II/LC3-I ratio in control PAEC (P < 0.001, n = 3, Fig. 10D), whereas the presence of NH4Cl did not further increase LC3-II/LC3-I ratio, indicating a degradation block (26) in control PAEC under increased ROS stress similar to PPHN-PAEC.
Fig. 10.
Hydrogen peroxide impairs tube formations and increases autophagy in normal PAEC. Compared with nontreated control PAEC (A) the tube formation is impaired after hydrogen peroxide (40 μM) treatment (B and C). Hydrogen peroxide increases LC3-II/LC3-I ratio in normal PAEC, and the presence of NH4Cl does not increase the LC3-II/LC3-I ratio, indicating a degradation block by hydrogen peroxide (D). Open bar, LC3-II/LC3-I ratio for nontreated normal PAEC; solid bar, LC3-II/LC3-I ratio for hydrogen peroxide-treated normal PAEC; hatched bar, LC3-II/LC3-I ratio for NH4Cl-treated normal PAEC; back-hatched bar, LC3-II/LC3-I ratio for hydrogen peroxide and NH4Cl-treated normal PAEC. *P < 0.05 compared with nontreated normal PAEC. #P < 0.05 compared with hydrogen peroxide-treated normal PAEC.
DISCUSSION
In the present study, we observed evidence of increased autophagy in PPHN. This was demonstrated by both increased LC3-II/LC3-I ratio and an increased percentage of GFP-LC3 punctate-positive cells in PPHN-PAEC when cells were transfected with EGFP-LC3 plasmid compared with control PAEC (19). Similarly, the increased LC3-II/LC3-I ratios were also observed in PPHN lung tissues compared with control lungs. The increased autophagy in PPHN-PAEC could not be explained simply by nutritional deprivation (35), as, in our results, the changes in FCS contents did not significantly affect the difference of autophagy between PPHN-PAEC and control PAEC. The increased autophagy is mainly due to defective autophagic degradation, as lysosomal inhibition did not increase the LC3-II/LC3-I ratio in PPHN-PAEC (26). Our results also showed that autophagy is proapoptotic and is associated with increased ROS production and impaired angiogenesis in PPHN-PAEC (Fig. 11). NOX inhibition decreased autophagy in PPHN-PAEC, whereas autophagy inhibition decreased gp91phox-p47phox association and NOX activity with consequent improvement in angiogenesis. These data together provide evidence for a cross talk between NOX-dependent ROS generation and autophagy in PPHN-PAEC.
Fig. 11.
The proposed relationship among autophagy, reactive oxygen species (ROS), and angiogenesis in PPHN-PAEC. PPHN-PAEC becomes a phenotype with increased ROS production that increases autophagy and leads to impaired angiogenesis. Increased autophagy in PPHN-PAEC also contributes to increased NOX activity. The increased NOX2 activity contributes to the increased ROS production in PPHN-PAEC.
Two autophagy inhibitors, 3MA and CQ, which work through different mechanisms, were used in this study to inhibit autophagy. 3MA is a class III PI3K inhibitor that blocks the formation of preautophagosomes, autophagosomes, and autophagic vacuoles (32), thereby decreasing autophagic flux. CQ abrogates the completion of auto-lysosome formation by increasing lysosomal pH and inhibits the completion of autophagy (19). Inhibiting autophagy in PPHN-PAEC by 3MA or CQ is associated with decreased apoptosis and increased in vitro angiogenesis, indicating that autophagy itself is proapoptotic and contributes to the impaired angiogenesis in PPHN-PAEC. Beclin-1 knockdown improved angiogenesis in PPHN-PAEC, which further confirms that increased autophagy does contribute to the impaired angiogenesis in our PPHN model.
We have previously reported that NAC and Apo decrease apoptosis and increase angiogenesis in PPHN-PAEC (44). Both NAC and Apo also decreased PPHN-PAEC autophagy in this study, which supports the hypothesis that autophagy is proapoptotic and contributes to impaired angiogenesis in PPHN-PAEC. Many angiogenic factors inhibit endothelial cell apoptosis, and increasing evidences suggest that the induction of endothelial cell apoptosis may counteract angiogenesis (3). Angiogenic factors are especially important in the growth of developing lungs. Inhibiting autophagy may offer some benefits to patients with PPHN in their recovery process.
In addition to decreasing autophagy in PPHN-PAEC, we also observed decreased ROS formation in PPHN-PAEC by both 3MA and CQ treatment. This finding indicates that there is an association between autophagy and ROS formation in PPHN-PAEC. However, we did not see increased NO formation with 3MA or CQ treatment, suggesting that improved angiogenesis and decreased ROS formation are not mediated by eNOS recoupling (20, 33). Our findings that beclin-1 knockdown decreased DHE fluorescence and improved angiogenesis in PPHN-PAEC further support the relationship between autophagy and ROS formation in PPHN-PAEC. The observation that H2O2 decreased angiogenesis and increased autophagy in control PAEC indicates an important cross talk between autophagy and ROS formation in the developing lungs.
The mechanism by which 3MA and CQ decrease ROS formation in PPHN-PAEC was explored by studying the interaction among NOX2 subunits. There was a decrease in the expression of p47phox, by 3MA and CQ treatments but an increase in p67phox expression detected by immunoblots. Our data suggest that the decreased association between p47phox and gp91phox, and probably also the decrease in p47phox expression, after 3MA and CQ treatments is the major mechanism for decreased NOX2 activity. It is known that a substantial proportion of NOX2 in the endothelial cells exists as a preassembled intracellular complex (22), and a lack of association between p47phox and gp91phox can abolish NOX2-mediated ROS formation (41). This finding suggests a possible mechanism by which autophagy inhibition decreases NOX activity in PPHN-PAEC.
There are several limitations to the present study. First, the animal model of PPHN we used did not allow us to study autophagic flux and prevented us from studying whether autophagy is also increased in the whole lung although the LC3-II/LC3-I ratios did show an increase in lung tissue harvested from PPHN lambs compared with controls (26). Second, information about the sheep genome is limited, which precludes us from designing adequate siRNAs to study autophagy without off-target effects. This may explain the limited beclin-1 knockdown we observed in our study using bovine sequence siRNA and was also a reason why we relied on the chemical inhibitors in this study. However, the progress in the sheep genome project (1) may soon provide information for our future studies.
The best way to elucidate the role of autophagy in PPHN would be to conduct studies in intact animal models. However, the tools for studying autophagic flux reliably in vivo remains limited (37). In addition, autophagy knockdown is an extreme event and might not be a reliable mimic of autophagy-deficient situations in physiological and pathological conditions. The newly invented zinc-finger nuclease-mediated gene knockdown may have the potential to create an appropriate animal model for our future studies, but reports in sheep models remain unavailable to date.
In conclusion, our study showed that autophagy is proapoptotic and contributes to impaired angiogenesis in PPHN (Fig. 10). We also observed that increased NOX activity contributes to increased autophagy in PPHN-PAEC, whereas increased autophagy reciprocally contributes to increased ROS formation probably via NOX2. The relationship between ROS and increased autophagy in PPHN-PAEC may be different from other conditions. We believe that established PPHN creates a phenotype of PAEC with increased ROS formation from both eNOS uncoupling (20) and upregulated NOX activity (44). The increased ROS formation in PPHN-PAEC may surpass the scavenging ability of intracellular antioxidant system. This may disturb lysosomal degradation with increased apoptosis and impaired angiogenic activity. PPHN is a disorder of developing lungs, and PAEC from this disorder may behave differently from PAEC from other developmental stages. This is the first report to demonstrate the role of autophagy in the impaired angiogenesis in developing lungs and provides modulation of autophagy as a potential therapeutic option in facilitating the growth of PPHN lungs.
GRANTS
The study was supported by Children's Research Institute Start-up fund (R.-J. Teng), National Institutes of Health Grants HL-080468S (Y. Shi), R03HD-65841 (G. Konduri), RO1HL-057268 (G. Konduri), and a grant from Advancing Healthier Wisconsin Foundation (G. Konduri).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.-J.T., J.D., S.W., T.G., A.E., Y.S., and G.G.K. conception and design of research; R.-J.T., J.D., S.W., T.G., and A.E. performed experiments; R.-J.T., J.D., S.W., A.E., Y.S., and G.G.K. analyzed data; R.-J.T., J.D., S.W., T.G., A.E., Y.S., and G.G.K. interpreted results of experiments; R.-J.T. prepared figures; R.-J.T. drafted manuscript; R.-J.T., J.D., S.W., T.G., A.E., Y.S., and G.G.K. edited and revised manuscript; R.-J.T., J.D., S.W., T.G., A.E., Y.S., and G.G.K. approved final version of manuscript.
REFERENCES
- 1. Archibald AL, Cockett NE, Dalrymple BP, Faraut T, Kijas JW, Maddox JF, McEwan JC, Hutton Oddy V, Raadsma HW, Wade C, Wang J, Wang W, Xun X. The sheep genome reference sequence: a work in progress. Anim Genet 41: 449– 453, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell 15: 344– 357, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol 22: 887– 893, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci 120: 4155– 4166, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16: 1040– 1052, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Dollberg S, Warner BW, Myatt L. Urinary nitrite and nitrate concentrations in patients with idiopathic persistent pulmonary hypertension of the newborn and effect of extracorporeal membrane oxygenation. Pediatr Res 37: 31– 34, 1995 [PubMed] [Google Scholar]
- 7. Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Diff 16: 966– 975, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Eskenlinen EL. New insights into the mechanisms of macroautophagy in mammalian cells. Int Rev Cell Mol Biol 266: 207– 247, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, Russell JA, Steinhorn RH. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 299: L109– L116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galluzzi L, Vicencio JM, Kepp O, Tasdemir E, Maiuri MC, Kroemer G. To die or not to die: that is the autophagic question. Curr Mol Med 8: 78– 91, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Gien J, Seedorf GJ, Balasubramaniam V, Markham N, Abman SH. Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor nitric oxide signaling. Am J Respir Crit Care Med 176: 1146– 1153, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glaumann H, Ahlberg J, Berkenstam A, Henell F. Rapid isolation of rat liver secondary lysosomes—autophagic vacuoles—following chloroquine administration. Exp Cell Res 163: 151– 158, 1986 [DOI] [PubMed] [Google Scholar]
- 13. Grover TR, Parker TA, Balasubramaniam V, Markham NE, Abman SH. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 288: L648– L654, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Hoyer LW, de los Santos RP, Hoyer JR. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest 52: 2737– 2744, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwata A, Riley BR, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem 280: 40282– 40292, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Karim MR, Kanazawa T, Daigaku Y, Fujimura S, Miotto G, Kadowaki M. Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy 3: 553– 560, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kim JH, Kim JH, Yu YS, Mun JY, Kim KW. Autophagy-induced regression of hyaloid vessels in early ocular development. Autophagy 6: 922– 928, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Klinosky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci 118: 7– 18, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clavé C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Dröge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fésüs L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, González-Estévez C, Gorski S, Gottlieb RA, Häussinger D, He YW, Heidenreich K, Hill JA, Høyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jäättelä M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovács AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, López-Otín C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Meléndez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Münz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nürnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Tallóczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcátegui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4: 151– 175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Konduri GG, Ou J, Shi Y, Pritchard KA., Jr Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 285: H204– H211, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest 115: 2679– 2688, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem 277: 19952– 19960, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Maiuri MC, Zalckvar E, Kinchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741– 752, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Massey AC, Zhang C, Cuervo AM. Chaperon-mediated autophagy in aging and disease. Curr Top Dev Biol 73: 205– 235, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Martinet W, De Meyer GR. Autophagy in atherosclerosis. Curr Atheroscler Rep 10: 216– 223, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 3: 542– 545, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069– 1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 140: 313– 326, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morin FC., 3rd Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 25: 245, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol 2: 211– 216, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Ni HM, Bockus A, Wozniak AL, Jones K, Weinman S, Yin XM, Ding WX. Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy 7: 54– 70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petiot A, Ogier-Denis E, Blommaart EFC, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′ kinase are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 275: 992– 998, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Pritchard KA, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric oxide synthase. J Biol Chem 276: 17621– 17624, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, Gilpin C, Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 128: 931– 946, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Rabinowitz J, White E. Autophagy and metabolism. Science 330: 1344– 1348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramakrishnan S, Nguyen TMB, Subramanian IV, Keleka A. Autophagy and angiogenesis inhibition. Autophagy 3: 512– 515, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Rubinsztein DC. Autophagy: where next?. EMBO Rep 11: 3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ryter SW, Choi AMK. Autophagy in the lung. Proc Am Thorac Soc 7: 13– 21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ryter SW, Lee SJ, Smith A, Choi AMK. Autophagy in vascular disease. Proc Am Thorac Soc 7: 40– 47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 17: 422– 427, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Selvakumar B, Hess DT, Goldschmidt-Clermont PJ, Stamler JS. Co-regulation of constitutive nitric oxide synthases and NADPH oxidase by the small GTPase Rac. FEBS Lett 582: 2195– 2202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sgonc R, Boeck G, Dietrich H, Gruber J, Recheis H, Wick G. Simultaneous determination of cell surface antigens and apoptosis. Trends Genet 10: 41– 42, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36: 2503– 2518, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 297: L184– L195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toogood PL. Inhibition of protein-protein association by small molecules: approaches and progress. J Med Chem 45: 1543– 1558, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Villamor E, Le Cras TD, Horan MP, Halbower AC, Tuder RM, Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 272: L1013– L1020, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol 99: 2034– 2040, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile LA, Shankaran S, Donovan EF, Oh W, Ehrenkranz RA, Fanaroff AA. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 105: 14– 20, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285: 10850– 10861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol 301: H647– H653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]