Abstract
The parabrachial and adjacent Kölliker-Fuse (PBN/KF) nuclei play a key role in relaying visceral afferent inputs to the hypothalamus and limbic system and are, thus, believed to participate in generating nausea and affective responses elicited by gastrointestinal (GI) signals. In addition, the PBN/KF region receives inputs from the vestibular system and likely mediates the malaise associated with motion sickness. However, previous studies have not considered whether GI and vestibular inputs converge on the same PBN/KF neurons, and if so, whether the GI signals alter the responses of the cells to body motion. The present study, conducted in decerebrate cats, tested the hypothesis that intragastric injection of copper sulfate, which elicits emesis by irritating the stomach lining, modifies the sensitivity of PBN/KF neurons to vertical plane rotations that activate vestibular receptors. Intragastric copper sulfate produced a 70% median change in the gain of responses to vertical plane rotations of PBN/KF units, whose firing rate was modified by the administration of the compound; the response gains for 16 units increased and those for 17 units decreased. The effects were often dramatic: out of 51 neurons tested, 13 responded to the rotations only after copper sulfate was injected, whereas 10 others responded only before drug delivery. These data show that a subset of PBN/KF neurons, whose activity is altered by a nauseogenic stimulus also respond to body motion and that irritation of the stomach lining can either cause an amplification or reduction in the sensitivity of the units to vestibular inputs. The findings imply that nausea and affective responses to vestibular stimuli may be modified by the presence of emetic signals from the GI system.
Keywords: semicircular canal, otolith organ, vomiting, nausea, motion sickness
the parabrachial nucleus (PBN) along with the ventrolateral extension of this region, the Kölliker-Fuse nucleus (KF) (7) plays a predominant role in relaying visceral signals to the forebrain (10). PBN/KF neurons project to the hypothalamus, limbic system, and other forebrain regions (6, 11, 12, 21, 53) and receive visceral inputs directly from area postrema and nucleus tractus solitarius (NTS) (25, 28, 33, 34, 37, 45, 46, 56). PBN/KF neurons respond to a variety of visceral inputs, including those from baroreceptors (27, 30, 31), pulmonary receptors (14, 19, 45), gastrointestinal (GI) receptors (2, 35, 60), and gustatory receptors (13, 17, 23). Some PBN/KF neurons also have respiratory related activity and modulate the respiratory cycle; these neurons are components of the pontine respiratory group (20). Because the PBN/KF complex is a relay through which visceral signals reach higher centers of the brain, this region is believed to be critical in generating affective responses and sensations, such as nausea following interoceptive stimuli (4). Evidence that PBN/KF neurons are part of the nausea-generating pathway includes observations that these neurons express the intermediate-early gene c-fos during emetic stimuli (15) and that lesions of this area affect behaviors in rodents that are believed to indicate the presence of malaise, including pica responses (29) and acquisition of conditioned taste aversions (44, 48, 50, 51, 57, 58).
Studies in a variety of species have shown that PBN/KF neurons also receive inputs from the vestibular nuclei (3, 47). Furthermore, experiments in nonhuman primates demonstrated that PBN/KF neurons respond to angular accelerations in semicircular canal planes (5), as well as linear accelerations that activate otolith organs, including off-vertical axis rotations and static tilts (40). Considering that PBN/KF neurons make connections with the limbic system and hypothalamus (6, 11, 12, 21, 53), as discussed above, these findings raise the possibility that the PBN/KF region plays a key role in triggering the nausea and affective responses that precede and accompany motion sickness.
The patterns of convergence of vestibular and visceral inputs on PBN/KF neurons have not been established. Furthermore, although PBN/KF neurons are likely part of the pathway that generates nausea in response to a variety of stimuli, previous experiments have not explored whether single units in this region integrate multiple emetic inputs and whether the presence of one emetic signal affects the processing of others. For example, it is not known whether the presence of a toxic compound in the stomach modifies the responsiveness of PBN/KF neurons to labyrinthine inputs, thereby affecting the susceptibility for motion sickness. The goal of the present study was to determine whether the administration of an emetic compound alters the responses of PBN/KF neurons to whole body rotations in vertical planes that activate the otolith organs and semicircular canals. We used the intragastric injection of copper sulfate, which evokes vomiting through irritation of the stomach lining (1, 8, 9, 18, 22, 24, 36, 54, 55), to stimulate GI afferents in these experiments. Transporters for copper sulfate are located in the intestine, but not the stomach, such that the compound can be placed intragastrically for a few minutes and then removed without being absorbed into the bloodstream (61). Thus, unlike drugs that are injected systemically and act through binding to receptors in circumventricular organs, copper sulfate can be used to provide a reversible emetic stimulus.
We tested the hypothesis that intragastric placement of copper sulfate increases the responsiveness of PBN/KF neurons to whole-body rotations that activate vestibular receptors, particularly for neurons with a tonic change in firing rate when copper sulfate was present in the stomach. In contrast, we presumed that copper sulfate injection would have little effect on the responses to vestibular stimulation of PBN/KF neurons without GI inputs, including those with a respiratory related discharge pattern or those with inputs from baroreceptors. The experiments were conducted on decerebrate animals, since the invasive procedures that were required preparing the animals for data collection, as well as for repeated administration of copper sulfate, precluded the use of a conscious preparation.
METHODS
All experimental procedures conformed to the American Physiological Society's “Guiding Principles for the Care and Use of Animals,” as well as the National Research Council's Guide for the Care and Use of Laboratory Animals, and were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. Data were collected from 16 purpose-bred adult cats (Liberty Research, Waverly, NY) of either sex, weighing 2.6 to 4.9 kg. The design of the experiments was similar to that in a recent study from our laboratory (52); as such, the description of the methodology is abbreviated below.
Surgical procedures.
Surgery was conducted under isoflurane anesthesia to intubate the trachea, cannulate both femoral veins, insert a transducer (Millar Instruments, Houston, TX) through the femoral artery into the abdominal aorta to record blood pressure, implant an intragastric catheter through an esophagostomy to administer copper sulfate, and secure both C5 phrenic nerves in bipolar silver cuff electrodes to record respiratory activity. The carotid arteries were dissected free of surrounding tissues and occluded bilaterally, and a ligature was placed around each artery to permit stretch of the carotid sinus (16, 52). The animals were placed in a stereotaxic frame with the head pitched-down 30° to vertically align the vertical semicircular canals, and they were supported using hip pins and a clamp placed on the dorsal process of an upper thoracic vertebra. A midcollicular decerebration was then performed, as was a craniotomy to expose the rostral aspect of the cerebellum, which was gently retracted and partially aspirated to visualize the region of the brain stem just caudal to the inferior colliculus.
During the surgery and subsequent recording session, atropine sulfate (0.10–0.15 mg/kg) was administered intramuscularly every 3 h to reduce airway secretions, and dexamethasone (2 mg/kg initial dose, 1 mg/kg subsequent doses) was injected intravenously every 6 h to reduce brain edema. Fluids, and, if necessary, phenylephrine (0.005–0.01 mg·kg−1·min−1), was infused intravenously to maintain blood pressure >90 mmHg. Rectal temperature was maintained at 37–38°C using a DC-powered heat lamp and pad. Anesthesia was discontinued after all surgery was complete, and animals were paralyzed using intravenous injections of pancuronium bromide (initial injection of 0.2 mg/kg, maintained by hourly injections of 0.1–0.2 mg/kg) and artificially ventilated with room air. Tidal volume and ventilation frequency were adjusted to maintain end-tidal CO2 at 4–5%. A bilateral pneumothorax was performed to reduce ventilation-related brain movements.
Data recording procedures.
Electrode penetrations were made 2.0–4.5 mm posterior to stereotaxic zero and 3.5–5.5 mm lateral to the midline to record activity from PBN/KF neurons, using a 5-MΩ tungsten microelectrode (FHC, Bowdoin, ME). Unit activity was amplified by a factor of 10,000, filtered with a band pass of 300–10,000 Hz, and sampled at 25,000 Hz using a Micro1401 mk 2 data collection system and Spike2 version 6 software (Cambridge Electronic Design, Cambridge, UK). When a unit was isolated, we first recorded its spontaneous activity along with blood pressure (sampled at 100 Hz) and phrenic nerve activity (amplified by a factor of 10,000, filtered with a band pass of 100–10,000 Hz, integrated with a 10-ms time constant, full-wave rectified, and sampled at 1,000 Hz). We then recorded the unit's responses during the decrease in blood pressure elicited by mechanical stretch of the carotid sinus (16, 52). We next examined the unit's activity while rotating the entire animal about the pitch (transverse) and roll (longitudinal) axes using a servo-controlled hydraulic tilt table (NeuroKinetics, Pittsburgh, PA), as described in detail in previous articles (32, 59). We first used 5° and 7.5° “wobble” stimuli, fixed-amplitude tilts, the direction of which moves around the animal at constant speed, to determine whether a unit's firing rate was modulated by vestibular stimulation (49). If a unit responded to wobble stimuli, we determined the plane of tilt that produced maximal modulation of the neuron's firing rate (response vector orientation). The direction of the response vector orientation lies midway between the maximal response direction to clockwise and counterclockwise wobble stimulation, because the phase differences between stimuli and responses are reversed during the two directions of rotation (49). Subsequently, tilts in a fixed plane at or near this orientation were used to study the dynamics of the vestibular response (response gain and phase across stimulus frequencies). Response dynamics were routinely determined over the frequency range of 0.05–0.5 Hz; for some units, 0.02 or 1-Hz rotations were also delivered. The amplitude of these stimuli was 2.5–5° at frequencies >0.2 Hz, and 5–10° at frequencies ≤0.2 Hz. As in previous studies, Bode plots generated from responses to fixed-plane stimuli were used to determine which vestibular end organs provided for the modulation of neuronal activity (16, 32, 42, 43, 52).
After the responses of a unit to vertical vestibular stimulation were characterized, 83 mg of copper sulfate dissolved in 10 ml of distilled water was injected into the stomach, as in a recent study (52). We recorded the effects of copper sulfate injection on the unit's firing rate for 5 min and then repeated the vestibular testing protocol outlined above. When stimuli were completed, the copper sulfate solution was aspirated from the stomach, and then a series of washes occurred using 10–15 ml of distilled water. In total, 60 ml of distilled water was injected and aspirated during the washing process to assure that the copper sulfate solution was completely removed. We waited at least 10 min after testing the effects of copper sulfate infusion on the responses of a neuron to vestibular stimulation before resuming recordings.
Near the end of the recording session, lesions were made at defined coordinates by passing a 0.1-mA negative current through the recording electrode for 60 s. Animals were subsequently killed using Euthasol Euthanasia Solution, and the brain stem was removed and fixed in 10% formaldehyde solution.
Data analysis procedures.
Following experiments, all data were subjected to spike sorting using Spike2 software to ensure that counts of neuronal activity were accurate. When spike shape or amplitude changed during the course of recording from a unit, such that we were not confident about the fidelity of the data, the spurious runs were discarded. Neural activity recorded during whole body rotations was binned (500 bins/cycle) and averaged over the sinusoidal stimulus period. Sine waves were fitted to responses with the use of a least-squares minimization technique (49). The response sinusoid was characterized by two parameters: phase shift from the stimulus sinusoid (subsequently referred to as phase) and amplitude relative to the stimulus sinusoid (subsequently referred to as gain). The signal-to-noise ratio for responses was also calculated (49). We used one primary criterion and two secondary criteria to determine whether neuronal activity was modulated by rotations (16, 32, 52, 59). First, responses were considered significant only if the signal-to-noise ratio was >0.5. Data meeting this criterion were considered to represent real modulation of neuronal activity when only the first harmonic was prominent and the responses were consistent from trial to trial.
Statistical analyses were performed using Prism 5 software (GraphPad Software, San Diego, CA). Pooled data are presented as means ± SE. Statistical significance was assumed if P < 0.05. A freezing microtome was used to cut the brain stem transversely at 100-μm thickness, and tissue sections were stained using thionine. Recording sites were reconstructed on drawings of sections with reference to the locations of electrolytic lesions, the relative positions of electrode tracks, and microelectrode depths.
RESULTS
Activity was recorded from 226 neurons in the PBN/KF nuclei during whole body rotations in vertical planes. Forty-five neurons were classified as cardiovascular units, including 21 units having activity synchronized to the cardiac cycle (as illustrated in Fig. 1, A and B) and 24 neurons whose activity increased (n = 15) or decreased (n = 9) over 30% during stretch of the carotid sinus, as shown in Fig. 1C. Sixteen units had activity synchronized with phrenic nerve discharges. The firing rate of 13 of these respiratory cells increased during inspiration (illustrated in Fig. 1D), one fired during expiration (illustrated in Fig. 1E), while the rhythmic activity of the other two neurons disappeared when the ventilator was transiently switched off, suggesting that their responses were due to activation of pulmonary afferents by lung inflation (38). The firing rate of 19 neurons increased (n = 6) or decreased (n = 13) >30% following the intragastric infusion of copper sulfate; these cells were classified as GI units, since the alteration in their activity was likely due to irritation of the stomach lining by copper sulfate. Figure 1F shows that the changes in the firing rate of GI units typically occurred 2–3 min following the administration of copper sulfate, and then their activity was sustained at the altered level. Twenty-nine neurons were classified as convergent units, as they had responses that combined two of the properties discussed above. Most (25/29) of the convergent units responded to stomach irritation by copper sulfate, 7 with increases and 18 with decreases in activity; 23 of these cells were also cardiovascular units, while the other 2 were respiratory neurons, whose activity increased during expiration. The additional four convergent units were cardiovascular neurons, whose activity was also synchronized with the respiratory cycle; the firing rate of three of these cells increased during expiration, whereas the respiratory related activity of the other cell disappeared when ventilation was discontinued, suggesting that it received pulmonary afferent input. The remaining neurons (117/226, 52%) were classified as “unknown units.”
Fig. 1.
Firing patterns of different unit types observed in the parabrachial and adjacent Kölliker-Fuse (PBN/KF), including cardiovascular (A–C), respiratory (D, E), and GI (F) units. A: record of arterial blood pressure (sampled at 100 Hz, top) and activity (sampled at 25,000 Hz, bottom) of a unit in lateral PBN with a cardiac-related firing pattern. B: averaged changes in blood pressure (top) and a poststimulus histogram showing associated changes in firing rate (bottom, bin width of 10 ms) of the same unit whose data are illustrated in A; 126 sweeps were pooled to generate traces. C: traces showing effects of mechanical stretch of carotid sinus (indicated by horizontal line) on arterial blood pressure (top) and activity of a unit in the medial PBN. A decrease in blood pressure occurred subsequent to the increase in the unit's firing rate, indicating that the excitation was evoked by afferent inputs from the carotid sinus and not by the change in blood pressure. D: activity of a KF neuron (bottom) that was synchronized to phrenic nerve discharges (top). E: activity of a neuron in the lateral PBN (bottom) that fired between phrenic nerve discharges (top). F: effect of copper sulfate infusion into the stomach (indicated by arrow) on arterial blood pressure (top) and activity of a unit in the medial PBN (bottom). A sustained increase in the neuron's firing rate occurred ∼2 min following the administration of copper sulfate, although there was no change in blood pressure.
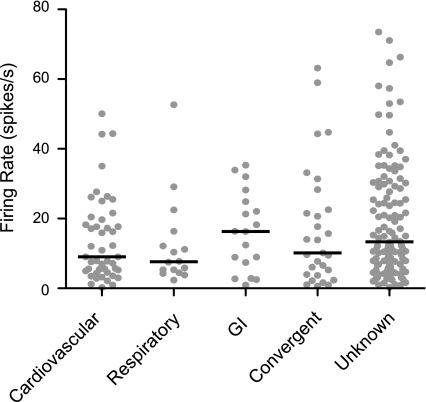
The mean spontaneous firing rate for all neurons was 17.3 ± 1.0 spikes/s. The spontaneous firing rates of each of the unit types are illustrated in Fig. 2. These firing rates were not demonstrated to be significantly different using a nonparametric one-way ANOVA (Kruskal-Wallis test, P = 0.2).
Fig. 2.
Spontaneous firing rates of different types of units tested for responses to whole body rotations. Gray symbols indicate data for each neuron, whereas horizontal lines designate the median firing rate of each unit type.
Responses of PBN/KF neurons to whole body rotation in vertical planes.
Approximately half (106/226, 47%) of the PBN/KF neurons examined responded to whole body rotations, including 25/45 (56%) of the cardiovascular, 5/16 (31%) of the respiratory, 16/19 (84%) of the GI, 21/29 (72%) of the convergent, and 39/117 (33%) of the unknown units. χ2-tests (P < 0.01) confirmed that GI and convergent units were more likely than respiratory or unknown units to respond to vertical-plane rotations. A caveat, however, is that the strong rhythmic activity of respiratory neurons could have distorted the responses of these cells to rotations, such that their activity did not appear to be modulated by the stimuli.
The response vector orientations for the neurons that responded to rotations are shown in Fig. 3. Two-thirds of the neurons (70/106) had response vector orientations that were closer to the pitch plane than to the roll plane, including 17/25 cardiovascular units, 3/5 respiratory units, 8/16 GI units, 13/21 convergent units, and 29/39 unknown units. In addition, a larger fraction of neurons (44/70, 63%) was excited by nose-up than by nose-down pitch (26/70, 37%).
Fig. 3.
Polar plots showing response vector orientations of different type of units: cardiovascular (A), respiratory (B), GI (C), convergent (D), and unknown (E). Response vector orientations were determined using wobble stimuli, usually delivered at 0.2 Hz. The response vector orientations were plotted using a head-centered coordinate system, with 0° corresponding to ipsilateral ear-down (IED) roll tilt, 90° to nose-down (ND) pitch, 180° to contralateral ear-down (CED) roll, and −90° to nose-up (NU) pitch.
We additionally delivered tilts in a fixed plane near the response vector orientation at 0.02–1 Hz to determine the dynamic properties of neuronal responses to rotations. Fig. 4 shows the responses of a cardiovascular (A) and an unknown (B) neuron to pitch rotations at 0.05–0.5 Hz. For both units, the gains of responses to tilts were similar across stimulus frequencies. However, whereas the responses of the cardiovascular unit (Fig. 4A) were in phase with the position of the tilt table, the responses of the other unit (Fig. 4B) lagged stimulus position. Bode plots illustrating the dynamic response properties over at least a stimulus decade of 42 neurons, including 11 cardiovascular, 1 respiratory, 10 GI, 15 convergent, and 5 unknown units, are shown in Fig. 5. Both response gain and phase were plotted with respect to stimulus position, such that a response whose phase leads stimulus position by 90° is synchronous with stimulus velocity. The gain of the response at the lowest frequency tested for a neuron was standardized at 1 spike·s−1·deg−1, so that the change in gain with increasing stimulus frequency was evident. As in previous studies (16, 42, 43, 52), neurons were categorized as having graviceptive responses (similar to otolith organ afferents) if their response gains remained stable (<3-fold increase) over a stimulus decade, and their response phases remained near stimulus position or lagged stimulus position. The majority of the PBN/KF neurons whose response dynamics were characterized (30/42, 71%) had such graviceptive responses. A few (4/42, 9%) of the neurons were characterized as having velocity responses (similar to semicircular canal afferents) because their response gains increased >5-fold per stimulus decade, and their response phases led stimulus position by ∼90° when rotations were delivered at frequencies ≥0.1 Hz. The remaining cells had response characteristics that deviated from those of vestibular afferents, typically response gains that increased appreciably as stimulus frequency was advanced (like velocity neurons) but response phases that remained near stimulus position or lagged stimulus position (like graviceptive neurons). These response properties were likely due to integration and transformation of vestibular signals by the central nervous system prior to being transmitted to the PBN/KF region.
Fig. 4.
Averaged responses of two units to 5° sinusoidal rotations in the pitch plane at 0.05–0.5 Hz. Each histogram contains 500 bins, such that bin width varies from 40 ms at 0.05 Hz to 4 ms at 0.5 Hz. A gray sine wave superimposed on each trace designates table movement. A: responses of a cardiovascular unit that occurred in phase with table movement. The number of sweeps averaged for each trace were: 32 in a, 12 in b, 8 in c, and 9 in d. B: responses of an unknown unit that lagged stimulus position; sine waves fit to the responses are indicated in red, so that response phases can be compared with table position (designated by a gray sine wave). The number of sweeps averaged for each trace were 19 in a, 9 in b, 4 in c, and 5 in d. ND, nose down; NU, nose up.
Fig. 5.
Bode plots illustrating the dynamic properties of responses of PBN/KF neurons to rotations in a fixed plane at multiple frequencies collected across all of the experiments. Response gain and phase are plotted with respect to stimulus position. A: bode plots for individual neurons; different unit types are indicated by lines with distinct colors: red, cardiovascular units; yellow, respiratory units; green, GI units; blue, convergent units; and gray, unknown units. Response gain at the lowest frequency for each unit (usually 0.05 Hz, but 0.02 Hz for 2 units and 0.1 Hz for 2 units) was standardized at 1 spike·s−1·degree−1, so that the change in gain with increasing stimulus frequency was evident. B: averaged data for each of the unit types (solid lines) and all neurons (dashed lines). Since a Bode plot was generated for only one respiratory neuron, averaged data are not provided. Error bars designate one SE.
Effects of intragastric copper sulfate administration on the responses of PBN/KF neurons to rotations in vertical planes.
The responses of 51 PBN/KF neurons to wobble stimuli were compared before and after the intragastric administration of copper sulfate; the other units were lost before the experimental protocol could be completed. The spontaneous firing rates of approximately two-thirds of these neurons (33/51) changed >30% after copper sulfate infusion, and for this analysis, these cells were classified as GI units (although 17 also responded to carotid sinus stretch or had cardiac or respiratory related activity). The spontaneous firing rates of the other 18 units were relatively unaffected by copper sulfate administration; they included eight cardiovascular, one respiratory, and nine unknown units.
Administration of copper sulfate often elicited dramatic changes in the magnitude of responses of neurons to whole body tilts. Most notably, 13 neurons that did not respond to 7.5° wobble rotations prior to copper sulfate infusion (median signal-to-noise ratio of 0.19) did so after the drug was administered (the median signal-to-noise ratio increased to 0.91), as illustrated in Fig. 6A. For these 13 neurons, the increase in signal-to-noise ratio resulting from the intragastric delivery of copper sulfate was significant (P < 0.001, Mann-Whitney U-test). The spontaneous firing rates of most (7/12) of these cells also changed >30% after copper sulfate was provided. In contrast, the responses of 10 neurons to wobble rotations (including nine GI units) were abolished following the intragastric delivery of copper sulfate, as shown in Fig. 6B. The median signal-to-noise ratios for responses to 7.5° wobble rotations decreased from 0.83 to 0.33 for these 10 cells; the differences were significant (P < 0.001, Mann-Whitney U-test).
Fig. 6.
Effects of copper sulfate administration on the averaged responses of two units to 7.5° wobble stimuli delivered at 0.2 Hz. Each histogram contains 500 bins; a gray waveform superimposed on each trace indicates the tilt table position, whereas a red waveform shows a sine wave fit to the response. In each panel, a indicates the response prior to intragastric copper sulfate, whereas b indicates the response after the drug was delivered. The shapes of five overlapped action potentials recorded from the units whose activity was binned in these histograms are illustrated in panels c and d; c shows action potential shape before copper sulfate administration, while d shows the action potential shape following administration. The spike shape was similar throughout the recording period, indicating that the same unit was sampled both before and after intragastric copper sulfate. A: responses of a neuron whose spontaneous firing rate decreased after copper sulfate administration. No modulation of unit activity was evident during rotations prior to intragastric copper sulfate (a, average of 11 sweeps, signal-to-noise ratio of 0.07), although a strong response was present afterwards (b, average of 8 sweeps, signal-to-noise ratio of 0.92). B: responses of a neuron whose spontaneous firing rate increased after copper sulfate administration. The robust modulation of unit activity evident before intragastric copper sulfate (a, average of 4 sweeps, signal-to-noise ratio of 2.14) was eliminated after the drug was injected (b, average of 15 sweeps, signal-to-noise ratio of 0.12).
Figure 7A shows the effect of intragastric copper sulfate on the gain of responses to wobble rotations. For this quantitative analysis, we included responses with signal-to-noise ratios <0.5, which were deemed to be insignificant, so that data for all neurons could be considered. The absolute value of the percentage change in gain is depicted; open symbols indicate neurons whose responses to wobble stimuli were augmented by the delivery of copper sulfate, whereas solid symbols designate neurons whose responses were diminished. Copper sulfate administration resulted in a 70% median change in the gain of responses of GI units to wobble stimulation; the response gains for 16 GI units increased, and those for 17 GI units decreased. In contrast, only a 25% median change in the gain of responses to wobble rotations was noted for neurons whose firing rate was not substantially altered by copper sulfate administration; the response gain increased for 10 of the neurons and decreased for the other eight. The percentage change in response gain for neurons with GI inputs was significantly larger than for neurons without GI inputs (P < 0.01, Mann-Whitney U-test).
Fig. 7.
A: effects of intragastric copper sulfate administration of the gain of responses to wobble stimulation. The absolute value of the percent change in gain following copper sulfate injection is indicated. Open symbols designate units whose response gain increased, whereas solid symbols indicate units whose response gain decreased. Data are segregated into two groups: those for units whose firing rate changed >30% following copper sulfate administration (GI input) and those whose firing rate remained stable (no GI input). Red circles indicate units with GI input whose spontaneous firing rate increased following copper sulfate administration, while black circles designate units whose firing rate decreased. Horizontal lines show median percentage changes in gain produced by intragastric copper sulfate. B: changes in response vector orientation produced by intragastric copper sulfate administration; data are provided only for the subset of neurons that responded to rotations before and after the drug was delivered. Horizontal lines show median values. C: bode plots showing average response dynamics for neurons before and after copper sulfate administration. Solid lines show responses prior to copper sulfate injection, whereas dashed lines indicate responses after drug delivery. Error bars indicate one SE. The average Bode plots include data from five neurons that responded to rotations only prior to intragastric copper sulfate and six neurons that only responded to rotations when the drug was present.
Figure 7B shows the effect of copper sulfate administration on the response vector orientations of PBN/KF neurons that responded to rotations before and after the drug was delivered. Intragastric copper sulfate altered the response vector orientation >45° for only five neurons (two GI units and three non-GI units). The median change in response vector orientation for GI and non-GI cells was 5° and 9°, respectively. There was no significant difference between these two groups (P = 0.66, Mann-Whitney U-test).
Figure 7C illustrates the properties of dynamic neuronal responses to fixed-plane rotations before and after the administration of copper sulfate. Solid lines show average data for neurons prior to copper sulfate administration, whereas dashed lines indicate responses following intragastric copper sulfate. The average response dynamics were similar before and after copper sulfate injection, although response gains for individual neurons changed appreciably, with some units responding to rotations only before (n = 5) or after (n = 6) the drug was provided.
Locations of neurons tested for responses to whole-body rotations.
The locations of the PBN/KF neurons tested for responses to rotations in vertical planes are illustrated in Fig. 8A; different symbols are used to distinguish each unit type. In general, unit types were distributed similarly across the medial and lateral subdivisions of PBN; an exception is that all but one neuron with respiratory related activity was located in the lateral subdivision of PBN or in KF. Fig. 8B shows the position of each unit with respect to stereotaxic zero, the midline, and the dorsal surface of the brain stem. On average, the neurons sampled were located at 4.5 ± 0.1 mm lateral to the midline, 3.3 ± 0.1 mm posterior to stereotaxic zero, and 1.2 ± 0.1 mm below the dorsal surface of the brain stem. Neurons that responded to whole body rotations were located more dorsally (1.0 ± 0.1 mm from the dorsal surface of the brain stem) than those that did not respond to tilt stimuli (1.4 ± 0.2 mm), as confirmed by a two-way ANOVA (factors were presence of a response to vestibular stimulation and the coordinates of unit locations) combined with Bonferroni post hoc tests (P < 0.001).
Fig. 8.
A: locations of PBN/KF neurons tested for responses to whole-body rotations. Locations of neurons are plotted on two transverse sections through the rostral (a) and caudal (b) half of PBN/KF, in accordance with Berman's atlas (7). Numbers at the top of each panel indicate distance in mm posterior (P) to stereotaxic zero. Different symbols are used to designate each unit type; red symbols indicate the neurons that responded to whole body rotations, whereas black symbols show units that failed to respond to tilt stimuli. B: coordinates of each recording site, based on histological reconstructions, with respect to stereotaxic zero (AP), the midline (ML), and the dorsal surface of the brain stem (Depth). Symbols are the same as in A. C: locations of the subset of PBN/KF neurons tested for the effects of copper sulfate administration on the responses to whole body rotations. Red symbols designate units whose response gain to wobble stimuli increased > 30% after intragastric copper sulfate, whereas blue symbols indicate units whose response gain decreased >30%; gray symbols show units whose responses to rotations were relatively unaffected. 5M, motor trigeminal nucleus; 5MT, motor roots of the trigeminal nerve; KF, Kölliker-Fuse nucleus; PBI, lateral parabrachial nucleus; PBm, medial parabrachial nucleus; scp, superior cerebellar peduncle; TAD, accessory dorsal tegmental nucleus; TDP, dorsal tegmental nucleus, pericentral division.
Fig. 8C indicates the locations of the subset of neurons tested for the effects of intragastric copper sulfate administration on responses to rotations in vertical planes. Red symbols show units whose response gains increased over 30% after copper sulfate infusion, blue symbols indicate units whose response gains decreased over 30%, and gray symbols designate units whose response gains were relatively unaffected (<30% change). Neurons did not appear to be segregated on the basis of the effects of copper sulfate on their responsiveness to rotations.
DISCUSSION
The most significant finding of this study was that for a majority of PBN/KF neurons, the gain of responses to rotations in vertical planes was altered substantially by the intragastric infusion of copper sulfate, a compound that readily evokes nausea and emesis through the irritation of the stomach lining (1, 8, 9, 18, 22, 24, 36, 54, 55). The effects of copper sulfate administration on responses to vestibular stimulation were particularly pronounced for neurons with a sustained change in their spontaneous firing rate after the drug was delivered. These data show that a subset of neurons whose activity is modulated by a nauseogenic stimulus delivered to the stomach also respond to body motion and that stomach irritation can either cause an amplification or reduction in the sensitivity of PBN/KF neurons to vestibular inputs.
We previously used methodology similar to that in these experiments to determine whether copper sulfate administration affected the responses to vestibular stimulation of NTS neurons (52). In contrast to the present results, intragastric copper sulfate had little effect on the responses of NTS neurons to labyrinthine inputs. In particular, copper sulfate administration produced only a 24% median change in the gain of responses to vertical tilts of NTS neurons with GI inputs (52), as opposed to a 70% median change for PBN/KF units. These median values were shown to be significantly different using a Mann Whitney U-test (P < 0.0001). Since strong integrative effects of stomach irritation and vestibular stimulation were not evident for NTS neurons (52), which relay visceral signals directly to the PBN/KF region (25, 28, 33, 34, 37, 45, 46, 56), it seems likely that the gating of vestibular inputs following intragastric copper sulfate infusion observed in this study occurs within the PBN/KF complex. This area is believed to play an essential role in producing nausea and affective responses during motion stimuli (4), but it is not required to elicit emesis (41). These observations raise the prospect that stomach irritation can affect motion sickness-related malaise (which is mediated through the connections of PBN/KF neurons), while having little impact on motion-induced vomiting [which is elicited by neurons in the medulla (41)]. Further studies, including those testing the combined effects of vestibular stimulation and stomach irritation on the activity of medullary neurons that coordinate the motor components of vomiting, will be required to test this premise.
Although copper sulfate infusion altered the responses to vestibular stimulation of a majority of PBN/KF neurons, in some cases, the responses were amplified, and in others, they were reduced. The effects could be so pronounced that a neuron only responded to vertical tilts before or after copper sulfate was administered. This diversity of effects of copper sulfate administration complicates the interpretation of the present findings, as it raises the prospect that stomach irritation can either increase or decrease affective responses and nausea during body motion. PBN/KF neurons project to many different locations in the brain; some have ascending projections to the hypothalamus, thalamus, limbic system, and forebrain structures (6, 11, 12, 21, 53), whereas others have descending projections to NTS and the medullary reticular formation (21, 28). It is feasible that the effects of copper sulfate administration on the responses of PBN/KF neurons to vestibular stimulation are related to which brain region a particular cell projects. Additional studies that examine this hypothesis could be very enlightening, but they will be difficult to perform. Antidromic stimulation of the projections of PBN/KF neurons to the telencephalon and diencephalon is impossible following decerebration, which entails sectioning all ascending axons at the midcollicular level. Such an experiment would be feasible in anesthetized animals, but the presence of anesthesia could alter the integrative effects of vestibular and stomach irritation on the activity of neurons. An approach that combines injection of retrogradely transported tracers into an animal (to determine where individual PBN/KF cells project) with single-unit recordings conducted on a later date following decerebration would be informative, particularly if the neurons sampled are marked with a method, such as juxtacellular labeling. Neurons with particular responses to intragastric copper sulfate could also have distinct pharmacological properties, and this possibility should also be explored in future studies.
Although the gain of responses to vertical tilts of PBN/KF neurons was typically altered by intragastric copper sulfate, the spatial and temporal properties of the responses were relatively unaffected. The responses of PBN/KF units to vertical plane rotations in this study suggested that their vestibular inputs came mainly from the subset of otolith organ receptors activated by rotations in the sagittal plane (pitch). However, individual vestibular system neurons are activated by body movements in every possible direction, and they receive inputs from otolith organs and semicircular canals (26). If additional pathways conveying vestibular signals to PBN/KF neurons were recruited following intragastric copper sulfate, or if a subset of pathways were suppressed, then shifts in response vector orientations and in the response parameters considered in a Bode plot should have occurred for the neurons tested. Instead, our data show that the balance of convergent labyrinthine inputs to PBN/KF units was unaffected by stomach irritation.
Several caveats must be considered when interpreting the current results. Copper sulfate infusion into the stomach often triggers brief transients in blood pressure, which may be related to the noxious nature of the compound (52). Thus, it is uncertain whether the changes in the responses of PBN/KF neurons to whole body rotation following intragastric copper sulfate were due to the nausea or pain elicited by the drug. Studies that make use of analgesic and antiemetic agents should be conducted in the future to distinguish between these possibilities. In addition, previous experiments in conscious nonhuman primates suggested that PBN/KF neurons receive inputs from both semicircular canals and otolith organs (5, 40). However, most cells sampled in the present study had graviceptive responses to whole body rotations, indicating that their vestibular inputs came primarily from otolith organs (42, 43). It is unclear whether the disparity in findings is due to species differences (cat vs. monkey) or to differences in preparation (conscious vs. decerebrate), since decerebration can profoundly affect how a population of neurons responds to vestibular stimulation (16). Although nonlabyrinthine graviceptors are present in the body (39), it seems doubtful that the small-amplitude tilts utilized in this study would have shifted the position of body viscera sufficiently to activate visceral receptors. Furthermore, the magnitudes of responses to high-frequency rotations were at least as large as those to low-frequency tilts, whereas high-frequency stimuli should have provided a weaker stimulus for visceral afferents than low-frequency rotations that better facilitated the shifting of the viscera in the body cavity. We have also previously documented that the small-amplitude rotations used in this study have little impact on arterial blood pressure, and thus should not have appreciably stimulated baroreceptors (52). Thus, it seems likely that the responses to whole body rotation of PBN/KF neurons in this study were due to activation of receptors in the inner ear.
Perspectives and Significance
This is the first report that visceral signals can substantially alter the responses of a population of neurons to vestibular inputs. In addition to eliciting reflex motor responses, vestibular stimulation can result in a variety of affective responses, including panic during unanticipated body movements and malaise during motion sickness, which are likely mediated through connections of the vestibular nuclei to the PBN/KF region (4). As such, the present results suggest that delivery of a nauseogenic stimulus to the stomach alters affective responses to vestibular stimulation. Further examination of this premise is worthwhile, as it may provide insights into treatment of conditions, such as anxiety disorders and motion sickness. It will also be informative to explore whether the gating of vestibular responses by visceral inputs is unique for PBN/KF neurons, or whether similar effects occur in the hypothalamus and limbic system. Little is known about the influences of the vestibular system on the activity of neurons in these areas, but since they are targets of projections from the PBN/KF region (6, 11, 12, 21, 53), the present results suggest that at least some hypothalamic and limbic system neurons should respond to inputs from the inner ear. Furthermore, studies are needed to determine whether copper sulfate administration affects the responsiveness of PBN/KF neurons to nonlabyrinthine inputs, particularly those that elicit nausea and vomiting. Such experiments would reveal whether stomach irritation alters the processing of all emetic inputs, or whether the effects are unique for vestibular signals.
GRANTS
This work was supported by Grant R01-DC03732 from the National Institute on Deafness and Other Communication Disorders of the National Institutes of Heath (NIH). Core support was provided by NIH Grant P30-DC05205.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.S., Y.S., and B.J.Y. performed experiments; T.S., Y.S., and B.J.Y. analyzed data; T.S., Y.S., and B.J.Y. interpreted results of experiments; T.S. and Y.S. prepared figures; T.S., Y.S., and B.J.Y. drafted manuscript; T.S., Y.S., and B.J.Y. edited and revised manuscript; T.S., Y.S., and B.J.Y. approved final version of manuscript; B.J.Y. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Bret Boyle, Michael Catanzaro, Lucy Cotter, Daniel Miller, Sarah Ogburn, and Nevin Sastry and for assistance in data collection and analysis.
REFERENCES
- 1. Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett 286: 123–126, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1581–R1593, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Balaban CD. Vestibular nucleus projections to the parabrachial nucleus in rabbits: Implications for vestibular influences on the autonomic nervous system. Exp Brain Res 108: 367–381, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Balaban CD, Jacob RG, Furman JM. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: Neurotherapeutic implications. Expert Rev Neurother 11: 379–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balaban CD, McGee DM, Zhou J, Scudder CA. Responses of primate caudal parabrachial nucleus and Kolliker-Fuse nucleus neurons to whole body rotation. J Neurophysiol 88: 3175–3193, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Berkley KJ, Scofield SL. Relays from the spinal cord and solitary nucleus through the parabrachial nucleus to the forebrain in the cat. Brain Res 529: 333–338, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Berman AI. The Brain Stem of the Cat. Madison, WI: University of Wisconsin Press, 1968 [Google Scholar]
- 8. Bountra C, Bunce K, Dale T, Gardner C, Jordan C, Twissell D, Ward P. Anti-emetic profile of a non-peptide neurokinin NK1 receptor antagonist, CP-99,994, in ferrets. Eur J Pharmacol 249: R3–R4, 1993 [DOI] [PubMed] [Google Scholar]
- 9. Brizzee KR, Marshall KR. Developmental studies on emetic response to tartar emetic and copper sulfate in the cat. Proc Soc Exp Biol Med 103: 839–842, 1960 [DOI] [PubMed] [Google Scholar]
- 10. Cechetto DF. Central representation of visceral function. Fed Proc 46: 17–23, 1987 [PubMed] [Google Scholar]
- 11. Cechetto DF, Calaresu FR. Central pathways relaying cardiovascular afferent information to amygdala. Am J Physiol Regul Integr Comp Physiol 248: R38–R45, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Cechetto DF, Ciriello J, Calaresu FR. Afferent connections to cardiovascular sites in the amygdala: A horseradish peroxidase study in the cat. J Auton Nerv Syst 8: 97–110, 1983 [DOI] [PubMed] [Google Scholar]
- 13. Cho YK, Li CS. Gustatory neural circuitry in the hamster brain stem. J Neurophysiol 100: 1007–1019, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen MI, Shaw CF. Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir Physiol Neurobiol 143: 127–140, 2004 [DOI] [PubMed] [Google Scholar]
- 15. De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48 h fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol 296: R902–R911, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Destefino VJ, Reighard DA, Sugiyama Y, Suzuki T, Cotter LA, Larson MG, Gandhi NJ, Barman SM, Yates BJ. Responses of neurons in the rostral ventrolateral medulla (RVLM) to whole-body rotations: Comparisons in decerebrate and conscious cats. J Appl Physiol 110: 1699–1707, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Lorenzo PM. Corticofugal influence on taste responses in the parabrachial pons of the rat. Brain Res 530: 73–84, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Endo T, Nemoto M, Minami M, Yoshioka M, Saito H, Parvez SH. Changes in the afferent abdominal vagal nerve activity induced by cisplatin and copper sulfate in the ferret. Biog Amine 11: 399–407, 1995 [Google Scholar]
- 19. Ezure K, Tanaka I, Miyazaki M. Pontine projections of pulmonary slowly adapting receptor relay neurons in the cat. NeuroReport 9: 411–414, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Feldman JL. Neurophysiology of breathing in mammals. In: Handbook of Physiology. The Nervous System. IV. Intrinsic Regulatory Systems of the Brain, edited by Bloom FE. Bethesda, MD: American Physiological Society, 1986, p. 463–524 [Google Scholar]
- 21. Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319: 229–259, 1984 [DOI] [PubMed] [Google Scholar]
- 22. Gardner CJ, Armour DR, Beattie DT, Gale JD, Hawcock AB, Kilpatrick GJ, Twissell DJ, Ward P. GR205171: A novel antagonist with high affinity for the tachykinin NK1 receptor, and potent broad-spectrum anti-emetic activity. Regul Pept 65: 45–53, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J Neurophysiol 101: 1598–1612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gonsalves S, Watson J, Ashton C. Broad spectrum antiemetic effects of CP-122,721, a tachykinin NK1 receptor antagonist, in ferrets. Eur J Pharmacol 305: 181–185, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Granata AR. Ascending and descending convergent inputs to neurons in the nucleus parabrachialis of the rat: An intracellular study. Brain Res 600: 315–321, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Green AM, Angelaki DE. Internal models and neural computation in the vestibular system. Exp Brain Res 200: 197–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han ZS, Gu GB, Sun CQ, Ju G. Convergence of somatosensory and baroreceptive inputs onto parabrachio-subfornical organ neurons in the rat: An electrophysiological study. Brain Res 566: 239–247, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Horn CC, De Jonghe BC, Matyas K, Norgren R. Chemotherapy-induced kaolin intake is increased by lesion of the lateral parabrachial nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 297: R1375–R1382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jhamandas JH, Aippersbach SE, Harris KH. Cardiovascular influences on rat parabrachial nucleus: An electrophysiological study. Am J Physiol Regul Integr Comp Physiol 260: R225–R231, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Jhamandas JH, Harris KH. Influence of nucleus tractus solitarius stimulation and baroreceptor activation on rat parabrachial neurons. Brain Res Bull 28: 565–571, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Jian BJ, Shintani T, Emanuel BA, Yates BJ. Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp Brain Res 144: 247–257, 2002 [DOI] [PubMed] [Google Scholar]
- 33. King GW. Topology of ascending brainstem projections to nucleus parabrachialis in the cat. J Comp Neurol 191: 615–638, 1980 [DOI] [PubMed] [Google Scholar]
- 34. Kobashi M, Adachi A. Projection of nucleus tractus solitarius units influenced by hepatoportal afferent signal to parabrachial nucleus. J Auton Nerv Syst 16: 153–158, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Lang IM, Medda BK, Shaker R. Differential activation of pontomedullary nuclei by acid perfusion of different regions of the esophagus. Brain Res 1352: 94–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lang IM, Sarna SK, Shaker R. Gastrointestinal motor and myoelectric correlates of motion sickness. Am J Physiol Gastrointest Liver Physiol 277: G642–G652, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Leslie RA, Gwyn DG. Neuronal connections of the area postrema. Fed Proc 43: 2941–2943, 1984 [PubMed] [Google Scholar]
- 38. Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol 443: 55–77, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCall AA, Yates BJ. Compensation following bilateral vestibular damage. Front Neurol 2: 88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCandless CH, Balaban CD. Parabrachial nucleus neuronal responses to off-vertical axis rotation in macaques. Exp Brain Res 202: 271–290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res 647: 255–264, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Miller DM, Cotter LA, Gandhi NJ, Schor RH, Cass SP, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp Brain Res 188: 175–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller DM, Cotter LA, Gandhi NJ, Schor RH, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of rostral fastigial nucleus neurons of conscious cats to rotations in vertical planes. Neuroscience 155: 317–325, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mungarndee SS, Lundy RF, Jr, Norgren R. Central gustatory lesions and learned taste aversions: Unconditioned stimuli. Physiol Behav 87: 542–551, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Otake K, Ezure K, Lipski J, Wong She RB. Projections from the commissural subnucleus of the nucleus of the solitary tract: An anterograde tracing study in the cat. J Comp Neurol 324: 365–378, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Papas S, Ferguson AV. Electrophysiological characterization of reciprocal connections between the parabrachial nucleus and the area postrema in the rat. Brain Res Bull 24: 577–582, 1990 [DOI] [PubMed] [Google Scholar]
- 47. Porter JD, Balaban CD. Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib Res 7: 63–76, 1997 [PubMed] [Google Scholar]
- 48. Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: Neophobia and conditioned taste aversion. Brain Res Bull 55: 359–366, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol 51: 136–146, 1984 [DOI] [PubMed] [Google Scholar]
- 50. Spector AC. Gustatory function in the parabrachial nuclei: Implications from lesion studies in rats. Rev Neurosci 6: 143–175, 1995 [DOI] [PubMed] [Google Scholar]
- 51. Spector AC. Gustatory parabrachial lesions disrupt taste-guided quinine responsiveness in rats. Behav Neurosci 109: 79–90, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Sugiyama Y, Suzuki T, Destefino VJ, Yates BJ. Integrative responses of neurons in nucleus tractus solitarius to visceral afferent stimulation and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol 301: R1380–R1390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Takeuchi Y, McLean JH, Hopkins DA. Reciprocal connections between the amygdala and parabrachial nuclei: Ultrastructural demonstration by degeneration and axonal transport of horseradish peroxidase in the cat. Brain Res 239: 583–588, 1982 [DOI] [PubMed] [Google Scholar]
- 54. Verbalis JG, Richardson DW, Stricker EM. Vasopressin release in response to nausea-producing agents and cholecystokinin in monkeys. Am J Physiol Regul Integr Comp Physiol 252: R749–R753, 1987 [DOI] [PubMed] [Google Scholar]
- 55. Wang SC, Borison HL. Copper sulphate emesis; a study of afferent pathways from the gastrointestinal tract. Am J Physiol 164: 520–526, 1951 [DOI] [PubMed] [Google Scholar]
- 56. Williams JB, Murphy DM, Reynolds KE, Welch SJ, King MS. Demonstration of a bilateral projection from the rostral nucleus of the solitary tract to the medial parabrachial nucleus in rat. Brain Res 737: 231–237, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Yamamoto T. Neural mechanisms of taste aversion learning. Neurosci Res 16: 181–185, 1993 [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in rats with excitotoxic brain lesions. Neurosci Res 22: 31–49, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Yates BJ, Jian BJ, Cotter LA, Cass SP. Responses of vestibular nucleus neurons to tilt following chronic bilateral removal of vestibular inputs. Exp Brain Res 130: 151–158, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Yuan CS, Barber WD. Parabrachial nucleus: Neuronal evoked responses to gastric vagal and greater splanchnic nerve stimulation. Brain Res Bull 27: 797–803, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Zimnicka AM, Ivy K, Kaplan JH. Acquisition of dietary copper: A role for anion transporters in intestinal apical copper uptake. Am J Physiol Cell Physiol 300: C588–C599, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]