Abstract
Consumption of high levels of fructose in humans and animals leads to metabolic and cardiovascular dysfunction. There are questions as to the role of the autonomic changes in the time course of fructose-induced dysfunction. C57/BL male mice were given tap water or fructose water (100 g/l) to drink for up to 2 mo. Groups were control (C), 15-day fructose (F15), and 60-day fructose (F60). Light-dark patterns of arterial pressure (AP) and heart rate (HR), and their respective variabilities were measured. Plasma glucose, lipids, insulin, leptin, resistin, adiponectin, and glucose tolerance were quantified. Fructose increased systolic AP (SAP) at 15 and 60 days during both light (F15: 123 ± 2 and F60: 118 ± 2 mmHg) and dark periods (F15: 136 ± 4 and F60: 136 ± 5 mmHg) compared with controls (light: 111 ± 2 and dark: 117 ± 2 mmHg). SAP variance (VAR) and the low-frequency component (LF) were increased in F15 (>60% and >80%) and F60 (>170% and >140%) compared with C. Cardiac sympatho-vagal balance was enhanced, while baroreflex function was attenuated in fructose groups. Metabolic parameters were unchanged in F15. However, F60 showed significant increases in plasma glucose (26%), cholesterol (44%), triglycerides (22%), insulin (95%), and leptin (63%), as well as glucose intolerance. LF of SAP was positively correlated with SAP. Plasma leptin was correlated with triglycerides, insulin, and glucose tolerance. Results show that increased sympathetic modulation of vessels and heart preceded metabolic dysfunction in fructose-consuming mice. Data suggest that changes in autonomic modulation may be an initiating mechanism underlying the cluster of symptoms associated with cardiometabolic disease.
Keywords: metabolic syndrome, autonomic nervous system, leptin, insulin resistance, spectral analysis, radiotelemetry
there are indications that the increase in fructose consumption in the form of high-fructose corn syrup in the last decades has paralleled the increased incidence of obesity and diabetes (1, 12). Consumption of high levels of fructose in humans and animals leads to insulin resistance, obesity, hypertension, autonomic dysfunction, and lipid and renal abnormalities (1, 5, 8, 10, 13). The cluster of metabolic and hemodynamic alterations, known as metabolic syndrome, has reached epidemic levels worldwide (1, 17, 48).
There is much information on the cardiovascular and metabolic effects of a high-fructose diet in animal models. Fructose-fed rats and mice show moderate hypertension and glucose intolerance, associated with increased levels of plasma insulin, cholesterol, and triglycerides (9, 13, 20, 23, 24, 31). There is consistent evidence for a role of the sympathetic nervous system and the renin angiotensin system (RAS) in fructose-induced cardiovascular and renal changes (5, 8, 13, 23, 41, 47). Our group demonstrated that only a 8-wk consumption of a fructose-enriched diet in mice was sufficient to induce prominent metabolic and cardiovascular changes, seen as glucose intolerance, as well as nocturnal hypertension (13, 14). These cardiometabolic changes were associated with an increase in systolic arterial pressure (SAP) variance and its low-frequency (LF) domain and increased plasma ANG II (13, 14, 41). We also demonstrated that rats subjected to fructose overload provided in the drinking water showed increased blood pressure and insulin resistance along with reduced cardiac vagal tone (5). In healthy humans, acute ingestion of fructose, but not water, significantly increased, heart rate, blood pressure, and SAP variability, while it decreased cardiovagal baroreflex sensitivity (BRS) (6).
Correlations between cardiovascular, autonomic, and metabolic dysfunctions in humans and animals have been demonstrated (5, 13, 14, 17, 19, 46). Van Gaal et al. (46) postulated that two common pathways in the pathogenesis of obesity and cardiovascular disease are insulin resistance and low-grade inflammation. Visceral obesity and insulin resistance increase cardiovascular risk by classical mechanisms, such as dyslipidemia, hypertension, and deficits in glucose metabolism. Dysfunction also occurs via hormonal and immunological changes induced by leptin, resistin, and adiponectin, all secreted from adipocytes. This may lead to increased oxidative stress, considered to be a pathogenic mechanism linking obesity and insulin resistance with endothelial dysfunction. Excess weight and obesity are associated not only with increased oxidative stress, but also with elevated systemic inflammation, activation of the coagulation cascade, disturbances in the RAS, and, most importantly, enhanced lipid and protein oxidation, resulting in the generation of oxidized low-density lipoprotein (46). Furthermore, Hellstrom (19) presented convincing evidence that the development of a diverse group of diseases, such as hypertension, diabetes, and heart disease, is favored by increased sympathetic neural outflow, resulting in endothelial dysfunction, dyslipidemia, inflammation, and insulin resistance. This is likely ameliorated by an opposing parasympathetic homeostatic shift. Studies in animal models also show that efferent vagal outflow inhibits proinflammatory cytokine production and systemic inflammation, identifying an immunoregulatory function of the vagal nerve (3, 21). Recently, Haensel et al. (18) in a review of 13 clinical studies involving healthy individuals or patients with cardiovascular, metabolic, or renal diseases, concluded that there was an inverse association between decreased heart rate variability (HRV) and subclinical inflammation. A key point is that in many clinical and/or experimental studies, the time points for examination of the relationship between autonomic function and cardiovascular and metabolic sequellae are usually limited to a fixed time point. There is little information on the time course of the changes, making it difficult to draw correlative conclusions.
Thus, we conducted a study using a mice model to examine the time course (from 2 wk to 2 mo) of metabolic, autonomic, and cardiovascular alterations induced by fructose overload. We used radiotelemetry for chronic 24-h blood pressure measurements and the autoregressive spectral method for blood pressure and heart rate analysis. We tested the hypothesis that fructose consumed in the drinking water causes time-dependent changes in blood pressure and autonomic function. We further evaluated the time course of metabolic changes by measuring plasma glucose, lipids, insulin, leptin, resistin, adiponectin, and glucose tolerance.
MATERIALS AND METHODS
General procedures.
Experiments were performed in male C57BL/6 mice (Harlan, Indianapolis, IN), 24–29 g (study beginning at 2 mo of age). Mice were housed individually at 22°C on a 12:12-h light-dark cycle (0500–1700, lights on). The experiment was begun after a 1-wk acclimatization period. Wright State University's Laboratory Animal Care and Use Committee approved all experiments.
Mice were given ad libitum access to a standard pellet diet (30% protein, 58% carbohydrate and 12% fat) and water or water containing fructose (100 g/l). Chow and fluid consumption were measured at the 60-wk time point. Caloric intake was calculated using the equations: 1 g chow = 2.89 kcal and 1 g fructose = 4.0 kcal (5).
After 7 days on the dietary regimen, telemetric catheters were inserted and cardiovascular measurements (n = 8 animals) were made at 15 days (F15 group) and 60 days (F60 group). A control group (n = 6) was conducted in parallel. For the surgical procedure, mice were anesthetized with ketamine/xylazine (120:20 mg/kg im). Catheters (model TA11PA-C20, Data Sciences International, St. Paul, MN) were inserted into the left common carotid artery, as previously described (10). Additional groups were used for evaluation of glucose tolerance at 15 and 60 days (n = 7 each group) and were killed by decapitation for blood collection.
Cardiovascular parameters and locomotor activity.
Cardiovascular parameters and locomotor activity were recorded by radiotelemetry (Data Sciences International). BP recordings were made at a high sampling rate (5 kHz) for 1 h during the light (0700–0800) and dark (1900–2000) light-dark periods. Continuous 24-h recordings (500 Hz) were made to determine the light-dark patterns. HR or pulse interval (PI) was calculated from the pressure signal. Twenty-four-hour locomotor activity was measured with the radiotelemetric system, which records animal movement (13, 14).
Variance and spectral analysis.
Overall variability of PI and SAP was assessed in the time domain by means of variance (standard deviation of mean2) and the root mean square of successive differences (RMSSD). PI and SAP fluctuations were assessed in the frequency domain using autoregressive spectral analysis, as described elsewhere (13, 33). Briefly, PI and SAP series were divided in segments of 350 beats and overlapped by 50%. A spectrum was obtained for each of the segments via the Levinson-Durbin recursion, with the model order chosen according to Akaike's criterion, ranging between 10 and 14. The oscillatory components were quantified in low- (LF: 0.1 to 1 Hz) and high- (HF: 1 to 5.0 Hz) frequency ranges. The LF band for SAP and PI oscillations was similar to that used by Janssen et al. (22). The power spectrum density was calculated for each recognizable component in the LF and HF bands by integrating the spectrum of the components. The power is expressed as LF and HF power, as described elsewhere (23, 33). When analysis showed the presence of very slow oscillations (< 0.1 Hz), which contributed to more than 80% of the segment variability, the segment was discarded from the analysis. Spontaneous BRS was calculated for each day/night recording period using the sequence analysis technique (2).
Glucose tolerance test.
The glucose tolerance test (GTT) was performed in controls and mice consuming fructose for 15 and 60 days. Animals were fasted for 6 h and injected with glucose (1.5 g/kg ip). Blood samples were taken from a tail cut at 0, 15, 30, 60, 90, and 120 min after the glucose load. Glucose concentrations were determined using an Accu-Check Advantage Blood Glucose Monitor (Roche Diagnostic, Indianapolis, IN) (13).
Metabolic hormonal measurements.
Blood was obtained by decapitation in controls and mice consuming fructose for 15 and 60 days (0900–1100). Plasma was immediately separated and stored at −80°C. Tests were performed by the University of Cincinnati Mouse Metabolic Phenotyping Center (Cincinnati, OH). Plasma insulin, leptin, resistin, and adiponectin levels were measured by radioimmunoassay using 20-μl aliquots with a multiplex system (Lincoplex Multiplexed Biomarker Immunoassays for Luminex Linco Research, St. Charles, MO). Total plasma cholesterol and triglycerides were determined by enzymatic assays (Thermo Fisher Scientific, Waltham, MA).
Statistical analysis.
Results are expressed as means ± SE. Data were compared using repeated-measures ANOVA followed by Newman-Keuls test for multiple comparisons. The Pearson correlation analysis was used to identify an association between variables. Differences were considered statistically significant when P < 0.05.
RESULTS
Body weight was similar between controls and fructose-fed mice at the beginning and end of the experimental protocol (Table 1). Twenty-four-hour chow consumption was lower and fluid consumption was higher in the F60 group (2.8 ± 0.2 g chow and 14 ± 1 ml fructose) compared with controls (4.5 ± 0.1 g chow and 6 ± 0.4 ml water). The total caloric intake (chow + fructose or chow alone) was similar between the F60 group (13.6 ± 0.4 kcal/day) and controls (13.0 ± 0.3 kcal/day).
Table 1.
Body weight and metabolic parameters in control, 15-day fructose, and 60-day fructose groups
| C (n = 6) | F15 (n = 8) | F60 (n = 8) | |
|---|---|---|---|
| Body weight, g | |||
| Initial | 26 ± 0.6 | 25 ± 0.3 | 26 ± 0.3 |
| Final | 31 ± 0.4# | 26 ± 0.5 | 32 ± 0.7# |
| Glucose, mg/dl | 174 ± 11 | 183 ± 14 | 219 ± 3*† |
| Cholesterol, mg/dl | 94 ± 6 | 105 ± 6 | 127 ± 4*† |
| Triglycerides, mg/dl | 79 ± 3 | 73 ± 3 | 97 ± 3*† |
| Insulin, pM | 81 ± 9 | 84 ± 13 | 158 ± 25*† |
| Leptin, pM | 90 ± 11 | 78 ± 34 | 146 ± 23*† |
| Resistin, ng/ml | 5.4 ± 0.3 | 6.3 ± 0.4 | 5.2 ± 0.8 |
| Adiponectin, ng/ml | 9039 ± 240 | 9181 ± 754 | 10573 ± 315 |
Values are expressed as means ± SE. Metabolic parameters were measured in plasma. C, control; F15, 15-day fructose; F60, 60-day fructose.
P < 0.05 vs. initial body weight in the same group.
P < 0.05 vs. C;
P < 0.05 vs. F15.
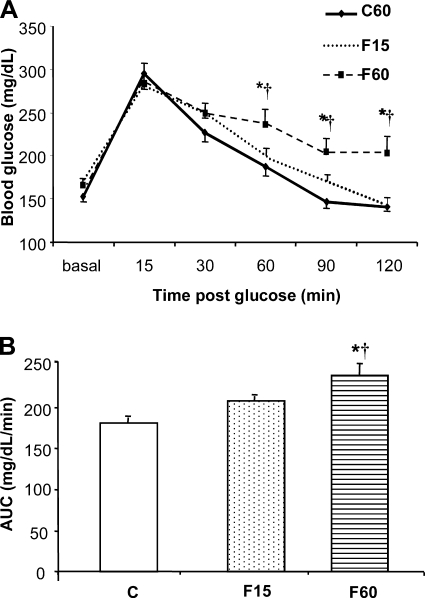
Metabolic parameters were unchanged after 15 days of fructose overload. However, after 60 days, plasma glucose, cholesterol, triglycerides, insulin, and leptin were increased compared with controls (Table 1). Plasma resistin and adiponectin were not significantly changed (Table 1). Handling of a glucose load was unchanged at 15 days of fructose but was impaired at 60 days (Fig. 1, A and B).
Fig. 1.
Effect of a high-fructose diet on glucose tolerance in mice. A: blood glucose levels during a glucose tolerance test in control (C, n = 6), 15-day fructose (F15, n = 8) and 60-day fructose (F60, n = 8) groups. Glucose was measured at basal, 15, 30, 60, 90, and 120 min after a glucose load (1.5 g/kg ip). B: glucose tolerance as estimated by area under time course curve (AUC). *P < 0.05 vs. C. †P < 0.05 vs. F15.
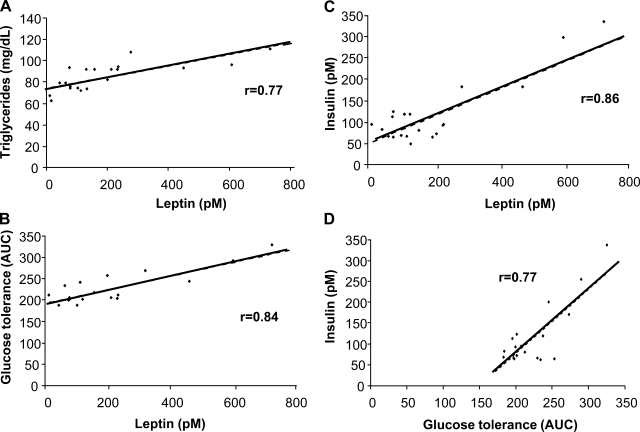
Correlation analyses were performed using all data from the 15- and 60-day groups (Fig. 2). Leptin was significantly correlated with triglycerides (r = 0.77), glucose tolerance (r = 0.84), and insulin (r = 0.86). Plasma insulin was correlated with glucose tolerance (r = 0.77).
Fig. 2.
Correlations of metabolic data collected from all fructose fed mice. Positive correlations were seen for leptin and triglycerides (A), leptin and glucose tolerance (B), leptin and insulin (C), and insulin and glucose tolerance (D).
Arterial pressure (AP) was recorded continuously for 24 h to determine diurnal patterns. There were light-dark rhythms for AP (+12%, C; +12%, F15; +14%, F60; % change from light to dark) and HR (+13%,C; +12%, F15; +17%, F60; % change from light to dark). Results showed that 15 or 60 days of fructose consumption increased SAP and MAP in both the light and dark periods. The HR was increased in light and dark periods in F15 group compared with C groups (Table 2).
Table 2.
Cardiovascular and behavioral parameters in control, 15 day-fructose, and 60 day-fructose groups
| C (n = 6) | F15 (n = 8) | F60 (n = 8) | |
|---|---|---|---|
| Dark (1700-0500) | |||
| SAP, mmHg | 122 ± 1 | 132 ± 2* | 132 ± 2* |
| DAP, mmHg | 97 ± 1 | 102 ± 2 | 102 ± 3 |
| MAP, mmHg | 110 ± 1 | 116 ± 2* | 117 ± 2* |
| AP pulse, mmHg | 23.7 ± 2 | 30.4 ± 3 | 30.5 ± 3 |
| HR, bpm | 571 ± 12 | 627 ± 16* | 592 ± 16 |
| Activity, counts/min | 15 ± 2 | 20 ± 3 | 18 ± 3 |
| Light (0500–1700) | |||
| SAP, mmHg | 109 ± 2# | 117 ± 2*# | 117 ± 3*# |
| DAP, mmHg | 85 ± 1# | 89 ± 2# | 87 ± 3# |
| MAP, mmHg | 98 ± 2# | 104 ± 2*# | 103 ± 2*# |
| AP pulse, mmHg | 21.9 ± 1 | 28.1 ± 2 | 28.7 ± 3 |
| HR, bpm | 504 ± 6# | 560 ± 13*# | 534 ± 8# |
| Activity, counts/min | 5 ± 1# | 5 ± 1# | 4 ± 1# |
Values are expressed as means ± SE. Blood pressure was recorded continuously for 24 h at 500 Hz.
P < 0.05 vs. C;
P < 0.05 vs. dark period in the same group.
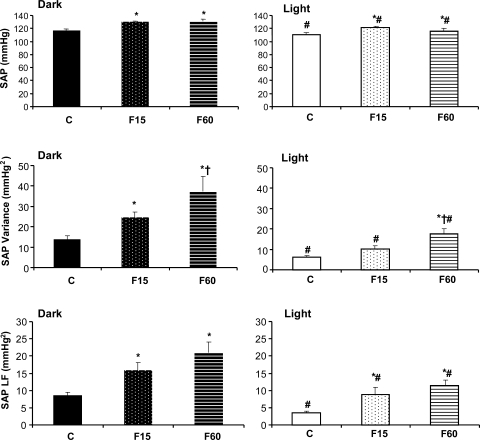
A high sampling rate was used to measure variance and spectral parameters (1-h recordings during the light and dark phases). Results obtained from this analysis corroborated the 24-h data, showing increases in SAP at 15 and 60 days (Fig. 3). The changes in AP after fructose overload could not be explained by increased locomotor activity, which was not altered (Table 2).
Fig. 3.
Systolic arterial pressure (SAP), SAP variance and SAP LF in control (C; n = 6), 15-day fructose (F15; n = 8), and 60-day fructose (F60; n = 8) groups. Blood pressure was recorded at 5 kHz during the dark (1900–2000) and light (0700–0800) periods. *P < 0.05 vs. C. †P < 0.05 vs. F15. #P < 0.05 vs. dark period in the same group.
PI and SAP variability were evaluated in time (variance) and frequency domains using autoregressive spectral analysis. SAP variance (SAP VAR) was increased in the 15-day fructose group during the dark period, while increases were seen at 60 days during the light and dark phases. Just 15 days of fructose consumption induced a marked increase in the LF component of SAP variability. This difference was also observed in the F60 group. Additionally, SAP VAR and LF were enhanced in the dark period compared with the light period in all of the groups (Fig. 3).
There was an increase in PI variance, LF component, and in the LF/HF ratio of PI variability in both fructose groups (F15 and F60) compared with controls. The RMSSD and HF component of PI variability were not changed by fructose consumption. There was no significant effect of circadian rhythm (light vs. dark) in PI variability for the groups (Table 3).
Table 3.
Pulse interval variability in time and frequency domains and spontaneously baroreflex sensitivity in control, 15 day-fructose, and 60 day-fructose groups
| C (n = 6) | F15 (n = 8) | F60 (n = 8) | |
|---|---|---|---|
| Dark (1900–1930) | |||
| Variance, ms2 | 19 ± 2.2 | 49 ± 13* | 51 ± 8.6* |
| RMSSD, ms | 7.3 ± 1.5 | 6.6 ± 0.4 | 7.6 ± 0.9 |
| LF normalized, UN | 21 ± 2.3 | 35 ± 4.5* | 41 ± 3.7* |
| HF normalized, UN | 27 ± 1.4 | 29 ± 2.9 | 25 ± 1.2 |
| LF/HF | 0.8 ± 0.1 | 1.7 ± 0.1* | 1.9 ± 0.2* |
| BRS, ms/mmHg | 2.9 ± 0.1 | 1.6 ± 0.1* | 1.6 ± 0.2* |
| Light (0700–0730) | |||
| Variance, ms2 | 17 ± 2.5 | 58 ± 12* | 92 ± 14*†# |
| RMSSD, ms | 6.6 ± 1.0 | 7.2 ± 0.9 | 6.7 ± 0.7 |
| LF normalized, UN | 24 ± 3.7 | 47 ± 4.1* | 46 ± 4.9* |
| HF normalized, UN | 34 ± 7.3 | 27 ± 3.9 | 34 ± 3.3 |
| LF/HF | 0.8 ± 0.1 | 2.2 ± 0.2* | 1.8 ± 0.3* |
| BRS, ms/mmHg | 2.8 ± 0.3 | 1.6 ± 0.4* | 1.8 ± 0.1* |
Values are expressed as mean ± SE.
BRS, baroreflex senstivity; RMSSD, root mean square of successive differences of the pulse interval; UN, unit normalized; LF, power of low frequency oscillations (0.1 to 1.0 Hz); HF, power of high-frequency oscillations (1.0 to 5.0 Hz).
P < 0.05 vs. C;
P < 0.05 vs. F15;
P < 0.05 vs. dark period in the same group.
The spontaneous BRS was attenuated in the dark and light periods in both fructose groups (F15 and F60) compared with controls. There was no significant effect of circadian rhythm (light vs. dark) on BRS (Table 3).
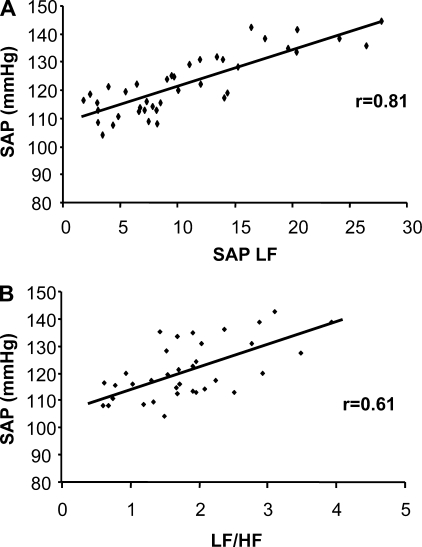
Correlations were performed using the spectral data from the fructose groups. There were positive correlations between LF SAP and SAP (r = 0.81) (Fig. 4A). The LF/HF of PI was also significantly correlated with SAP (r = 0.61) (Fig. 4B).
Fig. 4.
Correlations of SAP data collected from all fructose-fed mice. Positive correlations were noted during the light and dark periods between LF domain of SAP and SAP (A) and LF/HF ratio of PI and SAP (B).
DISCUSSION
The major aim of the study was to investigate the time course of metabolic, autonomic, and cardiovascular alterations induced by fructose overload in mice. In a previous study, we reported that consumption of high-fructose chow in mice produced nocturnal hypertension and autonomic imbalance, changes thought to be related to activation of the sympathetic and RAS systems (13, 14, 41). The results of the present study, which tested the effect of fructose given in the drinking water show 1) insulin resistance, increased plasma levels of cholesterol, triglycerides, and leptin after 60 days of fructose; 2) increase in SAP and MAP during the light and dark periods from day 15 of fructose; and 3) cardiac and vascular sympathetic increased modulation and spontaneous baroreflex attenuation from day 15 of fructose. Results show that chronic consumption of fructose induced physiological changes consistent with metabolic syndrome. The key finding was that dysfunction of cardiovascular autonomic control occurred prior to any metabolic changes.
To provide accurate and reproducible measures of autonomic function, it is important to record AP at high frequencies and over long periods. This was achieved in the present study via use of radiotelemetry. The method provides measures of AP during sleep and wakefulness cycles without human interference. A caveat in the method is that the catheter is implanted in the carotid artery, and this could interfere with baroreceptor function. However, numerous studies from our laboratory and others have documented the usefulness of the technique in measuring BRS under different physiological conditions (8, 13, 14, 44).
Using radiotelemetry, we observed that AP and HR were higher in the dark (awake period) over the light period (sleep period) in both control and fructose mice. This was seen with the 24-h recording (500 Hz), as well as the 1-h recording (5000 Hz). The important finding with respect to the cardiovascular system was the increase in light and dark AP levels in the fructose groups (15 and 60 days) compared with controls. The differences were evident in both the 1- and 24-h recordings. Locomotor activity in light and dark periods was similar between control and fructose groups, suggesting that the fructose-induced AP increase was not related to increased locomotor activity.
There is evidence that the LF components of SAP and PI are related to cardiac and vasomotor sympathetic modulation, respectively (37, 42). Aiming to understand the mechanisms involved in the AP increase in animals treated with fructose, we analyzed PI and SAP modulation. Although it seems paradoxical that the increase in total variance of PI was accompanied by an increase in LF component of HRV, other indices of parasympathetic modulation, such as HF component of HRV and RMSSD, did not change. Data suggest that the increase in LF component cannot be attributed to increased parasympathetic modulation, as described by Tank et al. (44). In our study, the increase in LF component of HRV may explain the increase in the total HRV in mice ingesting fructose. Additionally, the marked enhancement in the LF/HF ratio and the decrease in BRS in fructose mice at 15 and 60 days suggested a shift in autonomic nervous system balance, characterized by an increase in cardiac sympathetic modulation. These cardiac and BRS changes were not observed when mice were provided fructose in chow (60-day exposure) (13), suggesting an exacerbation of autonomic effects when fructose was given in the drinking water. Moreover, the increase in HR observed after 15 days of fructose may be related to the increased LF/HF ratio. In fact, we observed increased HR along with reduced vagal tone in female rats submitted to 60 days of fructose overload in drinking water (5). In healthy humans, acute ingestion of a fructose solution also enhanced HR, AP, and sympathetic modulation, while it decreased BRS (6).
Our findings clearly show that fructose modulates vasomotor sympathetic tone during the light and dark periods. The increase in the LF component of SAP in the fructose groups suggests a role for sympathetic overactivity in the AP increase. This was reinforced by the positive correlations between LF, SAP, and SAPVAR and also between LF/HF of PI and SAP. Farah et al. (13) also showed that 60 days of fructose treatment in mice increased AP, the LF component of SAP and SAP variance. The changes were specific for the dark period, which is different from the present study in that AP and sympathetics were increased in both light and dark periods. The difference in the results may be related to the mode of access, the rapid sensory activation provided by liquid fructose as opposed to the dietary administration, which would occur on a longer time frame. Similar increases in AP and sympathetic modulation were observed in humans in response to an oral fructose overload (6). Another means to examine sympathetic input is to study the central nervous system, specifically brain stem autonomic centers. This was accomplished using quantitative in situ hybridization for tyrosine hydroxylase (TH) mRNA, the rate-limiting enzyme for catecholamine synthesis. We found that TH mRNA expression was significantly increased in the brain stem locus coeruleus, a source for sympathetic innervation of the periphery. This was seen at the 15-day time point (data not shown). Similar changes in brain stem THmRNA were accompanied by increased LF of SAP (13) in mice submitted to 60 days of a fructose-enriched diet. Importantly, this autonomic imbalance in fructose fed-mice was correlated with renal dysfunction (8). Clinical studies have shown a correlation between elevation in SAP variance and target organ damage in hypertensive patients (15, 38), as well as with increased cardiovascular morbidity and mortality (25).
With regard to metabolic indicators, the changes occurred at the late, but not the early, time point. After 60 days of fructose overload, there were increases in plasma glucose (26%), cholesterol (44%), triglycerides (22%), insulin (95%), leptin (63%), as well as glucose intolerance. These results are supported by our previous study, which showed that liquid fructose intake in mice increased leptin and insulin (36). Studies in rats also showed that fructose consumption induced hypertension associated with moderate glucose intolerance, increased plasma insulin, cholesterol, and triglycerides (20, 23, 24). Consumption of fructose in addition to inducing insulin resistance causes unfavorable changes in lipid profiles. Studies in mice subjected to fructose overload demonstrated that hypercholesterolemia was accompanied by aortic atherosclerosis, demonstrating the tissue pathologies of the treatment (34). There is also evidence that insulin resistance, demonstrated in the present study by increased plasma glucose and insulin and changes in the glycemic response to glucose (GTT), increases the risk of cardiovascular mortality, perhaps via sympathetic activation (4, 11). Indeed, in humans, it was demonstrated that fructose infusion increased the release of epinephrine (16).
With regard to the metabolic pattern, there was a significant increase in plasma insulin and leptin in the F60 group. A stimulatory effect of fructose on plasma insulin was generally reported (32, 34, 36). However, a previous study by Farah et al. (13), reported unchanged insulin levels in fructose-fed mice. One explanation for the difference in results is the mode of fructose administration, chow vs. liquid. The insulin increase detected in the F60 group is likely related to the reduction in glucose tolerance, which was reinforced by the correlation between insulin and glucose tolerance.
There was also evidence for increased adiposity in the 60-day fructose group (data not shown). Body fat has been linked to insulin and leptin resistance, providing a possible mechanism. Adipose tissue is an active endocrine and paracrine organ that releases a large number of cytokines and bioactive mediators, such as leptin, adiponectin, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (28, 46). These substances negatively influence glucose and lipid homeostasis, blood pressure, coagulation, fibrinolysis, and inflammation, which may lead to endothelial dysfunction and atherosclerosis (28, 46). In the present study, correlation analysis showed that plasma leptin variability explained 60% of triglyceride variability, 74% of insulin variability, and 71% of glucose tolerance variability in our sample. Other studies have demonstrated similar correlations. For example, in men, insulin sensitivity was correlated with leptin, while in women, insulin sensitivity is influenced by a complex cadre of factors (30). Lee et al. (29) showed a correlation between changes in leptin and triglycerides in fructose-fed rats. More recently, Shapiro et al. (42) demonstrated that chronic fructose consumption in rats induces leptin resistance prior to increases in body weight, adiposity, leptin, insulin, or glucose. Fructose-induced leptin-resistance also accelerates dietary high-fat-induced obesity (42). Sakar et al. (40) identified a positive regulatory control loop between gut leptin and fructose, in which fructose triggers release of gastric leptin, which, in turn, modulates metabolic function in the liver. This loop appears to be a possible mechanism by which fructose rapidly becomes lipogenic, resulting in cellular pathologies.
Finally, it is important to emphasize that fructose-induced autonomic imbalance precedes the hormonal and metabolic changes. These data suggest that autonomic changes modulate hormonal and immune function, by inducing release of bioactive molecules, which are involved in the development of the profile changes (3, 21, 28, 46). The bigger differences are in autonomic balance, which likely lead to other clinically significant hemodynamic changes. Indeed, although the animals were not hypertensive in the light period (SAP > 140 mmHg or DAP >90 mmHg), there was an increase in MAP and SAP in the fructose groups compared with controls. Ribeiro et al. (39) demonstrated that insulin resistance caused by sucrose feeding is due to an important impairment of the hepatic parasympathetic nerve-dependent component of insulin action. In fact, autonomic imbalance has been associated with severe insulin resistance (45), which predates the overt development of type 2 diabetes mellitus by many years (35). Importantly, the impairment of the parasympathetic nervous system has been considered to be an etiological factor in the pathological process, rather than just a consequence of diabetes (7). Evidence showed decreased parasympathetic vagal activity in high-fructose-fed rats (35). Our group also demonstrated a positive correlation between cardiac parasympathetic dysfunction and insulin resistance in female fructose-fed rats (5).
In conclusion, results document the time course of the metabolic, hormonal, and autonomic responses to fructose consumed in the drinking water. The pattern of response shows that increased sympathetic modulation for vessels and heart preceded the development of metabolic dysfunction.
Perspectives and Significance
In recent years, there is increasing emphasis on a causal role of autonomic imbalance in the etiology of metabolic syndrome (27). In this imbalance, a decrease in parasympathetic activity seems to be the determining factor in the development of insulin resistance (31). Adipose, gut, and hepatic tissues that secrete metabolically important hormones are heavily innervated by sympathetic and parasympathetic fibers. Parasympathetic input modulates insulin sensitivity and opposes catabolic effects of sympathetic activation (26). Thus, our data provide evidence that autonomic imbalance, demonstrated by increased sympathetic modulation to the cardiovascular system (and, consequently, reduced parasympathetic modulation), could trigger hemodynamic and metabolic changes induced by fructose overload. Data suggest that autonomic imbalance may be a key mechanism underlying the cluster of cardiovascular and metabolic symptoms associated with cardiometabolic diseases.
GRANTS
The authors acknowledge the financial support of FAPESP (2006/51551-3, 2007/57595-5), CNPq (482520/2009-4, 306011/2010-7, 479766/2011-8), and National Institutes of Health Grant R-01-HL-093567 (M. Morris). M. C. Irigoyen and K. De Angelis are recipients of CNPq-BPQ fellowships. D. D. Serador was supported by the Foundation for the Improvement of Postsecondary Education, Ph.D. Exchange Program [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Programa de Doutorado com Estágio no Exterior (CAPES/PDEE) Process 010405-1].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.D.A., D.D.S., C.T.M., M.C.I., and M.M. conception and design of research; K.D.A., D.D.S., and C.T.M. performed experiments; K.D.A., D.D.S., C.T.M., M.C.I., and M.M. analyzed data; K.D.A., D.D.S., C.T.M., M.C.I., and M.M. interpreted results of experiments; K.D.A. and M.C.I. prepared figures; K.D.A., D.D.S., M.C.I., and M.M. drafted manuscript; K.D.A., C.T.M., M.C.I., and M.M. edited and revised manuscript; K.D.A., D.D.S., C.T.M., M.C.I., and M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the help of Cincinnati Mouse Metabolic Phenotyping Center (DK59630) for the conduct of all endocrine measurements, as well as the assistance of Dr. Yanfang Chen and Mary Key.
REFERENCES
- 1. Basciano H, Federico L, Adeli K. Fructose, insulin resistance and metabolic dyslipidemia. Nutr Metab 5: 1–14, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia GJ. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81, 1985 [PubMed] [Google Scholar]
- 3. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bressler P, Bailey SR, Matsuda M, DeFronzo RA. Insulin resistance and coronary artery disease. Diabetologia 39: 1345–1350, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Brito JO, Ponciano K, Figueroa D, Bernardes N, Sanches IC, Irigoyen MC, De Angelis K. Parasympathetic dysfunction is associated with insulin resistance in fructose-fed female rats. Braz J Med Biol Res 41: 804–808, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol 294: R730–R737, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Carnethon MR, Jacobs DR, Sidney S, Liu K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. Diabetes Care 26: 3035–3041, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Cunha TS, Farah V, Paulini J, Pazzine M, Elased KM, Marcondes FK, Cláudia Irigoyen M, De Angelis K, Mirkin LD, Morris M. Relationship between renal and cardiovascular changes in a murine model of glucose intolerance. Regul Pept 139: 1–4, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Dai S, McNeill JH. Fructose-induced hypertension in rats is concentration- and duration-dependent. J Pharmacol Toxicol Methods 33: 101–107, 1995 [DOI] [PubMed] [Google Scholar]
- 10. De Angelis K, Irigoyen MC, Morris M. Diabetes and cardiovascular autonomic dysfunction: application of animal models. Auton Neurosci 145: 3–10, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 334: 952–957, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Elliot SS, Keim NL, Stern JS, Teff K, Havel PT. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr 76: 911–922, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Farah V, Elased KM, Chen Y, Key MP, Cunha TS, Irigoyen MC, Morris M. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci 130: 41–50, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Farah V, Elased KM, Morris M. Genetic and dietary interactions: role of angiotensin AT1a receptors in response to a high-fructose diet. Am J Physiol Heart Circ Physiol 293: H1083–H1089, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Frattola A, Parati G, Cuspidi C, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens 11: 1133–1137, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Gabriely I, Hawkins M, Vilcu C, Rossetti L, Shamoon H. Fructose amplifies counterregulatory responses to hypoglycemia in humans. Diabetes 51: 893–900, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Bombelli M, Mancia G. Metabolic syndrome and cardiometabolic risk: an update. Blood Press 18: 7–16, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Haensel A, Mills PJ, Nelesen RA, Ziegler MG, Dimsdale JE. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 33: 1305–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hellstrom HR. The altered homeostatic theory: A hypothesis proposed to be useful in understanding and preventing ischemic heart disease, hypertension, and diabetes–including reducing the risk of age and atherosclerosis. Med Hypotheses 68: 415–433, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hsieh PS. Reversal of fructose-induced hypertension and insulin resistance by chronic losartan treatment is independent of AT2 receptor activation in rats. J Hypertens 23: 2209–2217, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic anti-inflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janssen BJ, Leenders PJ, Smits JF. Short-term and long-term blood pressure and heart rate variability in the mouse. Am J Physiol Regul Integr Comp Physiol 278: R215–R225, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Kamide K, Rakugi H, Higaki J, Okamura A, Nagai M, Moriguchi K, Ohishi M, Satoh N, Tuck ML, Ogihara T. The renin-angiotensin and adrenergic nervous system in cardiac hypertrophy in fructose-fed rats. Am J Hypertens 15: 66–67, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Katovich MJ, Reaves PY, Francis SC, Pachori AS, Wang HW, Raizada MK. Gene therapy attenuates the elevated blood pressure and glucose intolerance in an insulin-resistant model of hypertension. J Hypertens 19: 1553–1558, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension 36: 901–906, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat-functional implications. J Clin Invest 110: 1243–1250, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kreier F, Yilmaz A, Kalsbeek A, Romijn JA, Sauerwein HP, Fliers E, Buijs RM. Hypothesis: shifting the equilibrium from activity to food leads to autonomic unbalance and the metabolic syndrome. Diabetes 52: 2652–2656, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Lau DCW, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol 288: H2031–H2041, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Lee YC, Ko YH, Hsu YP, Ho LT. Plasma leptin response to oral glucose tolerance and fasting/re-feeding tests in rats with fructose-induced metabolic derangements. Life Sci 78: 1155–1162, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Lichnovská R, Gwozdziewiczová S, Chlup R, Hrebícek J. Serum leptin in the development of insulin resistance and other disorders in the metabolic syndrome. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 149: 119–126, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Lindmark S, Wiklund U, Bjerle P, Eriksson JW. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first-degree relatives of Type 2 diabetes patients and control subjects. Diabet Med 20: 399–405, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Luo D, Hou X, Hou L, Wang M, Xu S, Dong C, Liu X. Effect of pioglitazone on altered expression of Aβ metabolism-associated molecules in the brain of fructose-drinking rats, a rodent model of insulin resistance. Eur J Pharmacol 664: 14–19, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation 84: 482–492, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Merat S, Casanada F, Sutphin M, Palinski W, Reaven PD. Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet. Arterioscler Thromb Vasc Biol 19: 1223–1230, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Miller AW, Sims JJ, Canavan A, Hsu T, Ujhelyi MR. Impaired vagal reflex activity in insulin-resistant rats. J Cardiovasc Pharmacol 33: 698–702, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Morris M, Araujo IC, Pohlman RL, Marques MC, Rodwan NS, Farah VM. Timing of fructose intake: an important regulator of adiposity. Clin Exp Pharmacol Physiol 39: 57–62, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pagani M, Malfatto G, Pierini Casati R S, Masu AM, Poli M, Guzzetti S, Lombardi F, Cerutti S, Malliani A. Spectral analysis of heart rate variability in the assessment of autonomic diabetic neuropathy. J Auton Nerv Syst 23: 143–153, 1988 [DOI] [PubMed] [Google Scholar]
- 38. Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 5: 93–98, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Ribeiro RT, Lautt WW, Legare DJ, Macedo MP. Insulin resistance induced by sucrose feeding in rats is due to an impairment of the hepatic parasympathetic nerves. Diabetologia 48: 976–983, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sakar Y, Nazaret C, Lettéron P, Ait Omar A, Avenati M, Viollet B, Ducroc R, Bado A. Positive regulatory control loop between gut leptin and intestinal GLUT2/GLUT5 transporters links to hepatic metabolic functions in rodents. PLos One 4: e7935, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Senador D, Key M, Brosnihan KB, Irigoyen MC, Elased KM, Morris M. Cardiovascular interactions between losartan and fructose in mice. J Cardiovasc Pharmacol Ther 15: 68–77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol 295: R1370–R1375, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stauss HM, Mrowka R, Nafz B, Patzak A, Unger T, Persson PB. Does low frequency power of arterial blood pressure reflect sympathetic tone? J Auton Nerv Syst 54: 145–154, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Tank J, Jordan J, Diedrich A, Obst M, Plehm R, Luft FC, Gross V. Clonidine improves spontaneous baroreflex sensitivity in conscious mice through parasympathetic activation. Hypertension 43: 1042–1047, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Valensi P, Nguyen TN, Idriss S, Cazes P, Karam G, Paries J, Miossec P, Attali JR. Influence of parasympathetic dysfunction and hyperinsulinemia on the hemodynamic response to an isometric exercise in non-insulin-dependent diabetic patients. Metabolism 47: 934–939, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Van Gaal LF, Mertens IL, De Block DE. Mechanisms linking obesity with cardiovascular disease. Nature 444: 875–880, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Verma S, Bhanot S, Mcneill JH. Sympathectomy prevents fructose-induced hyperinsulinemia and hypertension. Eur J Pharmacol 373: R1–R4, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Wiernsperger N, Geloen A, Rapin JR. Fructose and cardiometabolic disorders: the controversy will, and must, continue. Clinics 65: 729–738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]