Abstract
Loss of the intestinal barrier is critical to the clinical course of heat illness, but the underlying mechanisms are still poorly understood. We tested the hypothesis that conditions characteristic of mild heatstroke in mice are associated with injury to the epithelial lining of the intestinal tract and comprise a critical component of barrier dysfunction. Anesthetized mice were gavaged with 4 kDa FITC-dextran (FD-4) and exposed to increasing core temperatures, briefly reaching 42.4°C, followed by 30 min recovery. Arterial samples were collected to measure FD-4 concentration in plasma (in vivo gastrointestinal permeability). The small intestines were then removed to measure histological evidence of injury. Hyperthermia resulted in a ≈2.5-fold elevation in plasma FD-4 and was always associated with significant histological evidence of injury to the epithelial lining compared with matched controls, particularly in the duodenum. When isolated intestinal segments from control animals were exposed to ≥41.5°C, marked increases in permeability were observed within 60 min. These changes were associated with release of lactate dehydrogenase, evidence of protein oxidation via carbonyl formation and histological damage. Coincubation with N-acetylcysteine protected in vitro permeability during hyperthermia and reduced histological damage and protein oxidation. Chelation of intracellular Ca2+ to block tight junction opening during 41.5°C exposure failed to reduce the permeability of in vitro segments. The results demonstrate that hyperthermia exposure in mouse intestine, at temperatures at or below those necessary to induce mild heatstroke, cause rapid and substantial injury to the intestinal lining that may be attributed, in part, to oxidative stress.
Keywords: heatstroke, small intestine, protein carbonyl, fluorescein isothiocyanate-dextran, BAPTA-AM
severe heatstroke in humans is characterized by multiple organ injury, cell death, and hemorrhage to a large number of organ systems (29). In the 1970s Graber et al. (13) reported that patients with heatstroke exhibit elevations in circulating endotoxin that suggested a breach in the intestinal barrier. Since this initial observation there have been numerous animal studies that have demonstrated that severe hyperthermia exposure results in elevations in intestinal permeability and endotoxin translocation (12, 18, 22, 36, 39, 40). This is generally held to be an important contributor to the morbidity and mortality of severe heat illness as the endotoxin released into the circulation or lymphatics activates the innate immune system, which can then contribute to the rapid progression toward multiorgan failure (4, 5, 24, 27). In addition, there is emerging evidence from other models of acute intestinal injury (e.g., ischemia-reperfusion) that the release of digestive enzymes into the submucosa from the upper regions of the small intestine may induce acute inflammatory responses as potent as endotoxin (38). Therefore, understanding the mechanisms and conditions underlying the permeability defect in the intestine is an important link to understanding the origins of the inflammatory response in hyperthermia and for developing future strategies for mucosal protection in at-risk populations.
The causes of the increased intestinal permeability due to hyperthermia are not well understood, but they have been attributed to both the direct influence of increased temperature (11, 16) and to secondary effects of intestinal ischemia that arise from diversion of splanchnic blood flow to vascular beds participating in heat exchange (16). In addition, both heat-induced injury to the epithelial lining (22) and regulated opening of epithelial tight junctions (11, 47) have been implicated as important underlying mechanisms of increased permeability.
Little is known about the influence of hyperthermia on the mouse intestine, even though the mouse has emerged in recent years as a highly relevant model for exploring the underlying pathophysiology of heatstroke (23, 25, 26) and for understanding the basic mechanisms regulating intestinal permeability in disease (10, 44). In this study, we tested the hypothesis that hyperthermia exposure in mice, at temperatures associated with mild heatstroke, causes significant elevations in in vivo gastrointestinal permeability and injury to the epithelial lining. Second, using an in vitro model of isolated small intestine, simulating heatstroke conditions, we tested the hypothesis that hyperthermia induces tissue injury to the intestinal lining via a mechanism dependent on oxidative stress. To address this hypothesis three different antioxidants, which suppress different aspects of oxidative stress, were used in the in vitro hyperthermic intestine, namely Tiron for superoxide formation (14), Trolox for lipid peroxidation (28), N-acetylcysteine (NAC) for thiol depletion (33).
METHODS
Chemicals and drugs used.
The following list of chemicals, their abbreviations, and sources were used in this study: medium 199 (Cellgro), l-glutamine (Lonza), sodium bicarbonate (Acros Organics), formaldehyde, fluorescein isothiocyanate (FITC)-dextran 4 kDa (FD-4, Sigma Aldrich), fluorescein-5-(6)-sulfonic acid trisodium salt (low-molecular-weight probe, Invitrogen), tetramethyl rhodamine isothiocyanate or TRITC-dextran (TD-4), Tiron (superoxide scavenger, Sigma Aldrich), Trolox (vitamin E analogue and general antioxidant, Sigma Aldrich), (NAC, a thiol donor and antioxidant, Sigma Aldrich), and 1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl ester (BAPTA-AM, an intracellular calcium chelator, Biomol).
All experiments were performed on adult C57BL/6J male mice (23–30 g; 8–13 wk of age) using procedures approved by the University of Florida Animal Care and Use Committee. The animals were housed at the University of Florida Animal Care Facility on a 12:12 light cycle, 22°C and 45% humidity.
In vivo permeability studies.
Mice were provided free access to both food and water before the experiment and were anesthetized with 60 mg/kg ip pentobarbital, diluted 1/10 in sterile saline and administered at ∼9:00 A.M. Under anesthesia, the mice were gavaged with 10 μl/g of 20.8 mM 4 kDa FD-4 into the middle of the esophagus. They were then held in an upright position for ≈5 min to avoid regurgitation of the probe. Mice were excluded if FD-4 was found in the oral-pharyngeal cavity, which could be checked post mortem with a UV light box. Next, 0.5 ml sterile saline was administered subcutaneously in the neck region. Core temperature was monitored with a YSI 400 rectal thermistor connected to a temperature monitor/PID servocontrol unit (Digi-Sense). Data were recorded in 15-s intervals with Digi-sense Software (Eutech). The mice were loosely taped in the supine position, and the incisors were pulled forward with suture to gently extend the neck. The tip of the tongue was pulled outward and to the side using a miniature bulldog clamp to ensure a patent upper airway. The tongue, oropharynx, and eyes were lubricated with petroleum jelly. The servocontrol unit was attached to a 150-Watt ceramic heat emitter, placed ≈20 cm above the animal. Ceramic heat emitters give long wavelength radiant heat (4–14 μmλ) outside the visible range, which penetrates deeply within the animal, thus avoiding extreme surface heat. After the drop in core temperature that occurs with anesthesia (≈34°C), the animals were brought to a baseline steady-state core temperature of 36°C for ≈10 min, the normal daytime core temperature of mice in their resting state (26). Then core temperature was either increased to 37°C (control animals) or 39.5°C for heatstroke animals over ≈1 h. In hyperthermia animals, core temperature was then incrementally increased by 0.5°C/30 min up to 42.4°C for 15 s (Tmax), whereas control animals were kept at 37°C for a matching time period. This Tmax for heated animals is known to induce mild, survivable heatstroke in awake mice (26). The hyperthermia mice were then allowed to recover at room temperature over 30 min, during which time core temperature fell to 37°C. At the conclusion of either protocol (≈4.5 h), a cardiac puncture was performed for measurement of plasma FD-4, and the intestines were removed and prepared for in vitro permeability assessment. Because the FD-4 was gavaged into the stomach, this method assessed “gastrointestinal permeability” and not intestinal permeability per se. In addition, no attempt was made to prevent excretion of FD-4 into the urine. In previous studies in rats (22) the renal arteries were ligated before heat stress to eliminate this pathway. Therefore, these measures of in vivo gastrointestinal permeability reflect the difference between the uptake of the probe from the intestine and the rate of glomerular filtration and excretion of FD-4 into the urine or other beds.
Procedures for in vitro intestinal permeability.
In vitro procedures for isolated intestine were performed in two different experiments 1) to evaluate the effects of hyperthermia on intestines from control animals and 2) to study the effects of heating living animals on the permeability of the isolated intestine, after heat exposure. Control mice were euthanized by carbon dioxide asphyxiation, and the entire small intestines were rapidly excised and placed in preoxygenated medium 199 (with glutamine and sodium bicarbonate but without phenol red). The protocol was modified from a previously developed procedure in the rat (22). Briefly, the intestinal lumen was gently rinsed with oxygenated medium 199 at room temperature until the contents were cleared. After cleaning was performed, a blunt glass rod was gently placed within the cavity and the gut was everted over the rod, taking care to not cause damage. The gut was then filled with oxygenated medium and tied off in segments ∼2 cm long with 2–0 suture. Each segment was exposed to ≈2 cmH2O pressure before being tied off to ensure equivalent initial transluminal hydrostatic pressure. Depending on the experiment, solutions were composed of fresh medium or fresh medium containing treatments such as antioxidants or Ca+2 chelators, as described in the text. This was included in the filling solutions to ensure treatments were loaded into the basal as well as the luminal sides of the tissue. Segments were then placed into 50-ml conical tubes, in media or media plus treatment compounds, while oxygenated with 95% O2-5% CO2 and were maintained at either 37°C or one of several hyperthermia temperatures, as described in the text. Buffer solutions included either FD-4 (0.3 mM) for experiments in control mice or TD-4 for experiments following heatstroke (to avoid interference with gavaged FD-4). After this preparation, the conical tubes were suspended in a circulating water bath and brought to the desired temperature, as monitored within the baths with a thermistor (Yellow Springs model 4610).
After the experiment, the volume of the solution inside the intestinal segments (100–300 μl) was carefully measured by collecting all of the fluid into preweighed tubes and calculating volume from weight and density. The square area of the luminal surface was standardized by applying a clear plastic sheet over each transected segment. Thirty grams of weight were distributed across the sheet to gently flatten it. In preliminary experiments, this approach resulted in a gentle stretch of the intestinal wall to the beginning of a plateau in its compliance curve, allowing for a reproducible measurement of area. Though this additional procedure had no impact on the overall effects of heat treatment, it greatly improved the reproducibility of the permeability measurements across segments. Fluorescence of the serosal fluid (inside the segment) was measured using a spectrofluorometer (SpectraMax M5, Molecular Devices), and the concentration of FD-4 or TD-4 was determined by comparison to standard curves. One series of experiments was done to prevent tight junction opening in the absence and presence of heat exposure by chelating intracellular Ca2+ using 100 μM BAPTA-AM, a dose based on previous studies (43).
Determination of oxidative stress.
Frozen samples of intestinal segments from in vitro experiments exposure to either 37°C or 41.5°C and with or without treatment with antioxidants were homogenized in PBS with an anti-protease cocktail to reduce protein degradation. After the homogenization, protein concentration was determined (Bradford Assay), and samples were diluted to achieve equivalent concentrations in each lane. The samples were then tested for protein carbonyl accumulation using a commercially available kit (Millipore Oxyblot Protein Oxidation Kit) using standard electrophoresis/Western blot methods: 150 V for 60 min followed by transfer to nitrocellulose at 275 mA for 90 min. The kit derivatizes carbonyls with 2,4-dinitrophenylhydrazine (DNPH) and then uses antibodies against DNPH for detection. After derivatization the membranes were washed and then treated with 1° and 2° antibodies, followed by repeated washes (1° antibody: rabbit anti-DNPH antibody; 2° antibody: goat anti-rabbit IgG, horseradish peroxidase-conjugated). The membranes were then developed using enhanced chemiluminescence (ECL)/Western blotting reagents. Ponceau (a short-term protein stain) was used for determination of even protein loading across all molecular weights and used as a reference source to identify the location of particular protein bands that were very sensitive to oxidation but may have drifted between gels. This allowed for better comparisons of specific bands between gels.
Lactate dehydrogenase assay.
Lactate dehydrogenase (LDH) activity assays were performed to evaluate cytotoxicity in the in vitro tissues taken from the control animals and exposed to heat. Luminal (outside of the gut sacs) fluid samples (50 μl) were collected after completion of the experimental protocol (for control tissues as well as NAC-treated tissues) for this test. A commercially available kit (Cayman Chemical) was used for this determination. LDH standard curves were obtained in all studies and were repeated in the presence or absence of NAC to detect for interactions within the assay for the NAC-treatment studies.
Histology.
In some in vitro studies and in vivo studies, tissues were collected for histological assessment after the experimental protocol. The tissues were fixed in 4% formaldehyde before staining with hematoxylin and eosin. Structural damage for in vitro segments was assessed using the scaling system from 1 to 5, adapted from Kamel et al. (21) and Chiu et al. (8): 1) Low amount of damage is seen with distinct structural components (epithelial layer and lamina propria). 2) Structural components can still be differentiated but the epithelial layer is noticeably separating from the lamina propria (layer of connective tissue below the epithelial layer containing vascular and lymph vessels). 3) Disorganization of the villi is beginning and differentiating between structural components is difficult. 4) Organization of the villi is chaotic. 5) Structure of villi is chaotic and many villi are completely destroyed down to the basal layers of tissue. Similarly, intestines were studied from in vivo experiments for control and hyperthermia mice. The intestines from in vivo heatstroke experiments were removed and fixed immediately after the 30-min recovery period. A slight variation of the 1–5 scoring system was used, based on the scaling system of Chiu et al. (8). Three transected segments from the duodenum, ileum, and jejunum were evaluated and results averaged from each animal. Samples were analyzed randomly by two graders in a blinded fashion during collection and the results averaged. Each histological segment was divided into four random areas, each given a ranking according to the scale above. The rankings for each area were then averaged to give an overall score for the segment of sample. This process was repeated for each sample.
Data analysis and statistics.
Permeability of intestinal segments was represented as transport of nanomoles of FITC-dextran/cm2 (i.e., normalized to intestinal surface area of the segment). This analytical method was taken from Lambert et al. (22): permeability = (concentrationserosal fluid·volumeserosal fluid)/mucosal surface area.
LDH release was measured by activity (represented as μU/mg tissue). The thermal load was reported in (°C·min) and was calculated as the integration of time (min) × (rectal temperature-40.4°C) when core temperature was ≥40.4°C (20, 22). This calculation of thermal load was used rather than the more recent calculation of thermal area, which sets a threshold in mice at 39.5°C (26). Our rationale was that we wanted to compare our heat exposures in mice directly with previous studies of in vivo intestinal permeability in rats, where the 40.4°C threshold was used (22). Second, our data will show that we did not see appreciable changes in intestinal permeability in vitro with temperature exposures below 40.5°C over 90 min. Therefore, we wanted to be able to compare the approximate temperature × time the animals were above this temperature range, which appears to be required for injury to occur to in vitro intestines. Analysis of gels was accomplished by using Image J software (NIH). For normalization of experiments, all samples were loaded onto a single gel for each set of experiments and controls. The densitometry measurements from seven relatively consistent bands were chosen across all lanes for a comparison of overall protein oxidation signal. This was done to reduce background noise. Data were expressed as fold changes compared with the first time point in a series (15 min).
Student’s t-test or ANOVA were used to describe main effects with Tukey's or least squares mean contrasts, where appropriate, used to evaluate specific effects between individual groups in multi-way ANOVA (SAS JMP software). All data are represented as means ± SE. For permeability measurements, each intestine produced 6–8 individual segments. A stratified randomization protocol was used to ensure that each experimental group represented an equivalent number of intestinal segments from each region to avoid bias from different regions (duodenal, ileal, or jejunal). Statistics were then performed on the grand mean values of the individual segments from each animal for each treatment; i.e., the n for each experiment represents the number of animals, not segments. P <0.05 was the minimum acceptable α value.
RESULTS
Effects of in vivo heat stress on gastrointestinal permeability.
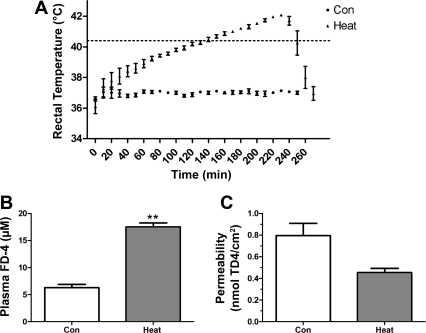
One group of mice were gavaged with FD-4 and then exposed to a hyperthermia protocol as shown in Fig. 1A. Core temperature was rapidly elevated to 39.5°C, over 1 h, and then increased at 0.5°C per 30 min until the target Tmax of 42.4°C was achieved. The average thermal load (20) was 111 ± 5.3°C·min. The survival rate over the whole heat stress protocol and recovery was 46% (6/13). Only the animals (n = 6) that survived the entire exposure and recovery period were compared against time-matched controls (n = 4), because the concentration of FD-4 in the plasma is time dependent. Animals exposed to hyperthermia showed elevated plasma FD-4 compared with controls (Fig. 1B, P < 0.01). However, when the small intestines were removed for in vitro measures of permeability, ∼3 h after reaching Tmax, no changes in permeability could be detected (Fig. 1C). This data reflects the influence of combined tight junction opening and injury in the in vivo permeability experiments. In contrast, the in vitro experiments suggest that the tight junctions were either closed, over the time required to test the segments, or mild injury was repaired.
Fig. 1.
A: average time profiles of core temperature during the heating protocol (Tmax: = 42.4°C). B: plasma levels of 4 kDa FITC dextran (FD-4) following in vivo heat treatment and 30 min recovery. **P < 0.01 compared with time-matched controls. C: intestinal permeability, measured in vitro, 4 kDa rhodamine-dextran (TD-4) following in vivo hyperthermia (P = 0.5) (n = 6 hyperthermia; n = 4 controls).
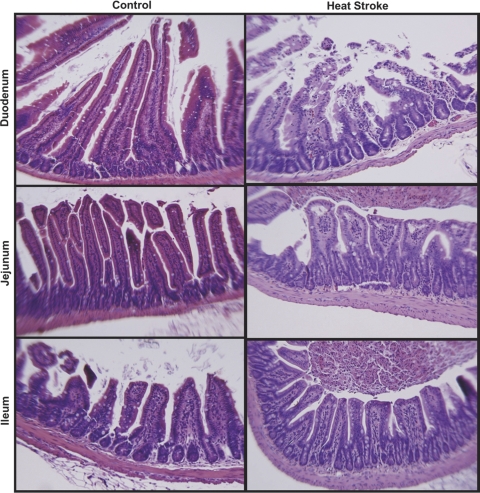
In a second set of in vivo heatstroke experiments in anesthetized mice (n = 6 in both control and heatstroke mice), the intestines were removed and fixed in formalin for histological evaluation immediately after the heating and recovery protocol. Histological results were compared with time-matched sham controls. A typical example can be seen in Fig. 2. The most severe injury was consistently observed in the duodenum. The average injury scores for each region of the intestine are expressed in Table 1, which confirms a predominant and consistent injury occurring primarily in the duodenal regions.
Fig. 2.
Representative hematoxylin and eosin staining of intestinal epithelium from specified regions of control mice (left) and heatstroke mice (right). There was consistent evidence of disorganized and blunted epithelium in the heatstroke animals, with more predominant evidence of injury in the duodenum.
Table 1.
Regional intestinal injury scores
| Controls | Heatstroke | *Significance | |
|---|---|---|---|
| Duodenum | 1.24 ± 0.37 | 2.59 ± 0.10 | < 0.0001 |
| Jejunum | 0.73 ± 0.18 | 1.24 ± 0.28 | NS |
| Ileum | 0.50 ± 0.12 | 0.55 ± 0.26 | NS |
Values are means ± SE; n = 6 animals in each group;
Two-way ANOVA, post least squares contrasts. NS, not significant
Susceptibility of in vitro small intestines from control animals to hyperthermia-induced changes in permeability.
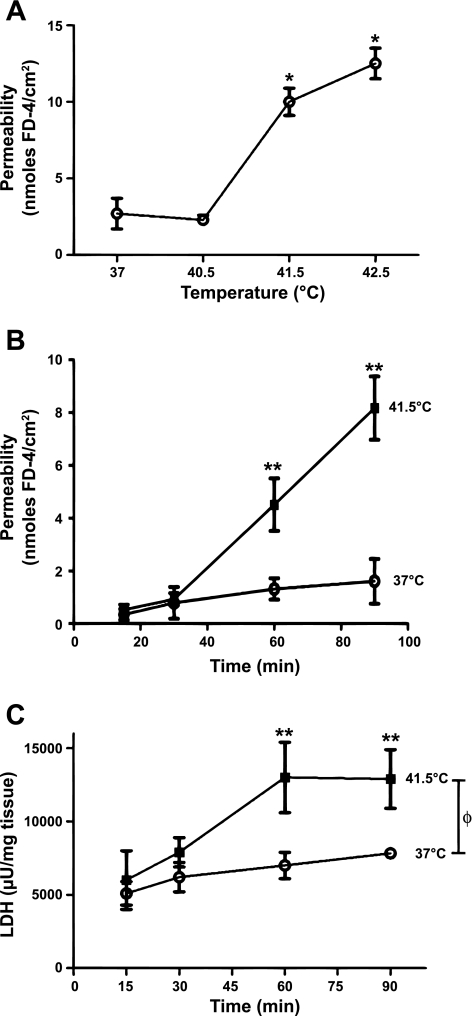
Permeability of isolated intestinal segments taken from control, untreated animals was significantly increased (P < 0.05) after exposure to 41.5°C or higher for 90 min compared with controls (Fig. 3A), whereas exposure to 40.5°C resulted in no significant increases in permeability. The thermal load of the in vitro tissue exposed to 41.5°C, where injury was first seen, was 99°C·min, which was roughly comparable to the thermal load that intestines would have been exposed to in the in vivo studies (111°C·min, as stated above). Exposure to 42.5°C showed a further apparent increase compared with 41.5°C treatment, though the differences did not reach statistical significance with this sample size. In a separate set of experiments, tissues exposed to 41.5°C, over a 90-min period were sampled at intermediate time points (Fig. 3B). Changes in permeability were first detectable between 30 and 60 min, whereas exposure for 15 min at 41.5°C was insufficient to increase permeability to a measurable extent. These results are nearly identical to previous studies in the rat (22).
Fig. 3.
A: comparison of intestinal permeability in in vitro intestinal segments when exposed to increasing bath temperatures (n = 4 mice in each); *P < 0.05 from 37°C. B: time course of changes in permeability in intestinal segments exposed to 41.5°C vs. 37°C (n = 4 mice at each time point). C: lactate dehydrogenase (LDH) release over time in segments exposed to 41.5°C or 37°C (n = 4 mice for each time point). For both B and C: ΦP < 0.01 from initial 15 min time point; *P < 0.05 for 41.5°C vs. 37°C at 90 min; +P < 0.05 from initial 15-min time point in 37°C-treated group.
LDH release from heated in vitro tissue taken from control animals.
Media taken from tissue bathing solutions during 37°C and 41.5°C exposures were used to measure for LDH release. As shown in Fig. 3C, 41.5°C exposure resulted in significant increases (P < 0.01) in LDH release after 60 min compared with 15 min exposure. LDH release was also significantly increased (P < 0.05) after 90 min of 37°C exposure, suggesting a small deterioration in control tissue at body temperature. The elevations in LDH in 37°C control experiments were not associated with elevations in permeability (see Fig. 3B). These results are also nearly identical to previous studies in the rat (22).
Oxidative stress in in vitro intestine from control animals treated with hyperthermia.
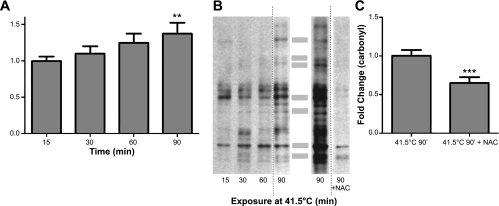
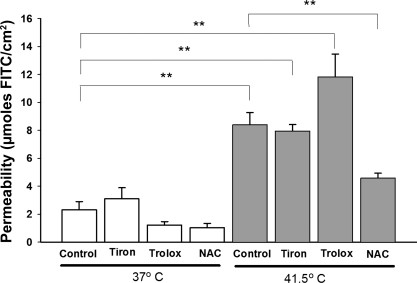
To evaluate the extent of oxidative stress during hyperthermia in the in vitro experiments, protein carbonyl formation was measured using Western analysis. Tissues exposed to 90 min at 41.5°C yielded a significant increase (P < 0.01) in protein oxidation (carbonyl formation) compared with 15 min at 41.5°C (Fig. 4, A and B). To test the hypothesis that the increase in permeability during hyperthermia is associated with increased oxidative stress, tissues were then treated during hyperthermia with the antioxidants Tiron (10 mM), Trolox (100 μM), or NAC (10 mM) (Fig. 5). Doses of these agents were taken from previous work by our laboratory and others (35, 45). Tiron and Trolox treatment did not protect against hyperthermia-induced permeability at 41.5°C. However, NAC treatment resulted in a significant protection to near-control levels. To ensure that the influence of NAC on in vitro permeability did not represent a Type I error due to low sample size, the sample size was increased to n = 8. There was no noticeable change in central tendency when these additional experiments were added. NAC treatment also significantly reduced the protein carbonyl formation in the heat-treated intestinal segments, as shown in Fig. 4, B and C.
Fig. 4.
Effects of hyperthermia on protein oxidation (carbonyl formation). A: densitometry averages in segments studied over time **P < 0.01 (n = 6 for each time point) B: left gel representative lanes from the same gel used for grouped densitometry data in A. Right gel is representative of experiments in heated (41.5°C) intestine, with and without coincubation with NAC. These 2 lanes are from a different gel from the series on the left. Dotted vertical lines show where the images were cut to exhibit different lanes from the same gel. The shaded bars in the middle are the approximate location of the bands that were used to generate a summed densitometry signals in each lane. C: grouped effects of NAC on heat-induced carbonyl formation. ***P < 0.001; n = 6 for each treatment.
Fig. 5.
Effects of antioxidant treatment of permeability in in vitro intestinal segments. **P < 0.01; n = 8 for all groups except Tiron and Trolox treatments, where n = 4.
Histological assessment of heated, control, and NAC-treated in vitro intestinal segments from control animals.
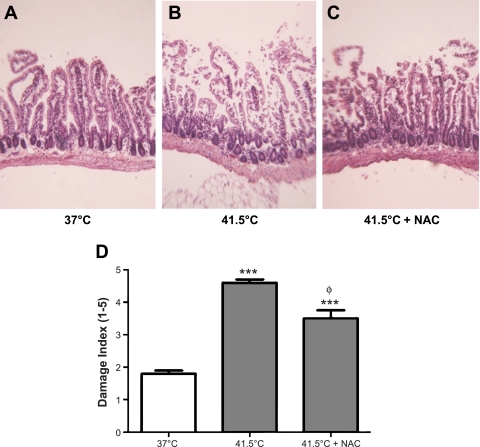
Representative histological hematoxolyn and eosin-stained pictures of in vitro tissues exposed to 37°C and 41.5°C for 90 min are shown in Fig. 6, A and B. Note that the in vitro segments lost some of their characteristic architecture compared with those intestines taken immediately from the living animal (compare Fig. 6 with Fig. 2). With the use of a standardized score index from 1 to 5, the average damage scores are shown in Fig. 6D. The extent of damage was significantly higher in tissues treated with 41.5°C compared with 37°C. NAC treatment significantly reduced the damage in hyperthermia-treated tissues compared with untreated control tissues, as shown in the image in Fig. 6C and the grouped data in Fig. 6D.
Fig. 6.
A: representative hematoxylin and eosin slides of effects of 90 min incubation of in vitro everted intestinal segments at 37°C compared with 41.5°C (B) and 41.5°C + NAC (C). D: grouped data of overall damage index of histological specimens from intestinal segments in control (n = 10), heated (n = 10), and NAC-heated (n = 8) animals. ***P < 0.001 compared with 37°C control; ΦP < 0.001 compared with 41.5°C control.
Effects of calcium chelation during hyperthermia on in vitro intestine from control animals.
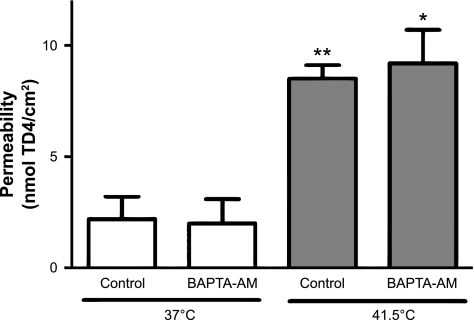
To test the hypothesis that an increase in intracellular calcium during hyperthermia is causing permeability dysfunction, possibly by opening regulatory tight junctions, in vitro segments undergoing hyperthermia or normothermia were treated with the Ca2+ chelator BAPTA-AM (100 μM). Ca2+ chelation had no effects on hyperthermia-induced intestinal permeability (Fig. 7).
Fig. 7.
Calcium chelation by BAPTA-AM (n = 4 in each group). *P < 0.05 and **P < 0.01 compared with 37°C control.
DISCUSSION
The results demonstrate that in the anesthetized mouse, exposure to elevated temperatures, sufficient to induce heatstroke-like symptoms (26), results in marked elevations in intestinal permeability and histological damage. These changes are very consistent with previous studies in other animal models, particularly in the rat (22), thus paving the way for future mechanistic studies using transgenic manipulations or other molecular approaches accessible in mice. Notably, in both in vivo and in vitro conditions, relatively brief hyperthermia exposure induced significant injury to the epithelium, as shown by histological evidence. In the in vitro intestine, hyperthermia also resulted in LDH release and oxidative damage. The injury seen in vitro was reduced significantly by incubation with NAC. Though epithelial tight junction opening may accompany or precede the epithelial injury during exposure to hyperthermia, as has been previously shown in cell culture (11), the time course and consistency of the histological evidence observed across both models suggests that the permeability defect is closely associated with extensive injury to the epithelial lining.
Although gastrointestinal permeability was clearly elevated during in vivo heatstroke, when the small intestines from these animals were removed and studied in vitro, there were no elevations in permeability. There are several plausible explanations. First, the >3-h recovery period following heat that was necessary for in vitro tissue preparation and permeability measurements may have been sufficient for the injury to repair itself. Rapid repair of local intestinal epithelial injury (i.e., restitution) is a well-known feature of the biology of the small intestine (3, 15). For example, following ischemia-reperfusion injury, human intestine restores the integrity of the epithelial lining within a 2- to 3-h window by pinching off damaged areas of the villus tip (15), and other processes of cell migration are employed as well. Second, isolated intestinal segments may be insensitive to the causes of permeability that occur in vivo, as discussed in more detail below. Third, in vivo intestinal permeability is regulated to some extent by the balance of pro- and anti-inflammatory cytokine signals within the mucosa (10, 44). Hyperthermia or heatstroke conditions result in large changes in the concentrations of circulating cytokines (5, 24, 25). These often include elevations in TNF-α, IFN-γ, and IL-1β (5, 24, 25), which are well-known inducers of epithelial tight junction opening (1, 44). Therefore, whereas in vivo these pro-inflammatory cytokines may cause an increase in permeability or contribute to injury, this effect would be diluted when tissues are bathed in media during in vitro experiments. Fourth, our measures of in vivo permeability are not restricted to the small intestine and may encompass permeability changes in the stomach or the large intestine, which would not be evident in the isolated small intestine. Furthermore, our in vivo permeability measurements have limitations. For example, they may reflect movement of the translocated dye into other tissue beds because of heat-induced changes in vascular permeability or into the urine via glomerular filtration. However, these effects would underestimate the in vivo intestinal permeability measurement. In contrast, if heat injury caused major reductions to kidney blood flow or injury to the glomerulus, it could limit the rate of excretion of the dye compared with controls and thus overestimate its translocation. However, we feel the consistency between the changes in in vivo permeability and the extent of histological evidence of injury, as well as the similar findings of Lambert et al. (22) in the rat where the renal arteries were ligated, makes this latter possibility unlikely to be a major factor over this time course.
Our results are almost entirely consistent with the previous classic work in the rat by Lambert et al. (22) except that these investigators did not see significant oxidative stress in isolated rat intestinal segments during hyperthermia. However, our results may complement their work with regard to the influence of specific antioxidants, as shown in Fig. 5. Lambert et al. used Tempol (a powerful superoxide scavenger and superoxide dismutase mimetic), ebselen (a glutathione peroxidase mimic), and nitro-l-arginine methyl ester (l-NAME, a nitric oxide synthase and nitric oxide synthase-derived-superoxide inhibitor) to block oxidative and nitrosative stress. None of these interventions impacted permeability. We utilized Tiron, a superoxide scavenger/superoxide dismutase mimetic, expected to behave like Tempol; it also had no effect. Ebselen is an effective antioxidant enzyme, but it requires an adequate level of reduced glutathione (GSH) to be effective. In GSH-depleted tissues, ebselen results in further oxidative stress and further thiol depletion (46). A parallel effect is seen with overproduction of glutathione peroxidase in transgenic mice, which results in a highly thermosensitive mouse phenotype (32). In contrast, our results show a powerful protective effect of NAC, known primarily as a thiol precursor, and a substrate for GSH synthesis (33). Hyperthermia induces a marked decrease in reduced thiols in many tissues such as the liver (41), and it is likely that GSH depletion is also occurring in the intestinal mucosa in these conditions. This would result in a strong sensitivity to NAC. Addition of the general antioxidant and vitamin E analog Trolox also had no effect in our hands, even though vitamin E has been shown to be effective in protecting rats during heatstroke (34).
To evaluate the relative importance of injury versus tight junction opening as the underlying cause of heat-induced barrier dysfunction in isolated intestinal segments, we performed experiments in the presence of an intracellular Ca2+ chelator BAPTA-AM. A key determinant of tight junction opening is the myosin-actin interactions resulting from elevations in intracellular [Ca2+] in the epithelial lining (42). Intracellular Ca2+ is expected to rise in hyperthermia, as cells initially release internal endoplasmic reticular Ca2+ stores in response to heat, followed by slow inward leakage of extracellular Ca2+ (7). Elevations in intracellular Ca2+ from ≈100 to 200 nM would be expected, based on observations in human colonic epithelium during heat (31). However, chelation had no effect on the permeability measured in the in vitro preparations. We conclude from this experiment that it is unlikely that tight junction opening via Ca2+ signaling is a prerequisite for the permeability defect seen in the in vitro preparations, which appear dominated by heat-induced injury.
In general, we found similar results in terms of both permeability and histological injury in the in vivo and in vitro preparations. However, the amount of injury appears to be larger in the in vitro intestinal segments compared with samples taken immediately from living animals (Fig. 2 vs. Fig. 6). There could be a number of explanations for this. For example, first it is likely that there is a substantial loss of the mucus layer in everted intestinal preparations caused by the isolation procedures. The mucus layer is known to have a myriad of protective functions against oxidative stress, protease activity, and other mediators of injury (2, 6, 37). Second, the epithelial surface is exposed to higher O2 levels during incubation than would occur in vivo. These could also contribute to ongoing oxidative stress. Furthermore, lack of a continuous stream of nutrients that would sustain intracellular redox state and thiol status may make the tissue more vulnerable. For example, the absence of significant cysteine in the bathing medium necessary for glutathione synthesis may account for the sensitivity to NAC in heat, as NAC functions as a substrate for glutathione synthesis (9). Finally, loss or dilution of normal cytokine signals such as IL-10 that are in the normal intestinal microenvironment may make the isolated intestinal wall particularly susceptible to other inflammatory pathways or to tight junction opening over extended periods (44). By whatever mechanism, it is clear that the histological architecture is greatly altered in the in vitro preparation (Fig. 6) compared with the in vivo preparation (Fig. 2).
Perspectives and Significance
Revealing the mechanisms and the timing of changes in intestinal permeability occurring as heatstroke progresses is key to ultimately understanding the origins of the inflammatory storm and multiorgan failure associated with severe hyperthermia. The results demonstrate that in the mouse, increases in intestinal permeability occur at core temperatures at or below conditions inducing heatstroke in humans (4, 19) and other species (5, 17, 25, 26) and may even occur under conditions of high fever or during exercise in warm environments. This work may enable future experiments that can take advantage of genetic mouse models, where different components of the immune system have been altered or different cells or proteins have been genetically labeled within the intestinal wall (30). In addition, it adds to the mounting data that oxidative stress is an important mediator of damage in heat-related illnesses, particularly with respect to intestinal injury. The finding that thiol oxidation and/or depletion may be an important underlying mechanism could provide a window for future therapeutic or nutritional interventions. The predominance of injury in the upper regions of intestine may provide a target organ for design of treatment paradigms. More experimentation is needed to fully elucidate the role of tight junctions in hyperthermia, because their importance could involve the initial steps of heat stress but is overshadowed by the severe damage due to prolonged oxidative stress and other forms of hyperthermic injury.
GRANTS
The work was supported by National Heart, Lung, and Blood Institute HLBI 53333 (to T. L. Clanton), an endowment from BK and Betty Stevens professorship (to T. L. Clanton), and support from the American Heart Association, Grant in Aid11GRNT7990119 (to T. L. Clanton). This work was in partial fulfillment of a Ph.D. degree for S. Ryan Oliver from the Ohio State University Biophysics Graduate Program. N. A. Phillips, V. L. Novosad, and E. E. Talbert were graduate students in Applied Physiology and Kinesiology, M. P. Bakos was an undergraduate intern in Applied Physiology and Kinesiology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.R.O. and T.L.C. conception and design of research; S.R.O., N.A.P., V.L.N., M.P.B., and E.E.T. performed experiments; S.R.O., N.A.P., V.L.N., M.P.B., and T.L.C. analyzed data; S.R.O. interpreted results of experiments; S.R.O., N.A.P., and T.L.C. prepared figures; S.R.O. drafted manuscript; S.R.O., N.A.P., V.L.N., M.P.B., E.E.T., and T.L.C. edited and revised manuscript; S.R.O. and T.L.C. approved final version of manuscript.
REFERENCES
- 1. Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 180: 5653–5661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev 73: 823–857, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87: 545–564, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bouchama A, Knochel JP. Heat stroke. N Engl J Med 346: 1978–1988, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Bouchama A, Ollivier V, Roberts G, Al Mohanna F, de Prost D, Eldali A, Saussereau E, El-Sayed R, Chollet-Martin S. Experimental heatstroke in baboon: analysis of the systemic inflammatory response. Shock 24: 332–335, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Brownlee IA, Knight J, Dettmar PW, Pearson JP. Action of reactive oxygen species on colonic mucus secretions. Free Radic Biol Med 43: 800–808, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Calderwood SK, Stevenson MA, Hahn GM. Effects of heat on cell calcium and inositol lipid metabolism. Radiat Res 113: 414–425, 1988 [PubMed] [Google Scholar]
- 8. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 101: 478–483, 1970 [DOI] [PubMed] [Google Scholar]
- 9. Circu ML, Rodriguez C, Maloney R, Moyer MP, Aw TY. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free Radic Biol Med 44: 768–778, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest 115: 2702–2715, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol 290: G204–G212, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Gathiram P, Gaffin SL, Brock-Utne JG, Wells MT. Time course of endotoxemia and cardiovascular changes in heat-stressed primates. Aviat Space Environ Med 58: 1071–1074, 1987 [PubMed] [Google Scholar]
- 13. Graber CD, Reinhold RB, Breman JG, Harley RA, Hennigar GR. Fatal heat stroke. Circulating endotoxin and gram-negative sepsis as complications. JAMA 216: 1195–1196, 1971 [DOI] [PubMed] [Google Scholar]
- 14. Greenstock CL, Miller RW. The oxidation of Tiron by superoxide anion: kinetics of the reaction in aqueous solution and in chloroplasts. Biochim Biophys Acta 396: 11–16, 1975 [DOI] [PubMed] [Google Scholar]
- 15. Grootjans J, Thuijls G, Derikx JP, van Dam RM, Dejong CH, Buurman WA. Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia-reperfusion. J Pathol 224: 411–419, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Hall DM, Baumgardner KR, Oberley TD, Gisolfi CV. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am J Physiol Gastrointest Liver Physiol 276: G1195–G1203, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Hall DM, Buettner GR, Matthes RD, Gisolfi CV. Hyperthermia stimulates nitric oxide formation: electron paramagnetic resonance detection of. NO-heme in blood. J Appl Physiol 77: 548–553, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol 280: H509–H521, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Hammami MM, Bouchama A, Al-Sedairy S, Shail E, AlOhaly Y, Mohamed GE. Concentrations of soluble tumor necrosis factor and interleukin-6 receptors in heatstroke and heatstress. Crit Care Med 25: 1314–1319, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Hubbard RW, Bowers WD, Matthew WT, Curtis FC, Criss RE, Sheldon GM, Ratteree JW. Rat model of acute heatstroke mortality. J Appl Physiol 42: 809–816, 1977 [DOI] [PubMed] [Google Scholar]
- 21. Kamel HM, Hume SP, Carr KE, Marigold JC, Michalowski A. Development of villous damage in mouse small intestine after local hyperthermia or irradiation. J Submicrosc Cytol Pathol 20: 185–193, 1988 [PubMed] [Google Scholar]
- 22. Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol 92: 1750–1761, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Leon L. The thermoregulatory consequences of heat stroke: are cytokines involved? J Therm Biol 31: 67–81, 2006 [Google Scholar]
- 24. Leon LR. Heat stroke and cytokines. Prog Brain Res 162: 481–524, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Leon LR, Blaha MD, DuBose DA. Time course of cytokine, corticosterone, and tissue injury responses in mice during heat strain recovery. J Appl Physiol 100: 1400–1409, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Leon LR, DuBose DA, Mason CW. Heat stress induces a biphasic thermoregulatory response in mice. Am J Physiol Regul Integr Comp Physiol 288: R197–R204, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Lim CL, Mackinnon LT. The roles of exercise-induced immune system disturbances in the pathology of heat stroke. Sports Med 36: 39–64, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Lucio M, Nunes C, Gaspar D, Ferreira H, Lima JLFC, Reis S. Antioxidant activity of vitamin E and Trolox: understanding of the factors that govern lipid peroxidation studies In vitro. Food Biophys 4: 312–320, 2009 [Google Scholar]
- 29. Malamud N, Haymaker W, Custer RP. Heat stroke; a clinico-pathologic study of 125 fatal cases. Mil Surg 99: 397–449, 1946 [PubMed] [Google Scholar]
- 30. Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 189: 111–126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mikkelsen RB, Reinlib L, Donowitz M, Zahniser D. Hyperthermia effects on cytosolic [Ca2+]: analysis at the single cell level by digitized imaging microscopy and cell survival. Cancer Res 51: 359–364, 1991 [PubMed] [Google Scholar]
- 32. Mirochnitchenko O, Palnitkar U, Philbert M, Inouye M. Thermosensitive phenotype of transgenic mice overproducing human glutathione peroxidases. Proc Natl Acad Sci USA 92: 8120–8124, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moldéus P, Cotgreave IA. N-acetylcysteine. Methods Enzymol 234: 482–492, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Niu KC, Lin KC, Yang CY, Lin MT. Protective effects of alpha-tocopherol and mannitol in both circulatory shock and cerebral ischaemia injury in rat heatstroke. Clin Exp Pharmacol Physiol 30: 745–751, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Oliver SR, Wright VP, Parinandi N, Clanton TL. Thermal tolerance of contractile function in oxidative skeletal muscle: no protection by antioxidants and reduced tolerance with eicosanoid enyzme inhibition. Am J Physiol Regul Integr Comp Physiol 295: R1695–R1705, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prosser C, Stelwagen K, Cummins R, Guerin P, Gill N, Milne C. Reduction in heat-induced gastrointestinal hyperpermeability in rats by bovine colostrum and goat milk powders. J Appl Physiol 96: 650–654, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Qin X, Sheth SU, Sharpe SM, Dong W, Lu Q, Xu D, Deitch EA. The mucus layer is critical in protecting against ischemia-reperfusion-mediated gut injury and in the restitution of gut barrier function. Shock 35: 275–281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmid-Schonbein GW. 2008 Landis Award lecture. Inflammation and the autodigestion hypothesis. Microcirculation 16: 289–306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shapiro Y, Alkan M, Epstein Y, Newman F, Magazanik A. Increase in rat intestinal permeability to endotoxin during hyperthermia. Eur J Appl Physiol Occup Physiol 55: 410–412, 1986 [DOI] [PubMed] [Google Scholar]
- 40. Singleton KD, Wischmeyer PE. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock 25: 295–299, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Skibba JL, Powers RH, Stadnicka A, Cullinane DW, Almagro UA, Kalbfleisch JH. Oxidative stress as a precursor to the irreversible hepatocellular injury caused by hyperthermia. Int J Hyperthermia 7: 749–761, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Stull JT, Blumenthal DK, Cooke R. Regulation of contraction by myosin phosphorylation. A comparison between smooth and skeletal muscles. Biochem Pharmacol 29: 2537–2543, 1980 [DOI] [PubMed] [Google Scholar]
- 43. Suzuki T, Hara H. Difructose anhydride III and sodium caprate activate paracellular transport via different intracellular events in Caco-2 cells. Life Sci 79: 401–410, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Wright VP, Klawitter PF, Iscru DF, Merola AJ, Clanton TL. Superoxide scavengers augment contractile but not energetic responses to hypoxia in rat diaphragm. J Appl Physiol 98: 1753–1760, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Yang CF, Shen HM, Ong CN. Intracellular thiol depletion causes mitochondrial permeability transition in ebselen-induced apoptosis. Arch Biochem Biophys 380: 319–330, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Yang PC, He SH, Zheng PY. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. J Gastroenterol Hepatol 22: 1823–1831, 2007 [DOI] [PubMed] [Google Scholar]