Abstract
Insulin resistance and obesity are components of the metabolic syndrome that includes development of cardiovascular disease and diabetes with advancing age. The thrifty phenotype hypothesis suggests that offspring of poorly nourished mothers are predisposed to the various components of the metabolic syndrome due to adaptations made during fetal development. We assessed the effects of maternal nutrient restriction in early gestation on feeding behavior, insulin and glucose dynamics, body composition, and liver function in aged female offspring of ewes fed either a nutrient-restricted [NR 50% National Research Council (NRC) recommendations] or control (C: 100% NRC) diet from 28 to 78 days of gestation, after which both groups were fed at 100% of NRC from day 79 to lambing and through lactation. Female lambs born to NR and C dams were reared as a single group from weaning, and thereafter, they were fed 100% NRC recommendations until assigned to this study at 6 yr of age. These female offspring were evaluated by a frequently sampled intravenous glucose tolerance test, followed by dual-energy X-ray absorptiometry for body composition analysis prior to and after ad libitum feeding of a highly palatable pelleted diet for 11 wk with automated monitoring of feed intake (GrowSafe Systems). Aged female offspring born to NR ewes demonstrated greater and more rapid feed intake, greater body weight gain, and efficiency of gain, lower insulin sensitivity, higher insulin secretion, and greater hepatic lipid and glycogen content than offspring from C ewes. These data confirm an increased metabolic “thriftiness” of offspring born to NR mothers, which continues into advanced age, possibly predisposing these offspring to metabolic disease.
Keywords: maternal nutrient restriction, thrifty phenotype, sheep
insulin resistance and obesity are components of the metabolic syndrome, which includes development of cardiovascular disease, the leading cause of death in the United States, and diabetes, the sixth leading cause of death, with advancing age (18, 23). Human epidemiologic studies have associated maternal undernutrition and fetal growth restriction during gestation with the development of a “thrifty phenotype” in offspring in later life (19). The “thrifty phenotype” hypothesis suggests that undernutrition during development leads to reallocation of energy and nutrients to favor development of organs critical for immediate survival (e.g., the brain and heart) at the expense of those organs and systems that are less critical for survival (e.g., pancreas and kidney) contributing to the eventual failure of those systems in adulthood (19). While an energetically thrifty phenotype may be beneficial in the face of food shortage, it constitutes a potentially dangerous strategy for individuals who in adult life experience an environment of great abundance of high-energy foods, as many do in the United States and other developed nations. With excessive food intake and a sedentary lifestyle, a thrifty individual is more likely to develop excessive adiposity (i.e., obesity) and its associated morbidities, such as cardiovascular disease and diabetes (19).
Early gestation is critical for placental growth, differentiation, and vascularization, as well as fetal organogenesis (39, 41, 47). We have previously demonstrated that feeding only 50% of NRC requirements from day 28 to 78 of gestation (gestation length = ∼150 days) results in a 30% fetal growth restriction at midgestation (54). When nutrient-restricted ewes fed 50% of NRC from 28 to 78 days of gestation, were realimented to 100% NRC from midgestation through lambing, male and female offspring were born at a similar size and weight as controls, but male lambs born to NR-realimented ewes gained more weight postnatally, reaching greater body weights by 4 mo of age (15). Furthermore, these adolescent male offspring were glucose intolerant, had less lean mass, and greater visceral fat mass at slaughter at 8 mo (young adult) of age relative to male offspring born from dams fed to requirements (15). These characteristics (insulin resistance, increased adiposity), resulting from a nutrient-restricted environment in utero, fit the thrifty phenotype hypothesis (19). However, evidence of programming into advanced age in precocial large animal models is lacking.
With the current increase in the incidence of obesity and diabetes, nonalcoholic fatty liver disease (NAFLD) is emerging as one of the most common causes of chronic liver disease; insulin resistance is considered to be an important cause of NAFLD (2). Because insulin resistance induces peripheral lipolysis and the delivery of free fatty acid to the liver, levels of potentially hepatotoxic free fatty acids are increased (46). Hepatocytes protect themselves by binding, transforming, catabolizing, and exporting excess free fatty acids. Peroxisome proliferator-activated receptor γ (PPARγ), one of the PPAR isoforms, seems to act as a sensor for the homeostasis of hepatic fatty-acid metabolism (12). Further, PPARγ agonists have been reported to increase insulin responsiveness (50) and to improve insulin sensitivity and histology (33).
The liver is responsible for maintaining circulating glucose levels within narrow limits and possesses the ability to synthesize glucose when blood levels are low, a process known as gluconeogenesis. This process is catalyzed by two rate-limiting enzymes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6P), which are regulated by hormones such as insulin, glucagon, and glucocorticoids (56). Liver PEPCK gene expression is increased in diabetes (22), and hepatic G6P gene mutations are linked to glycogen storage diseases (52).
Considering the importance of ageing in the advancement of metabolic disease in humans with a thrifty phenotype, it is important to evaluate whether characteristics of the thrifty phenotype are displayed in aged offspring (comparable to humans aged 50–60 yr) born to NR-realimented mothers. Therefore, the aim of this study was to evaluate voluntary feed intake, efficiency of body weight gain, body composition, glucose-insulin dynamics, and liver function in response to a bout of ad libitum feeding in aged female offspring of NR-realimented mothers.
MATERIALS AND METHODS
Animal care and feed treatments.
All methods were approved by the University of Wyoming Animal Care and Use Committee. In the fall of 2003, mature, multiparous ewes (Rambouillet/Columbia Cross) were bred by a single intact ram of the same breeding. From day 28 to 78 of pregnancy, dams were fed to either 50% of National Research Council (NRC) nutrient recommendations (32) (nutrient restricted, NR) or 100% of NRC nutrient recommendations (control, C) for maintenance of a pregnant ewe, as previously described (54). Beginning at day 79 of gestation, dams were realimented to 100% of NRC recommendations, so that both NR and C dams continued to receive 100% of NRC recommendations throughout the remainder of gestation and lactation. Gestation lengths and birth weights of singleton female lambs used in this study were similar across dietary groups averaging 150.2 ± 1.2 days and 5.14 ± 0.20 kg, respectively. Singleton female lambs born from these NR-realimented and C dams were commingled in a single group after weaning at 4 mo of age with diet details given by Burt et al. (11) and fed 100% of NRC recommendations up to 12 mo of age. From 1 yr of age through the time of testing, the ewes were maintained on NRC recommendations that were met by once a day feeding of alfalfa hay, and during late gestation and lactation, corn was added to meet energy requirements with a trace mineral block available ad libitum to meet mineral requirements. These female offspring were bred to produce lambs as 3-, 4-, and 5-yr olds.
At 6 yr of age (spring of 2010), ewes from both maternal treatments (NR, n = 4 and C, n = 4) were adapted from a hay and grain diet to the experimental ration at maintenance levels over a 2-wk acclimation period. The relatively low number of ewes per treatment group (n = 4) available for use in this study is a limitation and should be taken into account when assessing the data presented. The experimental diet consisted of a highly palatable, pelleted diet with the ration composition given by Long et al. (26) and was analyzed to contain 71.05% total digestible nutrients, 1.08 Mcal/kg net energy for gain, 1.64 Mcal/kg net energy for maintenance, 88.2% dry matter, 13.5% crude protein, and 4.05% fat on an as fed basis (ADM Alliance Nutrition, Quincy, IL). Pelleting the experimental nutrient-rich diet facilitates increased intake and thereby permits expression of differences in appetite. At the end of this acclimation period, ewes were weighed and removed from feed for 12 h and subjected to a frequently sampled intravenous glucose tolerance test (FSIGT) and then removed from feed and water for an additional 12 h and evaluated by dual X-ray absorptiometry (DEXA) to determine body composition (fat and lean tissue), as described below. The ewes were then returned to the same experimental ration, which was fed ad libitum for an 11-wk period.
During the feeding trial, ewes were housed in a single group (n = 8) under the cover of an open-front pole barn with free access to water and the pelleted feed available via an automated feeding behavior data acquisition system adapted for use in sheep (GrowSafe Systems, Airdrie, Alberta, Canada). Feed intake was continuously measured on the basis of the weight difference of the feed bunk (accuracy to 0.01 kg) at the beginning and end of each feeding episode for each individual animal, as determined by a unique electronic ear tag worn by each ewe. The feed bunk had an opening that permitted a single ewe's head to enter, allowing only one animal to consume feed at a time and her ear tag to be scanned identifying the ewe consuming feed at each episode. Feed was continuously available 24 h per day. Intake measurements were recorded throughout the entire 11-wk period. Body weight (BW) was measured every 2 wk. Also, a blood sample was collected from each ewe by venipuncture at 0800 into an evacuated blood collection tube containing heparin (BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ; 143 USP units sodium heparin per 10 ml) as anticoagulant every 2 wk prior to obtaining BW. Blood was kept cold and centrifuged within 30 min at 4°C and 1,000 g for 15 min. Plasma was collected and aliquoted prior to storage at −20°C pending analyses. At the end of the 11-wk feeding trial, ewes were again weighed and removed from feed for 12 h, and an additional FSIGT was performed; then the ewes were removed from feed and water for an additional 12 h before a DEXA was performed. Ewes were then returned to ad libitum consumption of the experimental diet for 48 h before necropsy.
Frequently sampled intravenous glucose tolerance tests.
An FSIGT was conducted to assess glucose and insulin dynamics both prior to and following the feeding trial, as previously described (17). A venous catheter (Abbocath, 16ga; Abbott Laboratories, North Chicago, IL) was placed aseptically into a jugular vein the afternoon prior to the FSIGT, which began the following morning. During this ∼12-h interval, animals were placed individually in adjacent pens and fasted, but provided with unlimited access to water. On the morning of the test, fasted baseline blood samples (6 ml) were taken at −15 min and immediately prior to intravenous glucose injection (250 mg/kg body wt, 50% dextrose; Vedco, St. Joseph, MO). Blood samples (6 ml) were then taken at 2, 4, 6, 8, 10, 12, 15, 20, 25, 30, 35, 40, 50, 60, 70, 80, 100, 120, 150, 180, 210, and 240 min following glucose injection (17, 26). Minimal model (MINMOD) analysis of the FSIGT is dependent on an adequate insulin response to the glucose load. As our sheep had plentiful endogenous insulin production (as is evident in their high insulin response to glucose injection), it was unnecessary to administer exogenous insulin during the FSIGT procedure (5). Heparinized plasma was collected following centrifugation (1,000 g for 15 min) at 4°C and stored frozen (−20°C) until time of hormone analysis. Parameters of the minimal model of glucose and insulin dynamics—insulin sensitivity (SI), glucose effectiveness (Sg), acute insulin response to glucose (AIRg), and disposition index (DI)—were determined by simultaneous fitting of glucose and insulin curves resulting from the FSIGT according to the following equations using MINMOD Millennium software (version 5.10, MINMOD) (8, 10): G′(t) = −(Sg+X)·G(t) + Sg·Gb, where G(t) is glucose at minute (t) and Gb is baseline glucose; and X′(t) = −P2·X(t) + P3·[I(t) − Ib], where X(t) is insulin action at minute (t), I(t) is insulin concentration at minute (t), Ib is baseline insulin concentration, P2 is loss rate of insulin action (X), and P3 is action of one unit insulin on glucose disposal per minute.
SI represents the acceleration of glucose clearance by insulin (SI = P3/P2), Sg is the rate of glucose clearance at basal insulin, AIRg is the insulin response measured in the first 10 min following glucose injection available to act on glucose clearance (via SI), and DI is an index of the absolute insulin action potential determined by the initial insulin secretion response and the tissue response (DI = AIRg × SI).
Dual energy X-ray absorptiometry.
To accurately determine body composition (fat and lean tissue), dual-energy X-ray absorptiometry (DEXA; GE Lunar Prodigy 8743; Madison, WI) was utilized as previously used in our laboratory and previously described and validated for sheep (16, 31, 36). Both prior to and following the feeding trail, ewes were deprived of food for 24 h and water for 12 h to minimize the chance of emesis and subsequent aspiration of gastric material, while under sedation with ketamine (22.2 mg/kg body wt), which was administered immediately prior to performing DEXA scans. The whole body scan mode was used for all animals, and scan times were approximately 3 min depending on the length of the animal. A single, blinded, and experienced investigator performed all DEXA scans and quantified % body fat. DEXA was calibrated and quality assurance tests were performed daily prior to measurement and according to the manufacturer's specifications and programmed acceptable limits.
Tissue collection.
At necropsy, ewes were sedated with ketamine (22.2 mg/kg body wt) and maintained under isoflurane inhalation anesthesia (4% induction, 1–2% maintenance) as routinely conducted in our laboratory (16). Ewes were then exsanguinated while under general anesthesia and selected organs (pancreas, liver, heart, kidneys, and lungs) and adipose tissue pools (perirenal, omental) were collected and weighed. Additionally, the right and left ventricles of the heart were dissected from the septum and the remainder of the heart and weighed, and their thickness was determined. Further, samples of liver tissue were collected from both left and right lobes, snap frozen in liquid nitrogen, and then stored at −80°C pending analysis.
Determination of hepatic lipid and glycogen content.
Tissue dry matter (DM) and percent total lipid (ether extract) was determined on duplicate 0.5-g samples of left and right lobe liver tissue by AOAC International procedures (3), as previously reported by Long et al. (27). Briefly, samples were weighed out onto dried filter paper, and the filter paper was folded to securely enclose samples. The samples were dried at 100°C for 24 h and then placed in an ether refluxer for 24 h. Weights were recorded between steps, and the difference in weights was used to calculate total lipid as a percent of DM. Final % lipid was calculated for both right and left lobes. Because of the fact that % lipid was similar for right and left lobes, they were averaged for each ewe and utilized for statistical analysis. All samples were analyzed in a single set and had a coefficient of variation (CV) of 3.1% for all liver samples for an animal. Glycogen content was determined using duplicate 0.1 g of hepatic tissue from the left lobe pulverized in liquid nitrogen. Glycogen was measured, as previously described (45), and is expressed as grams of glycogen per gram of wet hepatic tissue in a single assay with a CV of 5.4% between duplicate samples.
Western blot analysis procedures.
Western blot analysis was accomplished by methods previously described and utilized in our laboratory (16, 29). Hepatic tissue samples from the left lobe were pulverized in liquid nitrogen, and then 0.1 g of each sample was homogenized in a Polytron homogenizer with 400 μl of ice-cold buffer containing 137 mM NaCl, 50 mM Tris·HCl, 2% SDS, 1% Triton-100 solution, 10% glycerol, 2.5 mM EDTA, 1 mM CaCl2, 1 mM MgCl2, 2 mM Na3VO4, 100 mM NaF, and 1% protease inhibitor cocktail (Sigma, St. Louis, MO), at pH 7.4. Each homogenate was mixed with an equal volume of 2× standard SDS sample loading buffer and heated at 95°C for 5 min. Then, 5-μg samples of the protein extractions were separated by 10% SDS-PAGE gels, using a Mini-Protean IIITM gel electrophoresis system (Bio-Rad Laboratories, Hercules, CA, USA). After electrophoresis, the resolved proteins on the gel were transferred to nitrocellulose membranes in a transfer buffer containing 20 mM Tris-base, 192 mM glycine, 0.1% SDS, and 20% ethanol. Membranes were incubated in a blocking buffer consisting of 5% nonfat dry milk in TBST (0.1% Tween-20, 50 mM Tris·HCl, pH 7.6, and 150 mM NaCl) for 2 h. Membranes were incubated overnight at 4°C with rabbit anti-PEPCK (1:500 dilution in TBST with 5% milk), goat anti-G6P (1:500 dilution in TBST with 5% milk), and rabbit anti-PPARγ (1:800 dilution in TBST with 5% milk). PEPCK and 6GP antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and PPARγ antibody from Delta Biolabs (Gilroy, CA). After the primary antibody incubation, membranes were washed with TBST three times, for 10 min each. Membranes were incubated with horseradish-peroxidase-conjugated secondary antibody (Cell Signaling, Danvers, MA) for 1 h at room temperature. After three 15-min washes, membranes were visualized using ECL Western blotting reagents (Amersham Biosciences) and exposure to film (MR; Kodak, Rochester, NY). The density of bands was quantified using an Imager Scanner II and ImageQuant TL software (Amersham Bioscience), as previously reported (58). Each membrane was used for the detection of PEPCK, G6P, or PPARγ, then stripped and reprobed for β-actin antibody (Sigma, St. Louis, MO). Band density was normalized according to β-actin content.
Total RNA extraction and single-strand DNA synthesis.
Hepatic tissue samples from the left lobe were pulverized in liquid nitrogen. Total RNA was extracted from samples (50–100 mg) using TRIzol reagent (Invitrogen, Carlsbad, CA) treated with DNase I (Qiagen, Valencia, CA) and then purified by RNeasy mini column (Qiagen), according to the respective protocols and as previously utilized in our laboratory (29). One microgram of purified RNA of each hepatic tissue preparation was used to synthesize single-strand DNA using Promega ImProm- II Reverse Transcription System (Promega BioSciences, San Luis Obispo, CA), according to the kit protocol.
Real-time PCR.
All real-time PCR reactions were conducted using a Bio-Rad IQ5 real-time-PCR reaction system (Bio-Rad), as previously utilized in our laboratory (29). Reactions for each gene were run in duplicate. A temperature gradient PCR reaction was run for all primer sets before conducting real-time PCR to determine the optimal annealing temperature for all primer sets. According to gradient PCR, the optimal annealing temperature of all primer sets overlapped at 58°C. Correspondingly, the following protocol was designed and applied to all real-time PCR reactions: 1) 1 cycle at 95°C for 3 min; 2) 40 repeat cycles at 95°C for 10 s, followed by annealing at 58°C for 30 s; 3) 55.0°C–95.0°C, with melting temperature increasing 0.5°C for each 30 s. Fluorescence was detected at both step 2 and step 3. Real-time analysis was enabled at step 2, and melt curve data collection and analysis was enabled at step 3. An amplification efficiency of 90–105% is considered to be high in an optimized real-time PCR reaction. In our real-time-PCR experiments, all reactions reached 100% efficiency. Final data were analyzed through the 2-ΔΔCt method, in which 18s RNA was used as a reference gene to normalize all the selected gene expression data.
Primers.
PEPCK, G6P, and PPARγ primers were designed using Primer 3. 18s RNA primers were synthesized according to established methods (28). All primers were synthesized by Invitrogen (Carlsbad, CA). Primer sequences are as follows: PEPCK (forward,TCG AGA GCA GAG AGA TAC GG; reverse, TGA ACG CTT TCT CAA AAT CC ovine sequence accession no. EF062862), G-6P (forward, TGG AGT CTT TTC AGG CAT TG; reverse, CAG GAA GCA GGT GAT GAG AA bovine sequence accession no. 289179), PPARγ (forward, CCG-CAT-CTT-CCA-GGG-GTG-TC; reverse, CAA-GGA-GGC-CAG-CAT-CGT-GTA-AAT; ref. 25), and 18s RNA (forward, AGC CTG CGG CTT AAT TTG AC; reverse, CAA CTA AGA ACG GCC ATG CA).
Biochemical and hormone assays.
Plasma glucose was measured in triplicate by photo absorbance following the addition of glucose hexokinase reagent (Liquid Glucose Hexokinase Reagent Set, Pointe Scientific, Canton MI) using 96-well plates, as previously described (15). Mean intra-assay CV was 1.5%, and interassay CV was 4.0%. Plasma insulin was measured in duplicate in a single assay by commercial radioimmunoassay (RIA) kit previously validated for use in sheep (Siemens Healthcare Diagnostics, Deerfield, IL) (15). Intra-assay CV for insulin was <10%. Plasma cortisol was measured in duplicate in a single assay by previously validated commercial RIA (Siemens Healthcare Diagnostics, Deerfield, IL) (16) with an intra-assay CV <10%. Plasma leptin was measured in duplicate in a single assay by RIA (Multispecies leptin RIA; Linco Research, St. Charles MO) as previously described by Ford et al. (15) with an intra-assay CV <5%.
Statistical analysis.
Repeated variables were analyzed using a repeated-measures analysis of variance using ewe within treatment as the error term (SAS Institute, Cary, NC). Covariance structure was unstructured, and the model statement contained time, treatment, and their interaction. Differences in nonrepeated variables were determined using a general linear model of analysis of variance with treatment in the model statement. The least squared means method was used for post hoc mean separation following a significant (P < 0.05) preliminary F test. Differences were considered significant at P < 0.05 and tendencies at P < 0.10. Data are reported as means ± SE.
RESULTS
Over the 11-wk ad libitum feeding trial, average daily feed intake was greater (P < 0.001) in offspring from NR than C ewes (Table 1). Amount of time spent at the feeder per feeding episode was not different between treatment groups (NR: 150 ± 2 and C: 152 ± 2 s/episode; P = 0.46), although the amount of time spent with head down in the feeder (i.e., eating) per feeding episode was slightly, yet significantly (P = 0.02) less in offspring from NR ewes than C ewes (NR: 64 ± 1 and C: 67 ± 1 s, respectively; P = 0.02). Average number of feeding episodes per day was not different between groups (NR: 19.6 ± 0.4 and C: 20.1 ± 0.5; P = 0.42). In contrast, the quantity of feed removed from the feeder per unit time with head down was markedly greater in NR than C offspring (6.9 ± 0.3 and C, 3.6 ± 0.1 g/s; P < 0.001).
Table 1.
Changes in body weight, feeding parameters and glucose and insulin dynamics of 6-yr-old female offspring born to control and nutrient restricted mothers in response to the ad libitum feeding period
| C | NR | P value | |
|---|---|---|---|
| (n = 4) | (n = 4) | ||
| % increase in BW, kg | 29 ± 0.04 | 44 ± 0.02 | 0.002 |
| Average daily feed intake, kg feed/day | 2.07 ± 0.05 | 2.36 ± 0.05 | 0.001 |
| Feed efficiency, kg BW gain/kg feed | 0.11 ± 0.01 | 0.15 ± 0.01 | 0.014 |
| Insulin sensitivity (SI), ×10−4mIU−1·l·min−1* | 9.3 ± 1.5 | 5.7 ± 0.7 | 0.049 |
| Glucose effectiveness (SG), ×10−2 min−1* | 0.8 ± 0.1 | 1.7 ± 0.2 | 0.001 |
| Acute insulin response to glucose (AIRg), mIU·l−1·min* | 32.9 ± 7.7 | 144 ± 23.0 | 0.001 |
| Disposition index (SI × AIRg)* | 322 ± 99.1 | 727 ± 77.0 | 0.009 |
Data are expressed as means ± SE. BW, body weight; C, control; NR, nutrient restricted. *Because of a lack of significant differences between the first and second FSIGT (prior to and after the feeding challenge) values presented are averages of the two tests.
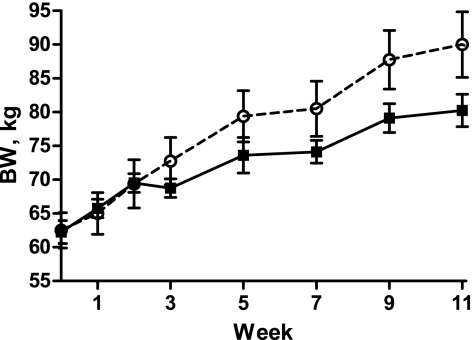
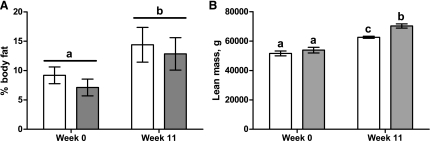
Body weight of NR (62.5 ± 2.6 kg) and C (62.3 ± 1.7 kg) offspring was similar prior to the ad libitum feeding, and both groups increased progressively in BW by the end of the study (Fig. 1, P < 0.001). However, NR offspring demonstrated a greater (P < 0.01) % increase in BW than C offspring after 2 wk of ad libitum feeding and had a greater (P < 0.01) efficiency of BW gain (kg BW gain/kg feed consumed) than C offspring (Table 1). There was a tendency for an interaction of treatment and week on lean mass (P = 0.09). NR and C ewes were not different in lean mass at the onset of the study (P = 0.30). Both NR and C offspring increased in lean mass over the 11-wk trial (P < 0.01), but at week 11, NR offspring had greater lean mass than C offspring (P < 0.01). Percent body fat also increased (P < 0.05) over the 11-wk trial in both groups but was not affected by maternal dietary treatment (Fig. 2).
Fig. 1.
Body weight (BW) of aged control (C, solid circles; n = 4) and nutrient restricted (NR) female offspring (open circles; n = 4) during ad libitum feeding. Both treatment groups increased in BW over time (P = 0.001), but NR offspring had greater overall BW than C (P = 0.002), and there was no significant interaction between week and treatment on BW (P > 0.50).
Fig. 2.
Body fat % (A) and lean mass (B) from dual-energy X-ray absorptiometry in aged female offspring of control (C; white bars; n = 4) and nutrient restricted (NR; gray bars, n = 4) ewes. a,b,cLetters that differ show significant difference, P < 0.05.
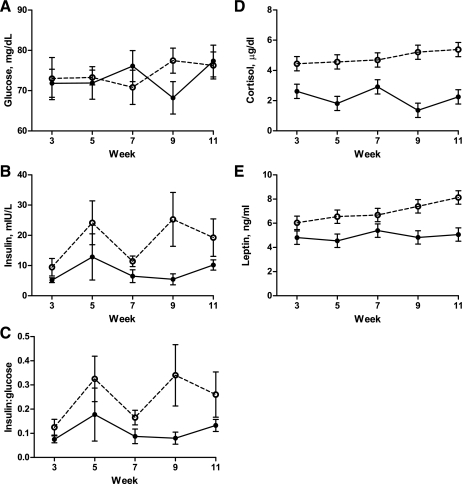
Fasted baseline plasma glucose and insulin concentrations obtained before the initial FSIGT conducted prior to the feeding trial and before the second FSIGT conducted after the feeding trial were similar in C and NR offspring averaging 79.8 ± 3.7 mg/dl and 3.9 ± 0.5 mIU/l before the initial FSIGT and 80.6 ± 4.0 mg/dl and 5.9 ± 0.8 mIU/l before the second FSIGT. Further, while fasted baseline glucose concentrations remained similar before and after the 11-wk ad libitum feeding period, fasted baseline insulin concentrations tended to increase (P < 0.10) from the first to the second FSIGT in both treatment groups. Biweekly glucose concentrations obtained from weeks 3 to 11 of ad libitum feeding remained similar between C and NR offspring (Fig. 3A), while plasma insulin and the insulin:glucose ratio were higher over this period in NR than C offspring (Fig. 3, B and C). During the feeding period, plasma cortisol was greater (P < 0.001) in NR offspring compared with C offspring (Fig. 3D). In addition, plasma leptin was increased (P < 0.01) in NR offspring compared with C offspring (Fig. 3F).
Fig. 3.
Plasma glucose (A), insulin (B), insulin to glucose ratio (C), cortisol (D), and leptin (E) of control (C) female offspring (closed circles; n = 4) and nutrient restricted (NR) offspring (open circles; n = 4) during ad libitum feeding. Baseline plasma glucose concentrations were not different between treatment groups, and there was no interaction of week and treatment on glucose concentrations (P > 0.50). Insulin and insulin:glucose ratio were greater overall in NR than C offspring (P = 0.001). However, there was no significant interaction of week and treatment for insulin or insulin:glucose ratio (P > 0.50). Plasma cortisol and leptin were greater (P < 0.0001 and P < 0.001, respectively) in NR offspring compared with C offspring.
All minimal model parameters (SI, Sg, AIRg, and DI) were unaffected by time over the 11-wk ad libitum feeding period. Therefore, data presented are an average of the measures obtained by FSIGT before and after the feeding trial (Table 1). Insulin sensitivity was lower (P < 0.05), and Sg was greater (P < 0.001) in NR than C offspring. Acute insulin response to glucose challenge was also significantly elevated in NR vs. C offspring (P < 0.001), resulting in a significantly greater DI for glucose disposal in NR offspring (P < 0.01).
At necropsy, heart weight, including right and left ventricular weight, average kidney weight and average lung weight were greater in NR than C offspring (Table 2). Further, the thickness of the right ventricle was greater (P < 0.05), and the left ventricle tended to be greater (P < 0.07) in NR vs. C offspring. When corrected for differences in ewe body weight, however, organ weights of C and NR offspring were found to be similar (Table 2).
Table 2.
Body weight and organ weights of 6-yr-old female offspring born to control and nutrient-restricted mothers at necropsy after an ad libitum feeding period
| Absolute Weight |
% of Body Weight |
|||||
|---|---|---|---|---|---|---|
| C (n = 4) | NR (n = 4) | SE | C (n = 4) | NR (n = 4) | SE | |
| Body weight, kg | 89.4a | 98.9b | 3.3 | |||
| Pancreas weight, g | 47.6 | 47.8 | 6.9 | 0.49 | 0.54 | 0.08 |
| Liver weight, g | 1409.01 | 1459.6 | 93.2 | 1.49 | 1.57 | 0.11 |
| Heart weight, g | 315.8a | 353.3b | 12.8 | 0.36 | 0.35 | 0.01 |
| Right ventricle weight, g | 65.6a | 76.1b | 1.8 | 0.08 | 0.07 | 0.01 |
| Left ventricle weight, g | 108.7a | 122.9b | 4.7 | 0.13 | 0.12 | 0.01 |
| Right ventricle thickness,cm | 5.19a | 6.89b | 0.5 | |||
| Left ventricle thickness, cm | 12.3 | 14 | 1.3 | |||
| Average kidney weight, g | 87.2a | 108.4b | 5.5 | 0.11 | 0.1 | 0.01 |
| Average lung weight, g | 345.2a | 386.1b | 14 | 0.39 | 0.39 | 0.03 |
| Perirenal adipose tissue, g | 3107.2 | 4298.7 | 673.8 | 4.29 | 3.44 | 0.56 |
| Omental adipose tissue, g | 3498.9 | 4188.8 | 558.3 | 4.21 | 3.91 | 0.52 |
| Subcutanous adipose tissue thickness, cm | 1.55 | 1.53 | 0.1 | |||
Values are expressed as means ± SE. a,bSignificantly different, P < 0.05.
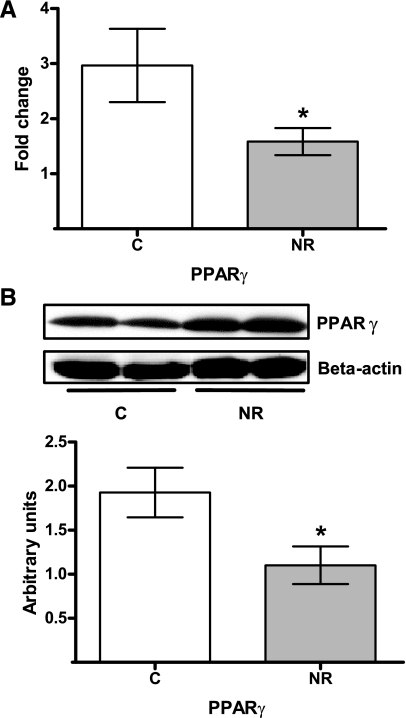
Liver weight was not different between NR (1,460 ± 93 g) and C (1,409 ± 93 g) offspring (P = 0.37). However, hepatic % lipid was greater (P < 0.01) in NR (1.73 ± 0.10%) than C (1.13 ± 0.08%) offspring. Hepatic glycogen content also tended to be greater in NR (100 ± 7.0 mg/g tissue) vs. C (80.5 ± 4.0 mg/g) offspring (P < 0.06). The mRNA (Fig. 4A) and protein (Fig. 4B) expression of PEPCK was also greater (P < 0.05) in NR compared with C offspring. Neither mRNA (Fig. 4A) nor protein expression (Fig. 4B) of G6P were affected by maternal nutrient restriction in the livers of NR vs. C offspring. Liver PPARγ mRNA (Fig. 5A) and protein (Fig. 5B) expression levels were lower (mRNA: 1.58 ± 0.25 vs. 2.96 ± 0.66; P < 0.05 and protein: 1.10 ± 0.34 vs. 1.92 ± 0.21; P < 0.05, respectively) in NR offspring compared with C offspring.
Fig. 4.
Relative mRNA expression (A) and protein levels (B) of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6 phosphatase (G-6P) to 18s RNA and β-actin expression, respectively, in livers from aged female offspring of control (C, white bars; n = 4) and nutrient-restricted (NR, gray bars; n = 4) dams. *P < 0.05.
Fig. 5.
Relative mRNA expression (A) and protein levels (B) of peroxisome proliferator-activated receptor-γ (PPARγ) to 18s RNA and β-actin expression, respectively, in livers from aged female offspring of control (C, white bars; n = 4) and nutrient-restricted (NR, gray bars; n = 4) dams. *P < 0.05.
DISCUSSION
These data demonstrate that a 50% global nutrient restriction during the first half of gestation in ewes leads to increased feed intake, increased efficiency of body weight gain, enhanced insulin secretion, and altered glucose metabolism in their aged female offspring. These data support the thrifty phenotype hypothesis, showing a relationship between nutrient restriction in pregnancy and characteristics of the resulting offspring, which favors energy thriftiness: more efficient acquisition and storage of food energy (19, 48). These characteristics are consistent with a predisposition for the development of metabolic disease (e.g., obesity, diabetes) (20).
To our knowledge, characteristics of the thrifty phenotype have not been assessed in aged offspring of the commonly studied nutrient-restricted ovine model. However, studies in aged rats have indicated a long-term programming of metabolic dysregulation of offspring due to a different model of maternal nutrient restriction during pregnancy. Aged male rats (17 mo old) born to protein-restricted dams had elevated basal glucose concentrations and greater insulin response to glucose challenge, indicating an insulin-resistant state (37). However, others have demonstrated inconsistent and sex-specific changes in body weight, body fat, and food intake in 18-mo-old rats exposed to maternal low protein at various periods during gestation and suggest that dietary challenge (e.g., exposure to high-fat diet) in postnatal life may be needed to bring about obesity and altered food intake in offspring of mothers experiencing maternal undernourishment (7). Studies following rats born from globally nutrient-restricted dams (30% restriction relative to ad libitum) to 12 mo of age have demonstrated increased food intake that was enhanced with the availability of a high-fat diet (53).
In other studies using sheep, 50% global nutrient restriction in ewes from day 30 to 80 of gestation induced hyperinsulinemia in 1-yr-old offspring (males and females combined) exposed to an obesogenic environment (i.e., reduced physical activity induced by reduced living space with ad libitum feeding) (42). However, a reduced daily feed intake was reported in offspring of nutrient-restricted dams. The investigators interpreted the lower feed intake in offspring of restricted dams as an appropriate adaptation to regulate energy balance in an environment of low-energy expenditure/high food availability, which was not observed in control offspring. The results presented by Sebert et al. (42) suggest that maternal nutrient restriction in the first half of gestation does not induce hyperphagia in offspring. However, these yearling offspring of restricted ewes exposed to the obesogenic environment were hyperinsulinemic relative to controls in the same environment, a likely indicator of insulin resistance and a predisposition for eventual metabolic disease (42). Furthermore, the study examined feed intake over a single 24-h period after moving animals to a novel environment of individual pens. Thus, this single measurement may have been reflective of differing early adaptations to a new environment more than in utero programming of appetite. Assessment of daily feed intake in a group setting, a more natural environment for sheep, over an extended period of time may be required to separate subtle differences in voluntary feed intake if they exist in offspring of nutrient-restricted dams.
The utilization of long-term, automated feed monitoring as in the present study enabled accurate measurement of voluntary feed intake in ewes maintained in a group social setting. Our results indicated greater offspring voluntary feed acquisition in aged offspring attributable to early pregnancy maternal nutrient restriction 6 yr prior to the study and may also differ from the study of Sebert et al. (42) due to differences in age at time of evaluation (1 vs. 6 yr old). Furthermore, in the present study, ewes from nutrient-restricted mothers consumed feed at a greater rate than controls with no difference in number of visits to the feeder or time spent at the feeder. Rate of food consumption likely reflects appetitive drive and may be a meaningful behavioral trait associated with greater risk of being overweight. In a study that evaluated body mass index and self-reported eating behaviors in a large human sample (30), both Japanese men and women who reported to eat quickly had increased odds ratio (1.84 and 2.09, respectively) for having a body mass index greater than 25 kg/m2.
Aged offspring of nutrient-restricted dams in the present study also gained more body weight per unit weight of feed consumed than control offspring, indicating an increased efficiency (i.e., thriftiness) of body tissue deposition when exposed to unlimited availability of high-quality feed. This thriftiness points toward an increased risk of obesity in these animals if the exposure to ad libitum feed were to continue indefinitely, as people of developed nations often experience throughout a lifetime. Feed intake regulation and efficiency of body weight gain could be mechanisms by which offspring of nutrient-restricted pregnancies, which experience metabolic dysregulation and are exposed to plentiful nutrition, become obese later in life (14, 48). Furthermore, programmed differences in intake and metabolism may contribute to many people's innate tendencies to stay thin or put on weight despite similar lifestyles.
However, offspring of nutrient-restricted dams in the present study did not have greater body fat than controls either before or after ad libitum feeding. Given their increased efficiency of gain and their low-moderate condition (∼8% body fat, typical of 6-yr-old ewes) at the onset of the study, it is likely that 11 wk of overeating was not sufficient time to allow for development of differences in body fat in this group of animals. Also, the present study evaluated body composition in old age, when lean mass is likely to be decreased relative to younger ages (44). Therefore, a greater proportion of body weight gain in these aged ewes may have been due to restoration of lean mass with ad libitum consumption of a high-quality diet, compared with a young animal, which would not yet have begun to lose its lean mass due to old age. It has been shown that ingestion of amino acids in elderly humans improves muscle protein syntheses (35), and this could be through increased intake of the branch-chained amino acid, leucine, which may help reverse impaired regenerative drive in sarcopenic muscle by enhanced anabolic pathways and inhibiting catabolic pathways (21) The lack of body fat differences by the end of the trial also may explain why the differences in insulin sensitivity and secretion between the nutrient-restricted and control offspring were not further exacerbated over this time period.
These data demonstrate that under fasting conditions both C and NR offspring exhibit similar circulating levels of glucose and insulin; however, in response to the FSIGT, marked differences were observed. Greater DI (both before and after ad libitum feeding) indicates an enhanced potential of NR offspring for insulin-mediated clearance of glucose from the blood and into body tissues, and in these animals, is due to significantly elevated insulin responsiveness to glucose challenge. However, if pancreatic β-cell insulin output were to become exhausted, as it is in the pathogenesis of type 2 diabetes, insulin-mediated glucose clearance would decline dramatically in the offspring of nutrient-restricted pregnancies due to their lower SI (49). An elevated insulin to glucose ratio also indicates that more insulin is required per unit glucose to maintain blood glucose concentrations within a normal range, which supports that body tissues of offspring of nutrient-restricted ewes have relatively low insulin sensitivity. Compensated insulin resistance occurs in response to decreases in peripheral tissue insulin sensitivity and is marked by hyperinsulinemia and enhanced insulin output in response to glucose in an effort by the body to maintain glucose homeostasis (1). This state of compensation can lead to pancreatic exhaustion, which then gives rise to glucose intolerance and diabetes (49).
Differences in glucose metabolism in the nutrient-restricted offspring also fit with their greater expression of hepatic PEPCK, an irreversible, rate-limiting enzyme, which catalyzes the first step of cytosolic hepatic gluconeogenesis (38). The minimal model parameter Sg (glucose effectiveness) is calculated on the basis of rate of endogenous glucose production estimated by the minimal model (8). Therefore, a higher Sg in offspring of nutrient-restricted dams matches the greater capability for production of glucose from the liver indicated by greater PEPCK expression levels. Thus, nutrient-restricted offspring may produce glucose more efficiently in the liver, which is matched by a more efficient basal disposal of glucose from the bloodstream and into body tissues. This notion also agrees with the apparently increased efficiency of weight gain observed in these ewes. Additionally, the unaltered mRNA and protein expression levels of G-6P, an enzyme that catalyzes the production of glucose from glucose-6-phosphate (4), suggests that more of the newly synthesized glucose-6-phosphate derived from the elevated gluconeogenic activity in hepatocytes of NR vs. C ewes could be directed toward glycogen synthesis and storage rather than being converted to glucose and subsequently released from hepatocytes. This is consistent with the observed trend for increased storage of hepatic glycogen in the liver of NR vs. C ewes, suggesting a heightened propensity for glucose storage in these offspring of nutrient-restricted dams, another example of a thrifty trait. This result is consistent with studies in rat offspring exhibiting greater hepatic glycogen stores following maternal low-protein diet during either early or late gestation (6). A similar picture of increased fetal liver glycogen has been described in term fetal baboons whose mothers received a 30% globally reduced food intake. This moderate level of maternal and fetal undernutrition led to decreased methylation of the PEPCK promoter associated with an increase in PEPCK immunoreactivity in the fetal liver (34).
Elevated hepatic lipid content of offspring from nutrient-restricted mothers is consistent with the lower expression of PPARγ, a transcriptional regulator that modulates adipocyte differentiation and fat metabolism. Overexpression of PPARγ has been shown to play an essential role in protection against nonalcoholic steatihepatites by redistribution of fatty acid from liver to adipose tissue (55). In a rat model of NAFLD, steatosis of the liver was improved with PPARγ agonist treatment by inducing fatty acid metabolic enzymes (43). PPARγ activation also reduces lipid accumulation in muscle (57). In a maternal nutrient restriction model in sheep, Hyatt et al. (24) reported an increase in liver triglyceride accumulation in association with elevated gene expression of PPARγ in overnourished 1-yr-old lambs born to undernourished mothers, indicative of changes in the rate of hepatic fatty acid oxidation. Our observation of reduced PPARγ expression in the livers of ad libitum fed 6-yr-old offspring from NR mothers may result from a reduced ability of these older overnourished animals to synthesize PPARγ in the face of increased hepatic fat deposition. While it has been reported that there was a positive correlation between genes of fatty acid oxidation and PPARγ in liver tissue of dairy cattle, it was also reported that there is a marked and progressive decrease in mRNA expression of hepatic PPARγ content with age (51). Although the pathogenesis and specific implications of nonalcoholic fatty liver disease in humans are still being uncovered, fat accumulation in the liver is clearly associated with obesity, insulin resistance, and dyslipidemia (13).
Perspectives and Significance
We have demonstrated that maternal nutrient restriction through early gestation resulted in increased insulin secretion in response to glucose, increased daily food intake and rate of intake, greater efficiency of body weight gain, and lower insulin sensitivity in aged sheep offspring during a period of ad libitum feeding. Further, these data show significant differences in feeding behavior, glucose/insulin dynamics, hepatic fat and glycogen content, and hepatic gluconeogenesis between offspring of nutrient-restricted and control dams, which point to mechanisms by which thrifty individuals are predisposed to overeating/obesity and metabolic disease with advancing age. Thus, the results of this study provide clear physiological evidence in a precocial large animal model of prenatal programming of energy metabolism and feeding behavior in old age. Finally, and perhaps most importantly, these data demonstrate that when fed only to requirements, NR offspring exhibited no differences in body weight, composition, or fasted circulating concentrations of glucose or insulin until allowed to consume unlimited amounts of feed, suggesting that diet per se has a potential role in mitigating disease, even after programming.
GRANTS
This project was supported in part by Wyoming National Institutes of Health IDeA Networks of Biomedical Research Excellence Grant P20-RR-016474.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.A.G., L.Z., Y.M., N.M.L., A.B.U., D.T.S., and S.P.F. performed experiments; L.A.G., L.Z., and N.M.L. analyzed data; L.A.G., L.Z., N.M.L., and S.P.F. interpreted results of experiments; L.A.G. and N.M.L. prepared figures; L.A.G., N.M.L., and P.W.N. drafted manuscript; L.A.G., L.Z., N.T., Y.M., N.M.L., A.B.U., D.T.S., P.W.N., and S.P.F. approved final version of manuscript; N.M.L. and P.W.N. conception and design of research; N.M.L., P.W.N., and S.P.F. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Christopher Dorozynski for DEXA evaluations of ewes, as well as the students and staff of the Center for the Study of Fetal Programming for their assistance in animal care and data collection.
REFERENCES
- 1. Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol 150: 97–104, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 346: 1221–1231, 2002 [DOI] [PubMed] [Google Scholar]
- 3. AOAC International Official Methods of Analysis of the Association of the Official Analytical Chemists. 15th ed Arlington, VA: AOAC International, 1990 [Google Scholar]
- 4.Azzout-Marniche D, Guadichon C, Blouet C, Bos C, Mathe V, Huneau JF, Tome D. Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats. Am J Physiol Regul Integr Comp Physiol 292: R1400–R1407, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Beard JC, Bergman RN, Ward WK, Porte D., Jr The insulin sensitivity index in nondiabetic man. Correlation between clamp-derived and IVGTT-derived values. Diabetes 35: 362–369, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Bellinger L, Langley-Evans SC. Fetal programming of appetite by exposure to a maternal low-protein diet in the rat. Clin Sci 109: 413–420, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Bellinger L, Sculley DV, Langley-Evans SC. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes 30: 729–738, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergman RN. Toward physiological understanding of glucose-tolerance—minimal-model approach. Diabetes 38: 1512–1527, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose-tolerance in man—measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68: 1456–1467, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. Minmod millennium: A computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5: 1003–1015, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Burt BE, Hess BW, Nathanielsz PW, Ford SP. Flock difference in the impact of maternal dietary restriction on offspring growth and glucose tolerance in female offspring. Soc Reprod Fertil Suppl 64: 411–424, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Fan CY, Pan J, Chu R, Lee D, Kluckman KD, Usada N, Singh I, Yeldandi AV, Rao MS, Maeda N, Reddy JK. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J Biol Chem 271: 24698–24710, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Farrell GC. The liver and the waistline: Fifty years of growth. J Gastroenterol Hepatol 24: S105–S118, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Twinn DS, Ozanne S. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav 88: 234–243, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. Maternal undernutrition during early gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci 85: 1285–1294, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Ford SP, Zhang LR, Zhu MJ, Miller MM, Smith DT, Hess BW, Moss GE, Nathanielsz PW, Nijland MJ. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: Prenatal consequences. Am J Physiol Regul Integr Comp Physiol 297: R835–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George LA, Uthlaut AB, Long NM, Zhang L, Ma Y, Smith DT, Nathanielsz PW, Ford SP. Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod Biol Endocrinol 8: 75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89: 2595–2600, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 60: 5–20, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Hales CN, Ozanne SE. For debate: Fetal and early postnatal growth restriction lead to diabetes, the metabolic syndrome and renal failure. Diabetologia 46: 1013–1019, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Han B, Tong J, Zhu MJ, MA C, Du M. Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satelite cells through mammalian target of rapamycin (mTOR) pathway. Mol Reprod Dev 75: 810–817, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Hasson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP): the gene and the enzyme. Annu Rev Biochem 66: 581–611, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Natl Vital Stat Rep 57: 1–134, 2009 [PubMed] [Google Scholar]
- 24. Hyatt MA, Gardner DS, Sebert S, Wilson V, Davidson N, Nigmatullina Y, Chan LLY, Budge H, Symonds ME. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction 141: 1119–1126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lomax MA, Sadiq F, Karamanlidid G, Karamitri A, Trayhurn P, Hazlarigg DG. Ontogenic loss of brown adipose tissue sensitivity to β-adrenergic stimulation in the ovine. Endocrinology 148: 461–468, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, Ford SP. Maternal obesity and high nutrient intake before and during gestation in the ewe results in altered growth, adiposity and glucose tolerance in adult offspring. J Anim Sci 88: 3546–3553, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Long NM, Prado-Cooper MJ, Krehbiel CR, DeSilva U, Wettemann RP. Effects of nutrient restriction of bovine dams during early gestation on postnatal growth, carcass and organ characteristics, and gene expression in adipose tissue and muscle. J Anim Sci 88: 3251–3261, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Lopez-Andreo M, Lugo L, Garrido-Pertierra A, Prieto MI, Puyet A. Identification and quantitation of species in complex DNA mixtures by real-time polymerase chain reaction. Anal Biochem 339: 73–82, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Ma Y, Zhu M, Zhang L, Hein SM, Nathananielsz PW, Ford SP. Maternal obesity and overnutrition alters fetal growth rate and cotyledonary vascularity and angiogenic factor expression in the ewe. Am J Physiol Regul Integr Comp Physiol 299: R249–R258, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maruyama K, Sato S, Ohira T, Maeda K, Noda H, Kubota Y, Nishimura S, Kitamura A, Kiyama M, Okada T, Imano H, Nakamura M, Ishikawa Y, Kurokawa M, Sasaki S, Iso H. The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: Cross-sectional survey. Br Med J 337: a2002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mercier J, Pomar C, Marcoux M, Goulet F, Theriault M, Castonguay FW. The use of dual-energy X-ray absorptiometry to estimate the dissected composition of lamb carcasses. Meat Sci 73: 249–257, 2006 [DOI] [PubMed] [Google Scholar]
- 32. National Research Council Nutrient requirements of sheep. Washington, D. C.: National Academies Press, 1985 [Google Scholar]
- 33. Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology 38: 1008–1017, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Nijland MJ, Mitsuya K, Li C, Ford S, McDonald TJ, Nathanielsz PW, Cox LA. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol 588: 1349–1359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolh SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286: E321–E328, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Pearce KL, Ferguson M, Gardner G, Smith N, Greef J, Pethick DW. Dual X-ray absorptiometry accurately predicts carcass composition from live sheep and chemical composition of live and dead sheep. Meat Sci 81: 285–293, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. Diabetes in old male offspring of rat dams fed a reduced protein diet. Intl J Exp Diabetes Res 2: 139–143, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Ann Rev Physiol 54: 885–909, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci 73: 1839–1851, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Sanson DW, West TR, Tatman WR, Riley ML, Judkins MB, Moss GE. Relationship of body-composition of mature ewes with condition score and body-weight. J Anim Sci 71: 1112–1116, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Schneider H. Ontogenic changes in the nutritive function of the placenta. Placenta 17: 15–26 1996 [DOI] [PubMed] [Google Scholar]
- 42. Sebert SP, Hyatt MA, Chan LLY, Patel N, Bell RC, Keisler D, Stephenson T, Budge H, Symonds ME, Gardner DS. Maternal nutrient restriction between early and midgestation and its impact upon appetite regulation after juvenile obesity. Endocrinology 150: 634–641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seo YS, Kim JH, Jo NY, Choi KM, Baik SH, Park JJ, Kim JS, Byun KS, Bak YT, Lee CH, Kim A, Yeon JE. PPAR agonists treatment is effective in a nonalcoholic fatty liver disease animal model by modulating fatty-acid metabolic enzymes. J Gastroenterol Hepatol 23: 102–109, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Sheffield-Moore M, Paddon-Jones D, Urban RJ. Amino acid supplementation and skeletal muscle metabolism in ageing populations. Horm Res 66: 93–97, 2006 [Google Scholar]
- 45. Shen QW, Jones CS, Kalchayanand N, Zhu MJ, Du M. Effect of dietary α lipoic acid on growth, body composition, muscle pH, and AMP-activated protein kinase phosphorylation in mice. J Anim Sci 83: 2611–2617 2005 [DOI] [PubMed] [Google Scholar]
- 46. Shulman GL. Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stegeman JHJ. Placental development in the sheep and its relation to fetal development. Bojdragen Tot Dierkunde 44: 1–72, 1974 [Google Scholar]
- 48. Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol 5: 604–610, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Talchai C, Lin HV, Kitamura T, Accili D. Genetic and biochemical pathways of beta cell failure in type 2 diabetes. Diabetes Obes Metab 11: 39–45, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet 354: 141–148, 1999 [DOI] [PubMed] [Google Scholar]
- 51. van Dorland HA, Richter S, Morel I, Doherr MG, Castro N, Bruckmaier RM. Variation in hepatic regulation of metabolism during the dry period and in early lactation in dairy cows. J Dairy Sci 92: 1924–1940, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Van Schaftingen EV, Gerin I. The glucose-6-phosphatase system. Biochem J 362: 513–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279: E83–E87, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, Murdoch WJ, Nijland MJ, Skinner DC, Nathanielsz PW, Ford SP. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod 69: 133–140, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Wu CW, Chu ES, Lam CN, Cheng AS, Lee CW, Wong VW, Sung JJ, Yu J. PPARgamma is essential for protection against nonalcoholic steatohepatitis. Gene Ther 17: 790–798, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Yabaluri N, Bashyam MD. Hormonal regulation of gluconeogenic gene transcription in the liver. J Biosci 35: 473–484, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome proliferator-activated reseptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes 50: 411–417, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Zhu MJ, Du M, Hess BW, Means WJ, Nathanielsz PW, Ford SP. Maternal nutrient restriction upregulates growth signaling pathways in the cotyledonary artery of cow placentomes. Placenta 28: 361–368, 2007 [DOI] [PubMed] [Google Scholar]