Abstract
One of the most consistent pathological conditions in the gastrointestinal tract with advancing age is malignancy, particularly gastrointestinal cancers, the incidence of which increases sharply with aging. Although the reasons for the age-related rise in colorectal cancer are not fully understood, we hypothesize that aging increases susceptibility of the colon to carcinogen(s)/toxicant(s), leading to an increase in cancer stem-like cells (CSLCs) that express cancer stem cell markers, in the colonic mucosa. The current study demonstrates that aging is associated with increased expression of several colon CSLC markers [CD44, CD166, and aldehyde dehydrogenase 1 (ALDH-1)] and a higher proportion of cells expressing these markers. Aging is also accompanied by increased expression of miR-21 in colon. These increases are further increased in response to the colonic carcinogen dimethylhydrazine (DMH). Aging is also associated with increased tyrosine-phosphorylated epidermal growth factor receptor (EGFR). Inhibition of EGFR using the EGFR inhibitor cetuximab abrogated the age-related increase in CD166 and ALDH-1 as well as miRNA (miR)-21. Our results provide new evidence that aging and DMH are associated with increases in CSLC biomarkers and miR21, each of which have been linked to colorectal cancer. EGFR inhibition attenuates these changes, indicating a role for EGFR in age- and mutagen-associated changes in CSLCs.
Keywords: aging, colon cancer, cancer stem cells, epidermal growth factor receptor, miRNAs
a primary conceptual challenge in the study of mammalian aging is the increased incidence of a variety of disorders and their intricate relationship to aging. One of the most consistent pathological conditions in the gastrointestinal tract with advancing age is malignancy, particularly colorectal cancer.
Over the last decade, there has been a growing body of evidence that supports the contention that cancer stem cells/cancer stem-like cells (referred to as CSLCs), identified by the expression of stem cell markers, are critically involved in the initiation, progression, and recurrence of cancer. Epithelial cancers, including colorectal cancer, are now believed to be diseases driven by a minor subpopulation of self-renewing CSLCs that also have the potential to invade and form distant metastasis (4, 5, 13). Biologically distinct and relatively rare populations of tumor-initiating cells or CSLCs have been detected by several methods and markers established in a variety of cancers, including the colon (1, 29). Currently, CSLCs are identified by specific surface epitopes. Colorectal CSLCs were initially characterized as cells expressing CD133, and subsequently as cells expressing other surface markers, including CD44, CD166, and epithelial cell adhesion molecule/epithelial-specific antigen (6). More recently, aldehyde dehydrogenase 1 (ALDH-1), which is a detoxifying enzyme, has been identified as a specific marker for normal and malignant human colonic stem cells (12). ALDH-1-positive cells, which are sparse and limited to the crypt bottom where stem cells reside, increase during transition of normal epithelium to adenoma or progression to carcinoma (12). However, the mechanisms that regulate the growth and development of CSLCs and their stemness are not well understood.
Members of the receptor tyrosine kinase family, that include epidermal growth factor receptor (EGFR)/ErbB-1, ErbB-2/HER-2, ErbB-3/HER-3, and ErbB-4/HER-4, are frequently implicated in experimental models of epithelial cell neoplasia as well as in human cancers (10, 26). Overexpression and/or activation of EGFR and/or member(s) of its family, particularly ErbB-2, has been associated with many human malignancies, including cancers of the lung, head and neck, brain, bladder, breast, prostate, stomach, and colon (10, 26). Overexpression of EGFR in some tumors is viewed as an indicator of poor clinical prognosis (8, 25). In addition, EGFR levels in premalignant lesions appear to be a sensitive predictor of the neoplastic potential of dysplastic tissues (9). Indeed, we have demonstrated that EGFR tyrosine kinase activity in macroscopically normal mucosa from patients with ulcerative colitis and adenomatous polyps (which are considered to be precancerous lesions) as well as those with colon cancer is considerably higher than in normal subjects (23). However, the highest increase is observed in mucosa from patients with frank colon cancer (23). Interestingly, aging, which is associated with increased incidence in colorectal neoplasia/cancer, is also associated with increased expression and activation of EGFR, ErbB2/HER-2, and ErbB-3/HER-3 (19, 20, 27). These increases are accompanied by increased CSLCs in the colonic mucosa (16, 30). Taken together, these observations suggest a close relationship between CSLCs and EGFR in the colonic mucosa. However, the mechanisms by which EGFR regulate CSLCs remain unknown.
Recent accumulating data implicate miRNAs (miRs) in the regulation of proliferation, apoptosis, differentiation, metabolism, and invasion (3). miRs are a class of endogenous small noncoding RNAs that control gene expression through binding to the seed sequence at the 3′-untranslated region of target mRNAs, resulting in translational repression or mRNA degradation (7). A given species of miR can perfectly or imperfectly base pair with multiple targets, allowing it to potentially regulate the translation of numerous mRNAs. It has been predicted that over 30% of the human protein coding genes are posttranscriptionally regulated by this mechanism (3, 7). Because miRNAs are reported to play a pleiotropic role in posttranscriptional regulation of numerous genes (3), a putative role for miRNAs in regulating cancer stem cell function is suggested. miR-21 is specifically significant in colon cancer due to its key role in several processes of tumor promotion, invasion, and metastasis and is therefore referred to as an “oncomiR” (a miRNA with oncogenic properties) (24, 41). Recent evidence has suggested a role for EGFR in regulating miR-21 (35).
Aging is associated with an increased risk for colon cancer (22). Neither the causative factor(s) nor the regulatory mechanisms for the age-dependent rise in colorectal cancer are fully known. We postulate that they could partly be due to increased susceptibility of the aging colon to carcinogen(s)/toxicant(s) leading to stimulation in CSLCs. We further hypothesize that EGFR and miR-21, each of which regulates the other, play pivotal roles in regulating the age-related rise in colorectal neoplasia/cancer. The current investigation was undertaken to test these hypotheses.
MATERIALS AND METHODS
Animal studies and tissue procurement.
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of Wayne State University. In the current investigation, male Fischer-344 rats aged 4–6 (young) and 22–24 (aged) mo were used in all experiments. They were purchased from the National Institute on Aging (Bethesda, MD) and maintained at the animal care facility of the Veterans Affairs Medical Center/Wayne State University in accordance with the policy of IACUC for at least 1 mo before the experiment. During this period, they had access to Purina rat chow and water ad libitum. Four to five animals from each age group were used in this investigation. The rats were either used without any intervention or injected intraperitoneally once per week for 4 wk with either 1,2-dimethylhydrazine (DMH) (30 mg/kg body wt), dissolved in 300 μl neutral buffered 10 mM NaHCO3, or vehicle (controls). They were killed 1 wk after the last injection. In some experiments, the animals were given a single injection (ip) of cetuximab (8 mg/kg; monoclonal antibodies to EGFR) or vehicle (controls) and killed 5 days later. Where appropriate, the animals were monitored and weighed two times a week.
All animals were killed by CO2 asphyxiation, following an overnight fast. The abdominal cavity was opened, and the colon was removed, rinsed with cold normal saline, opened, and cut longitudinally. After being washed thoroughly with cold PBS containing 10% antibiotic/antimycotic, a small portion of the distal colon was fixed in 10% buffered formalin, processed, and embedded in paraffin for immunohistochemical analysis. Mucosa from the rest of the tissue was obtained by scraping with glass slides, and aliquots were either processed immediately or frozen in liquid nitrogen and stored at −80°C.
Isolation of RNA and quantitative RT-PCR analysis.
Total RNA was extracted from rat intestinal mucosa using Trizol reagent according to the manufacturer's instructions. RNA concentration was measured spectrophotometrically at an optical density of 260 nm. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using the GeneAmp RNA PCR Kit (Applied Biosystems, Foster City, CA). Briefly, 1 μg of purified RNA was reverse-transcribed, and the transcribed RNA was diluted five times for quantitative PCR amplification of various cancer stem cell markers. Five microliters of complementary DNA products were amplified with SYBR Green Quantitative PCR Master Mix (Applied Biosystems). Table 1 provides the list and sequences of the rat specific primers used in the study. Reactions were carried out in an Applied Biosystems 7500 Real-Time PCR System, as described previously by Yu et al. (39). The quantitation of the marker gene was normalized to amplification of β-actin and subsequently expressed relative to untreated control.
Table 1.
List of different rat-specific PCR primers used in the study
| Gene | Direction | Primer Sequence |
|---|---|---|
| CD44 | Forward | tag ccc tga gaa agg ggt tt |
| Reverse | ttg ttg gct gca cag ata gc | |
| CD133 | Forward | acc tgc tga cat ttg cct ct |
| Reverse | gga tgg ctc gaa tgt tct gt | |
| CD166 | Forward | tca agg tgt tca agc aac ca |
| Reverse | gtt gcc gtc tgg gta act gt | |
| ALDH-1a | Forward | atc aag gaa gct gca gga aa |
| Reverse | tcg aca gca atg tcc aag tc |
ALDH, aldehyde dehydrogenase.
miRNA array and quantitation of miRNA-21.
Expression of 88 rat miRNAs associated with tumorigenesis was measured by real-time RT-PCR. Briefly, total RNA was extracted using TRIzol (Invitrogen) from the rat colonic mucosa. Small RNA was isolated using an miRNA isolation kit and reverse transcribed (200 ng) using the RT2 miRNA first strand kit (SABiosciences). Subsequently, real-time PCR was performed in 96-well plates using the Rat Cancer microRNA PCR Array plates (SABiosciences) and the SYBR Green master mix (SABiosciences) in an ABI 7500 instrument (Applied Biosystems). The miRNA PCR plate contained real-time PCR primers for 88 rat miRNAs, four normalizer small RNAs, and several quality controls. Data analysis was performed with the web-based software package, and the differences in miRNA levels were calculated using the ΔΔCt method. The criterion for differential expression was a more than fourfold change.
TaqMan microRNA.
Assays were used to quantitate miR-21 in the colonic mucosa of young and old rats according to the manufacturer's instruction (Applied Biosystems). Briefly, cDNA synthesis was carried out with the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems) using 10 ng total RNA as template. The miRNA sequence-specific RT-PCR primers for miR-21 and endogenous control RNU6B were purchased from Applied Biosystems. Real-time qRT-PCR analysis was carried out using an Applied Biosystems 7500 Real-Time PCR System. The PCR Master Mix containing TaqMan 2× Universal PCR Master Mix (No Amperase UNG) volume was processed as follows: 95°C for 10 min and then 95°C for 15 s, 60°C for 60 s for up to 40 cycles. Signal was collected at the endpoint of every cycle. The gene expression ΔCt values of miRNAs from each sample were calculated by normalizing with internal control RNU6B, and relative quantitation values were plotted.
Single cell isolation from the rat colonic mucosa.
Small portions (5–10 mg) of colonic mucosa was removed, washed extensively in 1× PBS containing 10% antibiotic/antimycotic, and subsequently incubated overnight in Dulbecco's minimum essential media (DMEM/F-12) containing 5% antibiotic/antimycotic at 4°C. The tissue was cut into fine pieces using a sterile scalpel and then digested with 1.5 mg/ml collagenase I (CO130–50 mg; Sigma Aldrich) and 20 μg/ml hyaluronidase I (H3506–100 mg; Sigma Aldrich) under gentle agitation for 2–3 h at 370C. The digested tissue was filtered through a 40-μM filter and centrifuged at 1,200 rpm for 5 min. The supernatant (containing dead cells as well as the fat cells) was discarded, and the cells (pellet) were washed three times with DMEM/F-12 media containing 5% antibiotic/antimycotic. The cells were suspended in previously defined stem cell media and plated in low-adhesion plates (14).
Formation of spheroid-like clusters by isolated colonic mucosal cells.
Spheroid-like clusters are formed by the CSLCs in stem cell media. However, cells isolated from tissues do not form spheroid, rather they form spheroid-like clusters. To examine the effects of aging as well as exposure to carcinogen DMH on the formation of spheroid-like clusters by the isolated colonic mucosal cells, the ability of these cells to form spheroid-like structures in suspension was evaluated as described by Liu et al. (17), with minor modifications. Briefly, the isolated cells were allowed to grow in nondifferentiating condition (stem-cell media) in ultra-low-attachment plates (Corning, Lowell, MA) for 5–10 days. The stem cell medium was supplemented with B27 (Life Technologies, Gaithersburg, MD), 20 ng/ml epidermal growth factor (Sigma, St. Louis, MO), 10 ng/ml fibroblast growth factor (Sigma), and antibiotic/antimycotic. By the end of 5 days, only the stem-like cells were able to survive in nonadherent conditions while the non-stem-like cells died off. Fresh stem cell media was provided to the surviving cells every 5 days. The cells were assayed for their ability to survive and form spheroid-like clusters. The spheroid-like clusters formed in 10 days were photographed at ×10 magnification.
Flow cytometric analyses of isolated mucosal cells.
Single cells isolated from the colonic mucosa of rats were subjected to direct and/or indirect immunofluorescence staining followed by flow cytometric analyses. Briefly, the cells were harvested and washed with PBS. One million cells were suspended in 95 μl of PBS containing 0.5% BSA for 10 min at room temperature followed by the addition of 5 μl of fluorescein isothiocyanate (FITC) fluorescent dye conjugated to CD44 antibodies (BD Pharmingen, San Jose, CA), incubated for 30 min in the dark, at room temperature. The samples were then washed and resuspended in PBS and analyzed using a FACS DiVa (BD Pharmingen). One million cells were also stained with FITC fluorophore-conjugated isotype control for CD44 antibodies.
Western blotting.
This methodology was used to examine the levels of different proteins and their activated states in the colonic mucosa in response to aging as well as DMH treatment. Briefly, aliquots of colonic mucosa from young and old rats were solubilized in lysis buffer (10 mM HEPES, pH 7.2, 150 mM NaCl, 2.5 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM EDTA, 25 μg/ml of aprotinin, leupeptin, and pepstatin A, 0.5% Triton X-100, and 0.5% Nonidet P-40). Following clarification at 11,000 g for 15 min at 40C, the supernatant was subjected to electrophoresis on a 12% SDS-PAGE. The electrophoresed proteins were transferred onto nitrocellulose membranes. The membranes were then probed with various antibodies. Protein bands were visualized by an enhanced chemiluminescence detection system and quantitated by densitometry. The membranes were stripped and probed for actin as an internal control. All Western immunoblots were performed at least three times using samples from different rats from each age group.
Statistical analysis.
Unless otherwise stated, data were expressed as means ± SD. Where applicable, the results were compared by using the unpaired, two-tailed Student's t-test, as implemented by Excel 2007 (Microsoft, Redmond, WA). P values <0.05 were considered statistically significant.
RESULTS
Aging is associated with increased expression of CSLC markers in colon.
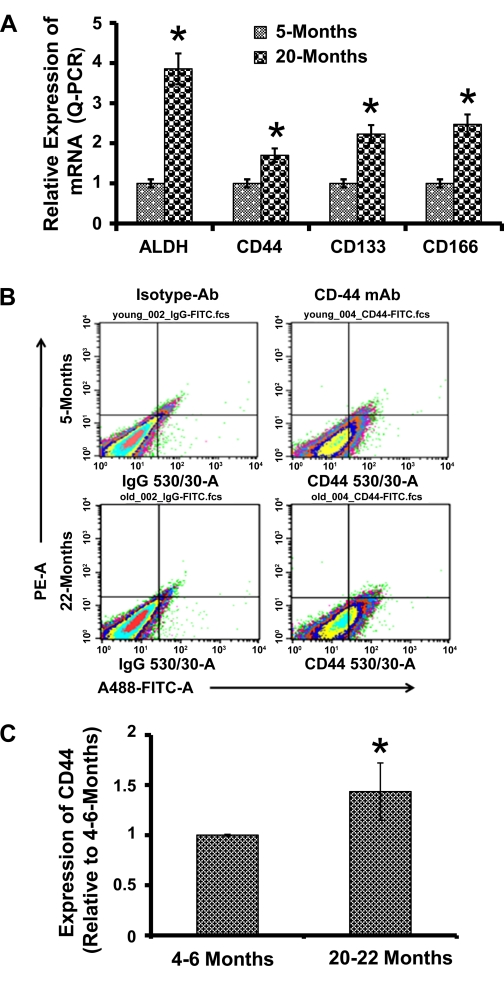
Considering that appearance of CSLCs might be one of the initial events in neoplastic transformation in solid tumors as well as in intestinal neoplasia, we investigated the status of CSLCs in normal-appearing colonic mucosa during aging in rats. Earlier, we reported the presence of CSLCs in normal-appearing mucosa in patients with adenomatous polyps and found them to increase with aging, as did the number of polyps (30). As a measure of age-related changes in colonic mucosal CSLCs, we examined the expression of mRNAs for CSLC-specific surface markers CD44, CD133, and CD166 and the cytoplasmic marker ALDH-1 in the normal colonic mucosa of young (4–5 mo old) and aged (22–24 mo old) Fischer-344 rats. As expected, the expression of colon CSLC markers, as determined by the respective mRNA levels, was found to be 1.6- to 3.8-fold higher in the colonic mucosa of aged (20 mo old) than in young (5 mo old) animals (Fig. 1A).
Fig. 1.
A: expression of cancer stem-like cell (CSLC)-specific markers in the colon mucosa of young (5 mo) and aged (20 mo) Fisher-344 rats. Representative scattered plots of flow cytometry analyses of CD44 (B) and histogram (C) showing the proportion of CD44-positive mucosal cells in 4- to 6-mo (young) and 20- to 22-mo (aged)-old Fisher-344 rats. Values (n = 3) represent the percentage of cells that are positive for CD44-fluorescein isothiocyanate (FITC). ALDH, aldehyde dehydrogenase; Ab, antibody. *P < 0.01 compared with the corresponding levels in 4- to 6-mo-old animals.
To further determine whether putative CSLCs in the normal colonic mucosa would exhibit properties of CSLCs, we isolated mucosal cells by collagenase/hyaluronidase treatment and subjected them to fluorescence-activated cell sorter (FACS) analysis. Mucosal cells isolated from the colon of aging rats exhibited a 50% higher population of cells expressing the cancer stem cell-specific marker CD44 compared with the corresponding young animals (Fig. 1, B and C).
Aging is associated with increased expression of miRNAs in colon.
Given the major roles of miRs in the regulation of protein expression, it is crucial to understand the contributions of miRs in the age-related rise in colorectal neoplasia. Profiling of miRs in the colonic mucosa of young (4 mo) and aged (24 mo) Fischer-344 rats revealed marked changes in the expression of several miRs in the colonic mucosa of aged rats that affect the expression of a number proteins, including EGFR and insulin-like growth factor-I receptor (Table 2). These miRs are thought to regulate the functional properties of CSLCs. We found the expression of several miRs in the colonic mucosa to either increase or decrease in aged animals. In the current investigation, however, we focused our attention to miR-21 since it is known to be upregulated in many solid tumors, including the colon (2), and has been shown to promote cell transformation by targeting the tumor suppressor programmed cell death 4 gene (PDCD4) (18). Our results show an ∼13-fold increase in the expression of miR-21 in the colonic mucosa of aged rats compared with their younger counterpart (Table 2).
Table 2.
Rat miR array for cancer showing changes (increase/decrease) in expression of several miRs in the colonic mucosa of 24-mo-old (aged) Fischer-344 rats over their 4-mo-old (young) counterparts
| Rat miRNA Array |
||
|---|---|---|
| miRs | Fold Increase/Decrease (24/4 mo) | Predicted Targets |
| miR-1 | −30.17 | IGF-I, TIMP3 |
| miR-9 | −7.44 | ALCAM, IGF-I |
| miR-19a | +10.97 | PTEN, TGFβR2 |
| miR-21 | +12.86 | PDCD4, PTEN, TGFβR2 |
| miR-32 | +46.05 | PTEN, DKK3, NOTCH1 |
| miR-133b | −52.53 | EGFR, IGFR1, SP-1 |
| miR-141 | +23.67 | PDCD4, PTEN, CTNND2 |
| miR-143 | 30.17 | ErbB3, IGFR1 |
| miR-145 | −149.6 | ErbB3/4, IGFR1, SP-1 |
miR, miRNA; IGF-I, insulin-like growth factor-I; TIMP, tissue inhibitor of metalloproteinase; PTEN, phosphatase and tensin homolog; TGFβR2, transforming growth factor receptor-β receptor 2; EGFR, epidermal growth factor receptor; IGFR1, IGI-I type 1 receptor; SP-1, surfactant protein-1.
Aging is associated with a higher proportion of CSLCs and increased susceptibility to carcinogen.
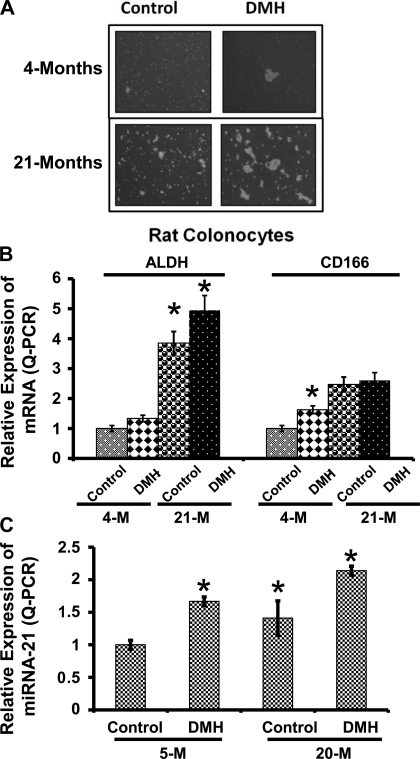
Because the aging colonic mucosa exhibited a higher proportion of CSLCs, we conducted the next set of experiments to determine whether exposure to the colonic carcinogen DMH would induce a further increase in CSLCs in aged animals. Mucosal cells were isolated from colon of young (4 mo old) and aged (21 mo old) rats and subjected to stem cell growth conditions to evaluate their ability to form spheroid-like clusters, a feature of CSLCs. We observed that the, under the current experimental condition, single cells isolated from aged colon (control and DMH-treated animals) demonstrated a higher survival than those from young animals. These cells also showed a greater potential to form bigger clusters (spheroids) compared with cells isolated from the colons of vehicle-treated or young rats (Fig. 2A). Additionally, we observed that, although administration of DMH increased the ability of isolated mucosal cells from both young and aged rats to form clusters/spheroid-like structures, the magnitude of this increase was higher in mucosal cells from DMH-treated aged than those from DMH-treated young animals (Fig. 2A). Expression of colon CSLC markers ALDH-1 and CD166 was also found to be similarly affected. Levels of ALDH-1 and CD166 mRNAs were found to be about 4- and 2.5-fold higher, respectively, in aged than in young animals. DMH caused a further increase in ALDH-1 mRNA in both age groups; DMH increased CD166 mRNA only in young animals (Fig. 2B).
Fig. 2.
A: phenotype of single cells isolated from 4-mo-old (young) and 20-mo-old (aged) Fischer-344 rats treated with or without dimethylhydrazine (DMH). The isolated cells show a differential potential to grow in stem cell media as well as to form spheroid-like clusters. B: relative expression of ALDH-1 and CD166 mRNA in 4- and 21-mo-old Fischer-344 rats. C: relative expression of miRNA (miR)-21 in the colonic mucosa of 5-mo-old (young) and 20-mo-old (aged) rats (n = 4–5 for each group). *P < 0.01 compared with 4- or 5-mo-old corresponding vehicle-treated controls.
The levels of miR-21 are known to be greatly elevated in colon tumors (2). Moreover, recent clinical findings have associated overexpression of miR-21 with a poor therapeutic response as well as poor prognosis in colon cancer patients (33). In view of our observations that the age-related rise in colonic mucosal CSLCs could be further stimulated by the colonic carcinogen DMH, we tested whether DMH increased miR-21 expression. The levels of miR-21 in the colon of older vehicle-treated animals (controls) were found to be ∼50% higher than in vehicle-treated young rats (Fig. 2C). DMH treatment caused a further increase in the expression of miR-21 in the colon of both age groups (Fig. 2C). The fact that the increase in expression of miR-21 is accompanied by a concomitant increase in expression of CSLC markers CD166 as well as ALDH-1 suggests that miR-21 may play a role in regulating colon CSLC.
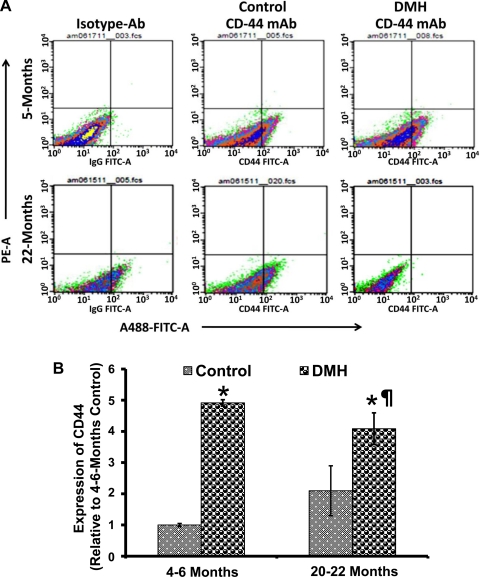
To further examine the DMH-induced changes in the proportion of CSLCs in the colonic mucosa of young and aged rats, freshly isolated mucosal cells were subjected to FACS analysis. The proportion of colonic mucosal cells expressing CD44 was found to be twofold higher in aged than in young animals, and administration of DMH in aged rats caused another twofold increase in the proportion of CD44-expressing cells compared with the young controls (Fig. 3, A and B). Our observations of increased expression of CSLC-specific markers in the colonic mucosa of aged rats and that this was associated with a parallel rise in the proportion of CSLC-specific population suggest that the increase in the CSLC population could be one of the driving forces for the age-related rise in colon cancer, which is increased further in response to carcinogen.
Fig. 3.
A: representative scattered plots of flow cytometry analyses of CD44-positive cells in 4- to 6-mo-old (young) and 20- to 22-mo-old (aged) Fisher-344 rats treated with DMH or vehicle. B: values represent the percentage of cells that are positive for CD44-FITC; they were derived from 4 independent experiments. mAb, monoclonal antibody. *P < 0.01 compared with the 4- to 6-mo-old (young) vehicle-treated rats. ¶P < 0.01 compared with the corresponding 20- to 22-mo-old (old) vehicle-treated animals.
The age-related rise in colonic CSLCs is associated with increased activation of EGFR.
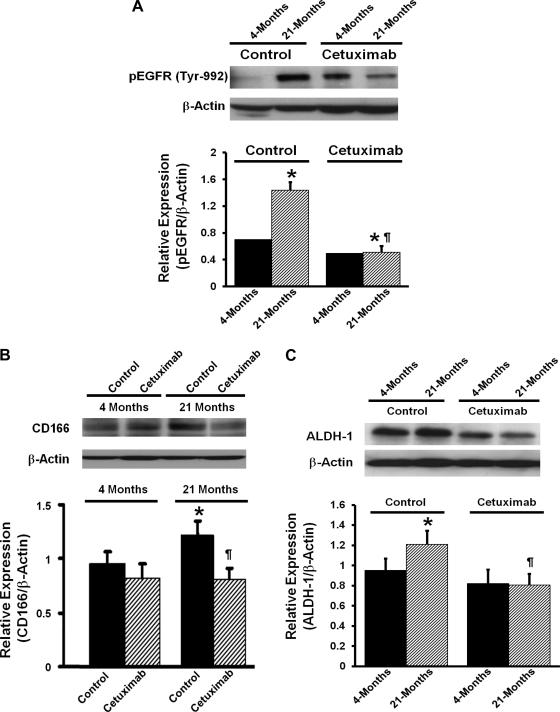
Earlier studies from this laboratory have demonstrated that aging is associated with increased activation of EGFR in the colonic mucosa and that this is further increased by DMH (20, 34). Because a similar phenomenon is also observed for colon CSLCs (23), our observations suggest a potential link between EGFR and CSLCs. The next experiment was therefore undertaken to determine whether EGFR regulates CSLCs in the colonic mucosa. We studied the effect of EGFR inhibition on the expression of CSLC markers (CD166 and ALDH-1) in the colonic mucosa during aging. Groups of young (4 mo) and aged (20–21 mo) male Fisher-344 rats were injected (ip) with cetuximab (8 mg/kg) or vehicle and killed 5 days later. Data from vehicle-treated animals (controls) revealed a 1.5-fold higher level of phosphorylated EGFR in the colonic mucosa of aged than young rats, indicating an age-related rise in EGFR activation in the colon (Fig. 4A). This increase was associated with a concomitant rise (40–50%) in the expression of CD166 and ALDH-1. Cetuximab administration caused a 68% inhibition in phosphorylated EGFR expression in the colonic mucosa of aged rats but produced only a marginal 15% reduction in young rats (Fig. 4A). The expression of CD166 and ALDH-1 was also markedly inhibited by cetuximab in the colonic mucosa of aged but not young animals (Fig. 4, B and C). Our observation that inhibition of tyrosine phosphorylation of EGFR is associated with attenuation of CSLC markers in the colon of aged rats suggests that activation of the EGFR signaling pathway can be functionally associated with the development of the CSLC phenotype in the colonic mucosa during aging.
Fig. 4.
Effects of cetuximab on epidermal growth factor receptor (EGFR) activation/phosphorylation (p) (A) and the expression of CD166 (B) and ALDH-1 (C) in the colonic mucosa of 4-mo-old (young) and 21-mo-old (aged) rats. Histograms show the relative expression of each parameter to the corresponding β-actin. *P < 0.01 compared with the 4-mo-old (young) vehicle-treated rats. ¶P < 0.01 compared with the corresponding 20- to 22-mo-old (old) vehicle-treated animals.
EGFR regulates CSLCs and miR-21 expression.
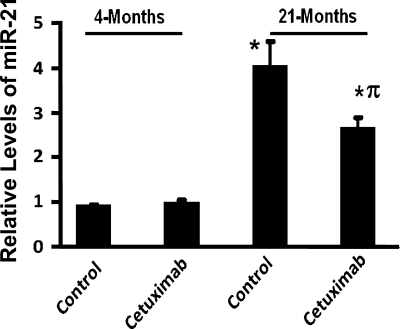
Recent evidence suggests a role for EGFR in regulating miR-21 (35). To determine whether the EGFR inhibitor cetuximab, which reduces the expression of CSLC markers, would affect the age-related increase in miR-21 expression in the colonic mucosa, we studied the effect of this inhibitor on miR-21 expression. Whereas cetuximab caused no apparent change in miR-21 expression in the colonic mucosa of 4-mo-old (young) rats, it produced a 40% reduction in the levels of miR-21 in aged rats compared with the corresponding vehicle-treated controls (Fig. 5). Taken together, the results suggest that EGFR plays a significant role in regulating the growth and maintenance of the CSLC population in the colon, and this may involve onco-mir miR-21.
Fig. 5.
Effects of cetuximab on miR-21 expression in the colonic mucosa of 4-mo-old (young) and 21-mo-old (aged) Fischer-344 rats. Animals were killed 5 days after a single ip injection of cetuximab (8 mg/kg) or vehicle. Data shown in histogram are from 3 independent experiments. *P < 0.01 compared with 4-mo-old vehicle-treated rats. πP < 0.01 compared with 21-mo-old vehicle-treated rats.
DISCUSSION
Cancers arise after the acquisition of multiple mutagenic events (31). Stem cells represent ideal cellular targets for the accumulation of precancerous damage, since the central properties of stem cell, long-life span, self-renewal, and differentiation enable mutations acquired in them to be propagated to both self-renewing progeny and downstream progenitors over the lifetime of the organism. Indeed, our recent observation that a linear increase in adenomatous polyps with advancing age (r2 = 0.92, P < 0.02) is associated with increased CSLCs in macroscopically normal mucosa (30) supports such a contention and further suggests that aging may predispose the colon to carcinogenesis. Moreover, the fact that CSLCs in the colonic mucosa increase with aging and with the number of adenomatous polyps suggests that CSLCs may play a pivotal role in regulating the processes of carcinogenesis.
Our current observation of a higher expression of several CSLC markers in the colonic mucosa of aged than in young animals, accompanied by an increased proportion of CD44 mucosal cells, further supports the contention that aging predisposes the colonic mucosa to processes of carcinogenesis. Moreover, the fact that these increases are further exacerbated by the colonic carcinogen DMH indicates a greater susceptibility of the aging colon to carcinogens/toxicants and may partly be responsible for the age-related rise in colorectal neoplasia/cancer.
Accumulating evidence suggests that miRNAs play critical roles in regulating biological processes like cell growth, tissue differentiation, cell proliferation, embryonic development, and apoptosis (32). In addition, it has been reported that miRNAs play a significant role in stem cell renewal and contribute to the stemness of CSLCs in cancers of breast, brain, and colon (11, 38, 40). More recently, miRNAs are being investigated as biomarkers of colorectal cancer as well as of aging (15, 37). A better understanding of the mechanisms of miRNA regulation of age-related genes in human should enable us to understand the complex processes of aging and the associated diseases like cancer. Noren Hooten et al. (28) investigated the miRNA expression profiles and differential expression of their targets with aging. The targets of the miRNAs that showed significant reductions with aging were miR-24, miR-128, miR-130a, miR-155, and miR-221 and were found to be involved in pathways that are commonly associated with cancer (28). We have conducted a similar miRNA expression profile and observed differential expression of miRNAs. We focused on miR-21 because of its oncogenic potential in colon cancer since it has been shown to promote cell transformation by targeting the tumor suppressor PDCD4 and phosphatase and tensin homolog (18). Moreover, overexpression of miR-21 has been linked to poor prognosis of colorectal cancer and also shows a strong association with the established prognostics factors for nodal and metastatic stages and directly with poor survival (33, 36). The colonic mucosa of aged rats showed higher expression of miR-21. Our current observation of a parallel rise in miR-21 and CSLCs in the colonic mucosa during aging and further increases of both of these parameters by the colonic carcinogen DMH suggest that miR-21 may also play a role in regulating CSLCs in the colonic mucosa. We, herein, report for the first time that upregulation of a miR is associated with aging and carcinogenesis.
Although the regulatory mechanisms for the age-related rise in CSLCs and miR-21 in the colonic mucosa are not understood, our current data suggest a role for EGFR signaling in regulating these processes. A number of observations support this postulation. Earlier, as has been observed in the current investigation, the age-related increase in the expression of several markers of CSLC in the colonic mucosa is associated with a concomitant increase in EGFR expression, and these increases are exacerbated by DMH (16, 34). Aging has also been shown to be associated activation of EGFR and its downstream signaling events (20, 21). Our current data further demonstrate that administration of cetuximab, monoclonal antibodies to EGFR that inhibit EGFR activation, not only inhibits the expression of CSLC markers CD166 and ALDH-1 but also miR-21 expression in the colonic mucosa in both young and aged animals, but the magnitude of inhibition of these parameters is much greater in aged than that noted in young animals. Whether other members of the EGFR, specifically HER-2 and HER-3, which are also activated in the aging colon (21) and are known to play critical roles in the development and progression of many epithelial malignancies, including the colorectal cancer, play a role in the development of the CSLC phenotype remain to be determined.
In conclusion, our study provides new evidence to demonstrate that aging is associated with an increase in the expression of several markers of CSLC and the proportion of CSLCs in the colonic mucosa and that they are further exacerbated by the colonic carcinogen DMH. These increases are also associated with a concomitant rise in miR-21 and EGFR in the colonic mucosa. The age-related rise in CSLCs and miR-21 could be inhibited by the EGFR-specific inhibitor cetuximab, suggesting a role for this growth factor receptor in modulating the age-dependent increase in CSLCs in the colonic mucosa and the carcinogen induction of colon CSLC phenotype during aging.
GRANTS
The work presented in this communication has been supported by grants to A. P. N. Majumdar from the National Institute on Aging (AG-014343 and AG-014343–12S1) and the Department of Veterans Affairs (VA Merit Review).
DISCLOSURES
None of the following authors have any conflict of interest: Jyoti Nautiyal, Jianhua Du, Yingjie Yu, Shailender S Kanwar, Edi Levi, Adhip P.N. Majumdar.
AUTHOR CONTRIBUTIONS
Author contributions: J.N., S.S.K., and A.P.M. conception and design of research; J.N., J.D., Y.Y., and S.S.K. performed experiments; J.N. and E.L. analyzed data; J.N., Y.Y., and E.L. interpreted results of experiments; J.N. prepared figures; J.N. drafted manuscript; J.N. and A.P.M. edited and revised manuscript; J.N., J.D., Y.Y., S.S.K., E.L., and A.P.M. approved final version of manuscript.
REFERENCES
- 1. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100: 3983–3988, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27: 2128–2136, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281–297, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol 26: 2828–2838, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell 1: 241–242, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA 104: 10158–10163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene 25: 6163–6169, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi H, Angeletti CA, Pingitore R, Pepe S, Basolo F, Bevilacqua G. Epidermal growth factor receptor (EGFr) expression in non-small cell lung carcinomas correlates with metastatic involvement of hilar and mediastinal lymph nodes in the squamous subtype. Eur J Cancer 31A: 178–183, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Grandis JR, Chakraborty A, Zeng Q, Melhem MF, Tweardy DJ. Downmodulation of TGF-alpha protein expression with antisense oligonucleotides inhibits proliferation of head and neck squamous carcinoma but not normal mucosal epithelial cells. J Cell Biochem 69: 55–62, 1998 [PubMed] [Google Scholar]
- 10. Gullick WJ. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med Bull 47: 87–98, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature 435: 974–978, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res 69: 3382–3389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 355: 1253–1261, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres (Abstract). Mol Cancer 9: 212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kashyap L. Can microRNAs act as biomarkers of aging? Bioinformation 5: 396–397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levi E, Misra S, Du J, Patel BB, Majumdar AP. Combination of aging and dimethylhydrazine treatment causes an increase in cancer-stem cell population of rat colonic crypts. Biochem Biophys Res Commun 385: 430–433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66: 6063–6071, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 27: 4373–4379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Majumdar AP. Regulation of gastrointestinal mucosal growth during aging. J Physiol Pharmacol 54, Suppl 4: 143–154, 2003 [PubMed] [Google Scholar]
- 20. Majumdar AP, Du J. Phosphatidylinositol 3-kinase/Akt signaling stimulates colonic mucosal cell survival during aging. Am J Physiol Gastrointest Liver Physiol 290: G49–G55, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Majumdar AP, Du J, Yu Y, Xu H, Levi E, Patel BB, Rishi AK. Cell cycle and apoptosis regulatory protein-1: a novel regulator of apoptosis in the colonic mucosa during aging. Am J Physiol Gastrointest Liver Physiol 293: G1215–G1222, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Majumdar APN, Basson MD. Effect of Aging on the Gastrointestinal Tract. New York, NY: Academic, 2006, p. 405–433 [Google Scholar]
- 23. Malecka-Panas E, Kordek R, Biernat W, Tureaud J, Liberski PP, Majumdar AP. Differential activation of total and EGF receptor (EGF-R) tyrosine kinase (tyr-k) in the rectal mucosa in patients with adenomatous polyps, ulcerative colitis and colon cancer. Hepatogastroenterology 44: 435–440, 1997 [PubMed] [Google Scholar]
- 24. Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature 467: 86–90, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Nathanson DR, Culliford A, Tt Shia J, Chen B, D'Alessio M, Zeng ZS, Nash GM, Gerald W, Barany F, Paty PB. HER 2/neu expression and gene amplification in colon cancer. Int J Cancer 105: 796–802, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Nautiyal J, Kanwar SS, Majumdar AP. EGFR(s) in aging and carcinogenesis of the gastrointestinal tract. Curr Prot Pept Sci 11: 436–450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nautiyal J, Rishi AK, Majumdar AP. Emerging therapies in gastrointestinal cancers. World J Gastroenterol 12: 7440–7450, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noren Hooten N, Abdelmohsen K, Gorospe M, Ejiogu N, Zonderman AB, Evans MK. microRNA expression patterns reveal differential expression of target genes with age. PLoS One 5: e10724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106–110, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Patel BB, Yu Y, Du J, Levi E, Phillip PA, Majumdar AP. Age-related increase in colorectal cancer stem cells in macroscopically normal mucosa of patients with adenomas: a risk factor for colon cancer. Biochem Biophys Res Commun 378: 344–347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell 132: 681–696, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Arch 452: 1–10, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, Liu CG, Calin GA, Croce CM, Harris CC. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. J Am Med Assoc 299: 425–436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmelz EM, Levi E, Du J, Xu H, Majumdar AP. Age-related loss of EGF-receptor related protein (ERRP) in the aging colon is a potential risk factor for colon cancer. Mech Ageing Dev 125: 917–922, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma A, Kudoh S, Croce CM, Harris CC. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Natl Acad Sci 106: 12085–12090, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slaby O, Svoboda M, Fabian P, Smerdova T, Knoflickova D, Bednarikova M, Nenutil R, Vyzula R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology 72: 397–402, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Yang L, Belaguli N, Berger DH. MicroRNA and colorectal cancer. World J Surg 33: 638–646, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 131: 1109–1123, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol 2: 321–328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H, Li W, Nan F, Ren F, Wang H, Xu Y, Zhang F. MicroRNA expression profile of colon cancer stem-like cells in HT29 adenocarcinoma cell line. Biochem Biophys Res Commun 404: 273–278, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Ziyan W, Shuhua Y, Xiufang W, Xiaoyun L. MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Med Oncol 28: 1469–1474, 2011 [DOI] [PubMed] [Google Scholar]