Abstract
Recently, we reported that nicotine in vitro at a low 1-μM concentration suppresses hyperexcitability of colonic dorsal root ganglia (DRG; L1-L2) neurons in the dextran sodium sulfate (DSS)-induced mouse model of acute colonic inflammation (1). Here we show that multiple action potential firing in colonic DRG neurons persisted at least for 3 wk post-DSS administration while the inflammatory signs were diminished. Similar to that in DSS-induced acute colitis, bath-applied nicotine (1 μM) gradually reduced regenerative multiple-spike action potentials in colonic DRG neurons to a single action potential in 3 wk post-DSS neurons. Nicotine (1 μM) shifted the activation curve for tetrodotoxin (TTX)-resistant sodium currents in inflamed colonic DRG neurons (voltage of half-activation changed from −37 to −32 mV) but did not affect TTX-sensitive currents in control colonic DRG neurons. Further, subcutaneous nicotine administration (2 mg/kg b.i.d.) in DSS-treated C57Bl/J6 male mice resulted in suppression of hyperexcitability of colonic DRG (L1-L2) neurons and the number of abdominal constrictions in response to intraperitoneal injection of 0.6% acetic acid. Collectively, the data suggest that neuronal nicotinic acetylcholine receptor-mediated suppression of hyperexcitability of colonic DRG neurons attenuates reduction of visceral hypersensitivity in DSS mouse model of colonic inflammation.
Keywords: dorsal root ganglia, hyperexcitability, mice, neuronal nicotinic acetylcholine receptor, hypersensitivity, colitis
several recent studies (1, 5, 19, 22, 23) in animal models of acute colonic inflammation have demonstrated an increase in the electrical excitability of colonic dorsal root ganglia (DRG) neurons due to altered ionic conductances and suggested a correlation between enhanced sensory neuronal excitability and visceral hypersensitivity. Visceral hypersensitivity has been shown to persist for several weeks during and following recovery from acute colonic inflammation in experimental animals injected with deoxycholic acid or 2,4,6-trinitrobenzenesulfonic acid with ethanol (11, 33). These data obtained from colitis animals correlate with the presence of hypersensitivity to colorectal distension stimuli in patients suffering from inflammatory bowel disease and with the presence of abdominal pain during the remission (24). Previously, we (1) reported that bath-applied nicotine at a low 1-μM concentration, suppresses the hyperexcitability of colonic DRG (L1-L2) neurons in dextran sodium sulfate (DSS)-treated C57Bl/J6 mice. Although controlled clinical trials indicate that transdermal nicotine patch significantly improves the histological and global clinical score of colitis, including abdominal pain (25, 34), the in vivo actions of nicotine in colonic inflammation are not well characterized. In agreement with anti-inflammatory effects mediated by neuronal neuronal nicotinic acetylcholine receptors (nAChRs; Ref. 3), Snoek et al. (32) recently reported that low doses of nicotine (0.25 and 2.50 μmol/kg) given intraperitoneally once per day decreased colonic cytokine production in the DSS-treated C57Bl/6 mice. However, nicotine failed to reduce the disease activity index in these animals.
The goals of the present study were to determine 1) whether hyperexcitability of colonic DRG neurons from inflamed mice persists following recovery from colonic inflammation and, if present, can be suppressed in vitro by nicotine, and 2) whether in vivo administration of nicotine alters hyperexcitability of colonic DRG neurons and visceral hypersensitivity in inflamed mice. The enhanced excitability of colonic DRG neurons during acute colitis is reflected in the altered ionic conductances of the DRG neurons (5, 23). Tetrodotoxin (TTX)-resistant sodium currents (4) that play an essential role in action potential generation have been found to be enhanced by ∼60% in the inflamed colonic DRG neurons due to the overexpression of Nav1.8 channels (13, 18). To address further the mechanism of the in vitro nicotine-induced suppression of hyperexcitability, we aimed to explore whether nicotine interaction with nAChRs leads to changes in properties of TTX-resistant sodium currents in inflamed colonic DRG neurons.
MATERIALS AND METHODS
Animals.
Adult male C57BL/J6 mice (Jackson Laboratory, Bar Harbor, ME) were used in our studies. Animals were housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care approved animal care facility with food available ad libitum. The rooms were on a 12-h light-dark cycle (lights on at 7:00 AM). Mice were 8–10 wk of age and weighed ∼25–30 g at the start of the experiment. All experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were performed under the Guidelines for the Care and Use of Laboratory Animals as promulgated by the U.S. National Institutes of Health.
Retrograde labeling of colonic DRG neurons.
The extrinsic sensory innervation to the colon consists of sympathetic and parasympathetic components the cell bodies of which are of small and medium (<40 pF) size within the L1-L2 and L6-S1 DRGs, respectively (28). In this study, we investigated colonic neurons from L1-L2 DRGs. To identify DRG neurons of colonic origin, we prelabeled colonic neurons as described by Abdrakhmanova et al. (1). Briefly, the animals were fasted for 4 h before surgical procedure. Mice were anesthetized by sodium pentobarbital (40 mg/kg) injected intraperitoneally, and a midline laparotomy was performed to gain access to the pelvic organs. The distal colon was exposed with cotton swabs and 1,1-dioctadecyl-3-3-3′3′-tetramethylindocarbocyanine (DiI; Invitrogen, Carlsbad, CA; 1.5% in DMSO) was injected into the colonic wall using a Hamilton syringe at four to five sites (volume = 1.0 μl/injection). The colon was placed back into the abdominal cavity. To avoid nonspecific labeling of surrounding tissues and organs, several precautions were taken during injection procedure: isolating of adjacent pelvic organs with gauze during dye injections; keeping the needle in place after the injection for 20 s and soaking up of dye reflux upon needle removal; and washing off any traces of dye from the organ surface with sterile saline before placing the organ into the pelvic cavity. Incisions were sutured in layers under sterile conditions, and mice were allowed to recover from anesthesia on a warm pad. After recovery from anesthesia, the mice were provided with free access to water, 12 h later to food, and monitored for signs of pain and discomfort.
DSS treatment and disease activity index evaluation.
To induce acute colon inflammation, treatment with DSS (40 kDa; MP Biomedicals, Solon, OH) was initiated 3–5 days after the surgery (8). DSS at 5% concentration was added to drinking water and administered for the total duration of 6–7 days ad libitum (10, 26, 35). To confirm signs of colon inflammation, we evaluated the “disease activity index” on a daily basis as described previously by Jin et al. (15) on combined assessment of weight loss, blood in stool, and its consistency, so that 0 corresponded to a condition with no weight loss, normal stool consistency, and no rectal bleeding; 1 was accompanied by 1–5% weight loss; 2 showed in addition to 5–10% weight loss, loose stool, and presence of blood in the stool; 3 for 10–15% weight loss; and 4 assigned when the weight loss was 15–20% of initial weight with gross bleeding. Blood in the stool was assessed using Hemoccult test (Beckman coulter, Brea, CA).
Nicotine administration.
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl)pyrrolidine-(+)-bitartrate salt] was purchased from Sigma-Aldrich (St. Louis, MO). Nicotine was dissolved in physiological saline (0.9% sodium chloride) and injected subcutaneously at the dose of 2 mg/kg b.i.d. (dose of nicotine is expressed as a free base of the drug). Nicotine administration was initiated 2 days before its coadministration with DSS.
Real-time PCR.
Quantitative real-time PCR assay for interleukin (IL)-1β was performed to confirm inflammatory changes on a MiniOpticon real-time PCR system (Bio-Rad, Hercules, CA). Ribosomal 18S RNA was used as the internal control. Colons were isolated from control and DSS-treated C57BL/J6 mice, and total RNA was extracted by the total RNA purification system (Invitrogen) according to the manufacturer's instructions. cDNA synthesis and subsequent polymerization was performed in a single step using the SensiMix One-Step kit (Quantace, Taunton, MA). The reaction mixture (50 μl) contained 200 nM forward primer, 200 nM reverse primer, 1× SensiMix One-Step buffer, 1× SYBR Green I solution, 10 Units RNase inhibitor, and 200 ng total RNA. With the use of the one-step SensiMix kit, reverse transcription was performed for 30 min at 42°C with an enzyme activation step for 10 min at 95°C. The PCR protocol consisted of 40 cycles of denaturation (15 s at 95°C), annealing (30 s at 58°C), and extension (30 s at 72°C). The cycle threshold value, Ct count, for each sample was normalized by subtracting it from the Ct value of the reference gene 18S to establish ΔCt (ΔCt = Il-1β Ct − 18S Ct) for each sample. The fold change was calculated as 2−ΔΔCt. All primers were designed using the Vector NTI software (Invitrogen, Carlsbad, CA), and the sequences in this study used were as follows: 5′-CCTGAACTCAACTGTGAAATGC-3′ (forward) and 5′-CGAGATTTGAAGCTGGATGC-3′ (reverse) for Il-1b (NM_008361), 5′-TCAAGAACGAAAGTCGGAGG-3′ (forward) and 5′-GGACATCTAAGGGCATCAC-3′ (reverse) for 18S (X00686).
Visceral hypersensitivity.
The number of abdominal constrictions in response to intraperitoneal injection of acetic acid (0.6%) was registered starting 3 min after the injection and continued for the following 20 min. Animals were killed immediately after the experiment. The total number of constrictions is expressed as means ± SE.
Isolation of DRG neurons.
The mice were anesthetized by sodium pentoparbtital (40 mg/kg ip) and killed by decapitation. The protocol for neuronal isolation was similar to that described by Jin and Gereau (14). Briefly, DRGs (L1-L2) were removed under dissecting microscope magnification and collected in cold (4°C) PBS without Ca2+ or Mg2+ (Invitrogen). Ganglia were incubated in 15 U/ml papain (Worthington, Lakewood, NJ) in HBSS (Invitrogen) for 18 min at 37°C. After this initial enzyme treatment, the ganglia were rinsed three times in HBSS and then incubated for 18 min with 1.5 mg/ml collagenase (Sigma-Aldrich) in HBSS at 37°C. After being washed three times with HBSS, ganglia were gently triturated with a flame-polished Pasteur pipette. The tissue fragments were centrifuged at 1,000 rpm for 5 min, and the pellet was resuspended in neurobasal culture media (Invitrogen) with 5% FBS (Invitrogen), 1% B27 (Invitrogen), 100 U/ml penicillin/streptomycin (Invitrogen), and 2 mM glutamax (Invitrogen). Cells were plated onto poly-d-lysine-coated 12 mm glass coverslips (Thermanox; Nalge Nunc, Napierville, IL) and maintained at 37°C in a 95% air-5% CO2 incubator overnight.
Electrophysiological recordings.
DRG neurons were studied within 1–2 days after being plated on the coverslips. The cells were transferred to an experimental chamber and bathed (1–2 ml/min) in a solution containing the following: 137 mM NaCl, 5.4 mM KCl, 2 mM MgCl2, 10 mM d-glucose, 10 mM HEPES, and 2 mM CaCl2 (pH adjusted to 7.4 with NaOH). Labeled neurons were identified using a specific filter for DiI (Olympus, Tokyo, Japan) under an inverted fluorescent microscope (Olympus IX50; Olympus). The patch electrodes, pulled from borosilicate glass capillaries (Sutter Instrument, Novato, CA), had a resistance of 2.5–3.5 MΩ when filled with internal solution. In voltage-clamp experiments, in majority of cells ∼85% of electrode resistance is compensated electronically. Pipette solution contained the following: 30 mM KCl, 100 mM potassium aspartate, 6 mM EGTA, 1 mM MgCl2, 10 mM HEPES, and 4 mM Mg-ATP (pH adjusted to 7.2 with KOH) for studies of action potentials or 20 mM CsCl, 110 mM CsMeSO3, 10 mM EGTA, 1 mM MgCl2, 10 mM HEPES, 0.1 mM CaCl2, and 2 mM Mg-ATP (pH adjusted to 7.2 with CsOH) when sodium currents were examined.
Generation of voltage-clamp and current-clamp protocols and acquisition of the data are carried out using pCLAMP 9.0 software (Molecular Devices, Sunnyvale, CA). To study the evoked action potential characteristics, currents of a 500-ms duration were applied in 20-pA steps in the current-clamp mode. Current steps to −20 pA (200 ms) were used to calculate the input resistance. To study the effect of nicotine on sodium currents, voltage-clamp recordings were carried out in whole cell configuration. Sodium currents were pharmacologically isolated by the using Cs-containing pipette solution and the presence of 100 μM CdCl in the extracellular solution and recorded using 30-ms depolarizing steps in 5-mV increments from −80 to +35 mV in neurons held at the membrane potential of −100 mV (4, 17). TTX-resistant sodium currents were isolated by perfusing with TTX (1 μM). Conductance (G) was determined as G = I/(Vm − Ex), where I in the current, Vm is the command voltage, and Ex is the equilibrium potential that was estimated to be +90 mV for sodium based on experimental solutions. Normalized average conductances (G/Gmax) were plotted against given membrane potential, and the activation curve was fitted using Origin software to a Boltzmann function to determine the voltage of half-activation, V0.5 (mV). Inactivation curves for sodium currents were measured using a two-pulse protocol (17): a 1-s prepulse between −120 and 0 mV, followed by a 30-ms pulse to 0 mV. Normalized current amplitudes (I/Imax) were plotted against conditioning membrane potential and fitted to Boltzmann function. Experiments were performed at room temperature (22–25°C) and recorded using an Axopatch 200B amplifier (Molecular devices).
Experimental solutions were applied on tested cells using a Y-ending application system as described in Hevers and Luddens (12) or via bath. pH of nicotine-containing solutions was adjusted to 7.4.
Chemicals.
All chemicals for patch-clamp experiments were obtained from Sigma-Aldrich (Atlanta, GA) with the exception of DiI (Invitrogen) and DSS (MP Biomedicals).
Statistical analysis.
Results are presented as the mean ± SE for the number of cells (n). Sigmaplot software was used for detection of differences in paired or unpaired t-tests, and Mann-Whitney rank sum test as appropriate, and values of P < 0.05 were regarded as significant.
RESULTS
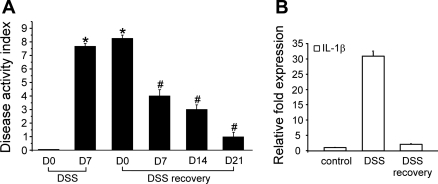
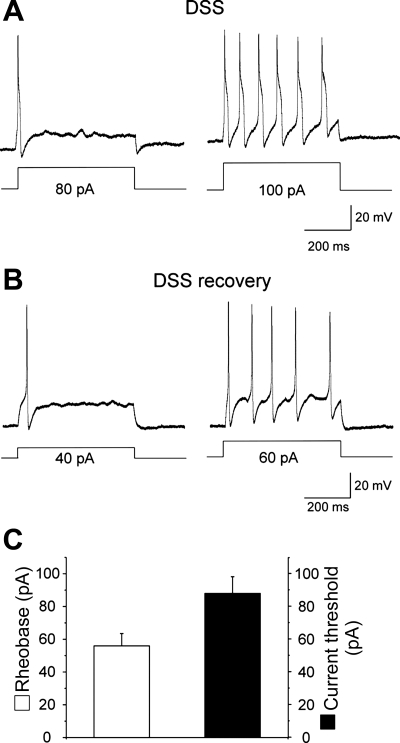
Recently, we (1) reported that nicotine at 1 μM concentration applied to the bath suppresses multiple action potential firing in colonic DRG (L1-L2) neurons in vitro from DSS-treated mice. To further distinguish between anti-inflammatory and antinociceptive effects of nicotine, we explored whether nicotine is able to suppress hyperexcitability of colonic DRG neurons during the recovery from DSS-induced colonic inflammation. Figure 1 shows that after 3 wk of recovery (day 21) from 5% DSS administration, both the disease activity index (A) and the levels of IL-1β in the colon tissue (B) significantly improved. However, colonic DRG neurons from these animals continuously exhibited signs of hyperexcitability similar to those during acute inflammation (Fig. 2, A and B). Three weeks post-DSS, colonic DRG (L1-L2) neurons fired single action potential (rheobase) at 56 ± 7 pA and multiple action potentials at 88 ± 10 pA (n = 8), plotted as a threshold in Fig. 2C (Table 1). Figure 2 shows representative traces of action potentials in acute inflammation and post-DSS treated DRG neurons at rheobase and current threshold. Figure 2B shows an action potential from a 21-day post-DSS neuron whereby the single action potential was registered at 40-pA current injection, and an increase of the amplitude of the injected current from 40 to 60 pA was sufficient to induce multiple action potentials in this neuron. The rheobase and current threshold for the 21-day post-DSS neurons was not significantly different from the acute inflamed cells (7 day DSS; Table 1). In comparison, the rheobase for acute DSS and following recovery was significantly lower than control values reported previously (1) and as shown in Table 1. Evaluation of the resting membrane potentials in colonic sensory neurons on day 21 of recovery from DSS-induced colonic inflammation showed that the values (−43.9 ± 2.7 mV; n = 6) were similar to those in neurons from control or inflamed mice [−42.2 ± 2.8 or −43.5 ± 2.7 mV, respectively; n was 12 in each group (1); Table 1]. In a similar manner, the input resistance (446 ± 37 MΩ; n = 4) of colonic DRG neurons 3 wk post-DSS was not significantly different from that in inflamed neurons (392 ± 86 MΩ; n = 4; t-test: P = 0.633).
Fig. 1.
Recovery from dextran sodium sulfate (DSS) administration. Normalization of the disease activity index (A) and levels of IL-1β in colon tissue (B) after 3 wk of recovery from DSS. Note gradual decrease of the disease activity index evaluated at 0, 7, 14, and 21 days (D) during the recovery from DSS-induced colonic inflammation. Samples of colon tissue shown as DSS recovery in B were collected on day 21 post-DSS. *Significant difference from day 0 of DSS administration. #Significant difference from day 0 of DSS recovery.
Fig. 2.
Persistence of hyperexcitability of colonic sensory neurons 3 wk post-DSS. A: action potential recordings at the rheobase and current threshold level in a representative neuron from inflamed mouse. B: action potential recordings at the rheobase and current threshold level in a representative postinflammatory neuron. Note the presence of multiple action potential firing typical for colonic dorsal root ganglia (DRG) neurons during acute colitis. C: averaged rheobase (open bar) and current threshold (closed bar) parameters in colonic sensory neurons on day 21 postinflammation.
Table 1.
Electrophysiological parameters of colonic DRG (L1-L2) neurons
| Rheobase, pA | Current Threshold, pA | RMP, mV | |
|---|---|---|---|
| DSS recovery | 56 ± 7 | 88 ± 10 | −43.9 ± 2.7 |
| DSS + nicotine (subcutaneous) | 211 ± 66 | NA | −45.0 ± 2.4 |
| DSS | 77 ± 13 | 103 ± 8 | −43.5 ± 2.7 |
| Control | 184 ± 26 | NA | −42.2 ± 2.8 |
Values are shown as means ± SE. Dextran sodium sulfate (DSS) and control data are determined previously (1).
RMP, resting membrane potential; NA, not applicable.
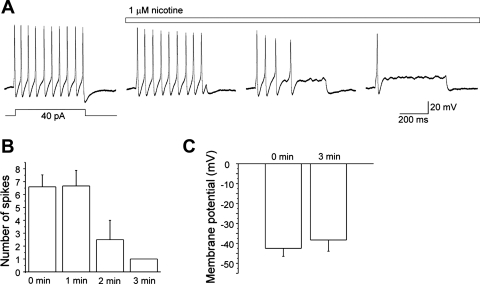
The next set of experiments was aimed to test whether bath-applied nicotine (1 μM), similar to that in DSS-induced acute colitis (1), can suppress multiple action potential firing in colonic sensory neurons during the recovery from DSS-induced colonic inflammation. Action potentials were elicited by current injections (500-ms duration) at the level of the current threshold initially in the absence of nicotine, followed by recordings in the presence of nicotine (1 μM) at intervals of 1 min. As demonstrated in Fig. 3, A and B, nicotine gradually reduced the number of action potentials fired per depolarization evaluated at the current threshold (n = 6). Increasing the amplitude of injected current (2-fold) did not result in recovery of multiple action potential firing in neurons incubated with nicotine for 3 min. No significant change in the membrane potential was observed in the absence and presence of nicotine (−42.5 ± 4.0 and −38.3 ± 5.6, respectively; n = 4; Fig. 3C).
Fig. 3.
Nicotine in vitro suppresses action potential firing in colonic sensory neurons isolated from mice 3 wk post-DSS. A: representative recordings of action potentials generated by a 500-ms current injection in the absence and presence of nicotine (1 μM) taken in a 1-min interval. B: time course of the suppressive effect of nicotine on action potential discharge. C: membrane potential in the absence and presence of nicotine.
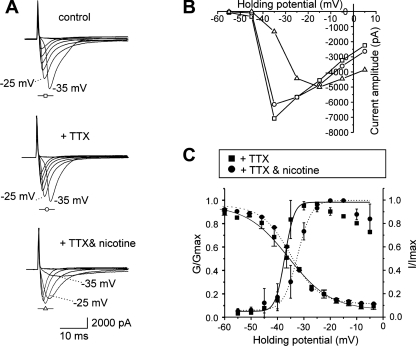
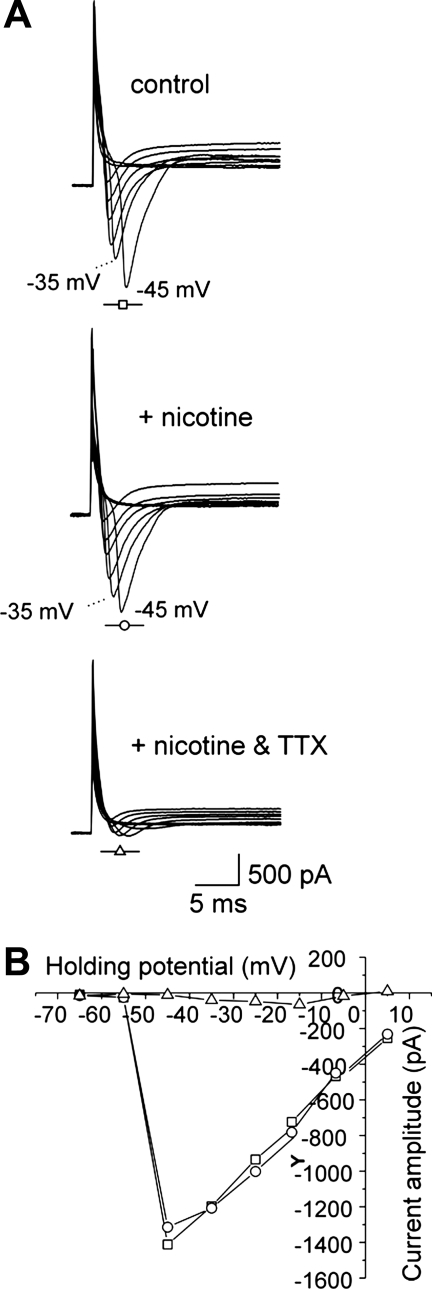
TTX-resistant sodium currents have been shown to be enhanced and mediate multiple action potential firing in inflamed colonic DRG neurons (4, 13, 18). To elucidate the mechanism of the nicotine-induced suppression of hyperexcitability in colonic sensory neurons, we tested the effect of bath-applied nicotine (1 μM) on TTX-resistant sodium current activation and inactivation. Interestingly, nicotine application (3 min) shifted the activation curve for TTX-resistant sodium currents to the right (V0.5 changed from −37.2 ± 1.0 to −32.4 ± 0.9 mV; n = 5) but did not alter the inactivation curve in colonic neurons from DSS-treated mice (Fig. 4; n = 5). In contrast, nicotine application did not affect sodium currents in control neurons that only expressed TTX-sensitive currents (Fig. 5; n = 3), suggesting that the effect of nicotine is specific for TTX-resistant sodium currents.
Fig. 4.
In vitro effect of nicotine on tetrodotoxin (TTX)-resistant voltage-gated sodium currents in inflamed colonic sensory neurons. A: representative currents elicited in inflamed neurons under control conditions (top), in the presence of 1 μM TTX (middle), and in 3 min after addition of 1 μM nicotine (bottom). B: current-voltage relation for the recordings shown in A. C: nicotine in vitro affected the activation but not the inactivation of TTX-resistant sodium currents in inflamed colonic neurons. G/Gmax, normalized average conductances.
Fig. 5.
Absence of the in vitro effect of nicotine on TTX-sensitive sodium currents in control colonic DRG neurons. A: representative currents elicited in uninflamed neuron under control conditions (top), in 3 min of incubation with 1 μM nicotine (middle), and after addition of 1 μM TTX (bottom). B: current-voltage relation for the recordings in A.
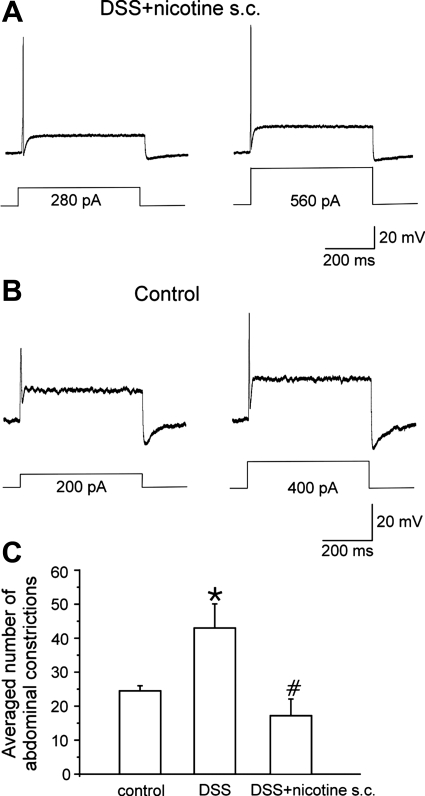
Next, we tested whether in vivo nicotine administration (2 mg/kg sc b.i.d) also suppresses hyperexcitability of colonic DRG (L1-L2) neurons in DSS(5%)-treated C57Bl/J6 mice. Current injections of 500-ms duration were used to study an activation of action potentials in prelabeled colonic sensory neurons. The minimal current required to elicit a single action potential (rheobase) in neurons from nicotine-treated inflamed mice was 211 ± 66 pA (n = 11) (Table 1), similar to that in noninflamed control animals (184 ± 26 pA; Fig. 6, A and B) but significantly higher than in DSS-treated animals (77 ± 13 pA; n = 12; t-test, P = 0.01). In 9 out of 11 tested neurons, only single action potential could be evoked upon a twofold increase of the amplitude of the injected current (2× rheobase). In a representative neuron shown in Fig. 6A, the single action potential was registered at 280 pA (rheobase), and twofold increase of the amplitude of the injected current from 280 to 560 pA did not induce multiple action potential firing unlike in inflamed neurons (1). Nicotine treatment did not alter the resting membrane potential (−45.0 ± 2.4 mV; n = 11) of colonic sensory neurons in inflamed mice (Table 1). The input resistance (321 ± 43 MΩ; n = 4) after nicotine administration was not significantly different from that for DSS mice neurons (392 ± 86 MΩ; n = 4; t-test: P = 0.497). These data suggest that subcutaneous nicotine administration suppresses the hyperexcitability of colonic DRG neurons induced by DSS-treatment.
Fig. 6.
Effect of in vivo nicotine administration (2 mg/kg sc b.i.d.) in DSS-treated mice. Action potential recordings induced by a current injection at the rheobase (left) and 2× rheobase (right) levels from a representative colonic sensory neuron in inflamed mouse treated with nicotine (A) and control animal (B). C: averaged number of abdominal constrictions in response to 0.6% acetic acid (ip) in control, inflamed and nicotine-treated mice. *Significant difference from control mice. #Significant difference from DSS-treated mice.
Acute colonic inflammation is suggested to modulate visceral sensitivity by increasing excitability of colonic sensory neurons (5, 22, 23). To test whether subcutaneous nicotine treatment (2 mg/kg b.i.d.) results in suppression of visceral hypersensitivity in DSS-treated mice, the number of abdominal constrictions in response to intraperitoneal injection of 0.6% acetic acid was evaluated over time period of 20 min in nicotine-treated inflamed mice and compared with that in DSS-treated and noninflamed control animals. DSS administration resulted in a significant increase of the number of abdominal constrictions compared with control (43.0 ± 7.0 vs. 24.5 ± 1.5; Mann-Whitney rank sum test: P = 0.008; n = 6 in each group; Fig. 6C). Nicotine treatment of inflamed mice significantly reduced the number of abdominal constrictions to the levels comparable to that in control animals (16.8 ± 4.9; n = 6). Taken together, these data suggest that subcutaneous nicotine treatment (2 mg/kg b.i.d.) reduces hyperexcitability and visceral hypersensitivity in a mouse model of DSS-induced acute colitis.
DISCUSSION
The results of this study performed in a mouse model of colonic inflammation suggest that 1) nicotine application suppresses hyperexcitability of colonic sensory neurons in postinflammatory mice; 2) nicotine induces a shift in activation curve of TTX-resistant sodium currents towards more depolarized potentials preventing multiple-spike action potential generation in inflamed colonic sensory neurons, and 3) in vivo subcutaneous nicotine administration reverses the hyperexcitability of inflamed colonic sensory neurons that correlates with suppression of hypersensitivity in acute DSS colitis.
Multiple action potential firing in response to a current injection at the amplitude above a rheobase is typical for colonic DRG neurons in animal models of acute colonic inflammation (1, 5, 22, 23). Our assessment of inflammatory signs in mice during the resolution from DSS administration revealed a significant reduction of the disease activity index and normal levels of IL-1β in their colon tissue following 3 wk of recovery from DSS treatment. However, the mice continued to exhibit hyperexcitability of their colonic sensory neurons. Likewise, Traub et al. (33) reported on the persistence of dorsal horn hyperexcitability and visceral hyperalgesia in rat after 4 wk of resolution from deoxycholic acid-induced colonic inflammation. In mice, colonic hyperalgesia evaluated by colorectal distension persisted for 14 days following ethanol-induced colonic inflammation (16). In contrast, Larsson et al. (20, 21) did not consistently register long-lasting inflammation-induced changes in colonic sensitivity to colorectal distension upon recovery from 3% DSS although there was a tendency towards visceral hyperalgesia in mice on day 10 post-DSS treatment.
Similar to the effect of nicotine in colonic DRG neurons from mice with acute DSS colitis (1), nicotine application at a low concentration of 1 μM reversed multiple action potential firing present in colonic DRG neurons from mice after recovery from DSS. Nicotine's effect developed gradually (∼3 min) and was not accompanied by significant changes in the membrane potential of the neurons.
Hyperexcitability of the DRG neurons following inflammation is suggested to involve significant remodeling of ion channel function (2). TTX-resistant sodium currents are enhanced and mediate multiple action potential firing in inflamed colonic sensory neurons (4). This is partly due to a shift in the voltage dependence of activation of TTX-resistant sodium currents. Beyak et al. (4) showed that colonic inflammation results in a shift of the V0.5 of TTX-resistant sodium currents from −26.6 to −36.6 mV. We found that 1 μM nicotine alters the voltage dependence of activation of TTX-resistant sodium currents in inflamed colonic DRG (L1-L2) neurons with a shift of V0.5 from −37.2 to −32.4 mV. Thus nicotine-induced activation of nAChRs leads to reversing the V0.5 of TTX-resistant sodium currents in inflamed colonic neurons back to values typical for control neurons that fire only a single action potential. The analysis of the threshold for multiple action potential firing at the current threshold level suggested similar values for acute inflammation and 3 wk post-DSS, being −26.4 ± 1.5 mV and −26.5 ± 2.0 mV (n = 6 in each group). According to activation data plotted in Fig. 4C, at this membrane potential nicotine reduced availability of TTX-resistant sodium currents by 25%, which may underlie a failure of regenerative multiple action potential firing in inflamed neurons. Under control conditions, TTX-sensitive sodium currents predominate in small DRG neurons (7). The fact that nicotine application does not alter TTX-sensitive sodium currents in control colonic neurons that fire only a single action potential is consistent with our previous data showing the absence of the effect of 1-μM nicotine application (3 min) on action potential firing in those neurons (1).
Our previous study (1) using α7-competitive antagonist, methyllycaconitine, and α7-knockout mice, suggested that nicotine-induced suppression of hyperexcitability of colonic DRG neurons in inflamed mice is not due to a direct effect of nicotine of sodium channels, develops gradually, and is mediated by α7-nAChRs. It has been reported that activation of α7-nAChRs elevates intracellular Ca2+, that leads to activation of numerous intracellular signaling pathways that regulate protein kinases A and C, CaMKII, phosphatidylinositol 3-kinase, ERK1/2, CREB, MAPK, Akt, phospholipases, Rac, and Rho (6, 9, 29). Phosphorylation and dephosphorylation have been reported to regulate gating of sodium channels including the TTX-resistant subtypes (31). It is possible that the suppressive effect of nicotine on hyperexcitability of colonic DRG neurons in colonic inflammation arises from downstream activation of intracellular pathway(s) that results in modulation of TTX-resistant sodium channel gating and merits further investigation.
In addition to increased TTX-resistant sodium currents, previous studies (5, 23) suggested that colonic inflammation induces suppression of potassium channels in colonic DRG neurons. Although no changes in input resistance were registered upon application of nicotine, it is possible that nicotine-induced suppression of multiple action potential firing in colonic DRG neurons involves an enhancement of potassium currents. Moreover, calcium channels are also known to play a role in action potential firing in nociceptive DRG neurons (7) and were demonstrated in a model of peripheral inflammation to be involved in neuronal hyperexcitability (30). This evidence from literature supports a necessity of evaluation of the effect of nicotine on potassium and calcium channels in colonic DRG neurons during acute inflammation and in the postinflammatory stage.
Nicotine application appears to shift multiple action potential activation to an activation of a single action potential not only in vitro, as we reported recently (1) but also upon in vivo administration. The majority of neurons from nicotine-treated (2 mg/kg sc b.i.d.) inflamed mice fired only a single potential even at the 2× rheobase current amplitude, making their excitability profile similar to that in control mice. In agreement with this finding, the neurons from nicotine-treated inflamed mice were characterized by a comparable rheobase (211 vs. 184 pA; Table 1) and resting membrane potential (−45 vs. −42 mV) to control neurons. It is an important finding of this study that nicotine administration reduced the number of abdominal constrictions induced by intraperitoneal injection of acetic acid in the DSS-treated mice to levels registered in control mice. This suggests that 1) subcutaneous nicotine administration reverses visceral hypersensitivity induced by an acute colonic inflammation, and 2) favors correlation between changes in visceral sensitivity and excitability of colonic sensory neurons.
In summary, in an acute and postinflammatory model of colitis, we demonstrated that nAChRs mediate suppression of hyperexcitability of colonic sensory. The present study also highlights the potential of in vivo treatment with nicotine towards its antinociceptive effects in colonic inflammation. Several receptors and ion channels, such as acid-sensing ion channels, Nav1.8 channels, purinergic P2X, 5-HT3, protease-activated receptor-2, and transient receptor potential vanilloid 1 and 4 receptors [reviewed by Robinson and Gebhart (27)], are currently being investigated for their role in mediating visceral hypersensitivity. Our findings define a potential role for neuronal nAChRs in modulation of visceral sensitization and identify these receptors as a novel target for treatment of abdominal pain induced by colonic inflammation.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-046367 (to H. I. Akbarali).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.R.A., M.I.D., and H.I.A. conception and design of research; G.R.A. and M.K. performed experiments; G.R.A. and M.K. analyzed data; G.R.A. and M.K. interpreted results of experiments; G.R.A. and M.K. prepared figures; G.R.A. drafted manuscript; G.R.A., M.I.D., and H.I.A. edited and revised manuscript; G.R.A., M.K., M.I.D., and H.I.A. approved final version of manuscript.
REFERENCES
- 1. Abdrakhmanova GR, AlSharari S, Kang M, Damaj MI, Akbarali HI. α7-nAChR-mediated suppression of hyperexcitability of colonic dorsal root ganglia neurons in experimental colitis. Am J Physiol Gastrointest Liver Physiol 299: G761–G768, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akbarali HI, EGH, Ross GR, Kang M. Ion channel remodeling in gastrointestinal inflammation. Neurogastroenterol Motil 22: 1045–1055, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bencherif M. Neuronal nicotinic receptors as novel targets for inflammation and neuroprotection: mechanistic considerations and clinical relevance. Acta Pharmacol Sin 30: 702–714, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol 287: G845–G855, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Beyak MJ, Vanner S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurones: the role of voltage-gated ion channels. Neurogastroenterol Motil 17: 175–186, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bitner RS, Nikkel AL, Markosyan S, Otte S, Puttfarcken P, Gopalakrishnan M. Selective alpha7 nicotinic acetylcholine receptor activation regulates glycogen synthase kinase3beta and decreases tau phosphorylation in vivo. Brain Res 1265: 65–74, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci 22: 10277–10290, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249, 1993. [PubMed] [Google Scholar]
- 9. El Kouhen R, Hu M, Anderson DJ, Li J, Gopalakrishnan M. Pharmacology of alpha7 nicotinic acetylcholine receptor mediated extracellular signal-regulated kinase signalling in PC12 cells. Br J Pharmacol 156: 638–648, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G, Caprilli R. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci 44: 1458–1475, 1999. [DOI] [PubMed] [Google Scholar]
- 11. Gschossmann JM, Liebregts T, Adam B, Buenger L, Ruwe M, Gerken G, Holtmann G. Long-term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig Dis Sci 49: 96–101, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Hevers W, Luddens H. Pharmacological heterogeneity of gamma-aminobutyric acid receptors during development suggests distinct classes of rat cerebellar granule cells in situ. Neuropharmacology 42: 34–47, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Phosphorylation of sodium channel Na(v)18 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci 28: 3190–3201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin X, Gereau RW., IV Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 26: 246–255, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin X, Malykhina AP, Lupu F, Akbarali HI. Altered gene expression and increased bursting activity of colonic smooth muscle ATP-sensitive K+ channels in experimental colitis. Am J Physiol Gastrointest Liver Physiol 287: G274–G285, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Kamp EH, Jones RC, Tillman SR, 3rd, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol 284: G434–G444, 2003. [DOI] [PubMed] [Google Scholar]
- 17. Kayssi A, Amadesi S, Bautista F, Bunnett NW, Vanner S. Mechanisms of protease-activated receptor 2-evoked hyperexcitability of nociceptive neurons innervating the mouse colon. J Physiol 580: 977–991, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. King DE, Macleod RJ, Vanner SJ. Trinitrobenzenesulphonic acid colitis alters Na 1.8 channel expression in mouse dorsal root ganglia neurons. Neurogastroenterol Motil 21: 880- e64, 2009. [DOI] [PubMed] [Google Scholar]
- 19. Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol 290: G451–G457, 2006. [DOI] [PubMed] [Google Scholar]
- 20. Larsson MH, Miketa A, Martinez V. Lack of interaction between psychological stress and DSS-induced colitis affecting colonic sensitivity during colorectal distension in mice. Stress 12: 434–444, 2009. [DOI] [PubMed] [Google Scholar]
- 21. Larsson MH, Rapp L, Lindstrom E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterol Motil 18: 144–152, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil 18: 936–948, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am J Physiol Gastrointest Liver Physiol 282: G1045–G1051, 2002. [DOI] [PubMed] [Google Scholar]
- 24. Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain 7: 529–535, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, and Sawe U. Transdermal nicotine for active ulcerative colitis. N Engl J Med 330: 811–815, 1994. [DOI] [PubMed] [Google Scholar]
- 26. Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology 129: 1967–1978, 2005. [DOI] [PubMed] [Google Scholar]
- 27. Robinson DR, Gebhart GF. Inside information: the unique features of visceral sensation. Mol Interv 8: 242–253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil 16: 113–124, 2004. [DOI] [PubMed] [Google Scholar]
- 29. Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol 31: 151–174, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Schaible HG, Nebe J, Neugebauer V, Ebersberger A, Vanegas H. The role of high-threshold calcium channels in spinal neuron hyperexcitability induced by knee inflammation. Prog Brain Res 129: 173–190, 2000. [DOI] [PubMed] [Google Scholar]
- 31. Scheuer T, Catterall WA. Control of neuronal excitability by phosphorylation and dephosphorylation of sodium channels. Biochem Soc Trans 34: 1299–1302, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Snoek SA, Verstege MI, van der Zanden EP, Deeks N, Bulmer DC, Skynner M, Lee K, Te Velde AA, Boeckxstaens GE, de Jonge WJ. Selective alpha7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. Br J Pharmacol 160: 322–333, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology 135: 2075–2083, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Assche G, Vermeire S, Rutgeerts P. Medical treatment of inflammatory bowel diseases. Curr Opin Gastroenterol 21: 443–447, 2005. [PubMed] [Google Scholar]
- 35. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546, 2007. [DOI] [PubMed] [Google Scholar]