Abstract
Afferent input contributes significantly to the pain and colorectal hypersensitivity that characterize irritable bowel syndrome. In the present study, we investigated the contributions of mechanically sensitive and mechanically insensitive afferents (MIAs; or silent afferents) to colorectal hypersensitivity. The visceromotor response to colorectal distension (CRD; 15–60 mmHg) was recorded in mice before and for weeks after intracolonic treatment with zymosan or saline. After CRD tests, the distal colorectum with the pelvic nerve attached was removed for single-fiber electrophysiological recordings. Colorectal afferent endings were located by electrical stimulation and characterized as mechanosensitive or not by blunt probing, mucosal stroking, and circumferential stretch. Intracolonic zymosan produced persistent colorectal hypersensitivity (>24 days) associated with brief colorectal inflammation. Pelvic nerve muscular-mucosal but not muscular mechanosensitive afferents recorded from mice with colorectal hypersensitivity exhibited persistent sensitization. In addition, the proportion of MIAs (relative to control) was significantly reduced from 27% to 13%, whereas the proportion of serosal afferents was significantly increased from 34% to 53%, suggesting that MIAs acquired mechanosensitivity. PGP9.5 immunostaining revealed no significant loss of colorectal nerve fiber density, suggesting that the reduction in MIAs is not due to peripheral fiber loss after intracolonic zymosan. These results indicate that colorectal MIAs and sensitized muscular-mucosal afferents that respond to stretch contribute significantly to the afferent input that sustains hypersensitivity to CRD, suggesting that targeted management of colorectal afferent input could significantly reduce patients' complaints of pain and hypersensitivity.
Keywords: irritable bowel syndrome, pelvic pain, visceral afferent sensitization, colorectal distension, single-fiber recording
intrarectal instillation of local anesthetic rapidly relieves discomfort and pain in patients with irritable bowel syndrome (IBS) (22, 23, 32), revealing that persistent afferent drive plays an important role in IBS. Significantly, referred abdominal (somatic) hypersensitivity is simultaneously relieved, confirming that afferent drive onto spinal dorsal horn neurons receiving convergent visceral and somatic input is necessary for the development of central sensitization. In support, there are increasing numbers of reports establishing that potential mediators of afferent sensitization, such as intestinal mucosal proinflammatory and lipotoxic lipids (14), mast cell products (1, 14, 21), and enteroendocrine cell products (20), are significantly increased in IBS (19). Furthermore, supernatants of colon biopsies and activated mucosal mast cells from patients with IBS directly excite rodent gastrointestinal afferent fibers (2, 10).
We and others have previously characterized the mechanosensitive properties of colorectal afferents in rodents (3, 7, 12, 13, 29), including their ability to sensitize (i.e., increase excitability) after exposure to inflammatory mediators (7, 12, 13). In addition to mechanosensitive afferent endings, so-called silent (or sleeping) nociceptors are present in the visceral innervation. Silent nociceptors, subsequently termed mechanically insensitive afferents (MIAs) because of their nonresponsiveness to mechanical stimulation, are characterized by their ability to acquire mechanosensitivity in pathophysiological conditions, thus contributing significant new peripheral input to the development of hyperalgesia (26, 33).
The estimated proportion of visceral MIAs has been both broad and inaccurate: from 35% to >90% (9, 28). We (8) recently characterized MIAs in the lumbar splanchnic (LSN) and pelvic nerve (PN) innervations of the mouse colorectum using an unbiased electrical search stimulus, documenting that they comprise ∼25% of the colorectal innervation. Importantly, after a brief exposure to inflammatory mediators, a significant proportion (71%) of PN MIAs became sensitized and acquired mechanosensitivity, a finding consistent with a previous report (25) of nonvisceral MIAs. Given the importance of MIAs to the development and maintenance of somatic hyperalgesia (26, 33), we hypothesized that activated colorectal MIAs contribute afferent input that underlies, at least in part, hypersensitivity to colorectal distension. Accordingly, we used a model of persistent colorectal hypersensitivity induced by intracolonic zymosan and characterized mechanosensitive and -insensitive PN afferent endings, positing that MIA proportions would be significantly reduced. Portions of these data have been reported in abstract form (6).
METHODS
All experiments were conducted on male C57BL/6 mice (20–30 g, Taconic, Germantown, NY), and protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Colorectal distension.
Surgical details have been previously reported (4, 29). Briefly, a pair of sterile stainless steel wire electrodes were implanted in the right abdominal musculature, tunneled subcutaneously, and externalized at the nape of the neck for access during testing. On testing days, a lubricated polyethylene balloon (1.5 cm long, ≈0.9 cm) was inserted transanally, and mice were placed in a plastic cylinder to limit movement. Electromyographic activity was recorded for 10 s before (resting) and during 10 s of phasic colorectal distension (15, 30, 45, or 60 mmHg). Each pressure was tested three times with 4 min between distensions. Responses to distension were quantified as the total area of electromyographic activity during balloon inflation minus resting activity.
Colorectal hypersensitivity.
After baseline responses to colorectal distension had been established (day 0), mice were anesthetized (isoflurane) and either vehicle (saline) or zymosan (0.1 ml at 30 mg/ml, Sigma-Aldrich, St. Louis, MO) was administered transanally via a 22-gauge feeding needle. Intracolonic treatment with saline or zymosan was performed daily for 3 consecutive days (days 0, 1, and 2), and colorectal hypersensitivity was assessed on days 3, 6, 10, 17, and 24.
Immunohistochemistry.
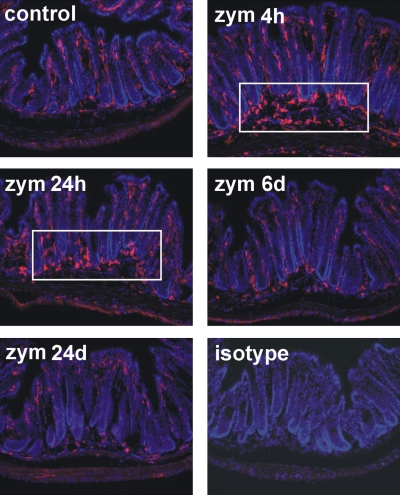
The distal colon was harvested at varying times after intracolonic treatment and fixed with 4% paraformaldehyde in 0.16 M phosphate buffer containing 14% picric acid (Sigma-Aldrich). After cryoprotection in 20% sucrose, fixed tissue was embedded in OCT compound (Sakura Finetek, Tokyo, Japan), frozen, and sectioned at 20 μm. Some tissue sections were incubated with rabbit anti-protein gene product (PGP)9.5 antibody (1:2,000, UltraClone, Yarmouth, UK) and then with Cy3-conjugated anti-rabbit IgG (1:200, Jackson Immunoresearch, West Grove, PA) for immunostaining of neuronal structures. Monocytes and macrophages were stained in other tissue sections using rat anti-F4/80 (1:1,000, Abcam, Cambridge, MA) and Cy3-conjugated anti-rat IgG (1:200), the specificity of which was verified by the absence of immunostaining with the isotype control antibody (rat κ monoclonal IgG2b, 1:1,000, Abcam; see Fig. 2).
Fig. 2.
F4/80-immunoreactive macrophages (amorphous cells in red) were mainly detected in the lamina propria of the mucosa in control tissue. Tissue sections were counterstained with 4′,6-diamidino-2-phenylindole (nuclei in blue). Four and twenty-four hours after the third daily intracolonic treatment with Zym, an accumulation of macrophages at the bottom of the crypts and in the submucosa in the distal colorectum was evident (boxed area), which resolved by day 6. The isotype control antibody, rat κ monoclonal IgG2b (1:1,000, Abcam, Cambridge, MA), showed no staining, indicating that the immunostaining with F4/80 antibody was not due to nonspecific binding of immunoglobulins to Fc receptors expressed on immune cells.
PGP9.5 immunostaining was quantified by setting a brightness threshold to the 8-bit grayscale images using ImageJ and calculating the area (proportion) of PGP9.5 immunoactivity relative to the colorectal mucosal and submucosal area. Thresholds were determined from the Gaussian distribution of background gray to achieve <0.001% possibility of erroneous inclusion of background into PGP9.5-immunopositive areas. To reduce variation between tissue slides, saline- (control) and zymosan-treated colorectums were placed on the same slides. Twenty images were randomly taken from each group and processed blind of treatment.
In vitro afferent fiber recording.
Details of the in vitrol afferent fiber recording have been previously reported (7, 8). Briefly, mice were euthanized via CO2 inhalation and exsanguination after the right atrium was perforated. The distal colorectum (∼3 cm) with the PN attached was transferred to oxygenated Krebs solution, to which was added nifedipine (4 μM) and indomethacin (3 μM). The colorectum was opened longitudinally along the antimesenteric border and pinned flat mucosal side up in a Krebs-filled tissue chamber. In the adjacent oil-filled recording chamber, the PN was teased into fine bundles (∼10 μm thick) for single-fiber electrophysiological recordings.
Colorectal afferent fibers were excited by suprathreshold electrical current stimulation (0.5-ms duration, 0.3Hz) using a round-tipped concentric electrode (external diameter: 0.55 mm and internal diameter: 0.125 mm, FHC, Bowdoin, ME) perpendicular to the mucosal surface (8). The electrode was moved systematically (∼1.5-mm steps) to examine the entire colorectal surface. Receptive endings were localized as the site requiring minimum stimulus intensity (i.e., stimulus threshold), after which they were tested for mechanosensitivity and classified as serosal, muscular, mucosal, muscular/mucosal, or MIA, as previously described (8), by probing with von Frey-like nylon monofilaments (0.4-, 1-, and 1.4-g force), mucosal stroking with nylon filaments (10-mg force), and circumferential stretch (0–170 mN, 35 s) using a servo-controlled force actuator (Aurora Scientific, Aurora, ON, Canada).
Data recording and analysis.
Afferent fiber activity was filtered (0.3–10 kHz), amplified (DAM 80, World Precision Instruments, New Haven, CT), digitized at 20 kHz (1,401 interface, CED, Cambridge, UK), and stored on a personal computer. Action potentials were discriminated based on principal component analysis using Spike2 software (CED). Serosal endings were tested by punctate probing with von Frey-like monofilaments at three forces (0.4, 1, and 1.4 g, 3-s duration); probing was conducted twice at each force, and responses were averaged as the number of action potentials/3 s. Stretch-sensitive muscular and muscular-mucosal afferents were tested with ramped circumferential stretch (0–170mN at 5 mN/s), corresponding to intraluminal pressures of 0–45 mmHg(7). Action potentials were evenly binned into three bins and plotted as responses to stretch. Spontaneous (baseline) activity was infrequent but when present was subtracted from stimulus-evoked responses. Data are presented as means ± SE. One- or two-way ANOVAs and Dunn's or Holm-Sidak post hoc pairwise multiple comparisons were performed with repeated measures as appropriate (SigmaPlot version 9.0, Systat Software, San Jose, CA). Differences in means and proportions were assessed by t-tests and χ2-tests or Fisher's exact tests, respectively. P values of ≤0.05 were considered significant.
RESULTS
Colorectal hypersensitivity and inflammation.
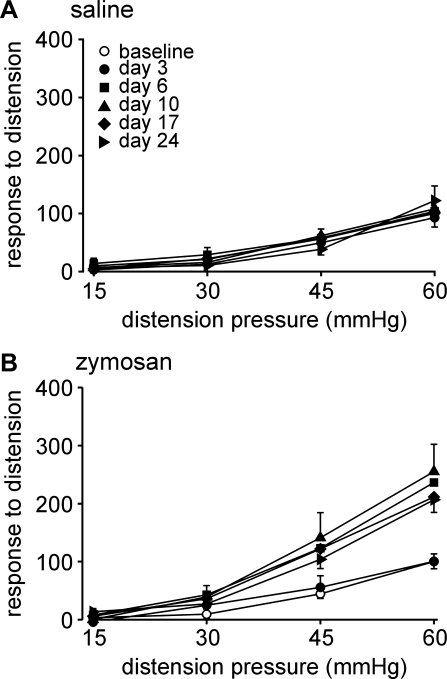
Visceromotor responses to colorectal distension, normalized to the percentage of the baseline response at 60 mmHg (Fig. 1), were recorded before [baseline (day 0)] and after (days 3, 6, 10, 17, and 24) intracolonic administration of saline or zymosan. Intracolonic saline treatment did not affect visceromotor responses to distension at any time of testing (F5,50 = 0.32; Fig. 1A), whereas zymosan induced significant colorectal hypersensitivity after treatment (F5,40 = 4.37, P = 0.003; Fig. 1B).
Fig. 1.
Visceromotor responses (VMRs) to colorectal distension (CRD). VMRs were normalized to the baseline (day 0) response at 60 mmHg. A and B: responses to CRD (15–60 mmHg) recorded over time from 11 saline (control)-treated mice (A) and 11 zymosan (Zym)-treated mice (B). VMRs were unchanged after saline treatment (F5,50 = 0.32) but significantly increased at all times except day 3 after Zym treatment (F5,40 = 4.37, P = 0.003).
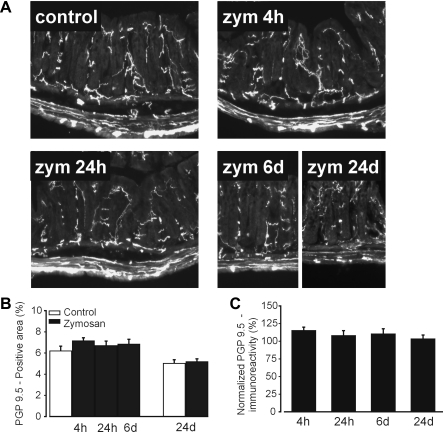
Previous reports (12, 29) have shown that intracolonic zymosan does not increase myeloperoxidase activity at any time after intracolonic treatment, and so we assessed whether zymosan produced macrophage-based inflammation. Figure 2 shows the accumulation of F4/80-positive macrophages at the bottom of crypts and in the submucosa 4 and 24 h after the third daily zymosan treatment, which resolved by day 6. To assess if there is a loss in the density of innervation after zymosan, colorectal tissue was immunostained with anti-PGP9.5, a pan-neuronal marker, which showed no difference between saline- and zymosan-treated colons at any time after treatment (Fig. 3).
Fig. 3.
Protein gene product (PGP)9.5 immunostaining of the distal colorectum. A–C: PGP9.5-immunoreactive nerve fibers in A were quantified as areas (proportions) with respect to the colorectal mucosal and submucosal area (B), which were normalized by control mean PGP9.5 proportions (C). Intracolonic treatment with Zym did not affect the density of PGP9.5 immunoreactivity.
Colorectal afferent classes.
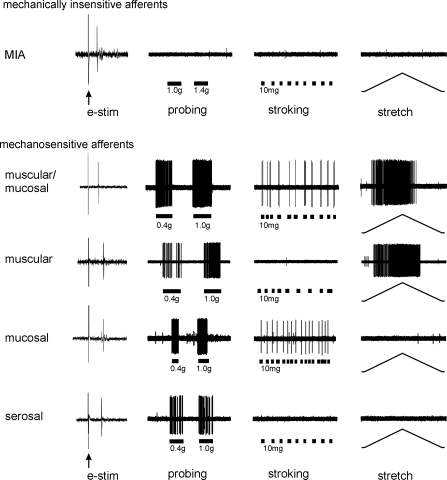
PN afferent endings identified by electrical stimulation were classed into four mechanosensitive classes and one mechanically insensitive class (8). Briefly, all mechanosensitive endings respond to blunt probing of the mucosal surface (Fig. 4) (3). In addition, muscular/mucosal endings respond to stroking of the mucosa and to ramped circumferential stretch; muscular endings respond to stretch (but not stroking) and mucosal endings respond to stroking (but not stretch). Serosal endings respond only to probing and not to either stroking or stretch. MIAs do not respond to any mechanical stimulus.
Fig. 4.
Classes of pelvic nerve afferent colorectal endings. Afferent endings were identified by electrical stimulation (e-stim; arrows indicate stimulus artifacts) and classified based on responses to mechanical stimuli. Mechanically insensitive afferents (MIAs) do not respond to mechanical stimuli. All four classes of mechanosensitive endings responded to probing (0.4–1.4 g). Muscular/mucosal (mus/mucos) endings also responded to mucosal stroking (10 mg) and circumferential stretch (0–170 mN). Muscular (mus) endings also responded to stretch, and mucosal (mucos) endings also responded to stroking. Serosal endings did not respond to either stroking or stretch.
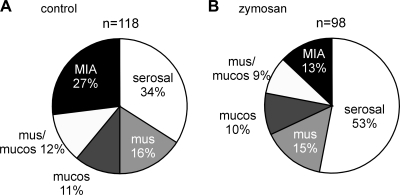
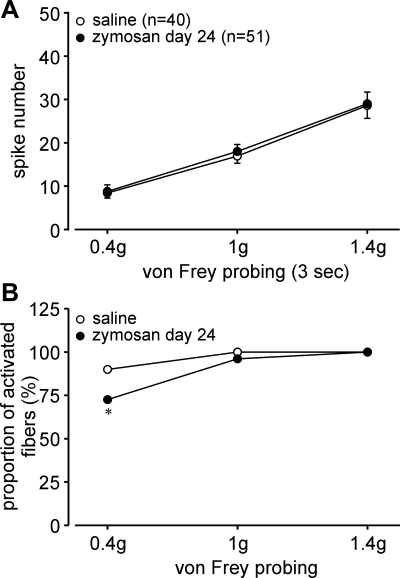
After the last colorectal distension test (day 24), zymosan-treated hypersensitive mice and saline-treated normosensitive mice were euthanized between days 25 and 28, the colorectums were removed, and PN receptive endings were studied in vitro. The proportions of afferent classes in saline-treated mice (Fig. 5A) were not different from our previous study (8) of naïve mice of the same strain. In contrast, the proportion of MIAs was significantly decreased to one-half and the proportion of serosal endings significantly increased in zymosan-treated mice relative to their saline-treated counterparts (both P < 0.05 by χ2-tests; Fig. 5B). The proportions of mechanosensitive muscular, muscular/mucosal, and mucosal afferents did not differ between saline- and zymosan-treated mice. Serosal endings were tested by punctate probing with von Frey-like monofilaments at three forces (0.4, 1, and 1.4 g, 3-s duration); probing was conducted twice at each force, and responses were averaged as the number of action potentials/3 s. The response magnitude of serosal afferents to probing after zymosan treatment (F1,267 = 0.144; Fig. 6A) did not differ from their saline-treated counterparts. However, the proportions of serosal afferents activated by probing significantly differed; probing with 0.4-g force activated a significantly smaller proportion of serosal afferents in zymosan-treated mice (73%) relative to saline-treated (90%) controls (P = 0.05 by Fisher's exact test, day 24; Fig. 6B). There were no differences between groups in the proportions of responders to von Frey-like probing at 1- and 1.4-g forces, which activated most, if not all, serosal afferents.
Fig. 5.
Proportions of colorectal pelvic nerve afferents after the intracolonic instillation of saline or Zym. A and B: proportions of afferent recorded from mice 24 days after saline (A) or Zym (B) treatment and tested for colorectal hypersensitivity (data shown in Fig. 1, A and B, respectively).
Fig. 6.
Responses of serosal afferents to graded probing (0.4, 1, and 1.4 g) in mice treated with saline or Zym. A and B: Zym did not affect the response magnitude (A) but did produce reduced proportions of fibers activated by 0.4-g probing (B). *P < 0.05.
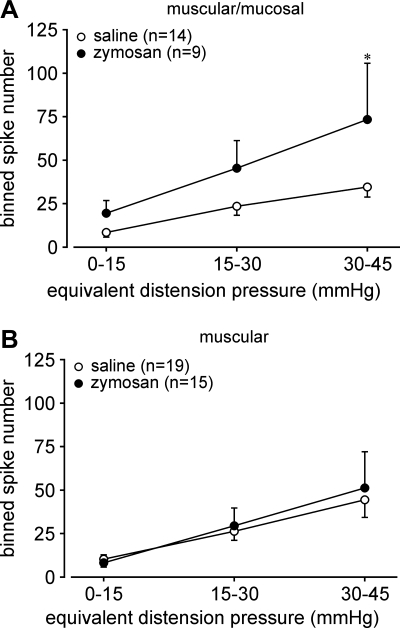
Muscular/mucosal afferents exhibited significantly greater responses to stretch (i.e., were sensitized) after zymosan treatment relative to saline-treated controls (F1,63 = 5.415, P < 0.03; Fig. 7A); responses of muscular afferents did not differ between the two treatment groups (F1,96 = 0.107; Fig. 7B). The threshold for response to stretch was defined as the force (in mN) at which the first action potential was produced (stretch-sensitive afferents were not spontaneously active) and did not differ between mice treated with saline or zymosan (muscular afferents: 43.6 ± 6.4 vs. 58.1 ± 9.3, P = 0.17 by t-test; muscular-mucosal afferents: 37.0 ± 7.0 vs. 34.0 ± 6.6, P = 0.78 by t-test). Responses of muscular-mucosal afferents to mucosal stroking (number of action potentials/10 consecutive strokes) were not different (P > 0.4) between saline- and zymosan-treated groups (14 ± 1.1 vs. 12.8 ± 1.3). Responses of muscular and muscular-mucosal afferents to probing were quantified in the same fashion as serosal afferents, revealing no significant differences between control and zymosan treatment groups (data not shown). Consistent with a previous report (3), the proportion of colorectal afferents with spontaneous (baseline) activity was small (<13%); there was no significant difference in either proportions (12.7% vs. 12.2%) or firing rate (0.52 ± 0.08 vs. 0.59 ± 0.18 Hz) between saline- and zymosan-treated groups. The vast majority (>93%) of afferents with spontaneous activity were either serosal or MIAs; very few stretch-sensitive afferents (1/57) and mucosal afferents (1/23) were spontaneously active. The proportions of spontaneously active serosal afferents (20% vs. 16%) and MIAs (22% vs. 15%) did not significantly differ between saline- and zymosan-treated groups (P > 0.6 by Fisher's exact test).
Fig. 7.
Effect of Zym on stretch-sensitive afferents. Stretch-sensitive muscular and muscular-mucosal afferents were tested with ramped circumferential stretch (0–170 mN at 5 mN/s), corresponding to intraluminal pressures of 0–45 mmHg (7). Action potentials were evenly binned into three bins and plotted as responses to stretch. A and B: responses of muscular/mucosal afferents to stretch were significantly increased after Zym treatment (A; F1,63 = 5.415, P < 0.03), whereas responses of muscular afferents to stretch were unaffected (B; F1,96 = 0.107). *P < 0.05.
DISCUSSION
These results support the importance and necessity of afferent input in persistent colorectal hypersensitivity. Recent reports (22, 23, 32) have documented that afferent drive contributes significantly to IBS patients' characteristic complaints of discomfort, pain, and hypersensitivity. We thus hypothesized that long-term sensitization of mechanosensitive afferents as well as PN MIAs contribute to prolonged colorectal hypersensitivity. Accordingly, we investigated the role of both mechanosensitive and -insensitive afferents in a model of persistent colorectal hypersensitivity induced by zymosan. The principal findings were that intracolonic zymosan produces long-lasting colorectal hypersensitivity, which 1) persisted throughout the time course of the experiments (24 days), 2) outlasted the initial brief monocyte-based inflammation (∼24 h); 3) was associated with sustained sensitization of muscular-mucosal afferents that encode mechanical colorectal distension as well as luminal stroking; and 4) was associated with a significant reduction in the proportion of PN MIAs with a complementary increase in the proportion of serosal afferents, suggesting that MIAs acquired a mechanosensitive phenotype (e.g., become “serosal”). It is also clear that colorectal tissue damage per se is not a necessary condition for either the development of colorectal hypersensitivity or sensitization of stretch-responsive afferents or MIAs.
Short-term sensitization of visceral afferents has been demonstrated both in vitro and in vivo by exposure to a variety of endogenous and exogenous molecules (27). Long-term sensitization of colorectal afferents has been proposed as a mechanism for sustained visceral hypersensitivity but has seldom been documented in the literature. In models in which long-term colorectal hypersensitivity develops after neonatal colon insult (e.g., Refs. 17 and 34), afferent sensitization in either whole nerve or sensory neuron recordings has been reported. Similarly, in a model of infectious colitis (11), sensitization of afferents and hyperexcitability of colonic dorsal root ganglion neurons were reported, but behavioral hypersensitivity was not assessed. The present study documents long-term sensitization (24 days) in a subgroup of stretch-responsive afferents in correlation with persistent colorectal hypersensitivity. Muscular-mucosal afferents exhibited significantly greater responses to stretch in colorectums taken from hypersensitive mice 24 days after zymosan (but not saline) treatment; responses from muscular afferents were unchanged. This is consistent with a previous in vitro study (12) in which muscular-mucosal but not muscular afferents were sensitized after the brief exposure of sensory endings to zymosan. Taken together, the present and previous results support contributions of sensitized muscular-mucosal mechanosensitive endings to both the initiation (short term) and maintenance (long term) of colorectal hypersensitivity.
Visceral MIAs have been previously suggested to be important in pathophysiological conditions and thus contributory to increased afferent input (9). We (8) recently documented that MIAs are abundant in the innervation of the mouse colorectum and, further, that MIAs acquire mechanosensitivity after a brief exposure to inflammatory mediators in vitro. The present study confirms that ∼25% of the PN innervation of the mouse colorectum is normally mechanically insensitive. However, it was unclear from previous work whether MIAs could contribute to the persistent colorectal hypersensitivity characteristic of IBS. In the present study, we observed a significant reduction in the proportion of MIAs in colorectums taken from zymosan-treated mice compared with saline-treated mice. This reduction in the proportion of MIAs is not due to bias in afferent fiber selection, as we systematically located and studied all excitable receptive endings in an unbiased manner using an electrical search strategy that bypasses transduction processes to excite afferent endings at their spike initiation zone (8). Examination of fiber density in the colorectum by PGP9.5 immunostaining revealed no fiber loss after zymosan treatment, suggesting that a reduction in the proportion of MIAs cannot be attributed to a selective loss of those receptive endings. In support, the proportion of functionally characterized serosal endings increased significantly to ≥50% of all PN endings in the colorectum, a proportion exceeding what has previously been reported, which is typically 35–40% (3, 8). Accordingly, we interpret the increase in the proportion of serosal endings after zymosan treatment to represent the conversion of mechanically insensitive into mechanically sensitive afferent endings. This is supported by the unchanged proportions of afferent classes other than MIAs and serosal afferents.
While the foregoing interpretation is further strengthened by the increased proportion of serosal endings that did not respond to low-intensity 0.4-g probing, evidence to date is indirect inasmuch as it is not possible at present to preidentify or select MIAs before a treatment and study them weeks later. Although apparently sensitized (i.e., some MIAs acquired a serosal-like phenotype and responded to high-intensity punctuate probing), no evidence that MIAs acquire sensitivity to stretch was found either in the present study or by a previous study (8). Thus, the functional role of sensitized MIAs in the context of prolonged visceral hypersensitivity to colorectal distension requires further investigation.
The distal colorectum is also innervated by afferents from the lumbar splanchnic/hypogastric (LSN) pathway (8), the sensitization of which could also contribute to colorectal pain and hypersensitivity. However, only a small proportion (6–10%) of LSN relative to PN (33–40%) (3, 8) afferents are stretch sensitive, suggesting that noxious colorectal distension is encoded principally by the PN pathway. In support, a recent study (16) demonstrated that responses to noxious colorectal distension are unaffected after LSN transection but absent after PN transection. Furthermore, a previous in vitro study (8) found that MIAs in the LSN pathway are less likely to be sensitized by inflammatory mediators than MIAs in the PN pathway (23% in LSN vs. 71% in PN). Although the present study focused on colorectal afferents in the PN pathway, we cannot rule out a contributing role of LSN afferents to visceral hypersensitivity, as a previous study (31) has suggested that the LSN pathway may encode noxious stimuli after colon inflammation.
Although functional changes in both mechanically sensitive and insensitive PN afferents weeks after colorectal insult were readily apparent, the underlying mechanism(s) for these persistent changes in colorectal afferents is unknown. Increasing evidence has implicated an involvement of the immune system in the initiation and perpetuation of peripheral nociceptor sensitization (18, 24). Zymosan reportedly activates antigen-presenting cells via Toll-like receptors (TLRs; TLR2 and TLR6) (30) and non-TLRs [e.g., dectin-1 (15)], which produce, in addition to proinflammatory cytokines [e.g., TNF-α (35)], anti-inflammatory cytokines (IL-10 and transforming growth factor-β) to induce immunological tolerance (5). Because changes in afferent sensitivity reported here and elsewhere were evident at significant times after recovery of acute colorectal insult, it is likely that the long-term sensitization of MIAs and stretch-sensitive afferents is related to interactions with the intestinal immune system and requires further investigation.
In summary, we systematically investigated the proportions and encoding characteristics of PN afferent endings in the mouse colorectum in a model of persistent colorectal hypersensitivity. Intracolonic zymosan treatment resulted in the sensitization of mechanosensitive muscular-mucosal afferents and a significant reduction in the proportions of colorectal MIAs with complementary increases in the proportions of serosal endings. Significantly, the sensitization of muscular-mucosal afferents and changes in MIAs persisted for weeks after the initial insult, suggesting long-lasting elevated input from stretch-responsive mechanoreceptors to encode colon distension as well as a persistent phenotypic switch of MIAs to acquire mechanosensitivity in situations involving colorectal hypersensitivity in the absence of apparent tissue damage or inflammation. The increased response magnitude clearly implicates mechanosensitive muscular-mucosal PN afferents in the development and maintenance of colorectal hypersensitivity. A significant proportion of mechanically insensitive PN afferents acquired mechanosensitivity but not sensitivity to the most physiological stimulus (stretch), and so their contribution to colorectal hypersensitivity remains an open question requiring additional study.
GRANTS
This work was supported by National Institutes of Health Grants R01-NS-019912, R01-DK-093525, and UL1-RR-024153.
DISCLAIMER
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources (NCRR) or National Institutes of Health. Information on the NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.F., J.-H.L., and G.F.G. conception and design of research; B.F., J.-H.L., E.S.S., T.T., and T.P.M. performed experiments; B.F., J.-H.L., and G.F.G. analyzed data; B.F., J.-H.L., T.P.M., and G.F.G. interpreted results of experiments; B.F., J.-H.L., and T.T. prepared figures; B.F. drafted manuscript; B.F., J.-H.L., E.S.S., and G.F.G. edited and revised manuscript; B.F., J.-H.L., and G.F.G. approved final version of manuscript.
ACKNOWLEDGMENTS
T. Tanaka was a visiting scholar from Takeda Pharmaceutical Company, Limited (Osaka, Japan).
The authors thank Michael Burcham for preparation of the figures.
REFERENCES
- 1.Barbara G, Stanghellini V, de GR, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Barbara G, Wang B, Stanghellini V, de GR, Cremon C, Di NG, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132: 26–37, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc 2: 2624–2631, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van DT, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest 116: 916–928, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng B, La JH, Tanaka T, Gebhart GF. Silent Afferents and Colorectal Hypersensitivity. Program No. 682.19/VV3.2010. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2010 [Google Scholar]
- 7.Feng B, Brumovsky PR, Gebhart GF. Differential roles of stretch-sensitive pelvic nerve afferents innervating mouse distal colon and rectum. Am J Physiol Gastrointest Liver Physiol 298: G402–G409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300: G170–G180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes PA, Brierley SM, Martin CM, Liebregts T, Persson J, Adam B, Holtmann G, Blackshaw LA. TRPV1-expressing sensory fibres and IBS: links with immune function. Gut 58: 465–466, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Ibeakanma C, Miranda-Morales M, Richards M, Bautista-Cruz F, Martin N, Hurlbut D, Vanner S. Citrobacter rodentium colitis evokes post-infectious hyperexcitability of mouse nociceptive colonic dorsal root ganglion neurons. J Physiol 587: 3505–3521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RC, III, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 133: 184–194, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajander K, Myllyluoma E, Kyronpalo S, Rasmussen M, Sipponen P, Mattila I, Seppanen-Laakso T, Vapaatalo H, Oresic M, Korpela R. Elevated pro-inflammatory and lipotoxic mucosal lipids characterise irritable bowel syndrome. World J Gastroenterol 15: 6068–6074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly EK, Wang L, Ivashkiv LB. Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J Immunol 184: 5545–5552, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyloh M, Nicholas S, Zagorodnyuk VP, Brookes SJ, Spencer NJ. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Front Neurosci 5: 16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res 971: 73–82, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 417–449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 7: 163–173, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Rhee PL, Kim G, Lee JH, Kim YH, Kim JJ, Rhee JC, Song SY. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil 18: 539–546, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Park JH, Rhee PL, Kim HS, Lee JH, Kim YH, Kim JJ, Rhee JC. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol 21: 71–78, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 47: 995–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain 7: 529–535, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 16: 1267–1276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol 54: 1109–1122, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Schmelz M, Schmid R, Handwerker HO, Torebjork HE. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 123: 560–571, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol 31–74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 71: 2046–2060, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X3 receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology 137: 2096–2104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol 1: 625–635, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Traub RJ. Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain. Neuroreport 11: 2113–2116, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci 19: 10184–10190, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu GY, Winston JH, Chen JD. Electroacupuncture attenuates visceral hyperalgesia and inhibits the enhanced excitability of colon specific sensory neurons in a rat model of irritable bowel syndrome. Neurogastroenterol Motil 21: 1302–e125, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Young SH, Ye J, Frazer DG, Shi X, Castranova V. Molecular mechanism of tumor necrosis factor-alpha production in 1→3-β-glucan (zymosan)-activated macrophages. J Biol Chem 276: 20781–20787, 2001 [DOI] [PubMed] [Google Scholar]