Abstract
Onset of the mitochondrial permeability transition (MPT) is the penultimate event leading to lethal cellular ischemia-reperfusion injury, but the mechanisms precipitating the MPT after reperfusion remain unclear. Here, we investigated the role of mitochondrial free Ca2+ and reactive oxygen species (ROS) in pH- and MPT-dependent reperfusion injury to hepatocytes. Cultured rat hepatocytes were incubated in anoxic Krebs-Ringer-HEPES buffer at pH 6.2 for 4 h and then reoxygenated at pH 7.4 to simulate ischemia-reperfusion. Some cells were loaded with the Ca2+ chelators, BAPTA/AM and 2-[(2-bis-[carboxymethyl]aono-5-methoxyphenyl)-methyl-6-methoxy-8-bis[carboxymethyl]aminoquinoline, either by a cold loading protocol for intramitochondrial loading or by warm incubation for cytosolic loading. Cell death was assessed by propidium iodide fluorometry and immunoblotting. Mitochondrial Ca2+, inner membrane permeability, membrane potential, and ROS formation were monitored with Rhod-2, calcein, tetramethylrhodamine methylester, and dihydrodichlorofluorescein, respectively. Necrotic cell death increased after reoxygenation. Necrosis was blocked by 1 μM cyclosporin A, an MPT inhibitor, and by reoxygenation at pH 6.2. Confocal imaging of Rhod-2, calcein, and dichlorofluorescein revealed that an increase of mitochondrial Ca2+ and ROS preceded onset of the MPT after reoxygenation. Intramitochondrial Ca2+ chelation, but not cytosolic Ca2+ chelation, prevented ROS formation and subsequent necrotic and apoptotic cell death. Reoxygenation with the antioxidants, desferal or diphenylphenylenediamine, also suppressed MPT-mediated cell death. However, inhibition of cytosolic ROS by apocynin or diphenyleneiodonium chloride failed to prevent reoxygenation-induced cell death. In conclusion, Ca2+-dependent mitochondrial ROS formation is the molecular signal culminating in onset of the MPT after reoxygenation of anoxic hepatocytes, leading to cell death.
Keywords: ischemia, reperfusion, cell death
cell and tissue injury after ischemia-reperfusion occurs to the liver during surgery, organ preservation, cardiac failure, and hemorrhagic shock. Cardinal events during ischemia include anoxia, substrate depletion, decreased ATP, and acidosis. Tissue acidosis in ischemia typically occurs as a consequence of hydrolysis of high energy phosphates, accumulation of lactic acid from anaerobic metabolism, and release of protons sequestered in acidic organelles. Although this acidosis is frequently considered detrimental, numerous studies demonstrate that naturally occurring acidosis protects liver and other tissues against ischemic necrosis (6, 12, 46, 47).
Cell death in anoxic tissue occurs after onset of reperfusion when tissue is reoxygenated and recovers normal pH. Numerous mechanisms have been proposed to explain reperfusion-induced cell death, including formation of ROS, Ca2+ overloading, and disruption of mitochondrial energy production (26, 28, 33). Despite multifactorial nature of anoxia/reoxygenation (A/R) injury mechanisms, restoration of pH after anoxia represents a major and independent factor contributing to cell death, a phenomenon termed the pH paradox (23), since reoxygenation at acidic pH prevents cell death and restoration of pH to 7.4 in the absence of reoxygenation causes nearly the same cell death as restoration of pH with reoxygenation (36). A key event contributing to this pH-dependent cell death after reoxygenation is onset of the mitochondrial permeability transition (MPT) (19, 36, 39).
Opening of high conductance permeability transition (PT) pores in the mitochondrial inner membrane causes the MPT. MPT onset conducts solutes with a molecular mass of up to 1,500 Da and causes mitochondrial depolarization and uncoupling of oxidative phosphorylation, which in turn leads to ATP depletion and subsequent necrotic cell death (20). Moreover, onset of the MPT is directly linked to apoptotic cell death, since the MPT causes mitochondrial swelling, rupture of the mitochondrial outer membrane, and release of cytochrome c and other proapoptotic molecules from the intermembrane space to the cytosol (13, 19, 25, 40, 45). The balance between glycolytic ATP generation and ATP loss after the MPT determines whether hepatocytes will develop necrotic or apoptotic cell death after A/R (19).
Multiple mitochondrial factors promote onset of the MPT, including pyridine nucleotide oxidation, alkalinization, and increased Ca2+, phosphate, and ROS. Previously, mitochondrial Ca2+ loading and subsequent generation of mitochondrial ROS were shown to be early events leading to the MPT and necrotic cell death when rat hepatocytes were exposed to oxidative stress induced by t-butylhydroperoxide (8). However, why the MPT occurs after A/R in hepatocytes is unknown. Accordingly, the aim of the present study was to characterize signaling events causing the MPT in reoxygenated hepatocytes. Our results indicate that increased levels of mitochondrial Ca2+ after reoxygenation stimulate mitochondrial ROS formation, which in turn triggers onset of the MPT and subsequent necrotic and apoptotic cell death.
MATERIALS AND METHODS
Hepatocyte isolation and culture.
Animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences, and protocols were approved by the Institutional Animal Care and Use Committee. Hepatocytes were isolated from overnight-fasted male Sprague-Dawley rats (200–300 g) by collagenase perfusion, as described previously (19), and resuspended in Waymouth's medium MB-752/1 containing 100 units/ml penicillin, 100 μg/ml streptomycin, 10% fetal calf serum, 100 nM insulin, and 100 nM dexamethasone. Cell viability after isolation, as determined by trypan blue exclusion, was routinely greater than 90%. For necrotic cell death and ROS measurements, aliquots (1 ml) of 1.5 × 105 cells were plated onto 24-well microtiter plates (Falcon, Lincoln Park, NJ). For immunoblotting analysis of apoptotic cell death, hepatocytes were plated on 35-mm culture dishes at a concentration of 106 cells. For confocal microscopic studies, 4.5 × 105 cells were cultured on 42-mm round glass coverslips in 60-mm culture dishes. All plates, dishes, and coverslips were coated with 0.1% Type 1 rat tail collagen. Hepatocytes were used after overnight culture in humidified 5% CO2 and 95% air at 37°C. Experiments were performed in Krebs-Ringer-HEPES buffer (KRH) containing (in mM) 15 NaCl, 5 KCl, 2 CaCl2, 1 KH2PO4, 1.2 MgSO4, and 25 HEPES (pH 6.2 or 7.4). To avoid confounding effects of insulin, dexamethasone, and serum from Waymouth's medium, and to better control pH, anoxic cells were reoxygenated in aerobic KRH. Under this condition, hepatocytes predominantly undergo necrotic cell death.
A/R in cultured rat hepatocytes.
Anoxia in the anaerobic chamber (Coy Laboratory Products, Ann Arbor, MI) was maintained under an atmosphere of 90% N2-10% H2 in the presence of a heated palladium catalyst to convert residual oxygen to water vapor. The anoxic buffer was prepared by equilibrating the KRH buffer (pH 6.2) overnight inside the anaerobic chamber before the experiments. Oxygen tension in the chamber and anoxic buffer was monitored by a gas analyzer (Model 10; Coy Laboratory Products) and was less than 0.001 Torr. To simulate the anoxia, substrate depletion, and tissue acidosis of ischemia, hepatocytes were incubated in KRH at pH 6.2 in the anaerobic chamber for 4 h. To simulate reperfusion and return to physiological pH, anaerobic KRH at pH 6.2 was replaced with aerobic KRH at pH 7.4. This model is widely used to study mechanisms of necrotic A/R injury in cells cultured from liver and other tissues (6, 19, 36). Some hepatocytes were treated with antioxidants, 0.6 mM desferal, 10 μM diphenylphenylenediamine (DPPD), 300 μM of apocynin, or 10 μM of diphenyleneiodonium chloride (DPI), beginning 20 min before and continuously after reoxygenation.
Hepatocellular loading of Rhod-2, calcein, tetramethylrhodamine methylester, and dichlorofluorescein.
To investigate the role of Ca2+ in A/R injury, hepatocytes cultured on 24-well plates were anaerobically incubated with Ca2+ chelators, 10 μM BAPTA/AM or 10 μM 2-[(2-bis-[carboxymethyl]aono-5-methoxyphenyl)-methyl-6-methoxy-8-bis[carboxymethyl]aminoquinoline (Quin 2)/AM, at 0 to 1°C (cold loading) for intramitochondrial loading or 37°C (warm loading) for cytosolic loading. After 20 min loading, the cells were rinsed with fresh anoxic KRH and further exposed to anoxia at 37°C for 220 min (total anoxia of 4 h). The cold loading protocol favors intramitochondrial localization of Ca2+ chelators, whereas warm loading leads to cytosolic localization without mitochondrial uptake (8, 42). To image temporal changes of mitochondrial Ca2+ and onset of the MPT, hepatocytes cultured on glass coverslips were coloaded with Rhod-2 and calcein. For intramitochondrial labeling of Ca2+ indicator, hepatocytes were anaerobically incubated with 10 μM Rhod-2/AM by cold loading. For cytosolic loading of calcein and Rhod-2, hepatocytes were incubated with 1 μM calcein/AM and 10 μM Rhod-2/AM by warm loading. To image mitochondrial membrane potential (ΔΨm) and ROS formation, hepatocytes on glass coverslips were coloaded with 100 nM of tetramethylrhodamine methylester (TMRM) and 10 μM dihydrodichlorofluorescein (H2DCF) diacetate in anoxic KRH at 30 min before reoxygenation, respectively, as described previously (21). Some hepatocytes were also cultured on 24-well plates and incubated in anoxic KRH with H2DCF diacetate to evaluate ROS formation using a fluorescence plate reader. H2DCF diacetate diffuses into the cells where endogenous esterases hydrolyze the acetate ester, trapping free dichlorofluorescein (DCF) inside the cells. ROS-dependent organic hydroperoxides convert nonfluorescent H2DCF to highly fluorescent DCF (9, 30). To monitor ROS generation and ΔΨm under the normoxic condition, hepatocytes were incubated for 3.5 h in aerobic KRH and then for 30 min with 10 μM H2DCF diacetate and 100 nM of TMRM.
Necrotic cell death.
Necrosis was assessed by propidium iodide (PI) fluorometry using a fluorescence plate reader (BMG Lab Technologies, Offenburg, Germany/Molecular Devices, Sunnyvale, CA), as described previously (19, 31). Briefly, hepatocytes were incubated in KRH containing 30 μM PI. Fluorescence from each well was measured at excitation and emission wavelengths of 530 nm and 590 nm, respectively. For each well, fluorescence at 20 min after addition of PI (A) was measured before anoxia and then at given times thereafter (X). Experiments were terminated by permeabilizing the plasma membrane with 375 μM digitonin. After 20 min, a final fluorescence (B) was measured. The percentage of nonviable cells (D) was calculated as D = 100 (X − A)/(B − A). Cell death assessed by PI fluorometry reflects necrotic death and correlates closely with trypan blue uptake and enzyme release (19, 21).
Apoptotic cell death.
To investigate apoptosis after A/R, hepatocytes after 4 h of anoxia were reoxygenated in hormonally defined medium (HDM) containing 20 mM fructose, 5 mM glycine, 100 nM insulin, 1 μg/ml transferrin, 0.3 nM selenium, and 1.5 μM free fatty acids in RPMI 1640 medium at pH 7.4 (22). Both fructose and glycine were added to anoxic hepatocytes 20 min before reoxygenation to supply a glycolytic substrate and to stabilize the plasma membrane, respectively (19, 22, 35). After 22 h of reoxygenation, apoptosis was evaluated by immunoblotting of caspase-3 and nuclear poly (ADP-ribose) polymerase (PARP). To examine apoptosis after TNF-α, hepatocytes were incubated for 24 h in HDM containing 10 μg/ml cycloheximide and 20 ng/ml TNF-α. Both fructose and glycine were removed from HDM for TNF-α experiments. Caspase-3 activation and PARP cleavage as an indicator of apoptosis were determined by immunoblotting.
Immunoblotting analysis of apoptosis proteins.
After reoxygenation or TNF-α treatment, hepatocytes were washed with cold phosphate-buffered saline and lysates were prepared in lysis buffer of 25 mM Tris·HCl (pH 8), 150 mM NaCl, 1% sodium deoxycholate, 0.1% SDS, Triton X-100, 5 mM EDTA (pH 7.5) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The cell lysates were centrifuged at 13,000 rpm for 15 min at 4°C, and the supernatant was collected thereafter. Protein concentrations were determined by the bicinchoninic acid protein quantification kit (Thermo Scientific, Rockford, IL). Protein samples of 15 μg were electrophoresed in 8–15% polyacrylamide gel and transferred electrophoretically to polyvinylidene fluoride membrane (Millipore, Billerica, MA). The membrane was blocked with 5% nonfat milk in phosphate-buffered saline Tween 20 and subsequently incubated with primary antibodies, including caspase-3, GAPDH, and PARP (Cell Signaling Technology, Danvers, MA), at 4°C overnight. The membrane was then incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Signals were detected using chemiluminescence detection kit (GE Healthcare, Arlington Heights, IL).
Confocal laser scanning microscopy.
Glass coverslips containing hepatocytes were mounted in a closed gas-tight cultivation chamber (Zeiss, Germany) inside the anoxic chamber. After 4 h of anoxia, the sealed chambers were mounted on the microscope stage. Temperature in the chamber was maintained at 37°C using a Zeiss temperature controller. Reoxygenation was subsequently induced by infusion of aerobic KRH buffer at pH 7.4. To monitor changes of mitochondrial Ca2+, ΔΨm, ROS formation, and onset of the MPT after reoxygenation, the red fluorescence of Rhod-2, TMRM, and green fluorescence of DCF and calcein were imaged using an inverted Zeiss LSM 510 laser scanning confocal microscope equipped with a 63× numerical aperture 1.4 oil-immersion planapochromat lens. Green fluorescing calcein and DCF were excited at 488 nm, and emission was imaged at 500–530 nm using a band pass emission filter. Red fluorescing Rhod-2 and TMRM were excited at 543 nm, and emission was collected at greater than 560 nm. Pinholes were set to Airy units of 1.0 in both channels. Laser power was set to below 0.3% to minimize photobleaching and photodamage during time-lapse imaging experiments. Changes of mitochondrial Rhod-2 fluorescence were analyzed by Adobe Photoshop (San Jose, CA).
Statistics.
Differences between means were compared by the Mann-Whitney test using a level of significance of P < 0.05. Data were expressed as means ± SE. All experiments were representative of at least three independent cell isolations.
RESULTS
Protection by intramitochondrial Ca2+ chelation against necrotic cell death after A/R to cultured rat hepatocytes.
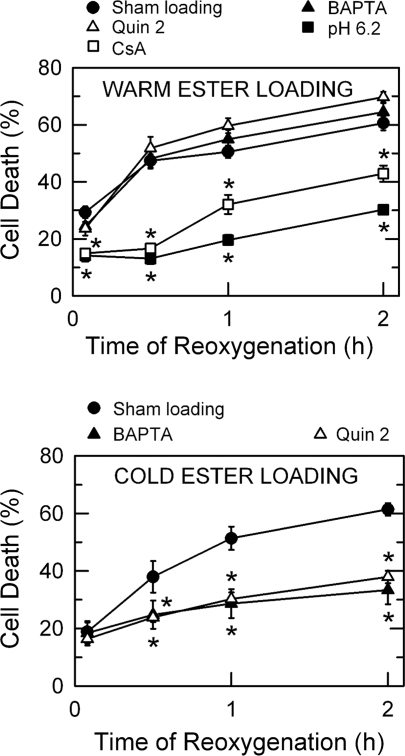
After 4 h of anoxia in KRH at pH 6.2, hepatocytes were placed in aerobic KRH at pH 7.4 to simulate the reoxygenation and restoration of physiologic pH after reperfusion. PI fluorometry showed that necrotic cell death increased from 29 ± 2.0% to 61 ± 2.5% within 2 h of reoxygenation (Fig. 1A), which was blocked after reoxygenation at pH 6.2 and after reoxygenation at pH 7.4 with 1 μM cyclosporin (CsA), an MPT blocker. These results confirmed that hepatocellular death after A/R is pH- and MPT dependent (18, 36).
Fig. 1.
Protection by intramitochondrial Ca2+ chelation against reoxygenation injury to hepatocytes. A: hepatocytes cultured in 24-well plates were incubated in anaerobic Krebs-Ringer-HEPES (KRH) buffer at pH 6.2 with 10 μM BAPTA/AM or 2-[(2-bis-[carboxymethyl]aono-5-methoxyphenyl)-methyl-6-methoxy-8-bis[carboxymethyl]aminoquinoline] (Quin 2)/AM at 37°C for the first 20 min of anoxia to chelate cytosolic Ca2+, as described in materials and methods. After 4 h of anoxia, cells were reoxygenated in aerobic KRH at pH 7.4 or at pH 6.2. In some experiments, hepatocytes were treated with 1 μM cyclosporin A (CsA) 20 min before and continuously after reoxygenation. Necrotic cell death was evaluated by propidium iodide (PI) fluorometry. B: hepatocytes were loaded with 10 μM of BAPTA/AM or Quin 2/AM at 0–1°C for 20 min to chelate mitochondrial Ca2+ and then subjected to anoxia at 37°C for 4 h. Cell death was assessed after reoxygenation. Values for each treatment group are means ± SE from 3 separate hepatocyte isolations for each group. *P < 0.05 vs. sham loading.
To investigate signaling events leading to reoxygenation-induced cell death, specifically the role of mitochondrial Ca2+, hepatocytes were loaded with Ca2+ chelators, 10 μM BAPTA or 10 μM Quin 2, either by a warm loading (Fig. 1A) or by a cold loading protocol (Fig. 1B). Cold ester-loading of BAPTA and Quin 2 results in localization into mitochondria (8, 42). In contrast, warm ester-loading produces nearly exclusive localization of loaded probes and chelators into the cytosol. When hepatocytes were loaded with Ca2+ chelators into the cytosol by warm loading, neither BAPTA nor Quin 2 prevented cell death after A/R (Fig. 1A). However, when BAPTA and Quin 2 were each introduced by cold loading, cell death after A/R was substantially decreased (Fig. 1B). Sham cold loading without chelators did not prevent cell death. Neither warm- nor cold loading of calcium chelators altered the baseline level of cell death (data not shown). Hence, these results suggest that a mitochondrial Ca2+ is contributing to necrotic death of hepatocytes after A/R.
Confocal imaging of mitochondrial Ca2+ and onset of the MPT after A/R.
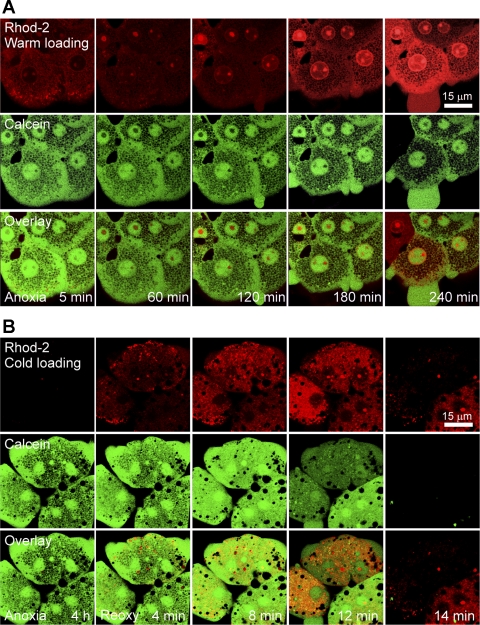
To further examine the involvement of mitochondrial Ca2+ in MPT-dependent reoxygenation injury and specifically to determine whether an increase of mitochondrial Ca2+ was a cause or an effect of MPT induction, hepatocytes cultured on glass coverslips were coloaded with Rhod-2 and calcein to monitor Ca2+ and the MPT, respectively. Mitochondrial localization of Rhod-2 was obtained by the cold loading protocol, and cytosolic localization of Rhod-2 and calcein was obtained by the warm loading protocol as described in materials and methods. During 4 h of anoxia, cytosolic Rhod-2 fluorescence increased progressively (Fig. 2A). However, the green fluorescence of calcein localized to the cytosol and nuclei, leaving mitochondria as dark voids, indicative of the closed state of PT pores in the mitochondrial inner membrane during anoxia. Nuclear labeling with Rhod-2 also occurred due to its binding to chromatin. Confocal imaging analysis of Rhod-2 after the cold loading protocol revealed that after 4 h of anoxia, intramitochondrial Rhod-2 fluorescence was barely detectable (Fig. 2B), consistent with Fig. 2A. After reoxygenation, intramitochondrial Rhod-2 fluorescence increased rapidly. Plot analysis of Rhod-2 fluorescence intensity showed that intramitochondrial Rhod-2 fluorescence increased to 154% of the value after 4 h of anoxia within 4 min of reoxygenation. Some mitochondria displayed a higher Rhod-2 fluorescence near the plasma membrane. After 4 min of reoxygenation, mitochondria, however, continued to exclude calcein, indicating that onset of the MPT had not yet occurred. After 8 min, intramitochondrial Rhod-2 fluorescence increased to 331% and most dark voids of calcein became blurry, suggesting a mitochondrial swelling or early onset of the MPT. Reoxygenation for 12 min further increased Rhod-2 fluorescence. At this time, the calcein fluorescence was markedly decreased in two hepatocytes and dark voids of calcein disappeared, indicating a widespread MPT onset in these cells. Later, all three cells completely lost calcein fluorescence, an event signifying the loss of plasma membrane barrier and necrotic cell death. These results demonstrate that intramitochondrial Ca2+ increases after reoxygenation, which precedes mitochondrial inner membrane permeabilization.
Fig. 2.
Mitochondrial Ca2+ loading precedes onset of the mitochondrial permeability transition (MPT) in hepatocytes after anoxia/reoxygenation (A/R). A: hepatocytes cultured on glass coverslips were incubated with 10 μM Rhod-2 and 1 μM calcein by the warm loading protocol to monitor cytosolic Ca2+ and onset of the MPT, respectively, as described in materials and methods. Confocal images were collected after 5, 60, 120, 180, and 240 min of anoxia. Note that Rhod-2 fluorescence progressively increased in the cytosol. During 4 h of anoxia, mitochondrial dark voids remained unfilled by green calcein fluorescence, indicating a closed state of permeability transition (PT) pores. B: hepatocytes cultured on glass coverslips were incubated with 10 μM Rhod-2 by the cold loading protocol to monitor mitochondrial Ca2+. Calcein was coloaded by warm ester-loading to monitor onset of the MPT. Confocal images were collected after 0, 4, 8, 12, and 14 min of reoxygenation (Reoxy). Mitochondrial Rhod-2 fluorescence increased rapidly after reoxygenation. At the end of anoxia and after 4 min of reoxygenation, MPT onset did not occur. After 12 min, mitochondrial dark voids of calcein fluorescence disappeared due to onset of the MPT.
Relation of mitochondrial Ca2+ to ROS formation after reoxygenation.
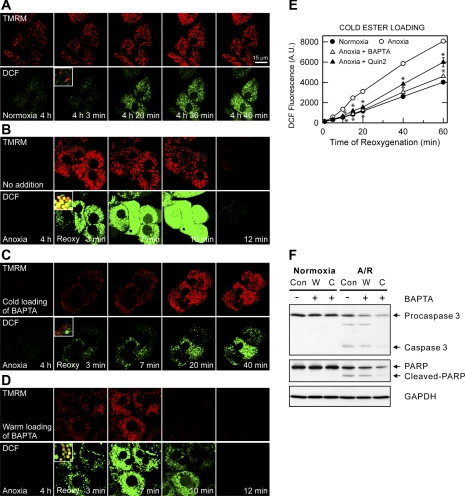
Mitochondrial ROS generation promotes the MPT (8, 21). Accordingly, we investigated the role of ROS in MPT-dependent cell death after A/R and, in particular, the temporal relationship between mitochondrial Ca2+, ROS, and MPT induction. ROS and ΔΨm were simultaneously visualized using a laser scanning confocal microscopy of DCF and TMRM, respectively (21) (Fig. 3, A–D). TMRM electrophoretically accumulates into mitochondria in response to the negative ΔΨm (18). For normoxic control, hepatocytes were incubated aerobically in KRH for 4 h. Mitochondria under the normoxic condition remained polarized (Fig. 3A, top). Normoxia generated ROS at a low and relatively constant rate, as determined by confocal microscopy of DCF fluorescence (Fig. 3A, bottom). After 4 h of anoxia, both TMRM and DCF were undetectable due to mitochondrial depolarization and lack of ROS generation during anoxia (Fig. 3B). Mitochondria began to repolarize and to take up TMRM within 3 min of reoxygenation but progressively lost ΔΨm thereafter. Most TMRM fluorescence disappeared after 12 min, indicative of mitochondrial depolarization after MPT onset. Reoxygenation of anoxic hepatocytes led to a markedly greater increase of ROS generation, compared with normoxic cells. Colocalization of red fluorescing TMRM and green fluorescing DCF demonstrated that ROS formation after 3 min of reoxygenation was confined predominantly to mitochondria (Fig. 3B, inset). An increase of mitochondrial ROS persisted during 7 min. In contrast with mitochondrial ROS, elevation of cytosolic ROS became noticeable after 7 min of reoxygenation, suggesting that mitochondrial ROS formation precedes cytosolic ROS generation after A/R. Cell surface blebs were also evident after 7 min. Like TMRM, DCF fluorescence disappeared after 12 min due to the failure of plasma membrane barrier and necrosis.
Fig. 3.
Suppression of reactive oxygen species (ROS) formation by intramitochondrial Ca2+ chelation. A: hepatocytes cultured on glass coverslips were subjected to 4 h of normoxia and then aerobically incubated in KRH for 40 min with 10 μM H2DCF diacetate and 100 nM tetramethylrhodamine methylester (TMRM) to monitor ROS generation and ΔΨm, respectively. Confocal images of green dichlorofluorescein (DCF) and red TMRM were simultaneously collected. An inset at bottom represents the merged image of red and green channel. Normoxic hepatocytes maintained ΔΨm and cell viability, and mitochondrial DCF fluorescence increased progressively but more slowly than after reoxygenation. B: confocal images of DCF and TMRM were collected after 4 h of anoxia and 3, 7, 10, and 12 min of reoxygenation. Mitochondria repolarized at 3 min but depolarized after 12 min. Reoxygenation progressively increased DCF fluorescence over 10 min. Note that ROS formation was predominantly in mitochondria during the early phase of reoxygenation. After 12 min, hepatocytes lost viability, as shown by the release of DCF fluorescence. C: hepatocytes were loaded with 10 μM BAPTA/AM by the cold loading protocol, as described in Fig. 1B. Cold loading of BAPTA prevented the loss of ΔΨm and cell death and markedly suppressed both mitochondrial and cytosolic ROS increase after reoxygenation. D: warm loading of BAPTA suppressed the cytosolic ROS increase but failed to prevent mitochondrial ROS increase, ΔΨm depolarization, and cell death. E: hepatocytes cultured in 24-well microplates were anaerobically loaded with 10 μM of BAPTA/AM or Quin 2/AM by the cold ester loading, and then labeled with H2DCF diacetate. DCF fluorescence was monitored after reoxygenation by using a multi-well fluorescence reader. As a normoxic control some hepatocytes were incubated in aerobic KRH at 37°C for 4 h. Values are means ± SE from 3 different cell preparations. *P < 0.05 vs. anoxia. F: hepatocytes were subjected to either 4 h of anoxia/22 h of reoxygenation (A/R) or normoxia. Some cells were treated with 10 μM BAPTA either by the warm (W) or by the cold (C) loading protocol. Apoptotic cell death was evaluated by immunoblotting of caspase-3 and poly (ADP-ribose) polymerase (PARP) cleavage and compared with the control (Con). AU, arbitrary units.
Chelation of intramitochondrial Ca2+ by cold loading of BAPTA substantially attenuated the reoxygenation-induced increase of both cytosolic and mitochondrial ROS and blocked mitochondrial depolarization and necrosis (Fig. 3C). Cold-loaded Quin 2 also significantly suppressed the ROS increase after reoxygenation (Fig. 3E). The extent of inhibition by Quin 2 was slightly less than that by BAPTA. Contrarily, warm-loaded BAPTA (cytosolic loading) did not suppress the increase of mitochondrial ROS formation, mitochondrial depolarization, and necrotic cell death after reoxygenation although this cytosolic Ca2+ chelation reduced the cytosolic ROS increase (Fig. 3D). Taken together, these results are consistent with the conclusion that ROS formation after reoxygenation is dependent on intramitochondrial Ca2+ and that increased mitochondrial Ca2+ after reoxygenation is an event upstream to increased ROS generation.
MPT onset can induce apoptosis by releasing proapoptotic factors that are normally sequestered in the mitochondrial intermembrane space (13, 40). Because intramitochondrial Ca2+ chelation inhibited MPT-dependent necrosis (Figs. 1B and 3C), we anticipated apoptosis inhibition by intramitochondrial Ca2+ chelation. Immunoblotting analysis of caspase-3 and PARP (2) showed a substantial apoptotic cell death after 22 h of reoxygenation, which was blocked by intramitochondrial Ca2+ chelation, but not by cytosolic Ca2+ chelation (Fig. 3F). Normoxic hepatocytes did not undergo apoptosis. Hence, these results demonstrate that intramitochondrial Ca2+ chelation prevents both necrotic and apoptotic cell death after reoxygenation.
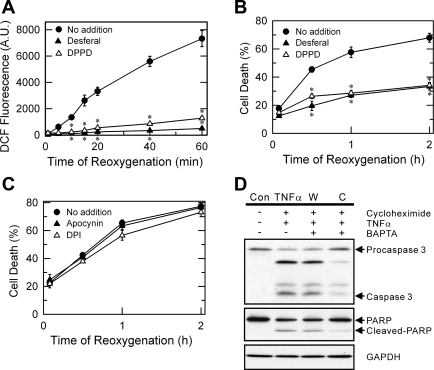
To further investigate the role of ROS in MPT-dependent cell death after A/R, hepatocytes were incubated with 0.6 mM desferal, an iron chelator that prevents hydroxyl radical formation by Fenton chemistry (44), or 10 μM DPPD, a lipid peroxidation inhibitor (30), beginning 20 min before and continuously after reoxygenation. Fluorometric analysis in H2DCF-loaded hepatocytes showed that both antioxidants abolished reoxygenation-induced ROS increase (Fig. 4A). Confocal imaging analysis indicated that reoxygenation with desferal or DPPD blocked mitochondrial ROS increase (data not shown). Consistent with DCF fluorometry, reoxygenation with each of these antioxidants blocked necrotic cell death after A/R (Fig. 4B).
Fig. 4.
Lack of cytoprotection by NADPH oxidase inhibitors. Hepatocytes cultured on 24-well microplates were subjected to A/R and labeled with H2DCF diacetate (A) and PI (B). Some hepatocytes were incubated with 0.6 mM desferal or 10 μM diphenylphenylenediamine (DPPD) beginning 20 min before and continuously after reoxygenation. ROS formation and necrotic cell death was evaluated by DCF and PI fluorometry, respectively, in the presence and absence of antioxidants. *P < 0.05 vs. no addition. C: hepatocytes cultured in 24-well microplates were subjected to 4 h of anoxia and incubated with 300 μM of apocynin or 10 μM of diphenyleneiodonium chloride (DPI). Cell death after reoxygenation was evaluated by PI fluorometry. Note the failure of cytoprotection by NADPH oxidase blockers. Values are means ± SE from 3 different cell preparations. D: hepatocytes were incubated with 20 ng/ml TNF-α for 24 h. Some cells were treated with 10 μM BAPTA either by the warm (W) or by the cold (C) loading protocol. Apoptotic cell death was determined by immunoblotting of caspase-3 and PARP cleavage. Control hepatocytes (Con) were incubated for 24 h without treatment.
ROS generation from extramitochondrial sources, such as cytosolic NADPH oxidase, could mediate hepatocyte cell death after A/R. To investigate whether an NADPH-like system is involved in reoxygenation-dependent ROS increase, necrotic cell death was fluorometrically evaluated in the presence and absence of 300 μM of apocynin or 10 μM of DPI, NADPH oxidase inhibitors (27, 38). As shown in Fig. 4C, inhibition of NADPH oxidase failed to prevent cell death after reoxygenation, suggesting a minor role of cytosolic NADPH oxidase in A/R injury to hepatocytes.
Similar to A/R injury, onset of the MPT culminates in TNF-α-mediated hepatocyte death (10, 37, 48). Because mitochondrial Ca2+ triggers MPT onset and subsequent cell death after A/R, we reasoned that chelation of intramitochondrial Ca2+ would also attenuate TNF-α-induced hepatocyte death. Chelation of intramitochondrial Ca2+ with cold-loaded BAPTA markedly suppressed TNF-α-induced apoptosis, as determined by immunoblotting of caspase-3 and PARP (Fig. 4D). However, chelation of cytosolic Ca2+ with warm-loaded BAPTA did not attenuate apoptotic cell death, confirming an integral role of mitochondrial Ca2+ in MPT-dependent hepatocellular death.
DISCUSSION
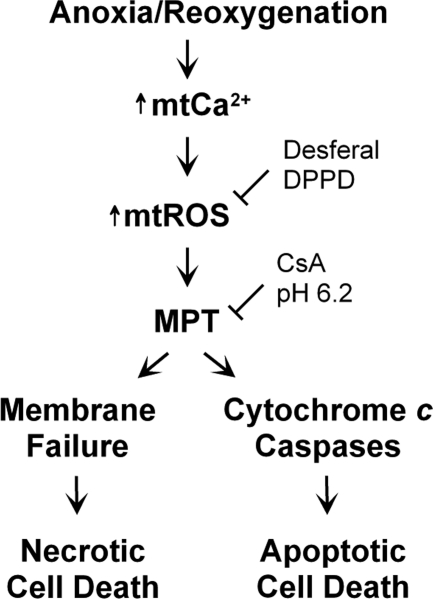
Naturally occurring ischemic acidosis protects cells and tissues against ischemic death, but normalization of pH after reperfusion paradoxically promotes cell death (6, 12, 46, 47). The MPT is a causative mechanism underlying both pH-dependent necrosis and apoptosis in reoxygenated hepatocytes (19, 36, 39). Opening of CsA-sensitive high conductance PT pores in the mitochondrial inner membrane initiates the MPT. Once PT pores open, mitochondria depolarize, oxidative phosphorylation becomes uncoupled, and large amplitude swelling occurs. As a consequence, ATP falls and irreversible cell death ensues. The MPT is directly linked to many pathological conditions (5, 10, 16, 21, 22, 36, 37, 44, 48). Cellular alterations actually inducing the MPT in intact cells and tissues remain largely unknown, although intramitochondrial Ca2+ loading promotes the MPT in isolated mitochondria (3). Here, we show that reoxygenation of anoxic hepatocytes causes a temporal sequence of mitochondrial Ca2+ loading, ROS production, MPT onset, and cell death (Fig. 5). This sequence appears to represent a chain of causation in reoxygenation-induced hepatocellular death after A/R.
Fig. 5.
Scheme of MPT induction and cell death after A/R. Reoxygenation of anoxic hepatocytes increases mitochondrial Ca2+ (mtCa2+), which in turn stimulates mitochondrial ROS (mtROS) generation. ROS generation subsequently promotes onset of the MPT, which is prevented by antioxidants like desferal and DPPD. CsA and acidotic pH also block pH-dependent onset of MPT after reoxygenation. After onset of the MPT, cells undergo either necrotic or apoptotic death, depending on the availability of glycolytic ATP. When cellular ATP is depleted, membrane failure and necrotic cell death ensues. However, when a glycolytic ATP is available after reoxygenation, necrosis is prevented and cytochrome c-dependent apoptotic death occurs instead.
After 4 h of anoxia at pH 6.2 (simulated ischemia), necrotic cell death occurred upon reoxygenation at pH 7.4 (simulated reperfusion), which was prevented by reoxygenation at pH 6.2 and at pH 7.4 with CsA (Fig. 1A), confirming the pH- and MPT dependence of cell death after A/R to hepatocytes (19, 36). Loading of intramitochondrial Ca2+ chelators prevented both necrotic and apoptotic cell death after reoxygenation, whereas loading of Ca2+ chelators into the cytosol did not (Figs. 1 and 3F). Quin 2 is a fluorescent chelator, and previous work shows that Quin 2 can be loaded into the cytosol and mitochondria of rat hepatocytes after warm and cold ester loading, respectively (8). The importance of mitochondrial Ca2+ in MPT-mediated cell death was further confirmed in TNF-α-induced apoptosis in hepatocytes (Fig. 4D).
Confocal microscopy of Rhod-2 and calcein revealed that mitochondrial Ca2+ increased substantially after reoxygenation, an event that preceded MPT onset and subsequent cell death (Fig. 2B). During anoxia, cytosolic Ca2+ increased progressively with little change of mitochondrial Ca2+ and onset of the MPT did not occur (Fig. 2A). The increase of cytosolic Ca2+ may be the consequence of ATP depletion and collapse of Na+ and K+ gradients that inhibit ATP-driven Ca2+ pumps and secondary ion exchangers, which translocate Ca2+ across the plasma membrane and into the endoplasmic reticulum. Moreover, mitochondrial depolarization during anoxia can block electrogenic mitochondrial Ca2+ uptake (15). After 4 min and 8 min of reoxygenation, Rhod-2 fluorescence increased to 154% and 331%, respectively, of values after 4 h of anoxia (Fig. 2B). Nearly all of the increase of Rhod-2 fluorescence after reoxygenation appeared to be mitochondrial, as judged by colocalization of mitochondrial calcein voids with Rhod-2. We repeatedly observed that, once intramitochondrial Rhod-2 fluorescence reached its maximal level after reoxygenation, Rhod-2 fluorescence then declined a few minutes later after which hepatocytes lost viability (data not shown). The late decrease of Rhod-2 fluorescence likely represents mitochondrial Ca2+ release into the cytosol as a consequence of MPT onset and loss of mitochondrial permeability barrier.
The importance of mitochondrial Ca2+ loading to induce the MPT is well documented in isolated mitochondria (11) and in oxidative stress and drug-induced toxicity to hepatocytes (8, 24). Ru360, an inhibitor of the mitochondrial Ca2+ uniporter, decreases reperfusion injury in the isolated perfused rat heart (14). Because the mitochondrial Ca2+ uniporter is primarily responsible for electrogenic Ca2+ uptake into mitochondria, repolarization of mitochondria after reoxygenation may be driving mitochondrial Ca2+ uptake. Although cytosolic Ca2+ increased after prolonged anoxia and thus might contribute to mitochondrial Ca2+ loading after reoxygenation, chelation of cytosolic Ca2+ did not protect hepatocytes against reoxygenation injury (Fig. 1A). Moreover, the MPT did not occur during 4 h of anoxia, suggesting a minimal role of increased cytosolic Ca2+ after anoxia in mitochondrial permeabilization. Rather, the increase of mitochondrial Ca2+ is likely associated with onset of the MPT after reoxygenation (Fig. 5).
In isolated mitochondria, ROS promote mitochondrial inner membrane permeabilization (3). Previous studies showed that mitochondrial formation of ROS contributes to A/R injury to cardiac myocytes (21) and t-butyl hydroperoxide-induced hepatocyte toxicity (8, 30). Confocal imaging of DCF and TMRM revealed that mitochondria were the primary site of ROS formation during the early phase of reoxygenation (Fig. 3B). Major sources of mitochondrial ROS generation in situ include Complex I and Q cycle ubisemiquinone in Complex III (43). A late increase of cytosolic ROS appears to be a consequence of the mitochondrial ROS increase since blockade of mitochondrial ROS also considerably reduced cytosolic ROS (Fig. 3C). Mitochondrial ROS exceeding a threshold level are released into the cytosol (50).
In the present study, DCF fluorometry showed that reoxygenation of anoxic hepatocytes markedly accelerated ROS generation, which was suppressed by intramitochondrial Ca2+ chelation (Fig. 3C). However, chelation of cytosolic Ca2+ did not affect the reoxygenation-induced mitochondrial ROS increase (Fig. 3D), suggesting that mitochondrial Ca2+, but not cytosolic Ca2+, promotes enhanced ROS formation after reoxygenation. Mitochondrial Ca2+ uptake has been shown previously to induce ROS formation and ROS-dependent MPT onset (1, 8, 32). The mechanisms underlying the mitochondrial Ca2+-induced ROS release are, however, unknown. Stimulation of mitochondrial respiration by Ca2+ has been proposed to generate more ROS by enhancing the electron flow in the mitochondrial electron transport chain (7). Additionally, it has been reported that ischemia-reperfusion depletes livers of mitochondrial antioxidant enzymes, including glutathione-S-transferase and SOD, which is reversed by amlodipine, a mitochondrial Ca2+ antagonist (34). Because oxidative stress stems from an imbalance between ROS and antioxidants, diminished antioxidant status would sensitize mitochondria to oxidative stress. Hence, impaired mitochondrial antioxidants by Ca2+ could be an important mechanism of Ca2+-dependent ROS increase. Future studies are warranted to elucidate the precise mechanisms underlying Ca2+-mediated ROS production in mitochondria.
MPT-dependent cell death after reoxygenation was abrogated by the antioxidants, desferal, and DPPD (Fig. 4, A and B). Reoxygenation with either of these antioxidants prevented the ROS increase and cell death, confirming that ROS generation is a key event responsible for reoxygenation-induced mitochondrial injury. Besides mitochondria, extramitochondrial enzymes, such as NADPH oxidase, cytochrome P-450, xanthine oxidase, cyclooxygenase, and lipooxygenase, can attribute to ROS production. Recently, Bhogal et al. (4) suggested that cytosolic NADPH oxidase as well as mitochondria may contribute to ROS increase after reoxygenation of hypoxic hepatocytes. However, in the present work, neither apocynin nor DPI, NADPH oxidase inhibitors, prevented cell death after reoxygenation, suggesting a minor role of cytosolic NADPH oxidase in A/R injury to hepatocytes (Fig. 4C). Moreover, blockade of cytosolic ROS did not prevent cell death after A/R, as visualized by confocal microscopy (Fig. 3D). Consistent with our results, Zhou et al. (49) have reported the importance of mitochondrial ROS by demonstrating that adenoviral delivery of mitochondrial MnSOD, but not cytosolic Cu/ZnSOD, to the liver significantly attenuates reperfusion injury to mouse livers. Nevertheless, our results do not exclude the possibility that cytosolic ROS increase is an additional factor promoting cell death.
Exposure of hepatocytes to oxidative stress with t-butyl hydroperoxide causes mitochondrial pyridine nucleotide oxidation, followed sequentially by mitochondrial Ca2+ loading and stimulation of mitochondrial ROS formation, leading to the MPT and cell death (8, 30). Why mitochondrial Ca2+ becomes elevated during the early phase of reoxygenation is unknown but oxidation of pyridine nucleotides during reoxygenation may be an important factor, which future studies will need to address.
Although MPT onset plays a critical role in reperfusion injury in vivo (41), efforts to improve liver function after ischemia-reperfusion have not been successful mostly due to the multifactorial nature of reperfusion injury. MPT blockers like CsA have a narrow range of therapeutic efficacy (36). Moreover, CsA-insensitive MPT can also develop when MPT induction increases (17). Our data suggest that overloading of mitochondrial Ca2+ and ROS is causal to ischemia-reperfusion injury to the liver and modulation of mitochondrial Ca2+ and ROS may have therapeutic potentials for ameliorating hepatic function after ischemia-reperfusion. The beneficial effects of mitochondrial Ca modulators and antioxidants have been reported in hepatic ischemia-reperfusion in vivo (29, 34, 49).
In conclusion, A/R increases mitochondrial Ca2+, which stimulates ROS generation, leading to the MPT and subsequent cell death of cultured rat hepatocytes. Because onset of the MPT is a causative event contributing to cell death after reoxygenation, interventions to block events upstream to MPT induction, such as mitochondrial Ca2+ loading and ROS formation, are potential strategies to prevent ischemic hepatocellular death after reperfusion.
GRANTS
This work was supported, in part, by National Institutes of Health Grants 5R01DK-079879 (to J.-S. Kim) and 5R01DK-073336, 5R01DK-037034, and 5P30CA-138313 (all to J. J. Lemasters).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.-S.K. and J.J.L. conception and design of research; J.-S.K. and J.-H.W. performed experiments; J.-S.K. and J.-H.W. analyzed data; J.-S.K. and J.J.L. interpreted results of experiments; J.-S.K. and J.-H.W. prepared figures; J.-S.K. drafted manuscript; J.-S.K. and J.J.L. edited and revised manuscript; J.-S.K., J.-H.W., and J.J.L. approved final version of manuscript.
REFERENCES
- 1. Battaglia V, Grancara S, Satriano J, Saccoccio S, Agostinelli E, Toninello A. Agmatine prevents the Ca2+-dependent induction of permeability transition in rat brain mitochondria. Amino Acids 38: 431–437, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Berger NA, Petzold SJ. Identification of minimal size requirements of DNA for activation of poly(ADP-ribose) polymerase. Biochemistry 24: 4352–4355, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: 1127–1155, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl 16: 1303–1313, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Black D, Bird MA, Samson CM, Lyman S, Lange PA, Schrum LW, Qian T, Lemasters JJ, Brenner DA, Rippe RA, Behrns KE. Primary cirrhotic hepatocytes resist TGFbeta-induced apoptosis through a ROS-dependent mechanism. J Hepatol 40: 942–951, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bond JM, Herman B, Lemasters JJ. Protection by acidotic pH against anoxia/reoxygenation injury to rat neonatal cardiac myocytes. Biochem Biophys Res Commun 179: 798–803, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Byrne AM, Lemasters JJ, Nieminen AL. Contribution of increased mitochondrial free Ca2+ to the mitochondrial permeability transition induced by tert-butylhydroperoxide in rat hepatocytes. Hepatology 29: 1523–1531, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Cathcart R, Schwiers E, Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134: 111–116, 1983 [DOI] [PubMed] [Google Scholar]
- 10. Chen X, Ding WX, Ni HM, Gao W, Shi YH, Gambotto AA, Fan J, Beg AA, Yin XM. Bid-independent mitochondrial activation in tumor necrosis factor alpha-induced apoptosis and liver injury. Mol Cell Biol 27: 541–553, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255: 357–360, 1988 [PMC free article] [PubMed] [Google Scholar]
- 12. Currin RT, Gores GJ, Thurman RG, Lemasters JJ. Protection by acidotic pH against anoxic cell killing in perfused rat liver: evidence for a pH paradox. FASEB J 5: 207–210, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33–42, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Garcia-Rivas GJ, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol 149: 829–837, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol 258: C755–C786, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Hatano E, Bradham CA, Stark A, Iimuro Y, Lemasters JJ, Brenner DA. The mitochondrial permeability transition augments Fas-induced apoptosis in mouse hepatocytes. J Biol Chem 275: 11814–11823, 2000 [DOI] [PubMed] [Google Scholar]
- 17. He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett 512: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Kim JS, Ohshima S, Pediaditakis P, Lemasters JJ. Nitric oxide protects rat hepatocytes against reperfusion injury mediated by the mitochondrial permeability transition. Hepatology 39: 1533–1543, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Kim JS, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology 124: 494–503, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304: 463–470, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 290: H2024–H2034, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40: 1170–1179, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Lemasters JJ. The mitochondrial permeability transition and the calcium, oxygen and pH paradoxes: one paradox after another. Cardiovasc Res 44: 470–473, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157, 1996 [DOI] [PubMed] [Google Scholar]
- 26. McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312: 159–163, 1985 [DOI] [PubMed] [Google Scholar]
- 27. Murillo MM, Carmona-Cuenca I, Del CG, Ortiz C, Roncero C, Sanchez A, Fernandez M, Fabregat I. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem J 405: 251–259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nayler WG, Poole-Wilson PA, Williams A. Hypoxia and calcium. J Mol Cell Cardiol 11: 683–706, 1979 [DOI] [PubMed] [Google Scholar]
- 29. Nicoud IB, Knox CD, Jones CM, Anderson CD, Pierce JM, Belous AE, Earl TM, Chari RS. 2-APB protects against liver ischemia-reperfusion injury by reducing cellular and mitochondrial calcium uptake. Am J Physiol Gastrointest Liver Physiol 293: G623–G630, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am J Physiol Cell Physiol 272: C1286–C1294, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Nieminen AL, Gores GJ, Bond JM, Imberti R, Herman B, Lemasters JJ. A novel cytotoxicity screening assay using a multiwell fluorescence scanner. Toxicol Appl Pharmacol 115: 147–155, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Panov A, Dikalov S, Shalbuyeva N, Hemendinger R, Greenamyre JT, Rosenfeld J. Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am J Physiol Cell Physiol 292: C708–C718, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Piper HM. Energy deficiency, calcium overload or oxidative stress: possible causes of irreversible ischemic myocardial injury. Klin Wochenschr 67: 465–476, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Pronobesh C, Dagagi AV, Pallab C, Kumar WA. Protective role of the calcium channel blocker amlodipine against mitochondrial injury in ischemia and reperfusion injury of rat liver. Acta Pharm 58: 421–428, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Qian T, Herman B, Lemasters JJ. The mitochondrial permeability transition mediates both necrotic and apoptotic death of hepatocytes exposed to Br-A23187. Toxicol Appl Pharmacol 154: 117–125, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol Cell Physiol 273: C1783–C1792, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Regueira T, Lepper PM, Brandt S, Ochs M, Vuda M, Takala J, Jakob SM, Djafarzadeh S. Hypoxia inducible factor-1 alpha induction by tumour necrosis factor-alpha, but not by toll-like receptor agonists, modulates cellular respiration in cultured human hepatocytes. Liver Int 29: 1582–1592, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Reinehr R, Becker S, Eberle A, Grether-Beck S, Haussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem 280: 27179–27194, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther 89: 29–46, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441–446, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology 47: 236–246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trollinger DR, Cascio WE, Lemasters JJ. Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem Biophys Res Commun 236: 738–742, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237: 408–414, 1985 [DOI] [PubMed] [Google Scholar]
- 44. Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology 48: 1644–1654, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weinberg JM. The cell biology of ischemic renal injury. Kidney Int 39: 476–500, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Zager RA, Schimpf BA, Gmur DJ. Physiological pH effects on posthypoxic proximal tubular injury. Circ Res 72: 837–846, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Zhao Y, Ding WX, Qian T, Watkins S, Lemasters JJ, Yin XM. Bid activates multiple mitochondrial apoptotic mechanisms in primary hepatocytes after death receptor engagement. Gastroenterology 125: 854–867, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia-reperfusion. Hepatology 33: 902–914, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757: 509–517, 2006 [DOI] [PubMed] [Google Scholar]