Abstract
Cell-surface-associated annexin A2 (CS-ANXA2) is a nonconventional “receptor” for progastrin; expression levels of both are elevated in colon cancers, and downregulation of either reduces tumorigenic potential of cells. We recently reported internalization of progastrin in target cells. Here, mechanisms mediating internalization of progastrin were examined. Initially, we confirmed that cell-surface ANXA2 mediates binding and internalization of progastrin in intestinal cells. Progastrin, covalently linked to sepharose beads, failed to activate p38MAPK/ERKs, suggesting internalization of progastrin was required for eliciting biological effects; importantly annexin A2 expression and availability of CS-ANXA2 were required for internalization of progastrin. Clathrin expression and formation of clathrin-coated pits were critically required for endocytotic internalization of progastrin; in the absence of clathrin, progastrin failed to activate p38MAPK/ERKs. Downregulation of caveolin had no effect on binding or internalization of progastrin. We therefore demonstrate for the first time that progastrin binds CS-ANXA2 and is rapidly internalized via clathrin-mediated endocytotic pathway, resulting in activation of MAPKinases. Targeting clathrin-mediated endocytosis of progastrin may thus inhibit previously reported co-carcinogenic/tumorigenic effects of progastrin on intestinal cells.
Keywords: caveolin, IEC-18 intestinal cells, clathrin-coated pits, chlorpromazine
accumulative evidence suggests that gastrins play an important role in proliferation/carcinogenesis of gastrointestinal and pancreatic cancers (45, 47, 68). Processing intermediates of gastrin, progastrin (PG), and glycine-extended gastrins (G-gly), are predominant forms of gastrins expressed by colon cancers (7, 55, 57, 65). PG exerts potent proliferative and anti-apoptotic effects in vitro and in vivo on target cells including intestinal epithelial (IEC) cells (2, 43, 59, 70, 71). Transgenic mice overexpressing PG are at high risk for developing colonic lesions in response to a colon-specific carcinogen, azoxymethane (8, 23, 58). Treatment with G-gly similarly increases risk for developing preneoplastic lesions (1). Normally, only processed forms of gastrins (G17, G34) are present in the circulation (68). In certain disease states, elevated levels of processing intermediates of gastrin (such as PG) are present in the circulation, which play a role in colon carcinogenesis (reviewed in Ref. 47).
PG stimulation of target cells activates p38 MAPK/ERKs, upstream of IKKα/β/NF-κBp65/β-catenin, in vitro and in vivo (48, 63, 64); activation of both NF-κBp65 and β-catenin is required for mediating growth effects of PG (64). Annexin A2 (ANXA2) is a non-conventional “receptor” for PG/gastrin peptides (60). Downregulation of ANXA2 attenuates growth-stimulatory effects of PG (48, 60) and is required for PG-stimulated activation/elevation of p38 MAPK/ERKs/NF-κB/β-catenin and stem cell markers DCAMKL+1/CD44 in target cells in vitro and in vivo (49). Intracellular co-localization of PG and ANXA2 was observed in colonic crypts of transgenic mice overexpressing PG (FabpPG mice) (63), suggesting internalization of PG. PG stimulation of embryonic kidney (HEK293) cells resulted in co-localization of PG/ANXA2 on plasma membranes, followed by rapid internalization, as punctate bodies, strongly suggesting endocytosis of PG (49). Although cell-surface, membrane-associated ANXA2 (CS-ANXA2) has been reported to bind several proteins/ligands (reviewed in Ref. 61), possible internalization/endocytosis of ANXA2/ligand complexes has not been examined. In this study, CS-ANXA2-dependent endocytosis of PG was confirmed in IEC cells. Binding of ligands (EGFs, transferrin) to membrane receptors (R), results in endocytosis of receptor/ligand complexes (20, 32), which is reportedly required for sustained activation of signaling pathways (31, 67). We and others have reported activation of MAPK/ERKs/NF-κB in response to ligand binding with ANXA2 (Refs. 63, 64; reviewed in Ref. 61). In the present study, we examined for the first time whether endocytosis of PG is required for activating MAPkinases.
NH2-terminal domains of ANXA2 contain binding sites for μ1/μ2 subunits of adaptor proteins, AP1/AP2, and are proposed to masquerade as transmembrane receptors and initiate clathrin-coated pit (CCP) formation at the cell surface (9). At the same time, ANXA2 associates with early endosomes (41). We therefore examined a possible role of clathrin in endocytosis of PG.
The results of our study demonstrate for the first time that clathrin mediates endocytosis of PG, which is required for intracellular signaling in response to PG. CS-ANXA2 was critically required for binding and endocytosis of PG, whereas caveolin played no role.
MATERIALS AND METHODS
Antibodies.
Antibodies used were anti-phospho (p)p65NF-κB(Ser276) (catalog no. 3037), anti-phospho p44/42 ERKs (catalog no. 9101), anti-phospho p38MAPkinase (catalog nos. 9211 and 2410), anti-clathrin (catalog no. 2410), anti-caveolin (catalog no. 3238), anti-CD44 (catalog no. 3570, clone no. 156-3C11) (Cell Signaling Technology, MA), anti-annexin A2 (catalog no. 610068) (BD Biosciences, San Jose, CA), anti-E-cadherin (catalog no. SC8426) (Santa Cruz Biotechnology. Santa Cruz CA ), anti-β-actin (catalog no. A 3854) (Sigma, St. Louis, MO), anti-rabbit Alexa fluor 594(catalog no. A11037), anti-rabbit Alexa fluor 488 (catalog no. A11034), anti-mouse Alexa flour 594 (catalog no. A11005), anti-mouse Alexa flour 488 (catalog no. A11029) (Invitrogen, Carlsbad, CA), anti-mouse IgG HRP (catalog no. NA931V), anti-rabbit IgG (catalog no. NA934) (GE Health Care, Piscataway, NJ), and anti-PG-Abs were generated and purified in our laboratory as described (59).
Cell culture.
Rat intestinal cell line IEC-18 (American Type Culture Collection, Manassas, VA) was grown as monolayer cultures as described previously (49, 59) in DMEM/F12 medium containing 10% fetal calf serum (FCS), 1% penicillin/streptomycin in a humid atmosphere at 37°C with 5% CO2. Cell lines were regularly monitored for absence of mycoplasma.
Immunoprecipitation and Western blotting.
Cells (4.0 × 106/ml) were seeded in 100-mm culture dishes in 5 ml of normal growth medium containing 10% FCS. After 24 h, medium was changed to serum-free medium (SFM) for 24 h and treated with rhPG (1–10 nM) for indicated times at 37°C. Cells were then washed with chilled phosphate-buffered saline (PBS), scraped with a rubber policeman into conical tubes, pelleted at 3,000 RPM for 5 min, and subjected to cell lysis as previously described (60). Lysates were pre-cleared for 1 h with 20 μl of normal rabbit serum/0.5 ml of lysate containing 500 μg of protein at 4°C with gentle agitation. Preequilibrated protein A sepharose (in 50 μl of lysate buffer) was added and gently agitated for 2 h at 4°C. Beads were pelleted and supernatant was incubated with either polyclonal or monoclonal antibodies (Abs) against PG or ANXA2 (0.5 μg/ml) O/N at 4°C with gentle agitation. Ab-bound complexes were immunoprecipitated (IP) with preequilibrated protein A sepharose beads (50 μl in lysase buffer) for 6 h at 4°C, followed by centrifugation and washing the beads three times to remove excess Abs. Cell/nuclear extracts were processed for gel electrophoresis and transferred to PVDF membranes as described (59). Blots were cut into horizontal strips containing target or loading control proteins and processed for Western blot (WIB) analysis, as described (59, 60). Antigen-antibody complexes were detected with chemiluminescence reagent kit (GE Healthcare). Membrane strips containing either target or loading control proteins were simultaneously exposed to autoradiographic film(s). Relative band density on scanned autoradiograms was analyzed densitometrically using imaging program (rsb.info.nih.gov/ij/download; NIH, Bethesda, MD) and expressed as a ratio of control loading protein (β-actin) in the corresponding samples.
Transfection of cells with double-stranded small interfering RNA oliglionucleotides.
Smart pool of target-specific small interfering RNA (siRNA) and nontargeting (control) siRNA pool were obtained from Dharmacon (Lafayette, CO). Cells, seeded in six-well dishes, were transfected with 1–1.5 μg of either specific or control siRNA using FuGENE 6 (Roche, IN). Transfected cells were propagated in normal medium containing 10% FCS for 48–72 h and processed for WIB as described above.
Membrane binding and internalization of PG/ANXA2.
Membrane binding and internalization assays were conducted essentially as described previously (49). Briefly, cells were seeded on glass coverslips, cultured O/N in complete growth medium, washed with PBS, and incubated with serum-free medium for 16–24 h. The quiescent cells were then stimulated for 0–15 min with 10 nM rhPG in the growth medium containing 0.1% serum at 37°C. In a few experiments, the serum-starved cells, as described above, were preincubated with either anti-ANXA2-IgG or control, preimmune IgG for 30 min at room temperature, as described previously (48, 60). We have previously reported that the anti-ANXA2-IgG used by us does not inhibit binding of PG to the cells but attenuates biological effects of PG on the cells (48). In all cases, the binding of PG, after indicated time periods, was terminated with ice-cold PBS, followed by fixation in acetone:methanol (1:1) for 20 min at −20°C. Fixed cells were washed with PBS, blocked with 5% BSA, and incubated at 4°C with rabbit anti-rhPG-Abs (1:200) and mouse anti-AnxA2-Abs (1:500). Excess antibody (Ab) was removed, and samples were incubated with cold anti-Rabbit-IgG coupled to Alexa Fluor 594 (for detecting PG) and anti-mouse-IgG coupled to Alexa Fluor 488 (for detecting ANXA2). Excess Ab was removed, and cells were incubated with 4′,6 diamidino-2-phenylintolde (DAP1) for 5 min to stain the nucleus. Coverslips were removed with anti-fade fixative (DAKO), and images were acquired as described below. To analyze the role of clathrin-coated pits (CCPs) in mediating internalization of PG/ANXA2 complexes, cells growing on glass coverslips were treated with 30 μM chlorpromazine (CHZ) [specific inhibitor of clathrin-mediated endocytosis (CME) (36)] before treatment with PG. As a negative control, an equivalent volume of the vehicle was added to the cells under similar conditions. At the end of the treatment period, cells were processed for staining with PG/ANXA2 (as described above). Possible co-localization of PG and/or ANXA2 with clathrin or Caveolin was determined by using specific Abs as listed above. Specific secondary Abs coupled with either Alexa Fluor 488 or 594 were used for detecting the indicated molecules as described above. The fluorescent images were captured with either Zeiss LSM 510 confocal microscope or Zeiss Epi-Fluorescence microscope model 3.0 Axioplan 2. Images were analyzed using METAMORPH version 6.0 software.
Fluorescent-activated cell scanning analysis for measuring the presence of CS-ANXA2.
Fluorescent-activated cell scanning analysis (FACScan) was conducted with ANXA2-Abs, essentially as described previously (44). Cells in culture, growing logarithmetrically at <60% confluence, were resuspended in PBS with 2% BSA at a concentration of 2 × 106 cells/ml at room temperature, followed by incubation with either anti-ANXA2-Abs (0.25 μg/ml) or preimmune (control) IgG for 3h at 4°C. Cells were washed two times with PBS at 4°C and incubated for 1 h with Alexa 488-conjugated anti-mouse Abs. Presence of cell surface ANXA2 was analyzed using FACScan (LSR II Fortessa, Becton, Dickson and Company, San Jose, CA), with the help of the flow Cytometry Core Facility at University of Texas Medical Branch Health.
DNA binding assay for measuring activated levels of NF-κB.
Activation of NF-κB was determined by using TransAM p65NFkB transcription factor assay kit, as per instructions of the manufacturer, as described previously (48, 49).
RESULTS
Presence of CS-ANXA2 on IEC-18 cells.
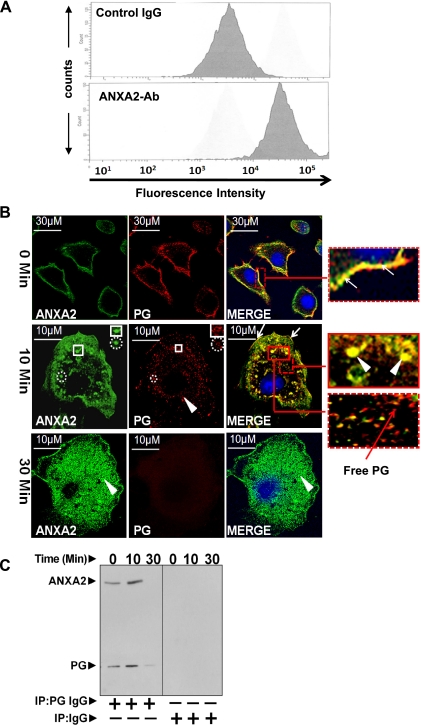
In the present studies, we chose IEC-18 (rat intestinal epithelial) cells, responsive to proliferative and anti-apoptotic effects of PG (59, 71), as an in vitro model since IEC-18 cells unlike HEK293 [used by our laboratory in previous studies (49)] have a much larger cytoplasm-to-nuclear ratio, allowing us to examine endocytosis more clearly. Our previous results suggested presence of CS-ANXA2 on cell lines responsive to PG. To substantiate this possibility, FACScan was used (Fig. 1A). Labeling with preimmune control IgG demonstrated background fluorescence. Intensity of labeling was significantly increased by >10-fold with anti-ANXA2-Ab, confirming the presence of CS-ANXA2 on IEC-18 cells.
Fig. 1.
A–C: association of progastrin (PG) with cell-surface-associated annexin A2 (CS-ANXA2), followed by internalization of PG. A: presence of CS-ANXA2 on intestinal epithelial (IEC)-18 cells was confirmed by FACScan. The fluorescence intensity of cells labeled with anti-ANXA2-Abs was increased >10-fold compared with cells labeled with control IgG. B and C: IEC-18 cells, cultured on glass coverslips, were stimulated with PG for 0–30 min and labeled with fluorescent Abs against ANXA2 (green) and PG (red) and imaged at ×60 by confocal microscopy. Representative images at 0, 10, and 30 min are presented in B (images at 10 and 30 min were computer enhanced). Association of PG with CS-ANXA2 on plasma membranes (0 min) and intracellularly (10 min) are presented in the insets after zooming the boxed areas. Arrows show association of PG/CS-ANXA2 on plasma membranes; arrow heads show co-localization of PG with ANXA2 intracellularly. Intracellular PG, free of ANXA2, is also shown in the inset with red arrows. Cells stimulated with 10 nM PG were processed for CO-IP with either preimmune control IgG or anti-PG-Abs and analyzed by WIB (C).
Association of PG with CS-ANXA2 followed by internalization.
ANXA2 co-immunoprecipitates (CO-IPs) with PG in cellular extracts of 1) colon cancer cells expressing autocrine PG (60) and 2) colonic crypts of transgenic mice (FabpPG), overexpressing PG (63). To further confirm and examine mechanisms of intracellular translocation of PG, we used IEC-18 cells, which do not express autocrine PG and are responsive to proliferative and anti-apoptotic effects of PG as described above (59, 71). At 0 min, PG-treated cells demonstrated strong co-localization of PG with CS-ANXA2 (Fig. 1B, top). Intracellular translocation of PG, bound to ANXA2, was observed in punctate bodies within 5–15 min of adding PG, suggesting endosomal localization of the complex. Representative images after 10 min of PG stimulation are shown in Fig. 1B, middle. Co-localized ANXA2/PG was observed on membranes (arrows) and intracellularly within punctate bodies (arrowheads) (as shown in merged images, presented as insets) (Fig. 1B). Submembranous ANXA2, not associated with PG, was also present, whereas PG free of ANXA2 was observed deep within the cells (Fig. 1B, inset), perhaps reflecting disassociated PG. To confirm association of PG/ANXA2 at 0 and 10 min after PG stimulation, cellular lysates were subjected to CO-IP with anti-PG-Abs and processed for Western immunoblot (WIB) analysis (Fig. 1C). Ratio of PG-to-ANXA2 remained similar over 10 min, but levels of bound PG/ANXA2 increased two- to threefold at 10 min, suggesting constant uptake of PG within this timeframe; pull-down assays with non-immune IgG were negative for PG and ANXA2 bands, confirming specificity of CO-IP experiments with anti-PG-Abs. At 30 min, no bands were observed in Western blots of CO-IP samples (Fig. 1C), and PG was not observed on either the plasma membranes or intracellularly (Fig. 1B, bottom), suggesting possible downregulation of CS-ANXA2 and/or lysosomal degradation of PG (as suggested by preliminary studies in our laboratory).
ANXA2 expression is required for binding and internalization of PG.
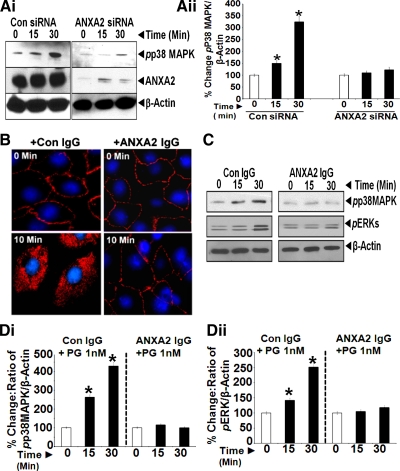
Intracellular PG, free of ANXA2, was observed after 10–15 min of adding PG (Fig. 1B, middle, inset), suggesting possible internalization of PG, independent of ANXA2. To examine this possibility, IEC-18 cells were treated with rat ANXA2 siRNA; control cells were treated with control siRNA. Representative results are presented in Fig. 2A. Downregulation of ANXA2 was confirmed (Fig. 2Ai). Cells treated with control siRNA demonstrated co-localization of PG/ANXA2 on membranes (0 min) and intracellularly (10 min) (Fig. 2Aii), as can be seen in the enhanced images of single cells, further zoomed in within the insets. Cells treated with ANXA2 siRNA were almost completely downregulated for PG binding and internalization (Fig. 2Aii, right). These data strongly suggest that ANXA2 expression is required for binding and internalization of PG.
Fig. 2.
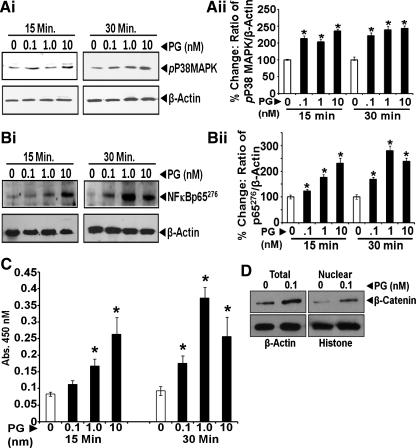
A: ANXA2 expression is required for binding and internalization of PG. IEC-18 cells were treated with either control or ANXA2 targeting siRNA; representative Western blots of ANXA2 levels in control (con) or ANXA2 siRNA-treated cells are presented in Ai. Cells thus treated were stimulated with 10 nM PG and processed by confocal microscopy. Representative images from three experiments are presented in Aii at ×40. Co-localization of PG and ANXA2 in the merged images is highlighted either as arrows (plasma membranes) or arrowheads (intracellularly). B: phosphorylation of ERKs in IEC-18 cells in response to PG. IEC-18 cells were treated with increasing concentrations of PG, and relative levels of activated (phosphorylated) ERKs (Bi and Bii), measured at 15 and 30 min after PG stimulation, by WIB. Representative Western blots from one of three similar experiments is presented in Bi, whereas data from all three experiments are presented as % change in the ratio of the kinase/β-actin in Bii as means ± SE. The % change was calculated as described in methods. *Significant difference vs. 0-min value (P < 0.05).
PG stimulates activation of p38MAPK/ERKs/NF-κBp65/NF-κB p65 in IEC-18 cells: role of annexin A2.
Several signaling pathways are activated in response to PG in vitro and in vivo in different target cells (2, 4, 14, 45, 47–49, 64), as diagrammatically presented in a recent paper (49). In the present studies, we confirmed whether the indicated kinases (p38 MAPK/ERKs) and transcription factors (NF-κB p65/β-catenin) are similarly activated/upregulated in IEC-18 cells in response to PG. PG (0.1–10 nM) stimulated activation (phosphorylation) of ERKs, p38 MAPK, and NF-κB p65 by two- to fourfold within 15–30 min (Figs. 2B and 3, A and B); representative Western blots from one of three experiments are presented at left of Figs. 2 and 3, A and B (Figs. 2Bi and 3, Ai and Bi). Data from all the experiments are presented as bar graphs at right of Figs. 2B and 3, A and B (Figs. 2Bii and 3, Aii and Bii). Activation of NF-κB was also measured in a DNA binding assay (Fig. 3C), which confirmed activation of NF-κB in IEC-18 cells in response to PG. Total cellular levels and nuclear levels of β-catenin were also increased in response to 0.1 nM PG (Fig. 3D), as previously reported for HEK-293 cells (49), confirming activation of β-catenin pathway in IEC-18 cells in response to PG. Downregulation of β-catenin (using siRNA targeting rat β-catenin) resulted in significantly reducing proliferative effects of PG on IEC-18 cells (data not shown), as previously reported for HEK-293 cells (49).
Fig. 3.
A and B: phosphorylation of p38MAPK and NF-κB in IEC-18 cells in response to PG. IEC-18 cells were treated with increasing concentrations of PG and relative levels of activated (phosphorylated) p38MAPK (Ai, Aii) and NFκBp65276 (Bi, Bii) measured at 15 and 30min after PG stimulation by WIB. In each case, representative Western blots from one of three similar experiments is presented at left of Ai and Bi, whereas data from all three experiments are presented as percent change in the ratio of the indicated kinase/β-actin at right of Aii and Bii as means ± SE. The percent change was calculated as described in methods. *Significant difference vs. 0-min value (P < 0.05). C and D: activation of NF-κB and increased nuclear translocation of β-catenin in response to PG in IEC-18 cells. Activation of NF-κB was further confirmed by an NF-κB DNA binding assay using nuclear extracts of PG-treated cells as described previously (49), and representative data from one of three experiments are presented as means ± SE in C. *Significant difference vs. 0-min values for the corresponding time points (P < 0.05). Activation of NF-κB was also examined in terms of relative levels of cellular β-catenin and increased nuclear translocation of β-catenin in PG-treated vs. control samples in D, since we have shown that β-catenin is downstream of NF-κB activation in response to PG (64). For WIB data presented for nuclear β-catenin, histone was used a loading control (D).
ANXA2 expression is required for measuring activation of p38MAPK in response to PG in IEC-18 cells.
In previous studies, our laboratory reported that IEC-18 cells, downregulated for ANXA2 expression, are not responsive to the growth effects of PG in vitro (60). In the present studies, we learned that downregulation of ANXA2 expression not only attenuated binding and internalization of PG as described above but also resulted in the loss of activation of p38 MAPK in response to PG (Fig. 4, Ai and Aii), once again confirming a critical role of ANXA2 in mediating biological effects of PG in IEC-18 cells.
Fig. 4.
A: ANXA2 expression is required for measuring activation of p38 MAPK in response to PG. IEC-18 cells, treated with either control or ANXA2 targeting siRNA, were stimulated with 1 nM PG from 0–30 min and processed by WIB for phosphorylated (p)p38MAPK and ANXA2. β-Actin levels were used as a loading control. Representative blot from two experiments are presented in Ai. Ratio of pp38MAPK-to-β-actin in cells treated with either control or ANXA2 siRNA are presented in Aii. The data in Aii are means ± SE of three blots from two experiments. *Significant difference vs. 0-min values (P < 0.05). B: freely available CS-ANXA2 is required for internalization of PG in the cells. IEC-18 cells in culture on glass slides were pretreated with either control (preimmune) IgG or anti-ANXA2-IgG (1:200) at equivalent concentrations as previously described (48, 60). Cells were pretreated with the antibodies for 30 min at room temperature, followed by stimulation with 10 nM PG for either 0 or 10 min. Cells thus treated were processed for epifluorescence microscopy. Representative images obtained at ×20 from two experiments were computer enhanced and are presented in B. Cells treated with either control or anti-ANXA2-IgG demonstrated the presence of PG associated with the cellular membranes. However, after 10 min of PG stimulation, internalization of PG was observed only in cells treated with control IgG, whereas cells treated with anti-ANXA2 IgG demonstrated the presence of PG on cellular membranes only, with no evidence of internalization of PG. Since anti-ANXA2-IgG likely binds CS-ANXA2, the absence of internalization of PG in cells treated with anti-ANXA2 antibodies suggests that freely available CS-ANXA2 is required for mediating internalization of PG in IEC-18 cells. C and D: internalization of PG is required for activation of p38MAPK/ERKs. IEC-18 cells were pretreated with either control IgG or anti-ANXA2-IgG as described above, followed by stimulation with 1 nM PG for 0–30 min, and the cells processed by WIB for phosphorylated (p) p38MAPK/ERKs; β-actin levels were used as a loading control. Representative blots from two experiments are presented in C. Ratio of target protein-to-β-actin, as described in text, is shown in Di (pp38MAPK) and Dii (pERK). Data in D are means ± SE of four blots from two experiments. *Significant difference vs. 0-min values (P < 0.05).
Role of CS-ANXA2 for mediating biological effects of PG.
Previously, we had reported that treatment of cells with anti-ANXA2-Abs attenuates biological effects of PG on the cells (48, 60), strongly suggesting that CS-ANXA2 is required for mediating biological effects of PG. In the present studies, we additionally examined whether antibodies against ANXA2 inhibit binding and/or internalization of PG, thus impacting biological effects of PG. IEC-18 cells, pretreated with anti-ANXA2-Abs demonstrated significant binding of PG to cellular membranes at 0 and 10 min (Fig. 4B), suggesting that the anti-ANXA2-Abs do not mask the binding sites for PG on the CS-ANXA2 molecules. However, internalization of PG was significantly attenuated in cells pretreated with anti-ANXA2-Abs (Fig. 4B, right), suggesting that anti-ANXA2-Abs inhibit the ability of CS-ANXA2 to facilitate internalization of PG in IEC-18 cells; control IgG, on the other hand, had no effect on binding or internalization of PG (Fig. 4B, left). We next examined whether loss of internalization of PG in anti-ANXA2-Ab-treated cells (as shown in Fig. 4B), impacts biological effects of PG in terms of activation of p38 MAPK/ERKs. IEC-18 cells were preincubated with either control IgG or anti-ANXA2-IgG and processed for measuring relative levels of pERKs and pp38 MAPK by WIB (representative data are presented in Fig. 4C). Ratio of phosphorylated kinase-to-β-actin at 0 min was arbitrarily assigned a 100% value. Percent change in the ratio of phosphorylated kinase-to-β-actin at 15 and 30 min, compared with that at 0 min, is presented as bar graphs from three experiments (Fig. 4, Di and Dii). As expected, rhPG increasingly stimulated activation (phosphorylation) of p38 MAPK and ERKs within 15–30 min in cells treated with control IgG, whereas cells treated with anti-ANXA2-Ab did not demonstrate significant activation of the kinases (Fig. 4, C and D). Results presented in Fig. 4, C and D, strongly suggest that freely available CS-ANXA2 (unbound to antibodies) is required for internalization of PG and that internalization of PG is required for measuring biological effects of the ligand.
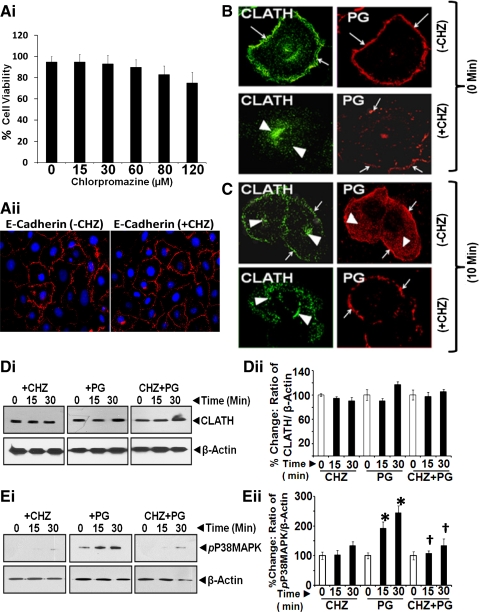
CME of PG is required for PG-stimulated activation of MAPKs. Endocytotic internalization of ligand/receptor complexes can be mediated via several pathways (29, 42), including formation of CCPs, which allows trafficking of the complex to early endosomes. We investigated a possible role of clathrin in internalization of PG. IEC-18 cells were treated with or without a specific inhibitor of CME (CHZ) (36). CHZ causes clathrin lattices to assemble on endosomal membranes and prevents CCP assembly at plasma membranes by controlling binding of adaptor protein AP2 to membranes (69). In initial experiments, we defined optimal doses of CHZ, which are nontoxic to the cells and do not inhibit the growth of IEC-18 cells in response to 1% FCS. Cells pretreated with either the vehicle control or increasing doses of CHZ (0–120 μM) were subjected to Trypan blue exclusion test. Percent cells, which were negative for Trypan blue staining (as a readout of viability) in a given field, were determined as described in the legend of Fig. 5Ai. Data from all three wells (means ± SE) are presented as bar graphs (Fig. 5Ai). CHZ at doses of 15–60 μM had no significant effect on cell viability; however, higher doses had ∼15–25% toxic effects on viability of the cells. The growth response of the cells to 1% FCS, in the presence of 15–60 μM CHZ, remained essentially similar to that measured in the absence of CHZ (data not shown), indicating that CHZ at doses of <60 μM had no significant effect on the viability of the cells. In preliminary studies, we learned that CHZ at doses of <15 μM were not as effective in inhibiting the presence of clathrin at the plasma membranes, whereas doses of 30–60 μM were effective in inhibiting the presence of clathrin molecules near the plasma membranes. We therefore chose 30 μM CHZ as an optimal dose in the present studies. To further confirm nontoxic effects of 30 μM CHZ on IEC-18 cells, cells pretreated with 30 μM CHZ for 60 min were processed for staining with anti-E-cadherin-Ab, and representative data from two experiments are presented in Fig. 5Aii. E-cadherin staining was essentially similar in cells treated with or without CHZ. Both PG and clathrin were observed near the plasma membranes, after adding PG to control, non-CHZ-treated, cells (at 0 min) (Fig. 5B, top). In CHZ (30 μM)-treated cells, clathrin was not present near the plasma membranes and remained localized in the perinuclear space (Fig. 5B, bottom). At 10 min after PG stimulation of control cells (Fig. 5C, top), significant internalization and intracellular staining of PG was evident; clathrin and PG were also present near the plasma membranes in control, PG-stimulated cells at 10 min (Fig. 5C, top). Absence of membrane-associated clathrin in CHZ-pretreated cells significantly attenuated endocytosis of PG at 10 min; however, PG remained associated with plasma membranes (presumably bound to CS-ANXA2) (Fig. 5C, bottom). These results for the first time provide evidence that internalization of PG is clathrin dependent, whereas binding of PG to CS-ANXA2 is clathrin independent (Fig. 5C, bottom). Relative expression of clathrin protein was not altered in the presence or absence of PG ± CHZ (Fig. 5, Di and Dii), confirming that absence of formation of CCPs in CHZ-treated cells resulted in attenuation of PG internalization (Fig. 5C). Importantly, PG-stimulated phosphorylation of p38 MAPK was also attenuated in CHZ-pretreated cells (Fig. 5, Ei and Eii), once again confirming that internalization of PG is required for activating MAPKinases, similar to results obtained with anti-ANXA2-Abs in Fig. 4, C–E.
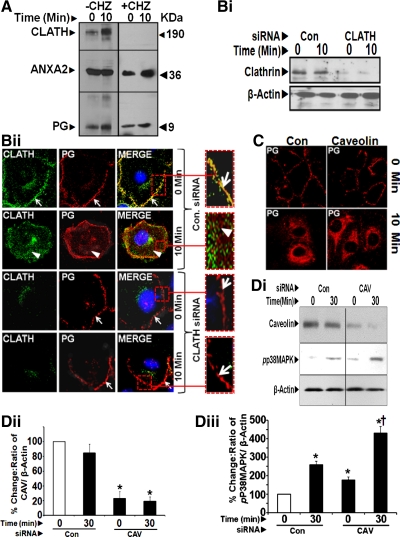
Fig. 5.
A: determination of optimal doses of chlorpromazine (CHZ) (inhibitor of clathrin-coated pit formation at the plasma membranes) for treating IEC-18 cells. IEC-18 cells were treated with increasing concentration of CHZ for 60 min and processed for staining with Trypan blue (0.2%). Percentage of cells that remained unstained by Trypan blue (Trypan blue exclusion test) was determined by counting the cells using a hemocytometer. Percent cell viability, thus measured in all six wells/dose, is presented as bar graphs in Ai. As can be seen, cells treated with doses of 15 and 30 μM CHZ remained as viable as control (0 μM) cells. Dose of 15 μM CHZ, however, was not as effective as 30 μM CHZ for completely inhibiting the presence of clathrin at the plasma membranes. We therefore chose 30 μM CHZ as an optimal dose for further investigations. To confirm nontoxic effects of 30 μM CHZ, IEC-18 cells were treated with 30 μM CHZ for 60 min and processed for staining with anti-E-cadherin-antibodies at 1:100 dilution. Cells treated with or without CHZ demonstrated a similar pattern of E-cadherin staining (Aii), confirming that the optimal dose of CHZ (30 μM) had no significant toxic effects on the cells. B and C: clathrin mediates endocytosis of PG in PG-stimulated IEC-18 cells. IEC-18 cells were pretreated with either vehicle control (−CHZ) or 30 μM chlorpromazine (+CHZ) and stimulated with PG for either 0 (B) or 10 min (C) and labeled with fluorescent Abs against clathrin (CLATH) and PG followed by confocal microscopy at ×60. Representative images (computer enhanced) from one of three experiments are presented. Arrows highlight presence of either PG (red) or clathrin (green) near plasma membranes. Arrowheads highlight either internalized PG in the absence of CHZ treatment or intracellular localization of clathrin in CHZ-treated cells. D and E: chlorpromazine (CHZ) inhibits activation of p38 MAPK. IEC-18 cells were treated with CHZ, PG, or CHZ + PG. Relative levels of clathrin were similar in all treatment groups (D). Representative Western blots are presented in Di; % change in ratio of clathrin-to-β-actin (calculated as described above) are presented as means ± SE of data from three experiments (Dii). CHZ alone had no effect on relative levels of pp38 MAPK (Ei and Eii, left), whereas PG alone significantly stimulated activation (phosphorylation) of p38 MAPK (Ei and Eii, middle). In CHZ-pretreated cells, PG failed to stimulate phosphorylation of p38 MAPK (Ei and Eii, right). Ei shows representative WIB from three experiments; Eii shows means ± SE of data from all three experiments. *Significant difference vs. CHZ alone (P < 0.05). †Significant difference vs. PG alone (P < 0.05).
To further assess association of PG with ANXA2 and clathrin, pull-down assays were conducted with anti-PG-Abs. In the absence of CHZ pretreatment, both clathrin and ANXA2 CO-IP with anti-PG-Abs (Fig. 6A). Even at 0 min, low levels of clathrin were associated with PG and ANXA2; CO-IP was increased several fold after 10 min of PG stimulation (Fig. 6A, left). PG and ANXA2 did not CO-IP with clathrin in CHZ-treated cells at 0 and 10 min (Fig. 6A, right). Results with CHZ treatment in Figs. 5, B–E, and 6A, were confirmed in cells downregulated for clathrin expression (using rat clathrin siRNA). Downregulation of clathrin was confirmed by WIB analysis of the cells (Fig. 6Bi). In control siRNA-treated cells, co-localization of clathrin and PG on the cellular membranes can be visualized at 0 min and to a lower extent at 10 min (as can be seen from the enhanced images of the insets in the top two panels of Fig. 6Bii). In clathrin siRNA-treated cells (bottom two panels in Fig. 6Bii), PG remained bound to ANXA2 on cell membranes at both 0 and 10 min, with significant attenuation of PG internalization at 10 min (Fig. 6Bii, bottom two panels).
Fig. 6.
A: CHZ treatment attenuates association of PG/ANXA2 with clathrin. Cells pretreated with or without CHZ, followed by PG stimulation, were processed for IP with anti-PG-Abs and WIB analysis. At 30 min, no bands were visible, suggesting degradation of PG after 15 min (data not shown). In CHZ-treated samples, PG/ANXA2 did not CO-IP with clathrin. B: downregulation of clathrin with clathrin siRNA attenuates internalization of PG. Representative Western blots from one of two experiments demonstrating loss of clathrin expression in IEC-18 cells treated with clathrin (CLATH) siRNA (Bi). IEC-18 cells, pretreated with either control (CON) or clathrin (CLATH) siRNA were stimulated with PG for 0–10 min and labeled with fluorescent antibodies against clathrin and PG and imaged at ×60. Representative images obtained with clathrin and PG-Abs are presented in Bii. Presence of PG and clathrin near the plasma membranes in control siRNA-treated cells is shown in insets at 0 min (arrows). Internalization of PG at 10 min in control siRNA-treated cells is also presented in the inset, after zooming in on the indicated area (arrowheads); in control siRNA-treated cells, intracellular clathrin can also be observed (arrowheads). C and D: caveolin (CAV) is not required for internalization of PG and signaling in response to PG. IEC-18 cells were pretreated with either control or caveolin-specific siRNA (C) and stimulated with PG for 0–10 min. Treated cells were processed for imaging with epifluorescence microscope after labeling with fluorescent antibodies against PG. Representative images presented in C are from one of two similar experiments. There was no difference in the internalization of PG at 10 min in control siRNA vs. caveolin siRNA-treated cells (C). Control and CAV siRNA-treated cells were also processed by WIB for the indicated proteins, and representative blots from one of two separate experiments are presented in Di. Western blot data from all the experiments are presented as % change in the ratios of caveolin-to-β-actin (Dii) and pp38 MAPK-to-β-actin (Diii). *Significant difference vs. 0-min values in control siRNA-treated samples (P < 0.05). †Significant difference vs. 0-min in caveolin siRNA-treated samples (P < 0.05). Loss of caveolin expression in caveolin siRNA-treated samples (Di and Dii), however, had no effect on activation of p38 MAPK (Di and Diii). If anything, relative levels of pp38 MAPK increased in caveolin siRNA-treated samples vs. control siRNA-treated samples for reasons unknown.
To examine a possible role of caveolin in endocytosis of PG, IEC-18 cells were pretreated with rat caveolin-specific siRNA. Cells treated with control siRNA demonstrated the expected pattern of membrane-associated PG at 0 min followed by intracellular localization at 10 min (Fig. 6C, left). This pattern of PG localization remained essentially similar in cells treated with caveolin siRNA (Fig. 6C, right). Downregulation of caveolin expression was confirmed by WIB (Fig. 6, Di and Dii). Relative levels of pp38 MAPK were measured as a readout of functional effects of PG in cells downregulated for caveolin expression (Fig. 6, Di and Diii). Surprisingly, relative levels of pp38 MAPK were increased at 30 min after PG stimulation in caveolin siRNA vs. control siRNA-treated cells (Fig. 6, Di and Diii) for unknown reasons. These results demonstrate that loss of caveolin expression has no effect on either binding, internalization, or activation of PG-stimulated signaling pathways.
DISCUSSION
Our studies confirm in situ co-localization of PG with CS-ANXA2 on intestinal epithelial cells. We learned for the first time that PG, after binding to CS-ANXA2, is rapidly internalized via CME and that internalization of PG is required for PG-stimulated activation of signaling pathways.
CS-ANXA2 has been reported on endothelial cells, skin keratinocytes, and several tumor cells (6, 17, 35, 44, 72). Functional significance of CS-ANXA2 in proliferation and metastasis of cancer cells is becoming increasingly evident (12, 13, 21, 25, 26, 33, 52–54, 73). Biochemical mechanisms mediating translocation of ANXA2 to cell surface of endothelial cells have been examined (10). Mechanisms by which ANXA2 translocates to cell surface of epithelial cells have also been recently examined. Wounding was reported to stimulate presentation of ANXA2 on the surface of human airway epithelial cells (46). It is possible that immortalization (as in the case of HEK293 and IEC-18 cells) or transformation (as in the case of cancer cells) is conducive to translocation and presence of CS-ANXA2 on epithelial cells. Exosomes, which are known to be increasingly secreted by cancer cells, contain annexins (5, 37, 66) and may represent the source of CS-ANXA2 and soluble ANXA2 measured in the conditioned medium and serum of cancer cells/patients (24, 27, 50). Exosomal ANXA2 was recently reported to be internalized by raft-mediated pathways in cancer cells and traffic to endosomes (66), providing strong evidence that extracellular ANXA2 can be internalized in cells by many different pathways. Based on the results of our studies, we speculate that ligand binding of CS-ANXA2 may also result in internalization of extracellular ANXA2 bound to the ligand via CME; the latter possibility needs to be confirmed in future studies.
CS-ANXA2 functions as a high-affinity receptor for several ligands including tenascin C-splice variants, angiotensin II, vitamin D, tPA, and PG (reviewed in Ref. 61; present studies). We have previously reported that downregulation of ANXA2 results in almost complete loss of proliferative effects of PG on the target cells (60). In the present studies, we demonstrate for the first time that CS-ANXA2 expression is not only required for binding PG to IEC cells but is also required for internalization of PG. Our present studies additionally suggest that CME plays a critical role in internalization of PG and that PG appears to remain associated with ANXA2 during endocytosis.
CME is a major pathway for internalization of receptors and receptor/ligand complexes (20, 31, 32, 51, 67). Ligand binding to receptors leads to recruitment of adaptor proteins (APs) and induces assembly of clathrin triskelions, a three-legged lattice of heavy and light chains of clathrin, resulting in formation of clathrin-coated vesicles or pits (CCVs/CCPs) (15). Selection of cargo into CCPs relies on internalization motifs present on receptor proteins, such as YxxΦ (28), which binds APs, such as AP-2 (composed of α, β2, μ2, and σ2 subunits) (40, 62). The NH2-terminal domain of ANXA2 contains two copies of YxxΦ motif, and in vitro interaction between μ2 and ANXA2 has been reported (9). The GTPase dynamin induces fission of CCPs, removing clathrin, and fuses with early endosomes (15). A homologous protein, ANXA1 present on cell surface of many cell types lacks the motifs for binding μ2 and hence does not traffic from plasma membranes to intracellular compartments (9). Interaction between AP-2 and ANXA2 can initiate assembly of CCPs and potentially result in internalization of ligands bound to ANXA2. In the present studies, both CHZ (an inhibitor of CCP formation) and clathrin siRNA attenuated internalization of PG (Figs. 5, A–D, and 6, A and B), confirming a critical role of clathrin in internalization/endocytosis of PG, which appears to remain associated with ANXA2. At the present time, it is not known whether CS-ANXA2 directly facilitates internalization of PG via CME or whether other unknown mechanism(s) are involved in facilitating internalization of PG via CME after binding with CS-ANXA2. It is, however, clear that availability of antibody-free CS-ANXA2 is required for internalization of bound PG (Fig. 4B).
Several clathrin-independent pathways are also involved in endocytosis of receptor/ligand complexes, including caveolin-dependent endocytosis and membrane rafts (42). Our results strongly suggest that caveolin 1 plays no role in internalization of PG (Fig. 6, C and D). It is, however, possible that some % of extracellular PG, with or without binding to ANXA2, is internalized via clathrin-independent pathways. Our studies so far strongly suggest that CME represents the major pathway by which PG bound to ANXA2 is endocytosed in target cells.
Only monomeric or dimeric ANXA2 bind clathrin and early endosomes; heterotetrameric ANXA2 (which includes two molecules of p11) does not associate with clathrin (9) or early endosomes (41). ANXA2 participates in both the recycling pathway (75) and the degradation pathway, leading to late endosomes and lysosomes (38). ANXA2, partially Tyr phosphorylated on early endosomes, regulates biogenesis of multivesicular intermediates destined for late endosomes and/or exosomes; mutation of Tyr-23 impaired association of ANXA2 to endosomes and exosomes (41, 66). Importantly, translocation and association of ANXA2 to the cell surface of pancreatic cancer cells was reported to require Tyr phosphorylation (74). ANXA2 is Tyr phosphorylated by Src kinases (3). Since PG upregulates Src kinase activity (4), it is possible that Src activation in response to PG facilitates internalization and trafficking of PG/ANXA2 complexes via the endocytotic pathway, which needs to be examined in appropriately designed experiments in the future.
PG activates several signaling molecules/transcription factors in target cells (including Src, PI3K, Akt, JAK2, STAT5/3, ERKs, p38 MAPK, NFκB, β-catenin, and Jagged1) (4, 14, 45, 49), as diagrammatically presented in a previous paper (49). Activation of p38 MAPK/ERKs/NF-κB p65/β-catenin was confirmed in IEC-18 cells in response to PG (Figs. 2B and 3, A–D). Results with anti-ANXA2-Abs, CHZ, and clathrin siRNA strongly suggest that internalization of PG is required for activating PG-mediated signaling pathways (Figs. 4, C and D, 5, B–E, and 6B). CME of many peptide/receptor complexes is required for activation of downstream signaling pathways, including MAPK/NF-κB (36). Effective signal transduction depends on internalization, as opposed to short-term signaling from cell surface (19). Internalization of GPCRs typically serves to terminate signaling; however, in some cases, endocytosis is required for receptor signaling (22, 67). CME is required for efficient receptor-mediated signal transduction of many cytokine receptors (18). CME of TGF-βRs increases TGF-β signaling, whereas membrane raft-mediated endocytosis promotes receptor degradation (11, 39). Targeting activated receptor Tyr kinases to clathrin-dependent endocytosis pathways, rather than non-clathrin endocytosis, was reported to be necessary for sustained signaling and growth response (16, 56). We reported sustained activation of NF-κB in response to PG (48), which was required for measuring growth effects of PG on pancreatic cancer cells (48). Based on our present findings, it appears likely that CME of PG may mediate previously reported (48) sustained activation of NF-κB, resulting in the observed growth response of the cells to PG.
Since ANXA2 lacks transmembrane domains, mechanisms mediating translocation of PG, bound to CS-ANXA2, from outer cellular membranes toward intracellular compartments has remained a puzzle. One report suggested that ANXA2 undergoes conformational changes at low pH, similar to that measured in the presence of calcium, allowing access of ANXA2 to hydrophobic part of plasma membrane (30). It has also been proposed that ANXA2 can masquerade as transmembrane receptors and initiate CCP formation at the cell surface (9) by associating with μ2 subunit of AP-2. It is also possible that association of ANXA2 with other transmembrane proteins such as CD44, ANXA2 receptor (ANXA2R; Ref. 34), and CD147-like proteins may facilitate internalization of PG bound to CS-ANXA2; these possibilities should be examined in future studies.
GRANTS
This work was supported by National Cancer Institute Grants CA-97959 and CA-114264 to P. Singh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.S. and C.K. performed experiments; S.S. analyzed data; S.S. interpreted results of experiments; S.S. and C.K. prepared figures; P.S. conception and design of research.
ACKNOWLEDGMENTS
The technical help of Carrie Maxwell and secretarial assistance of Cheryl Simmons are acknowledged.
REFERENCES
- 1. Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin(17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer 94: 307–313, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Baldwin GS, Hollande F, Yang Z, Karelina Y, Paterson A, Strang R, Fourmy D, Neumann G, Shulkes A. Biologically active recombinant human progastrin(6–80) contains a tightly bound calcium ion. J Biol Chem 276: 7791–7796, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bellagamba C, Hubaishy I, Bjorge JD, Fitzpatrick SL, Fujita DJ, Waisman DM. Tyrosine phosphorylation of annexin II tetramer is stimulated by membrane binding. J Biol Chem 272: 3195–3199, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Brown D, Yallampalli U, Owlia A, Singh P. pp60c-Src Kinase mediates growth effects of the full-length precursor progastrin1–80 peptide on rat intestinal epithelial cells, in vitro. Endocrinology 144: 201–211, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Choi DS, Park JO, Jang SC, Yoon YJ, Jung JW, Choi DY, Kim JW, Kang JS, Park J, Hwang D, Lee KH, Park SH, Kim YK, Desiderio DM, Kim KP, Gho YS. Proteomic analysis of microvesicles derived from human colorectal cancer ascites. Proteomics 11: 2745–2751, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Chung CY, Erickson HP. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J Cell Biol 126: 539–548, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ciccotosto GD, McLeish A, Hardy KJ, Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology 109: 1142–1153, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Cobb S, Wood T, Ceci J, Varro A, Velasco M, Singh P. Intestinal expression of mutant and wild-type progastrin significantly increases colon carcinogenesis in response to azoxymethane in transgenic mice. Cancer 100: 1311–1323, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Creutz CE, Snyder SL. Interactions of annexins with the mu subunits of the clathrin assembly proteins. Biochemistry 44: 13795–13806, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Deora AB, Kreitzer G, Jacovina AT, Hajjar KA. An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J Biol Chem 279: 43411–43418, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5: 410–421, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Díaz VM, Hurtado M, Thomson TM, Reventós J, Paciucci R. Specific interaction of tissue-type plasminogen activator (t-PA) with annexin II on the membrane of pancreatic cancer cells activates plasminogen and promotes invasion in vitro. Gut 53: 993–1000, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esposito I, Penzel R, Chaib-Harrireche M, Barcena U, Bergmann F, Riedl S, Kayed H, Giese N, Kleeff J, Friess H, Schirmacher P. Tenascin C and annexin II expression in the process of pancreatic carcinogenesis. J Pathol 208: 673–685, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Ferrand A, Bertrand C, Portolan G, Cui G, Carlson J, Pradayrol L, Fourmy D, Dufresne M, Wang TC, Seva C. Signaling pathways associated with colonic mucosa hyperproliferation in mice overexpressing gastrin precursors. Cancer Res 65: 2770–2777, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T, Walz T. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature 432: 573–579, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Goh LK, Huang F, Kim W, Gygi S, Sorkin A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol 189: 871–883, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hajjar KA, Jacovina AT, Chacko J. An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J Biol Chem 269: 21191–21197, 1994 [PubMed] [Google Scholar]
- 18. Henriques CM, Rino J, Nibbs RJ, Graham GJ, Barata JT. IL-7 induces rapid clathrin-mediated internalization and JAK3-dependent degradation of IL-7Ralpha in T cells. Blood 115: 3269–3277, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Hoeller D, Volarevic S, Dikic I. Compartmentalization of growth factor receptor signalling. Curr Opin Cell Biol 17: 107–111, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Ikari A, Takiguchi A, Atomi K, Sugatani J. Epidermal growth factor increases clathrin-dependent endocytosis and degradation of claudin-2 protein in MDCK II cells. J Cell Physiol 226: 2448–2456, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Jacovina AT, Deora AB, Ling Q, Broekman MJ, Almeida D, Greenberg CB, Marcus AJ, Smith JD, Hajjar KA. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2-dependent fibrinolysis. J Clin Invest 119: 3384–3394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jean-Alphonse F, Hanyaloglu AC. Regulation of GPCR signal networks via membrane trafficking. Mol Cell Endocrinol 331: 205–214, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Jin G, Ramanathan V, Quante M, Baik GH, Yang X, Wang SS, Tu S, Gordon SA, Pritchard DM, Varro A, Shulkes A, Wang TC. Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest 119: 2691–2701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji NY, Park MY, Kang YH, Lee CI, Kim DG, Yeom YI, Jang YJ, Myung PK, Kim JW, Lee HG, Kim J, Lee K, Song EY. Evaluation of annexin II as a potential serum marker for hepatocellular carcinoma using a developed sandwich ELISA method. Int J Mol Med 24: 765–771, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Kantara C, Sarkar S, Swiercz R, Ortiz I, Singh P. Over-expression of progastrin imparts tumorigenic/metastatic potential to immortalized embryonic cells and cancer cells: role of stem/progenitor cell markers and annexin A2. In: Proceedings of the Digestive Disease Week, Chicago, IL, May 2011, abstract no. 208, A-52 [Google Scholar]
- 26. Kesavan K, Ratliff J, Johnson EW, Dahlberg W, Asara JM, Misra P, Frangioni JV, Jacoby DB. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J Biol Chem 285: 4366–4374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim DM, Noh HB, Park DS, Ryu SH, Koo JS, Shim YB. Immunosensors for detection of annexin II and MUC5AC for early diagnosis of lung cancer. Biosens Bioelectron 25: 456–462, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Kozik P, Francis RW, Seaman MN, Robinson MS. A screen for endocytic motifs. Traffic 11: 843–855, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124: 997–1009, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lambert O, Cavusoglu N, Gallay J, Vincent M, Rigaud JL, Henry JP, Ayala-Sanmartin J. Novel organization and properties of annexin 2-membrane complexes. J Biol Chem 279: 10872–10882, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Lei JT, Martinez-Moczygemba M. Separate endocytic pathways regulate IL-5 receptor internalization and signaling. J Leukoc Biol 84: 499–509, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu AP, Aguet F, Danuser G, Schmid SL. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J Cell Biol 191: 1381–1393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Longerich T, Haller MT, Mogler C, Aulmann S, Lohmann V, Schirmacher P, Brand K. Annexin A2 as a differential diagnostic marker of hepatocellular tumors. Pathol Res Pract 207: 8–14, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Lu G, Maeda H, Reddy SV, Kurihara N, Leach R, Anderson JL, Roodman GD. Cloning and characterization of the annexin II receptor on human marrow stromal cells. J Biol Chem 81: 30542–30550, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Ma AS, Bel DJ, Mittal AA, Harrison HH. Immunocytochemical detection of extracellular annexin II in cultured human skin keratinocytes and isolation of annexin II isoforms enriched in the extracellular pool. J Cell Sci 107: 1973–1984, 1994 [DOI] [PubMed] [Google Scholar]
- 36. Marina-García N, Franchi L, Kim YG, Hu Y, Smith DE, Boons GJ, Núñez G. Clathrin- and dynamin-dependent endocytic pathway regulates muramyl dipeptide internalization and NOD2 activation. J Immunol 182: 4321–4327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol Cell Proteomics 9: 197–208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mayran N, Parton RG, Gruenberg J. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J 22: 3242–353, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McLean S, Di Guglielmo GM. TGF beta (transforming growth factor beta) receptor type III directs clathrin-mediated endocytosis of TGF beta receptor types I and II. Biochem J 429: 137–145, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Mettlen M, Loerke D, Yarar D, Danuser G, Schmid SL. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J Cell Biol 188: 919–933, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morel E, Gruenberg J. Annexin A2 binding to endosomes and functions in endosomal transport are regulated by tyrosine 23 phosphorylation. J Biol Chem 284: 1604–1611, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol 161: 673–677, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ottewell PD, Varro A, Dockray GJ, Kirton CM, Watson AJ, Wang TC, Dimaline R, Pritchard DM. COOH-terminal 26-amino acid residues of progastrin are sufficient for stimulation of mitosis in murine colonic epithelium in vivo. Am J Physiol Gastrointest Liver Physiol 288: G541–G549, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Ortiz-Zapater E, Peiró S, Roda O, Corominas JM, Aguilar S, Ampurdanés C, Real FX, Navarro P. Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am J Pathol 170: 1573–1784, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pannequin J, Bonnans C, Delaunay N, Ryan J, Bourgaux JF, Joubert D, Hollande F. The wnt target jagged-1 mediates the activation of notch signaling by progastrin in human colorectal cancer cells. Cancer Res 69: 6065–6073, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Patchell BJ, Wojcik KR, Yang TL, White SR, Dorscheid DR. Glycosylation and annexin II cell surface translocation mediate airway epithelial wound repair. Am J Physiol Lung Cell Mol Physiol 293: L354–L363, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Rengifo-Cam W, Singh P. Role of progastrins and gastrins and their receptors in GI and pancreatic cancers: targets for treatment. Curr Pharm Des 10: 2345–2358, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Rengifo-Cam W, Umar S, Sarkar S, Singh P. Antiapoptotic effects of progastrin on pancreatic cancer cells are mediated by sustained activation of nuclear factor-κB. Cancer Res 67: 7266–7274, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Sarkar S, Swiercz R, Kantara C, Hajjar KA, Singh P. Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem cell in response to progastrin in mice and HEK-293 cells. Gastroenterology 140: 583–595, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sarkar S, Maxwell CA, Luthra G, Singal A, Qiu S, Bauer VP, Okorodudu AO, Singh P. Annexina2 is increasingly expressed and released into the serum of patients positive for colonic growths/tumors in relation to disease progression: diagnostic implications. Gastroenterology 140, Suppl 1: S-341, 2011 [Google Scholar]
- 51. Schmid SL. Clathrin-mediated endocytosis: a universe of new questions. Mol Biol Cell 21: 3818–3819, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sharma M, Ownbey RT, Sharma MC. Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp Mol Pathol 88: 278–286, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Sharma MC, Sharma M. The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target. Curr Pharm Des 13: 3568–3575, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem 105: 370–380, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siddheshwar RK, Gray JC, Kelly SB. Plasma levels of progastrin but not amidated gastrin or glycine extended gastrin are elevated in patients with colorectal carcinoma. Gut 48: 47–52, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell 15: 209–219, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Singh P, Xu Z, Dai B, Rajaraman S, Rubin N, Dhruva B. Incomplete processing of progastrin expressed by human colon cancer cells: role of noncarboxyamidated gastrins. Am J Physiol Gastrointest Liver Physiol 266: G459–G468, 1994 [DOI] [PubMed] [Google Scholar]
- 58. Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology 119: 162–171, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Singh P, Lu X, Cobb S, Miller BT, Tarasova N, Varro A, Owlia A. Progastrin1–80 stimulates growth of intestinal epithelial cells in vitro via high-affinity binding sites. Am J Physiol Gastrointest Liver Physiol 284: G328–G339, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Singh P, Wu H, Clark C, Owlia A. Annexin II binds progastrin and gastrin-like peptides, and mediates growth factor effects of autocrine and exogenous gastrins on colon cancer and intestinal epithelial cells. Oncogene 26: 425–440, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Singh P. Role of annexin-II in GI cancers: interaction with gastrins/progastrins. Cancer Lett 252: 19–35, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol 10: 583–596, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Umar S, Sarkar S, Cowey S, Singh P. Activation of NF-kappaB is required for mediating proliferative and antiapoptotic effects of progastrin on proximal colonic crypts of mice, in vivo. Oncogene 27: 5599–5611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Umar S, Sarkar S, Wang Y, Singh P. Functional cross-talk between beta-catenin and NFkappaB signaling pathways in colonic crypts of mice in response to progastrin. J Biol Chem 284: 22274–22284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Van Solinge WW, Nielsen FC, Friis-Hansen L, Falkmer UG, Rehfeld JF. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology 104: 1099–1107, 1993 [DOI] [PubMed] [Google Scholar]
- 66. Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem 286: 30911–30925, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol 19: 436–445, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Walsh JH. Gastrin. In: Gut Peptides: Biochemistry and Physiology, edited by Walsh JH, Dockray GJ. New York; Raven, 1994 [Google Scholar]
- 69. Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol 123: 1107–1117, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918–1929, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu H, Owlia A, Singh P. Precursor peptide progastrin(1–80) reduces apoptosis of intestinal epithelial cells and upregulates cytochrome c oxidase Vb levels and synthesis of ATP. Am J Physiol Gastrointest Liver Physiol 285: G1097–G1110, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Yeatman TJ, Updyke TV, Kaetzel MA, Dedman JR, Nicolson GL. Expression of annexins on the surfaces of non-metastatic and metastatic human and rodent tumor cells. Clin Exp Metastasis 11: 37–44, 1993 [DOI] [PubMed] [Google Scholar]
- 73. Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li Y, Jiang JL, Zhang SH, Chen ZN. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci 101: 387–395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT, Anders RA, Illei PB, Van Eyk JE, Maitra A, Laheru D, Jaffee EM. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLos One 6: e19390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zobiack N, Rescher U, Ludwig C, Zeuschner D, Gerke V. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol Biol Cell 14: 4896–4908, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]