Abstract
Poorly synchronized activation of the ventricles can lead to impairment of normal cardiac structure/function. We reported previously that short term (4 h) left ventricular (LV) pacing-induced ventricular dyskinesis led to an inflammatory response localized to the epicardium. Results from this study demonstrated that neutrophils may play a major role in this inflammatory process. Neutrophil recruitment to a site of injury is a process that is highly dependent on an upregulation of cell adhesion molecules (CAM). The dependence of ventricular dysynchrony-induced inflammatory responses on CAM upregulation has not been explored. To gain further insight, we used a mouse model of LV pacing to evaluate the role of CAM in mediating the inflammatory response associated with ventricular dyskinesis. We first examined the effects of LV pacing in wild-type mice. Results demonstrate that 40 min of LV pacing increases ICAM-1 immunostaining as well as myeloperoxidase activity and tissue oxidative stress by twofold in early-activated myocardium. Matrix metalloproteinase-9 activity also increased in the same region by ∼3.5-fold. To determine the role of CAM, mice null for ICAM-1 or p-selectin were subjected to 40 min LV pacing. Results demonstrate that the inflammatory response seen in the wild-type mice was significantly mitigated in the ICAM-1 and p-selectin null mice. In conclusion, results demonstrate that CAM expression plays a critical role in the triggering of LV pacing-induced inflammation, thus providing evidence of a vascular mechanism underlying this response. The mechanisms that trigger an upregulation of myocardial CAM expression and, therefore, inflammation await further investigation since they suggest a specific involvement of vascular events.
Keywords: left ventricular pacing, inflammation
abnormal activation of the ventricles has emerged as a major contributor to the pathophysiology of heart failure. Perturbations in the normal sequence of left ventricular (LV) activation can create regions of early and late activation, leading to dysynchronous contraction and areas of dyskinesis (23). It has been shown that poorly synchronized activation of the LV can impair diastolic and systolic function, promote chamber remodeling, and lead to increased morbidity and mortality in patients with heart failure (22).
Mechanisms that trigger LV remodeling associated with ventricular dysynchrony are not fully understood. We reported previously the results of a study in which we induced abnormal wall motion (i.e., dyskinesis) on the anterior lateral wall of canine hearts by LV pacing. Results demonstrated that 4 h of epicardial LV pacing triggered increases in myeloperoxidase (MPO) activity, reactive oxygen species (ROS) generation, 92 kDa matrix metalloproteinase (MMP-9) activity, and collagen degradation at the early-activated site (anterior wall of the LV) (9). Neutrophils likely play a major role in this inflammatory-like response to ventricular dyskinesis by producing and/or releasing ROS and proteases that damage the myocardium. They also release inflammatory products that amplify the recruitment and activation of a greater number of neutrophils into the effected myocardium, thereby extending the severity of injury (10). In a follow-up large animal LV pacing study, we reported that the inflammatory response is localized exclusively to the epicardial layer and was associated with a 30% local loss of endocardial thickening (24). In this study, histological analysis demonstrates increases in neutrophil infiltration, as determined by CD-18, with colocalized MMP-9 staining in the early-activated sites (24), suggesting the role of neutrophils in mediating the inflammatory response. Altogether, these results indicate that short-term LV pacing-induced ventricular dyskinesis triggers a localized epicardial inflammatory response likely mediated by neutrophil infiltration, which leads to collagen damage. These events, if sustained over time, may explain the long-term remodeling observed in patients with ventricular dysynchrony.
Neutrophil recruitment to a site of injury is a process that is highly dependent on an upregulation of cell adhesion molecules (CAM). CAM comprises a large family of important members. Published literature has documented critical roles for ICAM-1 and p-selectin in the developmental role of myocardial inflammation following ischemia-reperfusion injury (4). Tissue infiltration by circulating neutrophils is a multistep process. The first step is initiated by neutrophils rolling on the luminal side of the endothelium, as mediated by the low-affinity receptors selectins (i.e., E- and p- selectins) (6). Binding of selectins stimulates outside-in signals in the neutrophils, increasing the affinity of the integrin family of receptors, which then bind to endothelial CAM such as ICAM-1 (6). This binding arrests the rolling neutrophils and subsequently allows transmigration to occur (6).
The dependence of ventricular dysynchrony-induced inflammatory responses on CAM upregulation has not been explored. To address this issue, rodent models of ventricular dysynchrony can be a useful tool to better understand the role of specific CAM in the setting of dyskinesis given the availability of mice null for CAM. Therefore, the major objectives of this study are to 1) implement a mouse model of acute ventricular dyskinesis, 2) examine the effects of LV pacing on local inflammation in wild-type mice, and 3) assess the role played by CAM in the development of dyskinesis-induced inflammation by using ICAM-1 or p-selectin null mice.
METHODS
Animal studies.
All procedures were approved by the University of California, San Diego Institutional Animal Care and Use Committee and conform to published National Institutes of Health guidelines for animal research.
Study groups.
To assess the effects of anesthesia and electrode insertion on myocardial inflammatory markers, a group of C57BL/6 wild-type male mice (n = 18) were instrumented but not paced. A second group of wild-type mice (n = 24) underwent instrumentation and LV pacing to determine whether dysynchrony induces similar inflammatory responses as seen in the canine model of LV pacing (9, 24). To examine the effect of dysynchrony on CAM and inflammatory responses, mice deficient in CAM (The Jackson Laboratory, ICAM-1: B6.129S4-Icam1tm1Jcgr/J or p-selectin null mice: B6.129S7-Selptm1Bay/J) were used. For both null models, one group of animals served as controls to study the effects of anesthesia and instrumentation on inflammatory markers (ICAM-1−/− mice, n = 10; p-selectin−/− mice, n = 5). The second group (ICAM-1−/− mice, n = 13; p-selectin−/− mice, n = 6) was instrumented and underwent LV pacing.
Surgical preparation.
Mice (22–26 g) were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally, intubated, and mechanically ventilated with room air (Kent Scientific Respirator). The heart was exposed via a thoracotomy through the 5th intercostal space. After pericardial dissection, a 36-gauge dual-insulated magnet wire (Belden CDT, Richmond, IN) with a hook on the end was inserted into the LV anterior wall via a 27-gauge needle to serve as a unipolar electrode. The chest was then closed with 6-0 silk before pacing. Another unipolar electrode was introduced into the abdominal muscles to serve as a ground. Surface electrocardiograms were recorded throughout the study.
Study design.
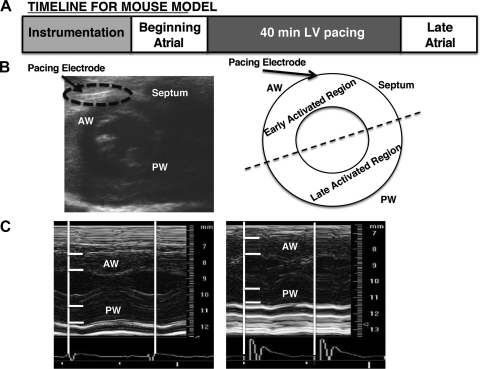
LV pacing was initiated within 5–10 min after instrumentation. During this time the animal was allowed to stabilize and regain normal sinus rhythm (∼500 beats/min). A square-wave, constant-voltage electronic stimulator (Grass Instruments, Quincy, MA) was used at a frequency 10–20% above baseline heart rate to suppress native sinus rhythm. LV pacing-induced electromechanical dysynchrony was verified by the widening of the QRS complex on the ECG or by the analysis of echocardiography images. LV dysynchrony was maintained for 40 min (this time point was calculated so that the number of beats of a murine heart is the same as the number of beats of a canine heart during 4 h). Figure 1A diagrams the experimental protocol we implemented in our studies. In a subgroup of animals, C57B6/L wild-type mice underwent 40 min of subthreshold pacing (voltage 10% below threshold).
Fig. 1.
Pacing preparation and experimental protocol. A: experimental design for mouse model of ventricular dysynchrony. Briefly, a unipolar pacing electrode was inserted into the left ventricle (LV) of the mouse. Successful implantation of the electrode was visualized by the 2-dimensional echo images (B) and the induction of ventricular dysynchrony was determined by M-mode echocardiography analysis of anterior and posterior wall changes in wall thickness during the cardiac cycle (C). Tissue samples were collected from the early-activated region and late-activated regions from all hearts and used for biochemical analysis. AW, anterior wall; PW, posterior wall.
In the nonpaced control group, recordings took place immediately after instrumentation (beginning atrial), at 40 min, and immediately after (late atrial). In the paced group, recordings took place immediately after instrumentation (beginning atrial), 40 min under LV pacing, and then immediately after under normal sinus rhythm (late atrial). Additional recordings took place in all animals between the beginning atrial and 40 min timepoints. Data from selected timepoints are shown.
Ventricular function.
In a subgroup of animals, mechanical dysynchrony was documented by echocardiography (Vevo 770, Visual Sonics, RMV 700-Series scanhead). A closed chest preparation was utilized to optimize echocardiography images. Animals were anesthetized with isoflurane at 5% and maintained at 1–1.5% throughout the study. Unipolar electrodes were inserted as above. Successful placement of the ventricular electrode was visualized using echo images (Fig. 1B). Ventricular dysynchrony was identified by the visual inspection of dysynchronous wall motion using EKV (ECG-Gated Kilohertz Visualization) software (online supplement). Typical B-mode imaging achieves up to 400 frames/s, whereas EKV imaging and image reconstruction can provide 1,000 frames/s. EKV imaging provides a higher temporal resolution than B-mode so cardiac wall abnormalities can be assessed. For EKV imaging, the transducer was attached to a fixed arm, and, over 6 min, synchronized electrocardiogram (ECG) signals and B-mode images were acquired and then processed for reconstruction of two-dimensional cine spanning one cardiac cycle (12, 15).
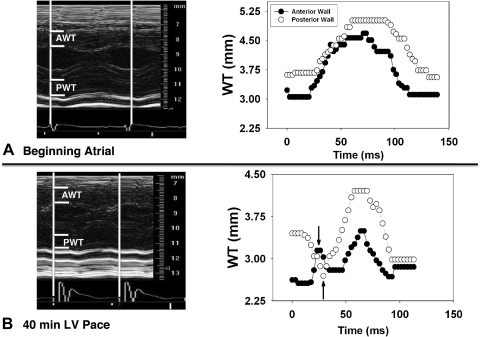
Additional documentation of ventricular dysynchrony was generated by analysis of M-mode echo recordings of LV anterior and posterior wall thickness using ImageJ. Briefly, the image pixels were calibrated with the scale within the echo image. Measurements (20–30) of the anterior wall thickness and posterior wall thickness were taken every 3 ms throughout one cardiac cycle (Figs. 1C and 2). Wall thickness was plotted as a function of time to determine whether early shortening occurred during pacing.
Fig. 2.
Representative M-mode echocardiogram in a mouse during normal sinus rhythm (A; n = 4) and 40 min of LV free wall pacing (B; n = 4). Dysynchrony is demonstrated during LV free wall pacing by the presence of early anterior wall thickening and early posterior wall thinning (arrows). AWT, anterior wall thickness; PWT, posterior wall thickness; WT, wall thickness.
Sacrifice protocol and tissue collection.
After 40 min of ventricular dysynchrony, the electrical stimulator was turned off. Animals were euthanized with pentobarbital sodium (200 mg/kg), and hearts were excised. Tissue samples were taken from the early-activated region (anterior wall) and late-activated region (posterior wall), as indicated by two-dimensional echo images (Fig. 1B) and stored at −80°C. In control animals, regions surrounding the pacing electrode were classified as early-activated region and the opposite wall as the late-activated region. In a subgroup of animals, tissue samples were snap-frozen in cryo-embedding media and stored at the −80°C.
Immunohistochemistry.
Using a cryotome, 7-μm sections of myocardium were obtained. A streptavidin/alkaline phosphatase-based protocol with Vector Red (Vector Labs) was used for immunohistochemical staining of the myocardium (26). Briefly, blocking was performed with 10% serum (from the animal source of the secondary antibody to be used) in 0.1% bovine-serum albumin/Tris-buffered saline. Primary ICAM-1 antibodies (BD Biosciences) were added and incubated for 60 min at room temperature. Preimmune sera of the animal species in which the primary antibody was developed were used as negative controls. Biotinylated immunoglobin G was used as a secondary antibody, and incubation was carried out for 60 min. This step was followed by incubation with alkaline phosphatase-conjugated streptavidin for 30 min.
MPO activity.
Tissue samples were homogenized in ice-cold buffer (50 mM KH2PO4 and 0.5% hexadecyltrimethylammonium bromide, pH 6.0). Homogenates were incubated on ice for 30 min and centrifuged at 4°C for 5 min. Following centrifugation, the supernatants were reacted with 0.8 mM tetramethylbenzidine (Sigma, St. Louis, MO) and 0.006% H2O2 in 50 mM KH2PO4. Kinetic absorbance measurements of MPO activity were immediately monitored at 655 nm (readings every 40 s for ∼20 min). Substrate cleavage rates were determined from the linear regions of the kinetic curve. Data were normalized to protein concentrations determined the BCA protein assay kit (Pierce, Rockford, IL) and are expressed as arbitrary units (AU).
Glutathione assay.
Reduced and oxidized glutathione, GSH, and GSSG, respectively, levels were determined as described previously (18). Briefly, tissue samples were homogenized in ice-cold homogenization buffer [154 mM KCL, 5 mM diethylenetriaminepentaacetic acid (DPTA), and 0.1 M potassium phosphate, pH 6.8]. After centrifugation, an aliquot was removed for protein determination using the BCA protein assay kit. Immediately after an aliquot was taken, one volume of cold acid buffer [40 mM HCl, 10 mM DPTA, 20 mM ascorbic acid, and 10% trichloroacetic acid (TCA)] was added to the homogenate. The suspension was centrifuged, and the resulting supernatant solution was centrifuged through a 0.45-μm microcentrifuge filter (Millipore, Bedford, MA). GSH and GSSG levels were determined using the fluorophore o-phtaladehyde. The GSH-to-GSSG ratio was normalized to protein concentration.
Gelatin zymography.
Samples were homogenized in 10 mM HEPES (pH 7.5), 150 mM NaCl, 0.2 mM EDTA, and 25% glycerol, with protease inhibitor cocktail (Sigma). Samples (10 mg of protein) were loaded onto a 10% SDS-PAGE gel substituted with 0.1% gelatin and then stained with 0.5% coomassie blue. MMP-2 and MMP-9 protein standards (Chemicon) were loaded on all gels. Bands of MMP-2 and/or MMP-9 gelatinolytic activity were digitally quantified with ImageJ, normalized to standards and expressed as arbitrary density units (ADU).
Data analyses and statistics.
Results were expressed as mean ± SE. Comparisons between means were analyzed, as appropriate, by Student's t-test, one-way ANOVA, or two-way ANOVA followed by Bonferroni post-hoc analysis. A value of P < 0.05 was considered statistically significant.
RESULTS
To validate the successful implementation of a mouse model of LV pacing, the electromechanical effects of LV pacing needed to be documented and verified. Figure 2 is a representative M-mode echocardiography image and a corresponding wall thickness analysis during the beginning atrial period (Fig. 2A) and at the 40-min LV pace period (Fig. 2B). The analysis of LV wall thickness, as described in the methods, before and during LV pacing demonstrated simultaneous contraction of the anterior and posterior walls during normal sinus rhythm. In contrast, there is early thickening in the anterior wall and early thinning of the posterior wall (as indicated by the arrows) with LV pacing. Analysis of video recordings in EKV mode using a short-axis view of the mouse heart evidence early-activated regions of the heart contracting before late-activated regions.
In animals that did not undergo echocardiography, the effects of LV pacing were documented by the widening of the QRS on the ECG. QRS duration was significantly greater during LV pace compared with normal sinus rhythm (67 ± 5 vs. 24 ± 2 ms; P < 0.001; paired t-test).
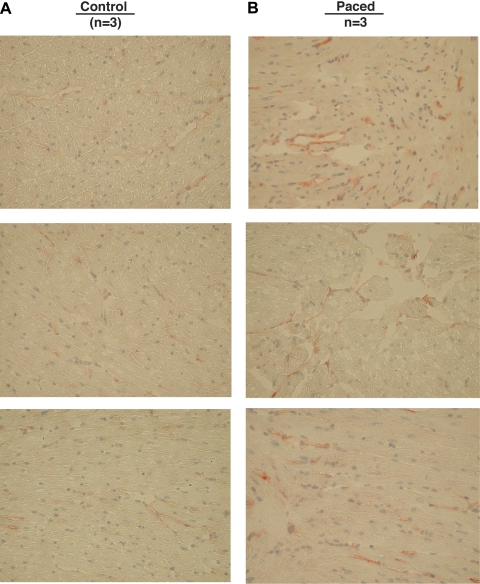
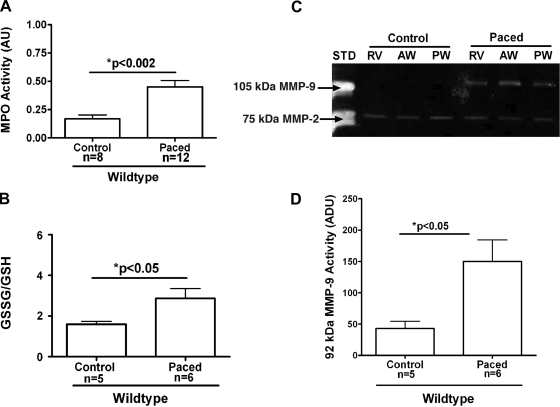
The effects of subthreshold pacing are shown in Fig. 3. Results indicate that subthreshold electrical stimulation in the absence of LV dyskinesis fails to induce neutrophil infiltration as measured by MPO activity. Figure 4 demonstrates representative images of ICAM-1 staining in wild-type animals. As can be observed, 40-min LV pacing increased endothelial ICAM-1 levels (Fig. 4B), as denoted by increases in Vector red staining, compared with controls (Fig. 4A). We next evaluated the inflammatory responses induced by LV pacing in wild-type animals to demonstrate that this rodent model of pacing can replicate the results seen in our published canine model of LV pacing studies. We measured MPO activity to assess inflammatory cell infiltration in the myocardium. As seen in Fig. 5A, there was a significant threefold increase in MPO activity in the early-activated region of paced versus control animals (0.45 ± 0.06 vs. 0.17 ± 0.03 AU; P < 0.002). This increase in MPO activity correlated with a significant increase in oxidative stress, as shown by the ratio GSSG/GSH, which is a measure of total tissue oxidative stress. Oxidative stress increased significantly in early-activated regions of paced animals compared with controls (2.9 ± 0.49 paced vs. 1.60 ± 0.14 control; P < 0.05; Fig. 5B). Gelatin zymography of tissue homogenates from the RV, anterior wall (early-activated region), and posterior wall (late-activated region) only revealed bands corresponding to the 105-kDa MMP-9 and 72-kDa MMP-2. A representative gelatin zymogram for a control and paced wild-type animal is shown in Fig. 5C. Densitometric analysis showed a significant threefold increase in 105-kDa MMP-9 activity in the early-activated regions of animals that underwent LV pacing (42.9 ± 11.7 ADU control vs. 150.2 ± 34.3 ADU paced; P < 0.02; Fig. 5D). No notable differences were identified in 72-kDa MMP-2 levels.
Fig. 3.
Effects of electrical stimulation on neutrophil infiltration in C57b6/L wild-type mice. Myeloperoxidase (MPO) activity in control (n = 4) and subthreshold LV paced animals (n = 4) is shown. AU, arbitrary units.
Fig. 4.
ICAM-1 levels induced by ventricular dyssynchrony in early-activated myocardium of wild-type C57BL/6. Representative images obtained from control animals (A; n = 3) and paced animals (B; n = 3) are shown.
Fig. 5.
Myocardial inflammatory responses induced by ventricular dysynchrony in early-activated regions of wild-type C57BL/6 mice. A: MPO activity in control (n = 8) and LV paced (n = 12) hearts. B: oxidative stress (GSSG/GSH) in control (n = 5) and LV paced (n = 6) hearts. C: representative gelatin zymogram of myocardial matrix metalloproteinase (MMP)-2 and MMP-9 levels in a control and LV paced heart. STD, human MMP -2/MMP-9 as standards; RV, right ventricle. D: densitometric analysis of 105 kDa MMP-9 zymographic activity in control hearts (n = 5) and paced hearts (n = 6). ADU, arbitrary density units.
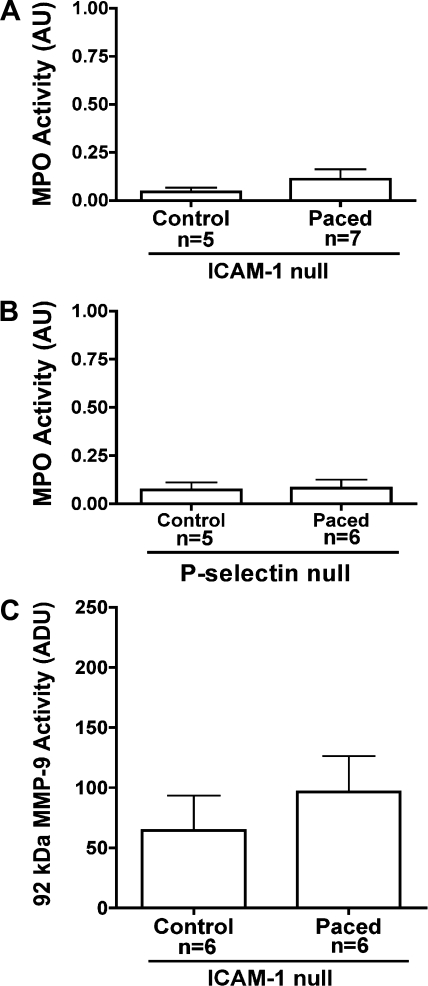
Given the differences seen in the inflammatory responses induced by LV pacing in wild-type animals, we evaluated the role of CAM, specifically ICAM-1 and p-selectin, using null mice. As seen in Fig. 6, LV pacing failed to significantly increase MPO activity in the early-activated region in the paced vs. control group for both ICAM-1 null (0.045 ± 0.02 AU control vs. 0.11 ± 0.05 AU paced; P = NS; Fig. 6A) and p-selectin null animals (0.07 ± 0.04 AU control vs. 0.08 ± 0.04 paced; P = NS; Fig. 6B). No significant increases were observed in 105-kDa MMP-9 activity in the control versus paced ICAM-1 null mice (64.5 ± 29.3 vs. 96.6 ± 29.9 ADU; P = NS; Fig. 6C). Results suggest that LV dyskinesis induces the expression of ICAM-1 and p-selectin, which allows for the triggering of an inflammatory cascade.
Fig. 6.
Inflammatory responses induced by ventricular dysynchrony in early-activated regions of ICAM-1 or p-selectin null mice. A: MPO activity in control (n = 5) and LV paced (n = 7) ICAM-1 null hearts. B: MPO activity in control (n = 5) and LV paced (n = 6) p-selectin null hearts. C: densitometric analysis of 105 kDa MMP-9 zymographic activity in control (n = 5) and LV paced (n = 6) ICAM-1 null hearts (n = 6).
DISCUSSION
Unique results from this study demonstrate that a mouse model of LV pacing can be used to study inflammatory responses triggered by ventricular dysynchrony. We were able to demonstrate significant increases in neutrophil infiltration, ROS generation, and MMP-9 activity in early-activated regions of paced wild-type mice. These inflammatory responses were notably mitigated in LV paced ICAM-1 or p-selectin null mice demonstrating the critical role played by these molecules in the triggering of responses.
We utilized a unipolar electrode implanted into the free wall to pace the LV. Using echocardiography, we were able to document the early activation of the LV free wall. Most importantly, in the mouse model, we managed to recapitulate the inflammatory/injury response seen in the canine model by subjecting wild-type mice to 40 min of LV pacing. Our large animal studies of LV pacing yielded approximately threefold increases in MPO activity, approximately fivefold increases in oxidative stress, and approximately twofold increases in MMP-9 activity in paced compared with control animals. In the mouse model of LV pacing, we documented similar upregulation of MPO activity (∼3-fold increases). Levels of oxidative stress and MMP-9 activity were also significantly upregulated (∼2-fold and 4-fold increases, respectively) in paced animals compared with control. Differences in the levels of tissue oxidative stress and MMP-9 activity may be due to differences in species or to the location and/or transmural nature of the responses. Of note is that these responses were not observed in animals subjected to subthreshold pacing.
To gain insight into the mechanisms responsible for triggering of the inflammatory responses and the possible dependence on vascular events, we paced CAM null animals. The upregulation of CAM allows for the physical interaction between inflammatory cells and the vascular endothelium needed for neutrophil transmigration to occur (4). In both the ICAM-1 and the p-selectin null mice, LV pacing failed to increase MPO activity. MMP-9 activity in the ICAM-1 null mice was also similar to nonpaced animals, thus confirming the importance of ICAM-1 and p-selectin in the induction of the myocardial inflammatory responses associated with LV pacing.
Given the localized nature of the inflammatory responses seen in the canine model, a local triggering event must occur. On the basis of alterations triggered by LV pacing in three-dimensional transmural strain patterns, there is a possibility that changes in the patterns of epicardial microvascular flow may lead to the enhanced expression of CAM, thus activating the acute phase of the inflammatory response. In this regard, the effects that mechanical forces exert on endothelial cell (EC) CAM gene expression and protein production have gained vast attention due to its relationship with atherogenesis (14, 16, 17). It has been shown that endothelial CAM expression can be modulated by mechanical stimuli, which is recognized through stress-sensitive promoter elements (13). Hemodynamic forces, such as fluid shear stress, are known to regulate vascular wall structure/function including EC cell shape, nitric oxide and prostacyclin production, EC proliferation, and CAM expression (2, 3, 7, 20). Studies have demonstrated that exposure of EC to normal laminar flow patterns produces low levels of CAM and enhances the production of antiadhesive factors (17, 20). However, when EC are exposed to altered patterns of flow, CAM expression/production significantly increases (7, 20). This perturbed flow-induced increase in CAM expression/production can then become the trigger for inflammation. Studies in large animals are warranted to examine the possibility that epicardial venular flow patterns may be perturbed in the setting of LV pacing, in a manner in which they may trigger inflammation. A select number of investigators have examined the vascular flow patterns that have been performed in beating canine myocardium using intravital microscopy (5, 11). These studies also allow the examination of neutrophil rolling and adhesion. However, studies would need to examine epicardial venular flows in the setting of ventricular dyskinesis in early-activated sites and the ensuing neutrophil adhesion and activation.
There is precedent for the use of mice to study ventricular dysynchrony. A recent study evaluated the influence of nonfailing ventricular dysynchrony on regional cardiac gene expression in mice (1). For this purpose, a custom miniature implantable mouse pacemaker capable of stimulating the murine heart at varying rates was used. In this study, they employed right ventricular free wall stimulation to generate ventricular dysynchrony for a period of 1 wk. Results demonstrated that LV pacing led to significant regional heterogeneity of gene expression involved in extracellular matrix remodeling, stem cell differentiation, myocardial stress responses, and hypertrophy. Of particular interest, the genes involved in matrix remodeling, osteopontin (OPN), matrilin-1, and latent transforming growth factor β-binding protein 2 increased with dysynchrony. Studies show OPN to be upregulated during stress, inflammation, and ROS generation, which then leads to ventricular remodeling (8, 21). Latent transforming growth factor β-binding protein 2 plays a structural role within elastic fibers and has important immunomodulatory and regulatory roles in cell growth (1, 19). Bilchick et al. (1) proved that ventricular dysynchrony generates a complexity of responses, which likely contribute to changes in cardiac structure/function.
In conclusion, results demonstrate that CAM expression plays a critical role in the triggering of the LV pacing-induced inflammation, thus providing evidence of a vascular mechanism for inflammation associated with local dyskinesis. The mechanisms that trigger an upregulation of epicardial inflammation await further investigation since they suggest a specific involvement of vascular events likely in the outer layers of the myocardium. Of interest is the possibility that LV dysynchrony induced changes in myocardial structure/function may be associated with altered microvascular flow patterns.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant HL-43617 and National Center for Minority and Health Disparities (NCMHD) P60 MD000220 (to F. Villarreal). Predoctoral support for K. G. Yamazaki was provided by NHLBI Training Grant T32-HL-107444 and postdoctoral support by National Institute of General Medicine IRACDA Grant GM06852.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.G.Y., D.M.R., and F.J.V. conception and design of research; K.G.Y., S.-H.I., R.L.T., and F.J.V. performed experiments; K.G.Y., R.L.T., and F.J.V. analyzed data; K.G.Y., S.-H.I., R.L.T., and F.J.V. interpreted results of experiments; K.G.Y. and F.J.V. prepared figures; K.G.Y. and F.J.V. drafted manuscript; K.G.Y., S.-H.I., R.L.T., D.M.R., and F.J.V. edited and revised manuscript; K.G.Y., S.-H.I., R.L.T., D.M.R., and F.J.V. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maria Rivas for technical assistance.
REFERENCES
- 1. Bilchick KC, Saha SK, Mikolajczyk E, Cope L, Ferguson WJ, Yu W, Girouard S, Kass DA. Differential regional gene expression from cardiac dyssynchrony induced by chronic right ventricular free wall pacing in the mouse. Physiol Genomics 26: 109–115, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Bongrazio M, Baumann C, Zakrzewicz A, Pries AR, Gaehtgens P. Evidence for modulation of genes involved in vascular adaptation by prolonged exposure of endothelial cells to shear stress. Cardiovasc Res 47: 384–393, 2000. [DOI] [PubMed] [Google Scholar]
- 3. Braddock M, Schwachtgen JL, Houston P, Dickson MC, Lee MJ, Campbell CJ. Fluid shear stress modulation of gene expression in endothelial cells. News Physiol Sci 13: 241–246, 1998. [DOI] [PubMed] [Google Scholar]
- 4. Briaud SA, Ding ZM, Michael LH, Entman ML, Daniel S, Ballantyne CM. Leukocyte trafficking and myocardial reperfusion injury in ICAM-1/P-selectin-knockout mice. Am J Physiol Heart Circ Physiol 280: H60–H67, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circ Res 69: 561–570, 1991. [DOI] [PubMed] [Google Scholar]
- 6. Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol 77: 487–495, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dancu MB, Berardi DE, Vanden Heuvel JP, Tarbell JM. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler Thromb Vasc Biol 24: 2088–2094, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48: 504–511, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia RA, Brown KL, Pavelec RS, Go KV, Covell JW, Villarreal FJ. Abnormal cardiac wall motion and early matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol 288: H1080–H1087, 2005. [DOI] [PubMed] [Google Scholar]
- 10. Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res 43: 860–878, 1999. [DOI] [PubMed] [Google Scholar]
- 11. Kajiya F, Yada T, Matsumoto T, Goto M, Ogasawara Y. Intramyocardial influences on blood flow distributions in the myocardial wall. Ann Biomed Eng 28: 897–902, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Leatherbury L, Yu Q, Chatterjee B, Walker DL, Yu Z, Tian X, Lo CW. A novel mouse model of X-linked cardiac hypertrophy. Am J Physiol Heart Circ Physiol 294: H2701–H2711, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Methe H, Balcells M, Alegret Mdel C, Santacana M, Molins B, Hamik A, Jain MK, Edelman ER. Vascular bed origin dictates flow pattern regulation of endothelial adhesion molecule expression. Am J Physiol Heart Circ Physiol 292: H2167–H2175, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Papadaki M, Eskin SG. Effects of fluid shear stress on gene regulation of vascular cells. Biotechnol Prog 13: 209–221, 1997. [DOI] [PubMed] [Google Scholar]
- 15. Pernot M, Fujikura K, Fung-Kee-Fung SD, Konofagou EE. ECG-gated, mechanical and electromechanical wave imaging of cardiovascular tissues in vivo. Ultrasound Med Biol 33: 1075–1085, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Resnick N, Gimbrone MA., Jr Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J 9: 874–882, 1995. [DOI] [PubMed] [Google Scholar]
- 17. Resnick N, Yahav H, Schubert S, Wolfovitz E, Shay A. Signalling pathways in vascular endothelium activated by shear stress: relevance to atherosclerosis. Curr Opin Lipidol 11: 167–177, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Senft AP, Dalton TP, Shertzer HG. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal Biochem 280: 80–86, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Shipley JM, Mecham RP, Maus E, Bonadio J, Rosenbloom J, McCarthy RT, Baumann ML, Frankfater C, Segade F, Shapiro SD. Developmental expression of latent transforming growth factor beta binding protein 2 and its requirement early in mouse development. Mol Cell Biol 20: 4879–4887, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91: 769–775, 2002. [DOI] [PubMed] [Google Scholar]
- 21. Singh M, Foster CR, Dalal S, Singh K. Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol 48: 538–543, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis 49: 26–41, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Vernooy K, Verbeek XA, Peschar M, Prinzen FW. Relation between abnormal ventricular impulse conduction and heart failure. J Interv Cardiol 16: 557–562, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Yamazaki KG, Villarreal FJ. Ventricular pacing-induced loss of contractile function and development of epicardial inflammation. Am J Physiol Heart Circ Physiol 300: H1282–H1290, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.