Abstract
Stimulation of cardiac sympathetic afferents during myocardial ischemia with metabolites such as bradykinin (BK) evokes sympathoexcitatory reflex responses and activates neurons in the external lateral parabrachial nucleus (elPBN). The present study tested the hypothesis that this region in the pons processes sympathoexcitatory cardiac reflexes through an ionotropic glutamate receptor mechanism. The ischemic metabolite BK (0.1–1 μg) was injected into the pericardial space of anesthetized and bilaterally vagotomized or intact cats. Hemodynamic and renal sympathetic nerve activity (RSNA) responses to repeated administration of BK before and after unilateral 50-nl microinjections of kynurenic acid (Kyn; 25 mM), 2-amino-5-phosphonopentanoic acid (AP5; 25 mM), and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzol(F)quinoxaline (NBQX; 10 mM) into the elPBN were recorded. Intrapericardial BK evoked significant increases in mean arterial pressure (MAP) and RSNA in seven vagotomized cats. After blockade of glutamate receptors with the nonselective glutamate receptor antagonist Kyn, the BK-evoked reflex increases in MAP (50 ± 6 vs. 29 ± 2 mmHg) and RSNA (59 ± 8.6 vs. 29 ± 4.7%, before vs. after) were significantly attenuated. The BK-evoked responses returned to pre-Kyn levels 85 min after the application of Kyn. Similarly, BK-evoked reflex responses were reversibly attenuated by blockade of glutamate N-methyl-d-aspartate (NMDA) receptors with AP5 (n = 5) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors with NBQX (n = 5). In contrast, we observed that the repetitive administration of BK evoked consistent reflex responses including MAP and RSNA before and after microinjection of 50 nl of the artificial cerebrospinal fluid vehicle into the elPBN in five animals. Microinjection of glutamate receptor antagonists into regions outside the elPBN did not alter BK-induced reflex responses. Microinjection of Kyn into the elPBN reversibly attenuated BK-induced reflex responses in four vagus intact animals. These data are the first to show that NMDA and AMPA ionotropic glutamate receptors in the elPBN play an important role in processing cardiac excitatory reflex responses.
Keywords: 2-amino-5-phosphonopentanoic acid; 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzol(F)quinoxaline; bradykinin; renal sympathetic nerve activity
activation of cardiac spinal (sympathetic) sensory nerve endings during myocardial ischemia by ischemic metabolites, such as thromboxane A2 and bradykinin (BK), evokes sympathoexcitatory reflex responses, including hypertension and tachyarrhythmias as well as increases in sympathetic outflow (11, 12, 14, 21, 53). Clinical and experimental studies (13, 35, 52, 60) have demonstrated that these reflexes significantly contribute to the development of lethal ventricular arrhythmias and heart failure, ultimately increasing morbidity and mortality of patients. Although several regions in the brain, including the rostral ventral lateral medulla (RVLM), nucleus of the solitary tract (NTS), and parabrachial nucleus (PBN), involved in the regulation of sympathoexcitatory reflexes have been identified (20, 22, 23, 56), details regarding the roles of these regions or exactly which region processes inputs from cardiac sympathetic afferents remain unclear.
The PBN, located in the dorsal lateral pons, is separated by the brachium conjunctivum into two main subregions: the lateral and medial PBN (2). The lateral PBN is reciprocally connected with the RVLM and NTS, well-recognized cardiovascular integrative centers (7, 21). Several studies (4, 38, 41) have shown that electrical stimulation of the medial and lateral subregions of PBN evokes pressor and tachycardia responses in cats and rats. Deactivation of neurons with kainic acid in the lateral PBN produces a small enhancement of baroreflex regulation of blood pressure but fails to alter baroreflex modulation of renal sympathetic nerve activity (RSNA) (30). Using c-Fos expression, we (19, 20, 22) have anatomically demonstrated that neurons in the elPBN are activated by the stimulation of cardiac sympathetic afferents. However, the importance of the lateral PBN in regulating sympathoexcitatory reflex responses is unknown. We speculated, in the present study, that the lateral PBN, particularly the external lateral PBN (elPBN) subdivision, serves an important role in processing reflex inputs from cardiac sympathetic afferents that ultimately lead to sympathoexcitatory reflex responses.
Glutamate is an important excitatory neurotransmitter throughout the central nervous system. Several sources of evidence have suggested that this excitatory amino acid in the lateral PBN may play an important role in mediating autonomic function, including cardiovascular regulation. For instance, glutamate-like immunoreactivity in the lateral and medial PBN has been detected (40). Microinjection of glutamate in the lateral PBN increases arterial blood pressure and RSNA in rats and cats (4, 26, 38). Immunohistochemical studies (17, 33) have shown that a group of neurons in the NTS projecting to the lateral PBN contain glutamate-like immunoreactivity. In addition, unloading the baroreceptors with an intravenous injection of nitroprusside significantly increases glutamate release in the PBN of rats, although the release of glutamate in the lateral PBN has not been evaluated (49). These data in aggregate suggest but do not prove that glutamate in the lateral PBN regulates cardiovascular function. Likewise, they do not address glutamate's actions in sympathoexcitatory reflex regulation.
Glutamate exerts its action through the activation of two types of receptors: ionotropic and metabotropic receptors. Several studies (8, 33, 63, 64) have supported the notion that ionotropic glutamate receptors participate in the lateral PBN neurotransmission, which can regulate cardiovascular function. In this regard, immunocytochemical and in situ hybridization studies (24, 25) have identified N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) ionotropic glutamate receptors in the lateral and medial PBN. Both receptor subtypes, mostly in the lateral PBN, can be activated pharmacologically in vivo and in vitro (33, 63). Furthermore, electrical stimulation of NTS-evoked PBN activity is eliminated by the selective blockade of NMDA and AMPA receptors in intact animals (33). The contribution of ionotropic glutamate receptors to processing the sympathoexcitatory reflex responses in the elPBN, however, has not been evaluated.
Because there is little or no information on the role of the lateral PBN, and more specifically the elPBN, in processing cardiovascular excitatory reflex inputs, the aim of the present study was to evaluate the role of the elPBN in processing reflex input from cardiac sympathetic afferents known to evoke sympathoexcitatory reflex responses. We tested the overall hypothesis that elPBN neurons process cardiac sympathoexcitatory reflex inputs through ionotropic glutamate receptor mechanisms, using neurophysiological and pharmacological approaches. We speculated that blockade of glutamate NMDA as well as AMPA receptor subtypes in the elPBN would attenuate sympathoexcitatory reflex responses during the activation of cardiac spinal afferents. A preliminary report of this study has been published (9).
METHODS
Animal Preparation
All experimental preparations and protocols were reviewed and approved by the Animal Care and Use Committee of the University of California (Irvine, CA). This study conformed with the American Physiological Society's “Guiding Principles in the Care and Use of Animals.” Thirty-two adult cats of either sex (2.3–4.9 kg) were anesthetized with an intramuscular injection of ketamine (20–30 mg/kg) followed by a bolus intravenous injection of α-chloralose (40–50 mg/kg) through the femoral vein. Additional α-chloralose (5–10 mg/kg iv) was given as needed to maintain an adequate depth of anesthesia, as assessed by observing the absence of a conjunctival reflex. The trachea was intubated, and respiration was maintained artificially (model 661 ventilator, Harvard, South Natick, MA). A femoral vein was cannulated to administer drugs and fluids. Systemic arterial blood pressure was monitored by a pressure transducer attached to a cannula inserted into the femoral artery. Arterial blood gases and pH were measured with a blood gas analyzer (ABL 5, Radiometer America, West Lake, OH) and were maintained within physiological limits (Po2: >100 mmHg, Pco2: 28–35 mmHg, pH 7.35–7.45) by adjusting the respiratory rate or tidal volume or by administering NaHCO3 (1 M iv). Body temperature was monitored with a rectal thermistor and was maintained at 36–38°C with a circulating water heating pad and heat lamp.
A catheter was inserted into the pericardium for the administration of BK, as previously described (14). In brief, a small incision through the left fourth or fifth intercostal space was made to expose the pericardium. A polyethylene-60 catheter with six perforations in its distal end was introduced into the pericardium for the intrapericardial application of BK and saline. Care was taken to eliminate any leaks from the pericardium by tightening a silk suture placed around the small hole in the pericardium, through which the catheter had been introduced. We observed no leakage over a 5-min period after the injection of 2 ml of saline. After this procedure, the ribs were approximated, and the chest was closed.
To eliminate the influence of vagal cardiac afferents and baroreflexes that could mask the reflex responses to stimulation of sympathetic afferents, a midline cervical incision was made to expose the cervical vagi bilaterally that were to be transected. In a separate group of vagus intact animals, we did not perform vagotomy to observe the influence of vagal cardiac afferents on BK-mediated cardiac reflexes.
RSNA Recording
Multiunit RSNA was recorded as previously described (14). We used a left flank retroperitoneal approach to isolate a branch of the renal sympathetic nerve from the surrounding connective tissue using a microscope (Zeiss). The nerve was covered with warm mineral oil and placed across one pole of a recording electrode, while the other pole of the recording electrode was grounded with a cotton thread to the animal. The recording electrode was attached to a high-impedance probe (model HIP511, Grass Tech, West Warwick, RI). The signal was amplified and filtered (100–3,000 Hz) with an alternating current amplifier (P511, Grass Tech). The amplified signal was monitored with an oscilloscope (model 2201, Tektronix, Beavertown, OR) and an audio amplifier (AM8B audiomonitor, Grass Tech) and processed with a Pentium computer through an analog-to-digital converter (CED micro 1401 mkII, Cambridge, UK) for displaying RSNA on a monitor and for subsequent offline analysis. Discharge activity of the renal sympathetic nerve was analyzed with data acquisition and analysis software (Spike 2). Electrical noise in the neural recordings was determined after the nerves were crushed at the end of the experiment. A window discriminator was set just above the noise level so that only renal nerve discharge signals were counted. Nerve discharge activity was rectified and averaged over a period of 10 s with software (Spike 2) and denoted as integrated RSNA.
Exposure of Dorsal Pons and Microinjection of Drugs
After renal nerve isolation, animals were mounted on a stereotaxic head frame (Kopf Instruments) with steel plugs introduced through the auditory meatus and bars holding the upper jaw to fix the head rigidly. A craniotomy was performed to expose the dorsal surface of the brain stem. To access the elPBN, we unilaterally removed the cerebellum to expose the surface of the pons. Microinjection was performed unilaterally with a single modified microdialysis probe (0.24-mm outer diameter, CMA/11) positioned by a micromanipulator (Kopf Instruments). The modified microdialysis probe was made by removing the microdialysis membrane of the probe before insertion (62). The tip of the probe was placed 10–11 mm rostral to the obex, 5 mm lateral to midline, at a 90° angle to the dorsal surface of the pons at a depth of 1.5–3 mm to reach the elPBN (2, 22) and was connected to a UMP3 microsyringe injector (WPI, Sarasota, FL), which contained a 10-μl Hamilton syringe. The injection volume was always 50 nl. In preliminary experiments, we obtained consistent repetitive sympathoexcitatory reflex responses evoked by the injection of BK (1–10 μg/ml, 0.1 ml) into the pericardial space after placing the modified microdialysis probe unilaterally in the elPBN (three cats). In another three cats, we observed inconsistent repetitive reflex responses induced by BK after positioning probes bilaterally in the elPBN. Thus, unilateral microinjection into the elPBN was used in the present study. The elPBN was identified provisionally by observing pressor responses to the injection of glutamate (40 mM) into the PBN and later confirmed through histological identification of 0.4% Chicago sky blue, which was mixed with all drugs to mark the sites of microinjection (21). At the end of each experiment, the animal was euthanized under deep anesthesia with an intravenous administration of 50 mg/kg of α-chloralose followed by saturated KCl. The brain stem was removed and submerged in 4% paraformaldehyde for at least 2 days. Frozen 60-μm coronal sections were then cut with a CM 1850 cryostat microtome (Leica). Microinjection sites were identified by the sky blue marker, as previously described (21).
Drugs Administered
This study used glutamate (40 mM), kynurenic acid [Kyn; 25 mM, a nonspecific glutamate ionotropic receptor antagonist (61)], 2-amino-5-phosphonopentanoic acid [AP5; 25 mM, an NMDA receptor antagonist (61)], and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzol(F)quinoxaline [NBQX; 10 mM, an AMPA receptor antagonist (45)]. Each drug was dissolved in artificial cerebrospinal fluid (aCSF; pH 7.4) mixed with Chicago sky blue (0.4%) for subsequent histological site verification (21). BK (1–10 μg/ml, 0.1 ml) was dissolved in physiological saline (pH 7.4). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO), prepared weekly, and stored in a −20°C freezer.
Experimental Protocols
Blockade of ionotropic glutamate receptors in the elPBN after vagotomy.
After completion of the surgical preparation, including bilateral vagotomy, a minimum of 60 min was allowed for the stabilization of arterial pressure. BK (1–10 μg/ml, 0.1 ml) was injected into the pericardial sack to evoke repetitive reflex increases in blood pressure and RSNA in response to BK. BK is produced during myocardial ischemia and participates in the activation of cardiac sympathetic afferents (15, 57). Intrapericardial saline (1 ml) was applied three times to wash out BK after its application. To prevent tachyphylaxis, recovery periods of at least 20 min were provided between consecutive stimuli. In this protocol, intrapericardial BK was applied seven times to induce consistent reflex responses over a period of 140 min. After the first two consecutive applications of BK, Kyn (7 cats) and aCSF (5 cats) as the vehicle control were microinjected into the elPBN. In a preliminary study using unilateral microinjections of 5, 10, and 25 mM Kyn into the elPBN, we observed that only 25 mM of Kyn consistently attenuated BK-mediated cardiac sympathoexcitatory reflex responses. Previous studies (28, 61) have demonstrated that this dose of Kyn markedly attenuates dorsal periaqueductal gray-evoked respiration and heart rate (HR) responses and eliminated RVLM neuronal responses to glutamate stimulation. In preliminary studies (two animals for microinjection in each side), we observed a similar attenuation of BK-evoked reflex responses after the microinjection of Kyn in the left and right sides of the elPBN [mean arterial pressure (MAP): −27 and −21 mmHg vs. −31 and −20 mmHg, left vs. right]. Hence, we chose 25 mM of Kyn and the left side for microinjection in the present study.
Blockade of glutamate receptors in the elPBN in vagus intact animals.
In four vagus intact animals, we microinjected Kyn (25 mM) into the elPBN to evaluate its role in processing reflex responses and the importance of ionotropic glutamate receptors in this region of the pons. The aCSF group mentioned above served as the time control for this protocol.
Blockade of ionotropic glutamate receptor subtypes in the elPBN.
We evaluated the effects of ionotropic glutamate NMDA receptor blockade with AP5 and AMPA receptor blockade with NBQX on the reflex responses to BK stimulation in three groups. In the first group of five animals, after two consecutive applications of BK, AP5 (25 mM) was microinjected into the elPBN, and the hemodynamic and RSNA responses to repeated intrapericardial BK stimulation were recorded. NBQX (10 mM) was microinjected into the elPBN, in a similar fashion as AP5, in five separate animals comprising the second group. Previous studies (45, 61) have demonstrated that these doses of AP5 and NBQX selectively and effectively block NMDA and AMPA receptors in the RVLM and the ventral respiratory group nucleus in the medulla. Finally, as an anatomic control, reflex responses to BK stimulation were evaluated in a third group of six cats in which antagonists were administered outside but adjacent to the elPBN.
Data Analysis
RSNA is expressed as the percent change from baseline activity to account for the variability in activity that occurs with multiunit nerve recordings. Baseline activity was determined by averaging RSNA over a 1-min period immediately preceding chemical stimulation. The response of RSNA to BK was determined by averaging RSNA during the entire period of each response, defined as the time during which sustained activity exceeded baseline activity by at least 3% (14).
Data are expressed as means ± SE. A Kolmogorov-Smirnov test was used to determine if the data were distributed normally. Normally distributed data in all protocols were compared with one-way repeated-measures ANOVA followed post hoc by a Tukey's test. Non-normally distributed data were compared with Friedman repeated-measures ANOVA on ranks followed by a Dunnett's post hoc test. All statistical calculations were performed with SigmaStat software (Jandel Scientific, San Rafael, CA). Values were considered to be significantly different when P < 0.05.
RESULTS
Response to Cardiac Afferent Stimulation After elPBN Ionotropic Glutamate Receptor Blockade
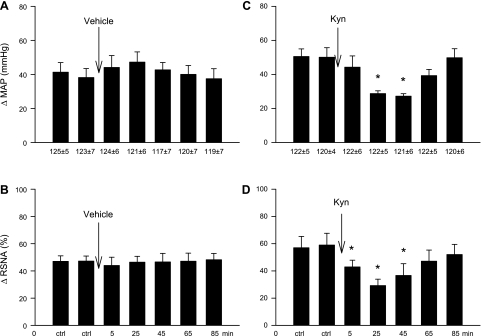
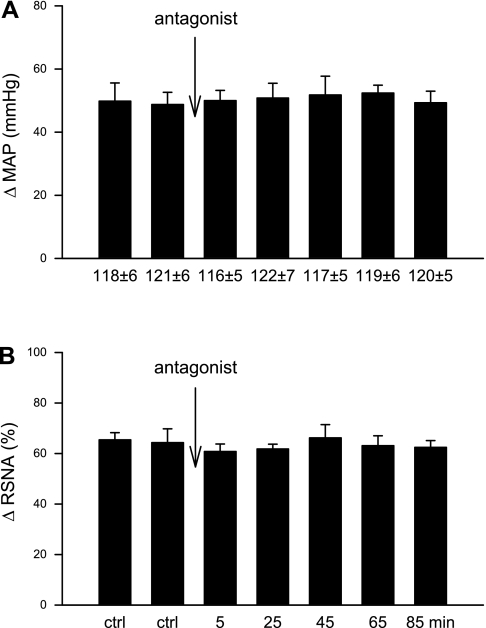
Intrapericardial BK administered every 20 min evoked consistent sympathoexcitatory reflex responses, including increases in MAP by 41 ± 5 mmHg and RSNA by 47 ± 4%, effects that were unaltered by the microinjection of vehicle into the elPBN in five vagotomized animals (Fig. 1, A and B). In contrast, in the second group of animals (n = 7), Kyn significantly attenuated MAP and RSNA responses to BK 25–45 min after the microinjection of Kyn into the elPBN (Fig. 1, C and D). Reflex responses to BK stimulation recovered 65–85 min after Kyn administration. Kyn did not alter baseline HR, MAP (Table 1), or RSNA. Intrapericardial BK slightly increased HR by 7 ± 1 beats/min from a baseline of 192 ± 10 beats/min (P > 0.05), a response that was not influenced by Kyn (7 ± 1 vs. 6 ± 1 beats/min, before vs. after Kyn). We observed similar increases in MAP in response to intrapericardial BK before and after probe insertion (49 ± 5 vs. 52 ± 5 mmHg, before vs. after) in control and Kyn-treated animals, indicating that insertion of the probe into the elPBN did not alter the function of this nucleus.
Fig. 1.
Bar graphs showing increases [changes in (Δ)] in mean arterial pressure (MAP) and integrated renal sympathetic nerve activity (RSNA) during the intrapericardial application of bradykinin (BK) before and after microinjection of 50 nl of vehicle in five animals (A and B) and kynurenic acid (Kyn; 25 mM) in seven animals (C and D) into the external lataral parabrachial nucleus (elPBN) of vagotomized animals. Values are means ± SE. *P < 0.05 compared with control (ctrl).
Table 1.
Baseline MAP and HR before and after the administration of antagonists
| MAP, mmHg |
HR, beats/min |
||||
|---|---|---|---|---|---|
| Number of Animals | Before | After | Before | After | |
| Vehicle | 5 | 125 ± 6 | 122 ± 7 | 197 ± 9 | 200 ± 9 |
| Kynurenic acid | 7 | 123 ± 7 | 121 ± 6 | 191 ± 9 | 193 ± 10 |
| 2-Amino-5-phosphonopentanoic acid | 5 | 121 ± 7 | 118 ± 6 | 198 ± 9 | 196 ± 10 |
| 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzol(F)quinoxaline | 5 | 115 ± 6 | 117 ± 6 | 201 ± 10 | 204 ± 10 |
Values are means ± SE. MAP, mean arterial pressure; HR, heart rate.
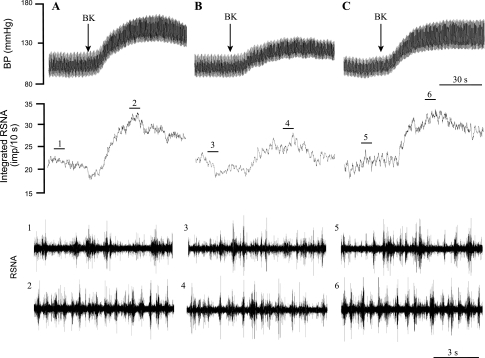
Representative tracings of arterial pressure and RSNA after intrapericardial BK before and after blockade of ionotropic glutamate receptors with Kyn in the elPBN are shown in Fig. 2. Intrapericardial BK increased MAP by 46 mmHg and integrated RSNA by 56% before Kyn and were attenuated to 29 mmHg and 32%, respectively, after Kyn (Fig. 2B). BK-induced sympathoexcitatory reflex responses recovered 85 min after the administration of Kyn (Fig. 2C).
Fig. 2.
Original records of arterial pressure, RSNA, and integrated RSNA responses to the intrapericardial application of BK before (A) and after (B) microinjection of Kyn into the elPBN in a cat after bilateral vagotomy. A: increase in MAP by 46 mmHg and integrated RSNA by 56% to initial BK (1 μg). B: blockade of glutamate receptors with Kyn attenuated responses to BK. C: reflex responses to BK during recovery 85 min after the administration of Kyn. Bars 1–6 show representative tracings of RSNA at the times indicated by the short line above the integrated RSNA recordings.
Response to Cardiac Afferent Stimulation After elPBN Ionotropic Glutamate Blockade in Vagus Intact Animals
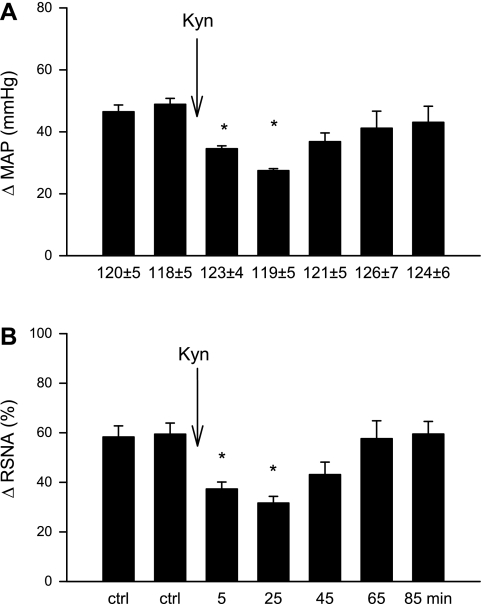
In four animals with vagal and sympathetic afferents, intrapericardial BK increased MAP by 49 ± 3 mmHg and RSNA by 59 ± 8%. Microinjection of Kyn into the elPBN attenuated BK-induced reflex responses (Fig. 3, A and B), which recovered 65–85 min after Kyn application. BK slightly increased HR by 10 ± 3 beats/min from a baseline of 199 ± 10 beats/min in this group, a response that was unaltered by Kyn application in the elPBN (10 ± 3 vs. 9 ± 2 beats/min, after vs. before Kyn, P > 0.05).
Fig. 3.
Increases (Δ) in MAP (A) and integrated RSNA (B) evoked by intrapericardial BK stimulation before and after microinjection of 50 nl Kyn (25 mM) into the elPBN of four vagus intact animals. Values are means ± SE. *P < 0.05 compared with control.
Influence of Selective Glutamate Ionotropic Receptor Blockade in the elPBN
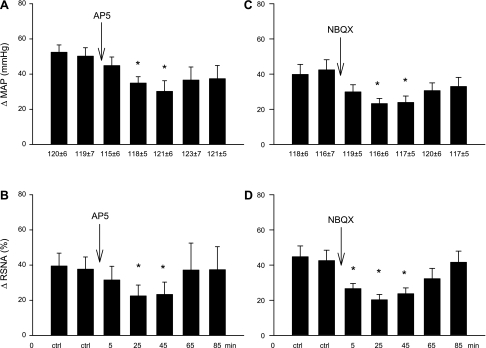
The increases in MAP and RSNA were reduced reversibly by unilateral elPBN microinjection of the NMDA receptor antagonist AP5 in five animals (Fig. 4, A and B) and by microinjection of the AMPA receptor antagonist NBQX in five separate animals (Fig. 4, C and D). Neither baseline blood pressure nor HR was altered by the administration of the glutamate receptor subtype antagonists (Table 1). Cardiac BK stimulation slightly increased HR by 8 ± 1 beats/min from a baseline of 197 ± 10 or 9 ± 2 from 202 ± 11 beats/min (P > 0.05), a response that was unaffected by AP5 (7 ± 2 vs. 8 ± 1 beats/min, after vs. before AP5) or NBQX (7 ± 2 vs. 9 ± 2 beats/min, after vs. before NBQX).
Fig. 4.
Increases (Δ) in MAP and integrated RSNA induced by the intrapericardial application of BK before and after microinjection of 50 nl of 2-amino-5-phosphonopentanoic acid (AP5; 25 mM) in five cats (A and B) and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzol(F)quinoxaline (NBQX; 10 mM) in five separate animals (C and D) into the elPBN. Values are means ± SE. *P < 0.05 compared with control.
Six microinjections of antagonists placed into regions surrounding the elPBN did not alter MAP and RSNA responses to repeated intrapericardial BK treatment (Fig. 5).
Fig. 5.
Changes in MAP (A) and integrated RSNA (B) induced by the intrapericardial application of BK before and after microinjection of glutamate receptor antagonists into regions outside the elPBN (n = 6). Values are means ± SE.
Anatomic Location of Microinjection Sites
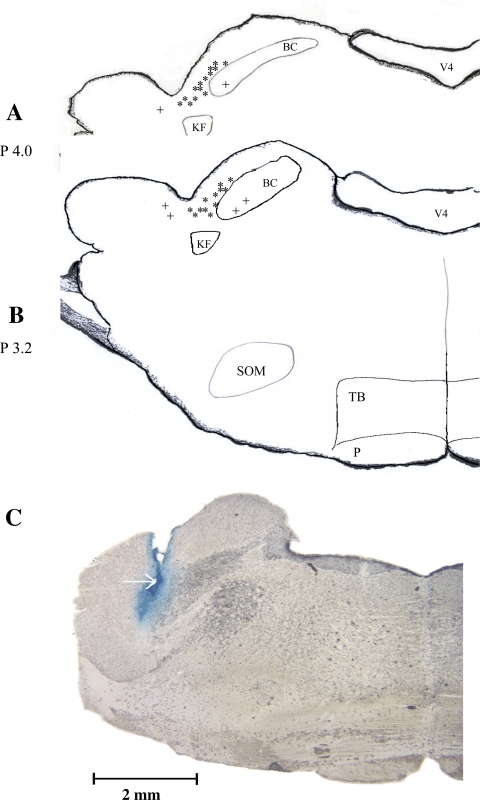
Figure 6, A and B, shows the microinjection sites of antagonists and vehicle in the present study. Twenty-six sites were observed to be inside the elPBN and six sites outside the elPBN, as identified by Berman's atlas for cats and in line with our previous studies (2, 20, 22). Figure 6C shows an original picture of the sites of microinjection in the elPBN.
Fig. 6.
A and B: composite map showing sections of the pons with sites of microinjections within (*, n = 26) and outside (+, n = 6) the elPBN. V4, fourth ventricle; BC, brachium conjuctivum; KF, Kolliker-Fuse nucleus; SOM, medial nucleus of the superior olive; TB, trapezoid body; P, pyramidal tract. C: original slide of the pons showing an actual microinjection site (arrow) in the elPBN of a cat. Scale bar = 3 mm. Coronal sections in A and B were adapted from Berman's atlas (2).
DISCUSSION
This is the first study to demonstrate that, through ionotropic glutamate receptor mechanisms, the elPBN processes inputs from cardiac sympathetic afferents that evoke sympathoexcitatory reflex responses, including increases in MAP and RSNA. In this regard, ionotropic glutamate receptor blockade in the elPBN with Kyn in both sympathetic and vagus afferent intact and vagotomized animals reversibly attenuated the excitatory reflexes evoked by cardiac spinal afferent stimulation with the ischemic metabolite BK. Glutamatergic blockade did not alter baseline arterial pressure and RSNA, indicating that this region does not exert tonic control of sympathetic outflow. Furthermore, antagonism of glutamate NMDA and AMPA ionotropic receptor subtypes in the elPBN attenuated the BK-evoked reflex responses. These data indicate that the elPBN functions as an important cardiovascular integrative center processing spinal afferent reflex input leading to the sympathoexcitatory reflex responses through both NMDA and non-NMDA ionotropic glutamate receptor mechanisms.
The PBN is located in the dorsolateral region of the rostral pons, flanking the brachium conjunctivum, which separates the PBN into two subregions: the lateral and medial PBN. Subregions of the PBN are reciprocally connected to the NTS and RVLM, two important cardiovascular integrative centers (7, 16, 21, 56). Although there is detailed information on the connections of the PBN with other cardiovascular centers, less is known about the influence of this nucleus on cardiovascular function. Electrical or glutamate stimulation of the lateral PBN increases arterial pressure and RSNA, which can be eliminated by depolarization blockade of neurons in the PBN with kainic acid (4, 26, 38, 41). In contrast, glutamate injected into the medial PBN evokes depressor responses in the rat (4). Thus, at least two populations of neurons in the PBN appear to be capable of evoking excitatory or inhibitory cardiovascular responses that are mediated through glutamatergic mechanisms.
Previous studies also have demonstrated that the lateral PBN receives visceral nociceptive information from the spinal cord. In this respect, Craig (5) has shown that dorsal horn lamina I–III neurons directly project to the lateral PBN in the cat and monkey. Other investigators (36) have demonstrated that many more lamina I neurons project to the PBN than to the thalamus. Since most neurons in lamina I of the spinal dorsal horn are nociceptive in function (3, 6), it is possible that the lateral PBN, and specifically the elPBN, may integrate cardiac sympathetic afferent input that passes through the spinal dorsal horn to the pons.
Much less is known about the role of the lateral PBN in regulating cardiovascular reflex responses. Jhamandas and colleagues (32) reported that neurons in the lateral PBN respond to baroreceptor stimulation. Also, activation of neurons within the lateral PBN with dl-homocysteic acid attenuates baroreflex control of arterial blood pressure and RSNA, suggesting that this region has the potential to regulate baroreflex input (30). Furthermore, blockade of the PBN with lidocaine enhances phenylephrine-evoked reflex bradycardia and nitroprusside-evoked reflex tachycardia (50). Other studies (27, 29) have shown that lidocaine in the lateral PBN attenuates chemoreflex-induced pressor responses. Lidocaine nonspecifically blocks both cell bodies and axons in passage, so it is unclear from these types of studies if the PBN is processing or simply transmitting responses from baroreceptors or other nuclei, such as the NTS. There are no investigations of the role of the lateral PBN in regulating cardiovascular reflex responses, although we (19, 20) have shown that the stimulation of cardiac sympathetic afferents activates neurons in the external lateral PBN, suggesting that this subregion of the lateral PBN has the potential to process input from spinal afferents. The present study provides the first evidence that neurons in the lateral PBN, specifically the elPBN, do regulate cardiovascular afferent input and more specifically sympathoexcitatory reflexes during the stimulation of cardiac afferents.
Glutamate, a ubiquitous excitatory neurotransmitter in the central nervous system, appears to serve an important role in the lateral PBN in the regulation of cardiac sympathoexcitatory reflexes. In this regard, glutamate-like immunoreactivity is present in the lateral and medial PBN (40). Glutamate injected into the lateral PBN, as noted above, also evokes cardiovascular excitatory responses. Finally, iontophoresis of glutamate into the PBN increases neuronal impulse activity, an action that can be eliminated by applying Kyn to the lateral PBN (33). The present study expands on the previous investigations by demonstrating a role for glutamate in the elPBN in processing BK-evoked cardiac sympathoexcitatory reflexes in vagus intact cats as well as in vagotomized cats.
Ionotropic glutamate NMDA and non-NMDA receptors regulate ligand-gated ion channels that mediate glutamatergic neurotransmission in the PBN (8, 33, 63, 64). Immunocytochemical and in situ hybridization studies (24, 25) have shown that both NMDA and AMPA receptors are located in the lateral PBN. Application of NMDA to the lateral PBN neurons in a brain stem slice causes membrane depolarization and the generation of a fast excitatory synaptic transmission within the PBN (33, 64). The increase in the PBN neuronal discharge induced by iontophoresis of glutamate in the PBN is attenuated by blockade of NMDA receptors with AP5 (33). NTS-evoked excitation of PBN neurons is reduced by antagonism of glutamate NMDA receptors in the lateral PBN, and excitatory NTS afferent input to the lateral PBN is reduced by blockade of APMA receptors (18, 33). Additionally, in vitro blockade of AMPA receptors with NBQX attenuates glutamate-induced excitation of lateral PBN neurons (63). Because neurons in the lateral PBN regulate respiratory as well as cardiovascular function (7, 38, 50), and cells in the medial PBN regulate gustatory activity (16), in vitro studies of neurons in these regions do not provide information about the importance of ionotropic glutamate receptors specifically with regard to their role in the regulation of cardiovascular reflex responses. The present study represents the first direct evidence that both glutamate NMDA and AMPA receptors in the elPBN serve an important role in the integration of afferent input that ultimately regulates cardiovascular function.
Processing cardiac sympathetic afferent input appears to be specific to the elPBN since glutamate receptor antagonism in areas surrounding this region did not alter the reflex responses. Previous studies (16, 27, 30, 50) have generally focused on the role of either lateral or medial PBN in the regulation of autonomic function. Although the lateral PBN can be further subdivided to the dorsal PBN, ventral PBN, central PBN, and elPBN (2, 19), there has been little investigation of which subdivision(s) participate(s) in cardiovascular reflex regulation. In this regard, one study (4) reported that glutamate microinjected into the elPBN could increase arterial pressure and HR, whereas glutamate in the dorsal, ventral, and central lateral PBN decreased blood pressure and HR. This study did not evaluate the role of these subregions in reflex regulation and thus documented the potential but not the actual cardiovascular regulatory roles of these subdivisions of the lateral PBN. Because we (19, 20) have previously observed that the stimulation of cardiac sympathetic afferents activates neurons primarily in the elPBN, the present study focused on the elPBN, which appears to have a clear role in processing cardiac afferent input that ultimately results in sympathoexcitation.
Our study supports observations by other investigators using lidocaine, kainic acid, and NMDA receptor blockade to show that ionotropic glutamate receptors in this nucleus do not contribute to tonic vasomotor or cardiomotor tone (26, 27, 50, 51).
Although it is clear from our protocols using vagotomy that sympathetic cardiac afferent input is regulated by glutamatergic mechanisms in the elPBN, we also wanted to study the preparation in a more natural state in which both vagal and sympathetic cardiac afferent systems were intact, which represents the situation in patients or experimental animal subjects experience myocardial ischemia. We speculated that it would be difficult to observe pressor reflex responses when BK was applied intrapericardially in intact animals because previous studies have documented that the epicardial application of BK activates cardiac vagal (34) as well as cardiac sympathetic afferents (12, 57, 59) to evoke inhibitory (42, 46) and excitatory (21, 55) reflex responses, respectively. Thus, activation of cardiac vagal afferents by chemical mediators of ischemia, such as H2O2, induces inhibitory reflexes that blunt excitatory reflexes engendered by the activation of sympathetic afferents (31). Interestingly, we found that intrapericardial BK stimulation provoked similar increases in arterial pressure and RSNA in vagotomized as well as vagus (and sympathetic afferent) intact animals and that Kyn in the elPBN similarly reduced these excitatory responses. Thus, the present data indicate that reflex input from the heart of intact animals with functioning sympathetic and vagal afferents is transmitted mainly through sympathetic afferents and is processed in the elPBN. The NTS receives input from the cardiac vagal and sympathetic afferents (56). As noted earlier, there are reciprocal connections between the NTS and the lateral PBN (33). Interactions between these two nuclei in processing cardiac vagal and sympathetic afferent stimuli deserve further study.
The antagonists attenuated reflex blood pressure and RSNA between 33% and 53% (Fig. 4, A and C) in the present study. This degree of attenuation has been observed in many studies (1, 28, 43, 61) of central neural regulation of cardiovascular and respiratory function after the microinjection of antagonists. For instance, Orer and colleagues (43) demonstrated that the bilateral microinjection of AP5 or NBQX into the lateral tegmental field attenuated vagal-induced excitatory responses in the inferior cardiac nerves by 38–66%. There are several factors that might explain this range of responses. Among others, these include the volume and dose of the antagonist injected, the speed of injection, unilateral versus bilateral injection, the half-life of the antagonist, the affinity of the antagonist for its receptor, the size of the nucleus and relevant neuron phenotype distribution within the nucleus that processes the afferent information, the presence of other neurotransmitters engaged in processing in the nucleus of interest, and the relative role of one nucleus versus other regions that participate in processing the reflex. In summary, given the large number of variables, it is not surprising that there is variability in the response to administration of a selective antagonist. However, the present study showed consistent reflex attenuations with unilateral injection of each antagonist, suggesting that glutamate and its ionotropic receptors play a role in processing cardiac sympathetic afferent reflex information in the elPBN.
In the present study, we did not observe any HR changes in response to microinjection of each antagonist or glutamate into the elPBN. BK-induced HR changes were also not affected by any of the antagonists. There are potentially two reasons for the absence of any HR responses. First, we used α-chloralose for anesthesia, which has a vagolytic effect that may mask HR responses to glutamate in the elPBN in our intact group (26). Second, in the vagotomized animal groups, the HR was relatively high, which also potentially could mask a HR response. Previous studies shown that glutamate in the PBN, including the elPBN, increases blood pressure and HR in rats (4), whereas it evokes an increase in blood pressure but no change in HR in cats (26).
Other neurotransmitters, including GABA and ACh, in the elPBN may play a role in processing the cardiac sympathoexcitatory reflexes. In this regard, microinjection of GABA into the elPBN causes depressor responses with an associated decrease in RSNA (44). Furthermore, blockade of GABAA receptors with bicuculline in the elPBN abolishes depressor responses and inhibition of RSNA induced by the application of estrogen to this region (51). An immunocytochemical and in situ hybridization study (48) has demonstrated that cells and processes containing ACh are present in the elPBN. Furthermore, there are cholinergic processes that project from the elPBN to the RVLM that appear to contribute to elevations in blood pressure (37). It is possible that, in addition to glutamate, these neurotransmitters may participate in processing of the cardiac-cardiovascular reflexes in the elPBN.
There are two potential limitations to the present study. First, damage caused by insertion of the modified microinjection probe may disrupt elPBN processing of cardiac sympathoexcitatory reflex inputs. However, previous studies (58, 61, 62) have shown that autonomic central nuclei function normally after the insertion of probes into the RVLM and periaqueductal gray. Furthermore, similar-sized microdialysis probes have been used in many studies (10, 39, 47, 54, 61, 62) to assess the function of central nuclei in association with measurement of neurotransmitter release in these regions. In the present investigation, we observed similar pressor responses to intrapericardial BK before and after probe insertion. We, therefore, believe that the elPBN functions well after insertion of the probe.
Second, unilateral blockade of the elPBN clearly attenuated cardiac-cardiovascular reflex responses. We opted not to perform bilateral microinjection to assess the overall role of the elPBN because of its size and significant rostral-caudal extension in cats (0.3–0.6 mm, lateral to midline, 1.0–1.8 mm, dorsal to ventral, 1.9–2.3 mm, caudal to rostral) (2, 22). It would be difficult to completely inhibit function of this entire region.
In summary, the present study provides the first evidence demonstrating that the elPBN participates in processing reflex input from cardiac sympathetic afferents that ultimately leads to sympathoexcitatory reflex responses. Glutamate NMDA and AMPA receptors in the elPBN are essential for integrating these reflex inputs in this brain region. These new data extend our understanding of the functional role of the elPBN, as an essential region in the pons that regulates sympathetic outflow.
GRANTS
J. Longhurst holds the Larry K. Dodge and Susan Samueli Endowed Chairs. This work was supported by National Heart, Lung, and Blood Institute Grant HL-66217 to L. W. Fu and J. C. Longhurst.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.-W.F. and J.C.L conception and design of research; L.-W.F. and Z.-L.G. performed experiments; L.-W.F. and Z.-L.G. analyzed data; L.-W.F. and Z.-L.G. interpreted results of experiments; L.-W.F. and Z.-L.G. prepared figures; L.-W.F. drafted manuscript; L.-W.F., Z.-L.G., and J.C.L. edited and revised manuscript; L.-W.F., Z.-L.G., and J.C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical assistance of Jesse Ho. The authors also thank the undergraduate students An Nguyen and Henry Chang for help with the experimental procedures.
REFERENCES
- 1. Anderson MK, Speck DF. Differential effects of excitatory amino acid receptor antagonism in the ventral respiratory group. Brain Res 829: 69–76, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Berman AL. The Brainstem of the Cat: a Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison, WI: Univ. of Wisconsin Press, 1968 [Google Scholar]
- 3. Cervero F, Tattersall JE. Somatic and visceral inputs to the thoracic spinal cord of the cat: marginal zone (lamina I) of the dorsal horn. J Physiol 388: 383–395, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chamberlin N, Saper C. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol 326: 245–262, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361: 225–248, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Craig AD, Kniffki KD. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol 365: 197–221, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Dutschmann M, Guthmann A, Herbert H. NMDA receptor subunit NR1-immunoreactivity in the rat pons and brainstem and colocalization with Fos induced by nasal stimulation. Brain Res 809: 221–230, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Fu LW, Guo ZL, Longhurst JC. Role of glutamate receptors in the latereal parabrachial nucleus in regulation of cardiac sympathoexcitatory reflexes (Abstract). FASEB J 25: 1078.–12., 2011 [Google Scholar]
- 10. Fu LW, Longhurst C. Electroacupuncture modulates vlPAG release of GABA through presynaptic cannabinoid CB1 receptor. J Appl Physiol 106: 1800–1809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu LW, Guo ZL, Longhurst JC. Undiscovered role of endogenous TxA2 in activation of cardiac sympathetic afferents during ischemia. J Physiol 586: 3287–3300, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu LW, Longhurst JC. Interactions between histamine and bradykinin in stimulation of ischaemically sensitive cardiac afferents in felines. J Physiol 565: 1007–1017, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu LW, Longhurst JC. Regulation of cardiac afferent excitability in ischemia. Handb Exp Pharmacol 185–225, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Fu LW, Phan A, Longhurst JC. Myocardial ischemia-mediated excitatory reflexes: a new function for thromboxane A2? Am J Physiol Heart Circ Physiol 295: H2530–H2540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu LW, Longhurst JC. Bradykinin and thromboxane A2 reciprocally interact to synergistically stimulate cardiac spinal afferents during myocardial ischemia. Am J Physiol Heart Circ Physiol 298: H235–H244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fulwiler C, Saper C. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319: 229–259, 1984 [DOI] [PubMed] [Google Scholar]
- 17. Gill CF, Madden JM, Roberts BP, Evans LD, King MS. A subpopulation of neurons in the rat rostral nucleus of the solitary tract that project to the parabrachial nucleus express glutamate-like immunoreactivity. Brain Res 821: 251–262, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Granata AR, Kitai ST. Intracellular study of nucleus parabrachialis and nucleus tractus solitarii interconnections. Brain Res 492: 281–292, 1989 [DOI] [PubMed] [Google Scholar]
- 19. Guo ZL, Li P, Longhurst J. Central pathways in the pons and midbrain involved in cardiac sympathoexcitatory reflexes in cats. Neuroscience 113: 433–444, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Guo ZL, Moazzami A, Longhurst J. Stimulation of cardiac sympathetic afferent activates glutamatergic neurons in the parabrachial nucleus: relation to neurons containing nNOS. Brain Res 1053: 36–48, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Guo ZL, Tjen-ALooi SC, Fu LW, Longhurst JC. Nitric oxide in rostral ventrolateral medulla regulates cardiac-sympathetic reflex: role of synthase isoforms. Am J Physiol Heart Circ Physiol 297: H1479–H1486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo ZL, Longhurst JC. Activation of nitric oxide-producing neurons in the brain stem during cardiac sympathoexcitatory reflexes in the cat. Neuroscience 116: 167–178, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Guo Z, Lai H, Longhurst J. Medullary pathways involved in cardiac sympathoexcitatory reflexes in the cat. Brain Res 925: 55–66, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Guthmann A, Herbert H. Expression of N-methyl-d-aspartate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei and in selected pontomedullary brainstem nuclei. J Comp Neurol 415: 501–517, 1999 [PubMed] [Google Scholar]
- 25. Guthmann A, Herbert H. In situ hybridization analysis of flip/flop splice variants of AMPA-type glutamate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei. Brain Res Mol Brain Res 74: 145–157, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Hade JS, Mifflin SW, Donta TS, Felder RB. Stimulation of parabrachial neurons elicits a sympathetically mediated pressor response in cats. Am J Physiol Heart Circ Physiol 255: H1349–H1358, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Haibara AS, Tamashiro E, Olivan MV, Bonagamba LG, Machado BH. Involvement of the parabrachial nucleus in the pressor response to chemoreflex activation in awake rats. Auton Neurosci 101: 60–67, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Hayward LF, Castellanos M, Davenport PW. Parabrachial neurons mediate dorsal periaqueductal gray evoked respiratory responses in the rat. J Appl Physiol 96: 1146–1154, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Hayward LF, Felder RB. Peripheral chemoreceptor inputs to the parabrachial nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 268: R707–R714, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Hayward LF, Felder RB. Lateral parabrachial nucleus modulates baroreflex regulation of sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 274: R1274–R1282, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Huang HS, Stahl G, Longhurst J. Cardiac-cardiovascular reflexes induced by hydrogen peroxide in cats. Am J Physiol Heart Circ Physiol 268: H2114–H2124, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Jhamandas JH, Aippersbach SE, Harris KH. Cardiovascular influences on rat parabrachial nucleus: an electrophysiological study. Am J Physiol Regul Integr Comp Physiol 260: R225–R231, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Jhamandas JH, Harris KH. Excitatory amino acids may mediate nucleus tractus solitarius input to rat parabrachial neurons. Am J Physiol Regul Integr Comp Physiol 263: R324–R330, 1992 [DOI] [PubMed] [Google Scholar]
- 34. Kaufman MP, Baker DG, Coleridge HM, Coleridge JCG. Stimulation by bradykinin of afferent vagal C-fibers with chemosensitive endings in the heart and aorta of the dog. Circ Res 46: 476–484, 1980 [DOI] [PubMed] [Google Scholar]
- 35. Kliks B, Burgess M, Abildskov J. Influence of sympathetic tone on ventricular fibrillation threshold during experimental coronary occlusion. Am J Cardiol 36: 45–49, 1975 [DOI] [PubMed] [Google Scholar]
- 36. Klop EM, Mouton LJ, Hulsebosch R, Boers J, Holstege G. In cat four times as many lamina I neurons project to the parabrachial nuclei and twice as many to the periaqueductal gray as to the thalamus. Neuroscience 134: 189–197, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Kubo T, Hagiwara Y, Sekiya D, Fukumori R. Evidence for involvement of the lateral parabrachial nucleus in mediation of cholinergic inputs to neurons in the rostral ventrolateral medulla of the rat. Brain Res 789: 23–31, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Lara JP, Parkes MJ, Silva-Carvhalo L, Izzo P, wid-Milner MS, Spyer KM. Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetized rat. J Physiol 477: 321–329, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li J, Mitchell JH. Glutamate release in midbrain periaqueductal gray by activation of skeletal muscle receptors and arterial baroreceptors. Am J Physiol Heart Circ Physiol 285: H137–H144, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Liu RH, Fung SJ, Reddy VK, Barnes CD. Localization of glutamatergic neurons in the dorsolateral pontine tegmentum projecting to the spinal cord of the cat with a proposed role of glutamate on lumbar motoneuron activity. Neuroscience 64: 193–208, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Mraovitch S, Kumada M, Reis DJ. Role of the nucleus parabrachialis in cardiovascular regulation in cat. Brain Res 232: 57–75, 1982 [DOI] [PubMed] [Google Scholar]
- 42. Neto FR, Brasil JC, Antonio A. Bradykinin-induced coronary chemoreflex in the dog. Naunyn Schmiedebergs Arch Pharmacol 283: 135–142, 1974 [DOI] [PubMed] [Google Scholar]
- 43. Orer HS, Gebber GL, Phillips SW, Barman SM. Role of the medullary lateral tegmental field in reflex-mediated sympathoexcitation in cats. Am J Physiol Regul Integr Comp Physiol 286: R451–R464, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Paton JF, Silva-Carvalho L, Thompson CS, Spyer KM. Nucleus tractus solitarius as mediator of evoked parabrachial cardiovascular responses in the decerebrate rabbit. J Physiol 428: 693–705, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Randle JC, Guet T, Cordi A, Lepagnol JM. Competitive inhibition by NBQX of kainate/AMPA receptor currents and excitatory synaptic potentials: importance of 6-nitro substitution. Eur J Pharmacol 215: 237–244, 1992 [DOI] [PubMed] [Google Scholar]
- 46. Reimann KA. Contrasting reflexes evoked by chemical activation of cardiac afferent nerves. Am J Physiol Heart Circ Physiol 239: H316–H325, 1980 [DOI] [PubMed] [Google Scholar]
- 47. Renno WM, Alkhalaf M, Mousa A, Kanaan RA. A comparative study of excitatory and inhibitory amino acids in three different brainstem nuclei. Neurochem Res 33: 150–159, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Ruggiero DA, Giuliano R, Anwar M, Stornetta R, Reis DJ. Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol 292: 1–53, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Saleh TM, Bauce LG, Pittman QJ. Glutamate release in parabrachial nucleus and baroreflex alterations after vagal afferent activation. Am J Physiol Regul Integr Comp Physiol 272: R1631–R1640, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Saleh TM, Connell BJ. Modulation of the cardiac baroreflex following reversible blockade of the parabrachial nucleus in the rat. Brain Res 767: 201–207, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Saleh TM, Connell BJ. Estrogen-induced autonomic effects are mediated by NMDA and GABAA receptors in the parabrachial nucleus. Brain Res 973: 161–170, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Schomig A, Richardt G, Kurz T. Sympatho-adrenergic activation of the ischemic myocardium and its arrhythmogenic impact. Herz 20: 169–186, 1995 [PubMed] [Google Scholar]
- 53. Schwartz P, Snebold N, Brown A. Effects of unilateral cardiac sympathetic denervation on the ventricular fibrillation threshold. Am J Cardiol 37: 1034–1040, 1976 [DOI] [PubMed] [Google Scholar]
- 54. Silva E, Hernandez L, Contreras Q, Guerrero F, Alba G. Noxious stimulation increases glutamate and arginine in the periaqueductal gray matter in rats: a microdialysis study. Pain 87: 131–135, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Staszewska-Woolley J, Woolley G. Participation of the kallikrein-kinin-receptor system in reflexes arising from neural afferents in the dog epicardium. J Physiol 419: 33–44, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tjen-ALooi S, Bonham A, Longhurst J. Interactions between sympathetic and vagal cardiac afferents in nucleus tractus solitarii. Am J Physiol Heart Circ Physiol 272: H2843–H2851, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Tjen-ALooi S, Pan HL, Longhurst JC. Endogenous bradykinin activates ischaemically sensitive cardiac visceral afferents through kinin B2 receptors in cats. J Physiol 510: 633–641, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tjen-ALooi SC, Li P, Longhurst JC. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: roles of endocannabinoids and GABA. J Appl Physiol 1793–1799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uchida Y, Murao S. Bradykinin-induced excitation of afferent cardiac sympathetic nerve fibers. Jpn Heart J 15: 84–91, 1974 [DOI] [PubMed] [Google Scholar]
- 60. Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med 358: 2024–2029, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Zhou W, Fu LW, Tjen-ALooi SC, Guo ZL, Longhurst JC. Role of glutamate in a visceral sympathoexcitatory reflex in rostral ventrolateral medulla of cats. Am J Physiol Heart Circ Physiol 291: H1309–H1318, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Zhou W, Fu LW, Guo ZL, Longhurst JC. Role of glutamate in rostral ventrolateral medulla in acupuncture-related modulation of visceral reflex sympathoexcitation. Am J Physiol Heart Circ Physiol 292: H1868–H1875, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Zidichouski JA, Easaw JC, Jhamandas JH. Glutamate receptor subtypes mediate excitatory synaptic responses of rat lateral parabrachial neurons. Am J Physiol Heart Circ Physiol 270: H1557–H1567, 1996 [DOI] [PubMed] [Google Scholar]
- 64. Zidichouski JA, Jhamandas JH. Electrophysiological characterization of excitatory amino acid responses in rat lateral parabrachial neurons in vitro. Brain Res 611: 313–321, 1993 [DOI] [PubMed] [Google Scholar]