Abstract
Mutations in SCN5A, the gene encoding the pore-forming subunit of cardiac Na+ channels, cause a spectrum of arrhythmic syndromes. Of these, sinoatrial node (SAN) dysfunction occurs in patients with both loss- and gain-of-function SCN5A mutations. We explored for corresponding alterations in SAN function and intracardiac conduction and clarified possible mechanisms underlying these in an established mouse long QT syndrome type 3 model carrying a mutation equivalent to human SCN5A-ΔKPQ. Electrophysiological characterizations of SAN function in living animals and in vitro sinoatrial preparations were compared with cellular SAN and two-dimensional tissue models exploring the consequences of Scn5a+/ΔKPQ mutations. Scn5a+/ΔKPQ mice showed prolonged electrocardiographic QT and corrected QT intervals confirming long QT phenotypes. They showed frequent episodes of sinus bradycardia, sinus pause/arrest, and significantly longer sinus node recovery times, suggesting compromised pacemaker activity compared with wild-type mice. Electrocardiographic waveforms suggested depressed intra-atrial, atrioventricular node, and intraventricular conduction in Scn5a+/ΔKPQ mice. Isolated Scn5a+/ΔKPQ sinoatrial preparations similarly showed lower mean intrinsic heart rates and overall slower conduction through the SAN to the surrounding atrium than did wild-type preparations. Computer simulations of both single SAN cells as well as two-dimensional SAN-atrial models could reproduce the experimental observations of impaired pacemaker and sinoatrial conduction in terms of changes produced by both augmented tail and reduced total Na+ currents, respectively. In conclusion, the gain-of-function long QT syndrome type 3 murine Scn5a+/ΔKPQ cardiac system, in overlap with corresponding features reported in loss-of-function Na+ channel mutations, shows compromised SAN pacemaker and conduction function explicable in modeling studies through a combination of augmented tail and reduced peak Na+ currents.
Keywords: sodium channel, voltage-gated, type V, α-subunit; long QT syndrome type 3; sinus node dysfunction
inherited mutations in SCN5A, the gene encoding the pore-forming subunit of the cardiac type Na+ channel, cause a spectrum of disease entities, Na+ channelopathies, which include multiple arrhythmic syndromes. Loss-of-function SCN5A mutations were first reported in patients with rare familial sinus bradycardic syndromes by Benson and colleagues (7). They were subsequently linked to familial sick sinus syndrome (17, 36, 39), for which 14 loss-of-function sick sinus syndrome-associated SCN5A mutations have so far been identified (23). However, sinus node dysfunction has also been reported in patients with gain-of-function SCN5A mutations associated with long QT syndrome type 3 (LQT3) in apparent overlap with the previous examples (41). This similarly presented as significantly depressed mean heart rates (HRs) (28) and episodes of sinus pause and arrest (16). These could exacerbate risks of lethal ventricular arrhythmias: in LQT3, QT prolongation associated with potentially life-threatening ventricular arrhythmias are most pronounced at lower HRs but virtually absent at high HRs (1, 4). Furthermore, sinus node dysfunction often occurs in association with other, acquired, heart conditions, such as heart failure and cardiac ischemia, that often accompany QT prolongation (47).
These similar clinical findings bearing on sinoatrial (SA) function, associated with contrasting SCN5A mutations, parallel previous reports of overlap syndrome involving ventricular arrhythmogenicity in LQT3 and Brugada syndrome (BrS) patients. Conversely, a number of mutations appear with more than one phenotype in different patients (24, 25, 33, 34). Thus, a single Na+ channel mutation involving the deletion of Lys1500 (ΔK1500) is associated not only with LQT3 but also BrS and conduction system disease (16). The D1795 insertion is similarly associated with both LQT3 and BrS and results in a 62% reduction of Na+ channel expression (3, 32). Finally, clinical phenotypes overlaping with those observed in BrS have also been reported in patients carrying the SCN5A-ΔKPQ mutation (27).
The present study examined SA function in gain-of-function murine Scn5a+/ΔKPQ hearts (20). These complement previous studies (18, 21) on SA node (SAN) function made in loss-of-function Scn5a+/− hearts. In so doing, it was possible to demonstrate a corresponding overlap syndrome involving SAN function in murine systems containing these gain- and loss-of-function variants. Murine systems have previously proven useful in studies of other conditions involving clinical electrophysiological abnormalities.
MATERIALS AND METHODS
Experimental animals.
All procedures were performed in licenced institutional premises under UK Home Office project licence No. 80/1974 and approved by a university ethics review board, all approved under the UK Animals (Scientific Procedures) Act (1986). Heterozygote Scn5a+/ΔKPQ and wild-type (WT) mice were maintained at room temperature and 12:12-h light-dark cycles and fed with sterile rodent chow with free access to water. Offspring from heterozygote Scn5a+/ΔKPQ and WT breeding pairs were genotyped, weaned, and used when of the correct age. Mice were bred on a 129/sv genetic background (Harlan, UK). The experiments examined electrophysiological characteristics of Scn5a+/ΔKPQ hearts at the levels of whole anesthetized animals, isolated hearts, and isolated tissue preparations. The experiments used mice aged 6–9 mo.
Experiments in anesthetized animals.
As we were studied apparently depressant effects on conduction in a system thought paradoxically to result in a gain-of-function mutation, our electrocardiographic experiments were made independently using two anesthetics to avoid interpretations based on anesthetic as opposed to genetic effects. The stock ketamine-containing anesthetic solution consisted of 1.8 ml of 100 mg/ml ketamine hydrochloride (Ketaset, Fort Dodge, UK), 0.35 ml of 23.32 mg/ml xylazine hydrochloride (Rompun, Bayer, Leverkusen, Germany), and 2.85 ml of sterile phosphate base solution. Mice were anesthetized at a dose of 0.10 ml/10 g body wt injected into the left peritoneal cavity 15 min before electrical recording. When avertin (2,2,2-tribromo-ethanol, Sigma, Poole, UK) was used as anesthetic, it was administered in a 24 mg/ml solution at a dose of 0.10 ml/10g body wt. The injection was given intraperitoneally into the left peritoneal cavity 5 min before electrical recording. Anesthetized mice were placed on a heating pad with continuous monitoring of body temperature for the following measurements.
Three-lead limb ECGs were recorded through subcutaneous needle electrodes using a Powerlab 26T system (AD Instruments, Hastings, UK). The resulting digital recordings (16 bit, 2 kHz/channel) were analyzed using the Chart version 6.0 program (AD Instruments) to obtain the signal-averaged ECG. T-wave durations and QT intervals were measured in lead II. As previously reported, the recorded ECGs returned to baseline either with or without a slow undershoot. When such an undershoot was absent, values of the QT interval were estimated as the interval between the onset of the QRS complex as indicated by the Q wave deflection or, where this was absent, the base of the R wave and the moment after the T wave peak when the first derivative of the voltage trace (dV/dt) became zero. When such undershoots were present, the end of the QT interval was estimated from the minimum value of the undershoot. QT intervals were then also corrected for HRs using the following formula: QTc = QT/(RR/100)1/2, where QTc is the corrected QT interval and RR is the R-R interval (26).
Measurements of sinus node recovery times (SNRTs) used an ultraminiature octapolar 1.1-Fr electrophysiology catheter connected to either recording or stimulating leads (EPR-800, Millar Instruments, Houston, TX) placed in the esophagus. Placement of these oesophageal electrodes was aided by an observation of approximately equal amplitudes of atrial and ventricular contributions to the electrical waveforms during continuous monitoring of transesophageal ECGs. Such determinations of SNRT were made after the simultaneous recording of the baseline surface and esophageal ECG for 5 min. This was then followed by esophageal pacing at a cycle length (CL) of 100 ms for 30 s before the protocols used to determine SNRT.
Experiments in isolated tissue preparations.
Isolated SAN preparations were obtained from mice after their death by cervical dislocation [Schedule I, UK Animals (Scientific Procedures) Act (1986)]. Multielectrode array recordings of extracellular potentials applied to such isolated SAN preparations permitted the construction of SAN activation maps and measurements of SAN CL and sinoatrial conduction time as previously described (21). The SA preparations were set up as previously described (22). After dissection of the SAN and surrounding atrial muscle, the preparation was placed endocardial surface up in a tissue bath and superfused with Tyrode solution [containing (in mM) 140 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 5 HEPES; pH 7.4 with NaOH (22)] maintained at a temperature of 37 ± 0.5°C and at a flow rate of 4∼5 ml/min. Electrical signals were obtained from the surface of this preparation by apposing a custom-made electrode array consisting of 64 separate electrodes (Teflon-coated silver wires, 0.125-mm diameter, Science Products) in a square 8 × 8 configuration with an interelectrode distance of 0.55 mm. The total dimensions of the entire array were thus ∼4 × 4 mm. As the mouse SAN has dimensions of ∼0.5 × 1 mm, considerably smaller than those of other animals in which the SAN has been studied (e.g., 3 × 5 mm in the rabbit), the array therefore covered the entire SAN as well as the surrounding atrium, extending to the crista terminalis, atrial septum, and the superior and inferior venae cavae. The multielectrode array was first placed upon the tissue. The position of the SAN was determined by locating the recording element that showed the earliest deflection and using this as a reference point for which to construct our isochronal map. Unipolar electrical recordings were then obtained using a large reference electrode located close to the array but not directly touching the tissue, acting as the indifferent pole.
The 64 recording electrodes were connected through shielded wires to two 32-channel amplifiers (SCXI-1102C, National Instruments, Newbury, UK). The sampling frequency for each channel was set at 2 kHz; at such sampling frequencies, our data storage capacity permitted the collection of records lasting not more than ∼20 s. Signals were continuously stored on disk and displayed onscreen using a custom-developed program written in Labview 7.0.
Propagation maps were then derived by offline analysis. Signals were displayed onscreen in sets of 8–16 electrograms. The activation time was determined as the point of maximal negative slope and marked with a cursor. After all significant waveforms in all leads were marked, the activation times were then displayed in a grid representing the layout of the original recording array. All activation times (in ms) were related to the timing of the first detected waveform. Isochrones were drawn manually around areas activated in steps of 5 ms.
Definition of arrhythmic events.
Sinus bradycardia and tachycardia were defined as ≥30% reductions or increases of the baseline HR, respectively, from the control condition. Sinus pause was defined as a spontaneous interruption in the regular sinus rhythm, with the pause lasting for a period not an exact multiple of the sinus cycle. Sinus arrest was defined as the cessation of SAN pacemaker activity; the ventricles may continue to beat under ectopic atrial, atrioventricular junctional, or idioventricular control.
Computer simulations.
The consequences of LQT3-associated Scn5a+/ΔKPQ mutations were explored using previously developed 1) cellular models of central and peripheral SAN cells (45) and 2) two-dimensional (2-D) tissue models of intact SAN-atrial tissue. This incorporated accurate representations of single SAN and right atrial (RA) cells (2) and a histologically reconstructed geometry of a single slice of the rabbit RA through the atrial muscle of the crista terminalis and the intercaval region including central and peripheral SAN areas (10, 12). These were represented by a regular Cartesian grid of 210 × 45 nodes at a high (40 μm) spatial resolution. This corresponds to two to four times the diameter of a cardiac myocyte. For each node, a flag variable was used to identify whether it possessed a SAN or RA cell type, as indicated by the immunohistochemical mapping data. The resulting 2-D anatomic partial differential equation (PDE) model of the intact SAN-atrium was solved using the explicit Euler method with a five-node approximation of the Laplacian operator. The effects of the Scn5a+/ΔKPQ Na+ channel mutation were modeled by modifying previous equations (45) for central and peripheral SAN cells. This incorporated additional equations for the late persistent Na+ channel current (INa,L) summarized above. In such modified models incorporating properties of both central and peripheral SAN cells, the Na+ current consists of two components: transient Na+ current (INa,T) and INa,L.
Equations by Zhang et al. (45) modeled the properties of central and peripheral SAN cells. Equations by Aslanidi et al. (2) modeled RA cells. The following additional formulations based on human ventricular cells (44) modeled INa,L within the SAN but not the atrium. Thus:
where ENa is the Na+ equilibrium potential, R is the universal gas constant, F is Faraday's constant, T is the absolute temperature, and [Na+]o and [Na+]i are extracellular and intracellular Na+ concentrations. The voltage-dependent opening rate constant of the prolonged Na+ current (αmL) was calculated as follows:
where Vm is membrance potential. The corresponding closing rate constant (βmL) was calculated as follows:
These give the time constant τmL of the activation variable mL as follows:
where
and
Similarly, the time constant (τhL) of the inactivation variable, hL, of the late Na+ current was calculated as follows:
in the following equation:
The conductance of INa,L (gNa,L) was given by
INa,L is then given by the following:
The 2-D model used was based on the SAN “gradient model” (2, 45). This incorporated established findings that cell size and, therefore, cell membrane capacitance (Cm) gradually increases from the SAN center to its periphery, varying from 20 to 65 pF. It also assumed a simple correlation between Na+ current density and cell capacitance. It further considered experimentally established regional differences in electrophysiological properties and gap junction coupling between the SAN center, periphery, and atrial tissue (12). The experimentally observed reduced excitability in the “conduction block zone” adjacent to the SAN on the same side as the atrial septum was modeled by reductions in the density of L-type Ca2+ current as previously described (10). Action potential (AP) conduction involving intercellular electrical coupling through gap junctions was modeled by adapting the diffusion equation to Vm in the form of the following PDE:
where t is time, ∇ is the spatial gradient operator, D is a diffusion coefficient that characterizes the electrotonic spread of voltage, and Itot is the total ionic current. The parameters used in both the cellular and tissue models have been validated in our previous studies. Use of these parameters in the 2-D tissue model (such as the value of D) was validated by the ability of the 2-D model to reproduce the experimentally observed sequence of AP initiation and conduction through the rabbit SAN and atrium. The 2-D anatomic PDE model of the intact SAN-atrium was solved using the explicit Euler method with a five-node approximation of the Laplacian operator. In numeric simulations, the time step was 0.005 ms and the space step was 0.04 mm. Corroborative simulations using a range of time and space steps confirmed that this gave accurate and convergent numeric solutions.
Statistical analysis.
All data are reported as means ± SE. One-way repeated-measures ANOVA was used to compare values of measurements obtained from the same heart before and after treatment. When ANOVA revealed the existence of a significant difference among values, the ANOVA test was applied to determine the significance of a difference between a selected group's means. Fisher's exact test was used as appropriate for categorical variables. P < 0.05 was taken as an upper limit to indicate a significant difference.
RESULTS
Electrocardiographic characteristics.
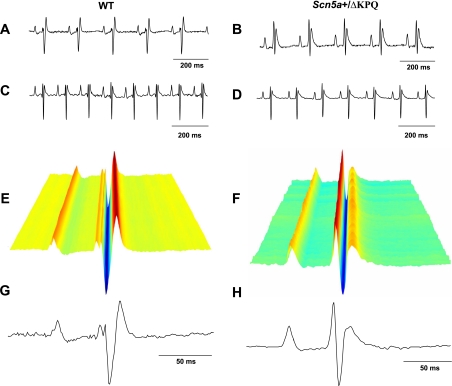
SAN automaticity was first characterized using ECG recordings and esophageal electrical pacing in anesthetized mice. Figure 1 shows typical results from lead II surface ECG recordings from 12 WT and 10 Scn5a+/ΔKPQ mice under ketamine anesthesia (A and B) and 19 WT and 19 Scn5a+/ΔKPQ mice under avertin anesthesia (C and D). Previous work has indicated that ketamine anesthesia provides reliable relaxation, sedation, and analgesia in mice but also exerts bradycardic effects (19). This was also observed in the present study. Accordingly, corroborating experiments used an alternative commonly used anesthetic, avertin. This exerted smaller effects on HRs. WT (Fig. 1, A and C) and Scn5a+/ΔKPQ (Fig. 1, B and D) recordings obtained in the presence of ketamine (Fig. 1, A and B) and avertin (Fig. 1, C and D) otherwise showed similar ECG waveforms, apart from longer basic CLs with ketamine. Waterfall representations confirmed the consistency of successively obtained ECG waveforms (Fig. 1, E and F). Sets of 200 individual ECG waveforms were accordingly used to derive averaged waveforms (Fig. 1, G and H) to determine specific electrocardiographic parameters. Scn5a+/ΔKPQ mice showed evidence of abnormal ventricular repolarization, as reflected in increased ECG QT and QTc intervals (after correction for RR intervals) compared with WT mice, consistent with a previous study (20) [QTc intervals in Scn5a+/ΔKPQ mice: 44.6 ± 2.2 ms with avertin (n = 19) and 45.4 ± 3.0 ms with ketamine (n = 10) and QTc intervals in WT mice: 34.5 ± 2.3 ms with avertin (n = 19) and 33.0 ± 2.2 ms with ketamine (n = 10), P < 0.01; Table 1]. They also showed longer PR intervals and QRS durations, suggesting conduction abnormalities.
Fig. 1.
ECG recordings (lead II) from anesthetized wild-type (WT) mice (A, C, E, and G) and Scn5a+/ΔKPQ mice (B, D, F, and H). A–D: representative recordings of surface ECG recordings under ketamine (A and B) and avertin (C and D) anesthesia. E–H: waterfall plots (E and F) showing individual ECG recordings used to obtain averaged ECG recordings (G and H).
Table 1.
Summary of ECG parameters under avertin and ketamine anesthesia
| Genotype | Number of Mice | Heart Rate, beats/min | P Wave, ms | PR Interval, ms | QRS Interval, ms | QT Interval, ms | Corrected QT Interval, ms |
|---|---|---|---|---|---|---|---|
| Ketamine | |||||||
| WT | 12 | 260.6 ± 18.6 | 15 ± 0.5 | 46.2 ± 0.6 | 14.4 ± 0.4 | 51.3 ± 3.7 | 33.0 ± 2.2 |
| Scn5a+/ΔKPQ | 10 | 269.7 ± 13.3 | 18.2 ± 1.2* | 50.1 ± 1.0† | 17.1 ± 0.8† | 68.0 ± 4.0† | 45.4 ± 3.0† |
| Avertin | |||||||
| WT | 19 | 410.4 ± 14.5 | 12.3 ± 0.7 | 44.2 ± 1.1 | 11.6 ± 0.5 | 42.0 ± 2.9 | 34.5 ± 2.3 |
| Scn5a+/ΔKPQ | 19 | 390.4 ± 12.5 | 13.5 ± 0.5 | 47.8 ± 0.8* | 14.7 ± 0.7† | 56.0 ± 3.2† | 44.6 ± 2.2† |
Values are means ± SE.
WT, wild type.
P < 0.05 vs. control;
P < 0.01 vs. control.
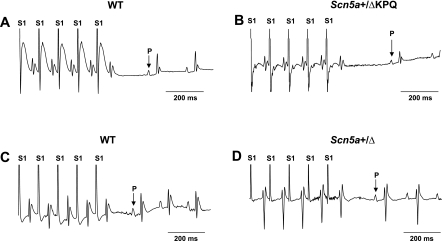
Further assessments of SAN function evaluated SNRTs by applying a burst pacing protocol over a 30-s stimulation period by esophageal electrical pacing. Figure 2 shows the resulting traces from WT (A and C) and Scn5a+/ΔKPQ (B and D) hearts under ketamine (A and B) and avertin (C and D) anesthesia. Scn5a+/ΔKPQ animals gave SNRT values of 433.2 ± 29.5 ms (n = 10) and 233.2 ± 23.9 ms (n = 6) under ketamine and avertin anesthesia, respectively. These were significantly greater than the respective WT values of 293.8 ± 17.6 ms (n = 12) and 159 ± 0.6 ms (n = 5) (ketamine: P < 0.001 and avertin: P < 0.05). Similarly, use of ketamine rather than avertin resulted in significantly greater SNRTs in both Scn5a+/ΔKPQ (P < 0.05) and WT (P < 0.05) mice. These findings suggest depressed SAN function in Scn5a+/ΔKPQ mice that could translate into the occurrence of bradycardic episodes reported in LQT3 patients, which might, in turn, contribute to the risks of developing ventricular arrhythmogenesis.
Fig. 2.
Electrocardiographic recordings (lead II) obtained during determinations of sinus node recovery time in the form of a resumption of atrial P waves after sequences of S1 stimuli in a burst pacing protocol in WT (A and C) and Scn5a+/ΔKPQ (B and D) mice under ketamine (A and B) and avertin (C and D) anesthesia.
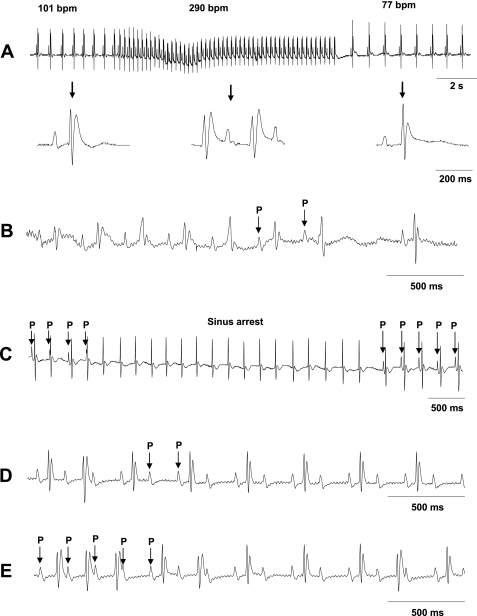
Finally, we explored for arrhythmic events implicating alterations in either SAN or atrioventricular node function both under baseline ECG recording conditions and after burst pacing. Different Scn5a+/ΔKPQ mice but not WT mice showed episodes of 1) sinus bradycardia (Fig. 3A) and tachycardia, defined, respectively, as episodes containing ≥30% reductions or increases in baseline HR (43); 2) sinus pause, defined as a spontaneous interruption in the regular sinus rhythm lasting for a period that was not an exact multiple of the sinus cycle (Fig. 3B); 3) episodes of ectopic, atrial, or junctional rhythms as well as periods during which there was sustained cessation of SAN pacemaker activity (Fig. 3C); and 4) episodes of atrioventricular block (Fig. 3, D and E). Table 2 shows the frequencies of these abnormal conduction phenomena both before and after burst pacing. Fisher exact tests suggested that their incidences did not differ between WT and Scn5a+/ΔKPQ mice with either anesthetic before burst pacing. However, burst pacing combined with the use of ketamine, but not avertin, anesthesia unmasked increased incidences of sinus bradycardia and atrioventricular block in Scn5a+/ΔKPQ mice.
Fig. 3.
Examples of lead II ECG recordings of arrhythmic events in Scn5a+/ΔKPQ mice. A: bradycardic episodes occurring before and after a period of normal sinus rhythm. Insets show that the corresponding detailed ECG waveforms were normal. bpm, beats/min. B: sinus pause occurring after a sequence of seven normal P wave deflections. C: sinus arrest showing an episode in which P waves were absent. D: persistent second-degree 2:1 atrioventricular block in which QRS complexes failed to follow alternate P waves. E: episodes of third degree atrioventricular block showing dissociation between P wave and QRS deflections.
Table 2.
Frequencies of sinoatrial and atrioventricular node arrhythmic events under ketamine and avertin anesthesia in WT and Scn5a+/ΔKPQ mice
| Number of Mice | Sinus Bradycardia | Sinus Tachycardia | Sinus Pause or Arrest | II to III Atrioventricular Block | |
|---|---|---|---|---|---|
| Baseline | |||||
| Ketamine | |||||
| WT | 12 | 1/12 | 2/12 | 0/12 | 0/12 |
| Scn5a+/ΔKPQ | 10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Avertin | |||||
| WT | 19 | 1/19 | 0/19 | 0/19 | 0/19 |
| Scn5a+/ΔKPQ | 19 | 1/19 | 0/19 | 0/19 | 1/19 |
| After pacing | |||||
| Ketamine | |||||
| WT | 12 | 0/12 | 0/12 | 0/12 | 0/12 |
| Scn5a+/ΔKPQ | 10 | 6/10† | 0/10 | 3/10 | 4/10* |
| Avertin | |||||
| WT | 5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Scn5a+/ΔKPQ | 6 | 0/6 | 0/6 | 1/6 | 0/6 |
Values are means ± SE.
P < 0.05 vs. control;
P < 0.01 vs. control.
Mapping of impulse generation and conduction in isolated SA preparations.
SAN pacemaker activity and SA and atrial conduction properties were then compared in WT and Scn5a+/ΔKPQ hearts using isolated SA preparations. A custom-made 64-electrode array simultaneously recorded extracellular potentials successfully from most of the 64 sites from the SAN and surrounding atrial muscle. The position of the SAN was identified as the earliest point at which the spontaneously recorded electrical activity took place by comparing the AP latencies at the different points and determining the earliest point in which spontaneous electrical activity was recorded. This varied between hearts, but with no discernible differences in pattern between WT and Scn5a+/ΔKPQ mice. Furthermore, preliminary explorations of a series of cardiac cycles over recording times of ∼1 s demonstrated that, although there were variations in the detailed pattern of the current spread between cycles, there was persistent overlap between regions showing the earliest APs. Thus, we did not detect electrophysiological evidence of beat-to-beat variations in the precise position of the principal pacemaker site. Areas with electrical activity with similar latencies were color coded and separated by isochrones corresponding to intervals of 0–5, 5–10, and 10–15 ms separating the onset of firing and response.
Electrophysiological properties of Scn5a+/ΔKPQ SA preparations.
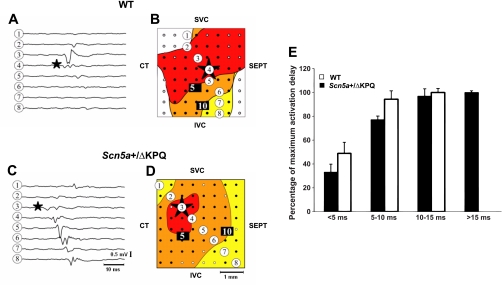
Figure 4 shows typical records of extracellular potentials (A and C) and activation maps (B and D) from the endocardial surface of WT (A and B) and Scn5a+/ΔKPQ (C and D) SA preparations. The stars in Fig. 4 indicate the pacemaker sites at which electrical activity was initiated. AP traces at different recording points are exemplified by the traces labeled 1–8 in Fig. 4, A and C, and were mapped onto the correspondingly numbered positions shown in the activation map in Fig. 4, B and D. This process was used to construct activation maps in which isochrones separated their different time intervals after the initiation of SAN pacemaker activity. These revealed a spreading pattern, in which a significant number of records suggested a slower conduction toward the septum compared with conduction toward the crista terminalis. Comparison of these revealed substantially larger areas of tissue enclosed by the earliest isochrone (0–5 ms) suggesting substantially slower activation sequences in Scn5a+/ΔKPQ hearts compared with WT hearts (Fig. 4, B and D). Figure 4E shows findings of this kind that were obtained from 12 WT hearts and 10 Scn5a+/ΔKPQ hearts. The results shown in Fig. 4E summarize the number of electrodes that showed excitable activity at different times after the initiation of each electrical cycle. This was expressed as a percentage of the total number of electrodes that eventually showed electrophysiological activity and thereby provided normalized indications of the extent of spread of excitation at different times. There was a significant lag in the initial spread of excitation shown by Scn5a+/ΔKPQ hearts compared with WT hearts.
Fig. 4.
Comparison of array recording results from typical WT (A and B) and Scn5a+/ΔKPQ (C and D) preparations, showing individual traces (A and C) obtained from the selected sequence of sites 1–8 as marked on the activation map (B and D) reflecting the slower spread of electrical activity in Scn5a+/ΔKPQ preparations. The following labels were included to orient the specimen: SVC, superior vena cava; IVC, inferior vena cava; CT, crista terminalis; SEPT, atrial septum. Points on the recording arrays in which recordings were obtained are marked as solid circles in B and D. The point showing the earliest deflection, likely representing the sinoatrial node (SAN) is marked with a star on both the electrical traces and the activation map. E: quantification of propagation maps by determining the number of recording sites as a proportion of the total number of sites in which electrical activity could be recorded in Scn5a+/ΔKPQ and WT preparations at different times after the initiation of activation demonstrating the slower spread of such activity in Scn5a+/ΔKPQ preparations.
The results shown in Table 3 further quantify this spread of excitation. Table 3 shows measurements of the time intervals between electrical activity at the pacemaker site and activity observed in strategic, identifiable, anatomic regions. Results were based on observations obtained in 10 Scn5a+/ΔKPQ preparations and 12 WT preparations mapped over 15–20 s/preparation. The conduction velocity from the SAN to different anatomic points in the atria, namely, the atrial septum and RA, was given by the following simple formula: conduction velocity = conduction time/conduction distance. Conduction distance was defined as the distance from the position of the multielectrode showing the earliest evidence of electrical activity to the anatomic point in question. Although we did not allow for the detailed nature of the propagation pathway, these measurements gave us a useful estimate of the rate of conduction. Scn5a+/ΔKPQ preparations showed a significantly depressed sinus rate (222.7 ± 24.3 beats/min, n = 10) compared with WT preparations (331.2 ± 22.7 beats/min, n = 12), consistent with the evidence for SAN depression indicated in the previous electrocardiographic results in anesthetized intact SA preparations (19). Scn5a+/ΔKPQ preparations showed significantly greater excitation latencies and therefore slowed conduction velocities for the spread of excitation between the SAN and atrial septum.
Table 3.
Summary of mapping electrical propagation properties in isolated WT and Scn5a+/ΔKPQ sinoatrial preparations
| To the Septum |
To the Right Atrium |
|||||
|---|---|---|---|---|---|---|
| Number of Mice | Heart Rate, beats/min | CT, ms | CV, cm/s | CT, ms | CV, cm/s | |
| WT | 12 | 331.2 ± 22.7 | 6.8 ± 0.8 | 21 ± 2.1 | 8.1 ± 0.7 | 18 ± 1.5 |
| Scn5a+/ΔKPQ | 10 | 222.7 ± 24.3† | 9.3 ± 0.3† | 15 ± 1.4* | 8.5 ± 0.9 | 18 ± 2.7 |
Values are means ± SE.
CT, conduction time; CV, conduction velocity.
P < 0.05 vs. control; **P < 0.01 vs. control.
Computation analysis predicts overlapping loss- and gain-of-function changes in the SAN with Scn5a+/ΔKPQ.
The experimental findings thus associated Scn5a+/ΔKPQ with a complex set of physiological changes compromising both pacemaker function and SAN and atrial conduction. The final simulation experiments explored for simplified explanations for such changes in terms of altered Na+ channel properties that have been associated with the genetically modified systems under study. They explored both the effects of prolonged tail Na+ currents (INa,L) reported in earlier analyses of Scn5a+/ΔKPQ mutations (20) as well as of altered maximum Na+ currents (INa,T) associated with the loss-of-function Scn5a+/− system previously described (21). They thus modeled the consequences of 1) a loss of Na+ channel function, which would result in a reduction in maximum Na+ current, as occurs in Scn5a+/− animals; 2) a gain of Na+ channel function, which would result in prolonged INa,L, as suggested by Ref. 20 on the basis of ventricular properties; and 3) an overlap situation in which there was both a loss of Na+ channel expression and a modification of the properties of the Na+ channels still present. The computational approach was particularly useful in being able to model both peripheral and central SAN cells as well as to explore the resulting properties in full 2-D systems. The results of the different combinations of conditions that were most consistent with the third possibility above are summarized below.
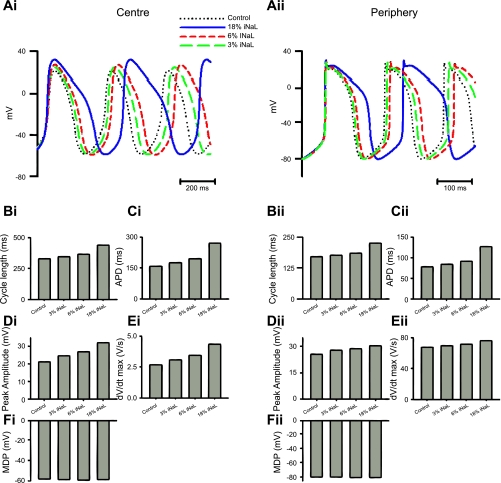
This analysis was first performed at the single cell level for central (Fig. 5,i) and peripheral (Fig. 5,ii) SAN cells. The simulations computed AP waveforms (Fig. 5A), CLs (Fig. 5B), AP durations (APDs; Fig. 5C), peak AP amplitudes (Fig. 5D), maximum rates of voltage change during the AP [(dV/dt)max], which, in turn, would provide an indication of alterations in conduction velocity at the multicellular level (E), and minimum diastolic potential (MDP; Fig. 5F). These demonstrated that a combination of both increased CL and reduced (dV/dt)max require simulations involving both reduced INa,T and the introduction of INa,L.
Fig. 5.
Effects of varying persistent late Na+ current (INa,L) on SAN activity. i and ii: results of simulations from central (i) and peripheral (ii) SAN cells. A–F: corresponding effects on action potentials (APs; A), cycle lengths (CLs; B), AP durations (APD; C), peak amplitudes (D), maximum change in voltage over time [(dV/dt)max] values (E), and maximal diastolic potential (MDP; F).
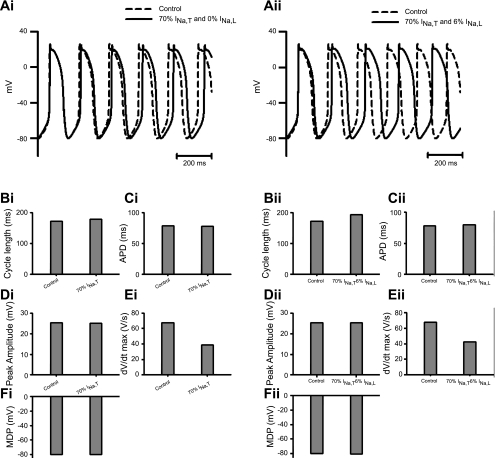
Thus, first, computations testing the consequences of adding prolonged INa,L at magnitudes of 0%, 3%, 6%, and 18% of the normal (100%) total WT INa,T in the presence of a normal INa,T (Fig. 5) demonstrated 1) increased CL, APD, AP peak, and (dV/dt)max but not MDP in central SAN cells (Fig. 5,i) and 2) similarly increased CL and APD but not MDP but had less marked effects on AP peaks and (dV/dt)max in the SAN periphery (Fig. 5,ii). It thus predicted slowed HR and prolonged APDs but not altered conduction properties. Second, reductions in INa,T to 70% of its WT level in the absence (0%) of prolonged INa,L replicated the reductions in (dV/dt)max but did not predict prolongations in CL and APD and reductions of AP peak and MDP (Fig. 6,i) in the peripheral SAN cell (Fig. 6). They thus only replicated the conduction velocity but not the HR changes. Finally, however, computations performed for the peripheral SAN cell (Fig. 6) that both reduced INa,T to 70% of its WT level and introduced prolonged INa,L with a magnitude of 6% of INa,T predicted both increases in CL and APD without affecting the AP peak and reductions in (dV/dt)max (Fig. 6,ii). They thus replicated both the changes in conduction velocity and the altered HRs.
Fig. 6.
Effects of reducing transient Na+ current (INa,T) to 70% of its control value in the absence (i) and presence (ii) of INa,L of 6% magnitude on SAN activity using a peripheral SAN cell model. A–F: corresponding effects on APs (A), CLs (B), APDs (C), peak amplitudes (D), (dV/dt)max values (E), and MDPs (F).
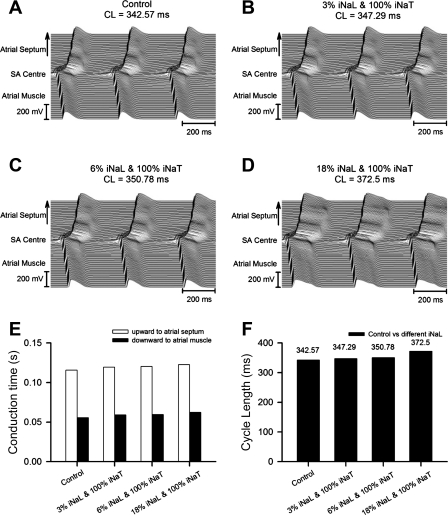
Further 2-D simulations of an AP first initiated in the center of the SAN and then propagating in both directions toward the atrial septum and RA and crista terminalis similarly required both marked reductions in INa,T and the presence of low (∼3–6%) levels of INa,L to simulate the changes in both conduction times and CL reported by the present experiments.
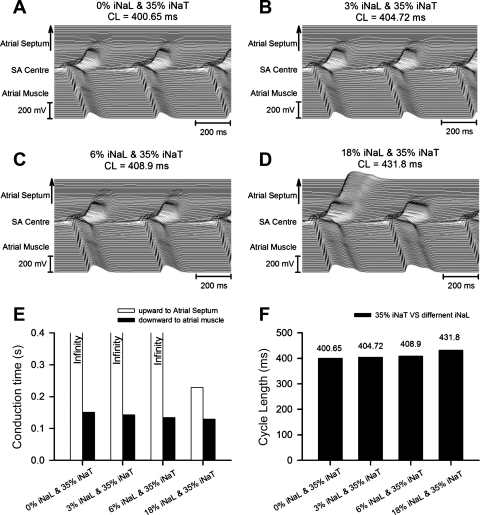
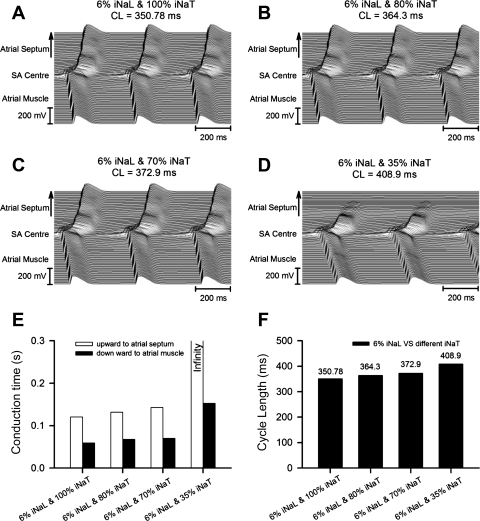
Thus, first, progressively increasing INa,L from 0% (Fig. 7A) to 3% (Fig. 7B), 6% (Fig. 7C), and 18% (Fig. 7D) of control INa,T in the presence of a normal (100%) INa,T neither significantly altered conduction times nor produced large changes in CL (which were <10%). Second, a combination of reducing INa,T to 35% of its normal value and increasing INa,L from 0% (Fig. 8A) to 3% (Fig. 8B), 6% (Fig. 8C), and 18% (Fig. 8D) of control INa,T (Fig. 8E) reproduced both an SA exit block leading to large increases in conduction time from the SAN to atrial septum and also, although less so, from the SAN to RA as well as marked changes in CL (Fig. 8F). Finally, progressive reductions in INa,T from 100% (Fig. 9A) to 80% (Fig. 9B), 70% (Fig. 9C), and 35% (Fig. 9D) of its control value in combination with INa,L maintained constant at 6% of the control INa,T (Fig. 9, E and F) significantly prolonged both conduction times and CLs.
Fig. 7.
Effects of varying INa,L to levels corresponding to 0% (A), 3% (B), 6% (C), and 18% (D) of a constant, control (100%) level of INa,T on AP conduction in a two-dimensional (2-D) tissue model of the SAN coupled to the atrial septum (upward direction) and atrial muscle (downward direction). E and F: calculated conduction times (E) from the SAN center to the atrial septum or atrial muscle and the CLs (F) with the corresponding INa,L values.
Fig. 8.
Effects of INa,T reduced to 35% of its control value and different values of INa,L corresponding to 0% (A), 3% (B), 6% (C), and 18% (D) of the control INa,T on AP conduction in a 2-D tissue model of the SAN coupled to the atrial septum (upward direction) and atrial muscle (downward direction). E and F: calculated conduction times (E) from the SAN center to the atrial septum or atrial muscle and the CLs (F) with the corresponding INa,L values.
Fig. 9.
Effects of a contribution from INa,L of magnitude 6% of the control INaT in combination with varying INa,T of 100% (A), 80% (B), 70% (C), and 35% (D) of the control INa,T on AP conduction in a 2-D tissue model of the SAN coupled to the atrial septum (upward direction) and atrial muscle (downward direction). E and F: calculated conduction times (E) from the SAN center to the atrial septum or atrial muscle and the CLs (F) with the corresponding INa,L values.
DISCUSSION
Human LQT3 syndrome is associated with gain-of-function alterations in the cardiac Na+ channel. Yet it has also been associated with arrhythmic features attributable to sinus node dysfunction, thereby overlapping phenotypes normally associated with loss of Na+ channel function (41). Such overlaps have been reported clinically with ΔKPQ1505–1507 (28), D1790G (5), E1784K (42), 1795insD (8, 38), and ΔK1500 mutations (16). Corresponding physiological findings have been replicated in WT murine hearts in which increased slowly inactivating late Na+ current components produced by the sea anemone Anemonia sulcata toxin increased CLs, PR intervals, and SNRTs in addition to prolonging electrocardiographic QT intervals and resulting in ventricular tachycardia. These phenomena were inhibited by inhibiting late Na+ currents using ranolazine (43).
The present experiments went on to demonstrate such overlapping phenotypes in a murine system replicating genetic (ΔKPQ1505–1507) changes known to underlie LQT3 for the first time. They first verified the hypothesis that murine hearts carrying the gain-of-function Scn5a+/ΔKPQ variant modeling LQT3 (20, 29, 37) can show bradycardic phenotypes resembling those associated with a loss of Na+ channel function (21). They then explored the physiological basis for these changes at the SAN and atrial tissue levels, thereby implicating changes in both pacemaker and conduction function in these phenomena.
Thus, the ECG experiments demonstrated significantly altered SAN pacemaker function in Scn5a+/ΔKPQ mice. Admittedly intact WT and Scn5a+/ΔKPQ preparations showed similar baseline HRs, slower with ketamine than avertin anesthesia, confirming previous results (19). However, Scn5a+/ΔKPQ but not WT hearts showed episodes of sinus bradycardia, sinus pause, and sinus arrest. They also showed significantly longer SNRTs after burst pacing than WT hearts, reflecting more greatly suppressed SAN pacemaker function after overdriven conditions. The ECG experiments also demonstrated conduction abnormalities in the form of episodes of second- and third-degree heat block in Scn5a+/ΔKPQ but not WT hearts. Scn5a+/ΔKPQ hearts showed prolonged PR intervals and QRS durations compared with WT hearts.
Experiments using isolated preparations then directly attributed these changes to SAN pacemaker function and conduction at the tissue level. Isolated WT preparations showed HRs similar to those reported in intact preparations on an earlier occasion (21). In contrast, Scn5a+/ΔKPQ preparations showed lower mean intrinsic HRs than did the corresponding intact preparations, or either isolated or intact WT preparations, consistent with their possible correction by autonomic activity. Multiarray electrode mapping experiments revealed a spreading pattern of excitation from the SAN. A number of records demonstrated a slower conduction toward the septum than toward the crista terminalis. This was in general agreement with previous reports of block zones between the leading SAN pacemaker site and the interatrial septum, which have been observed in several species (9, 13). Such delayed activation in the SAN septal margin has been previously observed by optical mapping methods in the mouse heart (15, 30, 40) and in other mammals (13, 14, 31, 35). They also demonstrated an overall depressed impulse conduction compared with WT preparations. The latter values were in agreement with previous studies (15, 21, 40) using both microelectrode and optical mapping methods. The slowing in Scn5a+/ΔKPQ preparations was apparent in their greater excitation latencies and therefore slowed conduction velocities for the spread of excitation between the SAN and septum, although not the RA. These findings from ECG and isolated SAN experiments together suggest slowed intra-atrial, AV node, and intraventricular conduction in Scn5a+/ΔKPQ mice in the absence of significant SA exit block.
Finally, modeling experiments both at the single cell level and in a 2-D SAN-atrial tissue model then explored simple hypotheses explaining these experimental findings. These considered both contributions from prolonged INa,L, reported in earlier analyses of Scn5a+/ΔKPQ (20), and altered maximum INa,T. They explored the general effects of variations in INa,L and INa,T reflecting the Scn5a+/ΔKPQ mutation upon the key parameters of CL, APD, AP peak, and (dV/dt)max as well as conduction times between strategic structures. This involved the use of a rabbit model, for which the required extensive quantitation was available (2, 10, 45). Nevertheless, such an approach had previously successfully reproduced experimental findings of depressed HRs and slowed AP propagation through the SAN and from the SAN to atria in a complementary Scn5a+/− murine atrial model (21). Furthermore, the incorporation of both gain- or loss-of-function changes in such rabbit models of SAN function had successfully reproduced experimental findings arising from the corresponding mutations in the murine heart (10). This approach followed from previous experimental studies that associated Scn5a variants modeling LQT3 with 1) loss of Na+ channel function resulting from negative and/or positive shifts in the respective steady-state inactivation and activation curves, either of which would reduce INa,T window current; and 2) gain of function in the form of increased INa,L (6, 8, 11, 16, 39, 42), previously shown computationally to increase pacemaker CL with 1995insD (39) in a single cell SAN model. However, the latter study had not investigated the ability of the SAN to pace surrounding atrial muscle. This approach also complemented previous simulations of the effects of reducing INa,T on SAN function (46) that had not clarified either the mechanistic links between the gain of function of INa,L or the combined effects of reduced INa,T and augmented INa,L.
These simulations demonstrated that alterations in INa,L alone did alter CL but failed to affect (dV/dt)max, a major determinant of conduction, in the SAN periphery. Conversely, reducing INa,T alone reduced (dV/dt)max but did not produce such increases in CL. However, the combined reduction in INa,T and introduction of INa,L reduced both pacemaker function and (dV/dt)max. Extending these experiments using a 2-D model for the intact SAN and atrial tissue provided detailed predictions of conduction to both the atrial septum and atrial tissue. They similarly demonstrated that simulation of the altered conduction times and CLs observed experimentally required both marked reductions in INa,T and the presence of low (∼3–6%) levels of INa,L.
In summary, while showing the electrocardiographic QT and QTc prolongations expected of human LQT3, murine ΔKPQ1505–1507 hearts demonstrated both compromised SAN function and slowed atrial conduction, in common with findings associated with the loss-of-function Scn5a+/− mutation (21). These features directly parallel similar overlaps between human LQTS and BrS. These could be explained through systematic simulation experiments in terms of a combination of reduced total INa,T that might reflect reduced Na+ channel expression in combination with INa,L associated with Scn5a+/ΔKPQ. The findings thus provide a possible physiological basis for the overlaps observed in the corresponding human conditions.
GRANTS
The work was supported by Chinese Nature Science Foundation Project Grants 30470634 (to M. Lei) and 30371571 and 30672209 (to Y. Zhang), the Chinese Scholar Research Council (to J. Wu and L. Cheng), The Wellcome Trust (to M. Lei, C. L.-H. Huang, A. Grace, and H. Zhang), The British Heart Foundation (to M. Lei, C. L.-H. Huang, and A. Grace), the Medical Research Council (to C. L.-H. Huang), a CVT research grant (to M. Lei), the Biotechnology and Biological Sciences Research Council (to J. A. Fraser and Y. Zhang), and The Helen Kirkland Trust (to A. Grace).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.W., Y.Z., H.Z., and M.L. performed experiments; J.W., Y.Z., X.Z., L.C., A.A.G., J.A.F., H.Z., and C.L.-H.H. analyzed data; J.W., L.C., W.J.L., and J.A.F. prepared figures; Y.Z., C.L.-H.H., and M.L. drafted manuscript; Y.Z., C.L.-H.H., and M.L. edited and revised manuscript; X.Z., L.C., W.J.L., A.A.G., J.A.F., H.Z., C.L.-H.H., and M.L. interpreted results of experiments; H.Z., C.L.-H.H., and M.L. conception and design of research; C.L.-H.H. and M.L. approved final version of manuscript.
REFERENCES
- 1.Ackerman MJ, Khositseth A, Tester DJ, Hejlik JB, Shen WK, Porter CB. Epinephrine-induced QT interval prolongation: a gene-specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc 77: 413–421, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Aslanidi OV, Boyett MR, Dobrzynski H, Li J, Zhang H. Mechanisms of transition from normal to reentrant electrical activity in a model of rabbit atrial tissue: interaction of tissue heterogeneity and anisotropy. Biophys J 96: 798–817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baroudi G, Chahine M. Biophysical phenotypes of SCN5A mutations causing long QT and Brugada syndromes. FEBS Lett 487: 224–228, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Beaufort-Krol GC, van den Berg MP, Wilde AA, van Tintelen JP, Viersma JW, Bezzina CR, Bink-Boelkens MT. Developmental aspects of long QT syndrome type 3 and Brugada syndrome on the basis of a single SCN5A mutation in childhood. J Am Coll Cardiol 46: 331–337, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Benhorin J, Taub R, Goldmit M, Kerem B, Kass RS, Windman I, Medina A. Effects of flecainide in patients with new SCN5A mutation: mutation-specific therapy for long-QT syndrome? Circulation 101: 1698–1706, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature 376: 683–685, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, Strieper MJ, Rhodes TH, George AL., Jr Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest 112: 1019–1028, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezzina C, Veldkamp MW, van Den Berg MP, Postma AV, Rook MB, Viersma JW, van Langen IM, Tan-Sindhunata G, Bink-Boelkens MT, van Der Hout AH, Mannens MM, Wilde AA. A single Na+ channel mutation causing both long-QT and Brugada syndromes. Circ Res 85: 1206–1213, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res 47: 658–687, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Butters TD, Aslanidi OV, Inada S, Boyett MR, Hancox JC, Lei M, Zhang H. Mechanistic links between Na+ channel (SCN5A) mutations and impaired cardiac pacemaking in sick sinus syndrome. Circ Res 107: 126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deschenes I, Baroudi G, Berthet M, Barde I, Chalvidan T, Denjoy I, Guicheney P, Chahine M. Electrophysiological characterization of SCN5A mutations causing long QT (E1784K) and Brugada (R1512W and R1432G) syndromes. Cardiovasc Res 46: 55–65, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Dobrzynski H, Li J, Tellez J, Greener ID, Nikolski VP, Wright SE, Parson SH, Jones SA, Lancaster MK, Yamamoto M, Honjo H, Takagishi Y, Kodama I, Efimov IR, Billeter R, Boyett MR. Computer three-dimensional reconstruction of the sinoatrial node. Circulation 111: 846–854, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fedorov VV, Hucker WJ, Dobrzynski H, Rosenshtraukh LV, Efimov IR. Postganglionic nerve stimulation induces temporal inhibition of excitability in rabbit sinoatrial node. Am J Physiol Heart Circ Physiol 291: H612–H623, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Fedorov VV, Schuessler RB, Hemphill M, Ambrosi CM, Chang R, Voloshina AS, Brown K, Hucker WJ, Efimov IR. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ Res 104: 915–923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glukhov AV, Fedorov VV, Anderson ME, Mohler PJ, Efimov IR. Functional anatomy of the murine sinus node: high-resolution optical mapping of ankyrin-B heterozygous mice. Am J Physiol Heart Circ Physiol 299: H482–H491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant AO, Carboni MP, Neplioueva V, Starmer CF, Memmi M, Napolitano C, Priori S. Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J Clin Invest 110: 1201–1209, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groenewegen WA, Firouzi M, Bezzina CR, Vliex S, van Langen IM, Sandkuijl L, Smits JP, Hulsbeek M, Rook MB, Jongsma HJ, Wilde AA. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res 92: 14–22, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hao X, Zhang Y, Zhang X, Nirmalan M, Davies L, Konstantinou D, Yin F, Dobrzynski H, Wang X, Grace A, Zhang H, Boyett M, Huang CL, Lei M. TGF-β1-mediated fibrosis and ion channel remodeling are key mechanisms in producing the sinus node dysfunction associated with SCN5A deficiency and aging. Circ Arrhythm Electrophysiol 4: 397–406, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Hart CY, Burnett JC, Jr, Redfield MM. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol 281: H1938–H1945, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Head CE, Balasubramaniam R, Thomas G, Goddard CA, Lei M, Colledge WH, Grace AA, Huang CL. Paced electrogram fractionation analysis of arrhythmogenic tendency in ΔKPQ Scn5a mice. J Cardiovasc Electrophysiol 16: 1329–1340, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lei M, Goddard C, Liu J, Leoni AL, Royer A, Fung SS, Xiao G, Ma A, Zhang H, Charpentier F, Vandenberg JI, Colledge WH, Grace AA, Huang CL. Sinus node dysfunction following targeted disruption of the murine cardiac sodium channel gene Scn5a. J Physiol 567: 387–400, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei M, Jones SA, Liu J, Lancaster MK, Fung SS, Dobrzynski H, Camelliti P, Maier SK, Noble D, Boyett MR. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol 559: 835–848, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lei M, Zhang H, Grace AA, Huang CL. SCN5A and sinoatrial node pacemaker function. Cardiovasc Res 74: 356–365, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Makita N. Phenotypic overlap of cardiac sodium channelopathies: individual-specific or mutation-specific? Circ J 73: 810–817, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Makiyama T, Akao M, Shizuta S, Doi T, Nishiyama K, Oka Y, Ohno S, Nishio Y, Tsuji K, Itoh H, Kimura T, Kita T, Horie M. A novel SCN5A gain-of-function mutation M1875T associated with familial atrial fibrillation. J Am Coll Cardiol 52: 1326–1334, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol Heart Circ Physiol 274: H747–H751, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Moss AJ, Windle JR, Hall WJ, Zareba W, Robinson JL, McNitt S, Severski P, Rosero S, Daubert JP, Qi M, Cieciorka M, Manalan AS. Safety and efficacy of flecainide in subjects with Long QT-3 syndrome (ΔKPQ mutation): a randomized, double-blind, placebo-controlled clinical trial. Ann Noninvasive Electrocardiol 10: 59–66, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss AJ, Zareba W, Benhorin J, Locati EH, Hall WJ, Robinson JL, Schwartz PJ, Towbin JA, Vincent GM, Lehmann MH. ECG T-wave patterns in genetically distinct forms of the hereditary long QT syndrome. Circulation 92: 2929–2934, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, Vos MA, Dewerchin M, Benndorf K, Collen D, Carmeliet E, Carmeliet P. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med 7: 1021–1027, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Nygren A, Lomax AE, Giles WR. Heterogeneity of action potential durations in isolated mouse left and right atria recorded using voltage-sensitive dye mapping. Am J Physiol Heart Circ Physiol 287: H2634–H2643, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Opthof T, de Jonge B, Jongsma HJ, Bouman LN. Functional morphology of the pig sinoatrial node. J Mol Cell Cardiol 19: 1221–1236, 1987 [DOI] [PubMed] [Google Scholar]
- 32.Remme CA, Verkerk AO, Nuyens D, van Ginneken AC, van Brunschot S, Belterman CN, Wilders R, van Roon MA, Tan HL, Wilde AA, Carmeliet P, de Bakker JM, Veldkamp MW, Bezzina CR. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation 114: 2584–2594, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Remme CA, Wilde AA, Bezzina CR. Cardiac sodium channel overlap syndromes: different faces of SCN5A mutations. Trends Cardiovasc Med 18: 78–87, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 103: 89–95, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Shibata N, Inada S, Mitsui K, Honjo H, Yamamoto M, Niwa R, Boyett MR, Kodama I. Pacemaker shift in the rabbit sinoatrial node in response to vagal nerve stimulation. Exp Physiol 86: 177–184, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Smits JP, Koopmann TT, Wilders R, Veldkamp MW, Opthof T, Bhuiyan ZA, Mannens MM, Balser JR, Tan HL, Bezzina CR, Wilde AA. A mutation in the human cardiac sodium channel (E161K) contributes to sick sinus syndrome, conduction disease and Brugada syndrome in two families. J Mol Cell Cardiol 38: 969–981, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Thomas G, Killeen MJ, Grace AA, Huang CL. Pharmacological separation of early afterdepolarizations from arrhythmogenic substrate in ΔKPQ Scn5a murine hearts modelling human long QT 3 syndrome. Acta Physiol (Oxf) 192: 505–517, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Berg MP, Wilde AA, Viersma TJW, Brouwer J, Haaksma J, van der Hout AH, Stolte-Dijkstra I, Bezzina TCR, Van Langen IM, Beaufort-Krol GC, Cornel JH, 2nd, Crijns HJ. Possible bradycardic mode of death and successful pacemaker treatment in a large family with features of long QT syndrome type 3 and Brugada syndrome. J Cardiovasc Electrophysiol 12: 630–636, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Veldkamp MW, Wilders R, Baartscheer A, Zegers JG, Bezzina CR, Wilde AA. Contribution of sodium channel mutations to bradycardia and sinus node dysfunction in LQT3 families. Circ Res 92: 976–983, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Verheijck EE, van Kempen MJ, Veereschild M, Lurvink J, Jongsma HJ, Bouman LN. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res 52: 40–50, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Chen Q, Li H, Towbin JA. Molecular genetics of long QT syndrome from genes to patients. Curr Opin Cardiol 12: 310–320, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Wei J, Wang DW, Alings M, Fish F, Wathen M, Roden DM, George AL., Jr Congenital long-QT syndrome caused by a novel mutation in a conserved acidic domain of the cardiac Na+ channel. Circulation 99: 3165–3171, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Cheng L, Lammers WJ, Wu L, Wang X, Shryock JC, Belardinelli L, Lei M. Sinus node dysfunction in ATX-II-induced in-vitro murine model of long QT3 syndrome and rescue effect of ranolazine. Prog Biophys Mol Biol 98: 198–207, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Xia L, Zhang Y, Zhang H, Wei Q, Liu F, Crozier S. Simulation of Brugada syndrome using cellular and three-dimensional whole-heart modeling approaches. Physiol Meas 27: 1125–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Holden AV, Kodama I, Honjo H, Lei M, Varghese T, Boyett MR. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am J Physiol Heart Circ Physiol 279: H397–H421, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Zhao Y, Lei M, Dobrzynski H, Liu JH, Holden AV, Boyett MR. Computational evaluation of the roles of Na+ current, iNa, and cell death in cardiac pacemaking and driving. Am J Physiol Heart Circ Physiol 292: H165–H174, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Zicha S, Fernandez-Velasco M, Lonardo G, L'Heureux N, Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res 66: 472–481, 2005 [DOI] [PubMed] [Google Scholar]