Abstract
The purpose of this study was to determine if tonic restrain of blood pressure by nitric oxide (NO) is impaired early in the development of hypertension. Impaired NO function is thought to contribute to hypertension, but it is not clear if this is explained by direct effects of NO on vascular tone or indirect modulation of sympathetic activity. We determined the blood pressure effect of NO synthase inhibition with Nω-monomethyl-l-arginine (l-NMMA) during autonomic blockade with trimethaphan to eliminate baroreflex buffering and NO modulation of autonomic tone. In this setting, impaired NO modulation of vascular tone would be reflected as a blunted pressor response to l-NMMA. We enrolled a total of 66 subjects (39 ± 1.3 yr old, 30 females), 20 normotensives, 20 prehypertensives (blood pressure between 120/80 and 140/90 mmHg), 17 hypertensives, and 9 smokers (included as “positive” controls of impaired NO function). Trimethaphan normalized blood pressure in hypertensives, suggesting increased sympathetic tone contributing to hypertension. In contrast, l-NMMA produced similar increases in systolic blood pressure in normal, prehypertensive, and hypertensive subjects (31 ± 2, 32 ± 2, and 30 ± 3 mmHg, respectively), whereas the response of smokers was blunted (16 ± 5 mmHg, P = 0.012). Our results suggest that sympathetic activity plays a role in hypertension. NO tonically restrains blood pressure by ∼30 mmHg, but we found no evidence of impaired modulation by NO of vascular tone contributing to the early development of hypertension. If NO deficiency contributes to hypertension, it is likely to be through its modulation of the autonomic nervous system, which was excluded in this study.
Keywords: hypertension, nitric oxide, nervous system, autonomic, nervous system, sympathetic, nitric oxide synthase
nitric oxide (NO) is arguably the most important metabolic modulator of blood pressure (BP); our previous studies suggest that NO tonically restrains BP by at least 30 mmHg in healthy young adults (6). Not surprisingly, there has been great interest in determining if impaired NO function plays a role in hypertension. Reduced NO production (5) or bioavailability (1), or impaired NO-mediated vasodilation (17), have been implicated in the development or maintenance of hypertension (2). There is, indeed, significant evidence that NO function is impaired in hypertension, but the precise mechanism underlying this problem is less clear. There are at least two mechanisms by which NO can lower BP. NO, derived from endothelial cells or from nitrergic nerves, tonically produces vasodilatation. NO also interacts with the sympathetic nervous system at multiple levels and, through this interaction, can modulate BP. In particular, several lines of evidence suggest that NO tonically suppresses sympathetic tone (22, 31), and impaired NO function could contribute to the sympathetic activation observed in hypertension. Conversely, sympathetic activation can impair NO function (9). It is possible, therefore, that these interactions between NO and the sympathetic nervous system, rather than a primary impairment of NO-mediated dilation, contribute to hypertension.
NO is generated from l-arginine by nitric oxide synthase (NOS). Systemic inhibition of NOS is a widely used tool to assess the tonic role of NO on BP regulation. Systemic NOS inhibition produces an increase in BP (13) that is very similar between normotensive and hypertensive subjects (10). However, any increase in BP induced by NOS inhibitors is counteracted by the baroreflex. Therefore, this approach underestimates the real importance of NO. Furthermore, it cannot differentiate between the direct effects of NO on BP mediated by its vasodilatory effects, from its indirect sympatholytic actions.
We have developed a paradigm that allows us to selectively examine the vasodilatory effects of NO that tonically restrain BP without the confounding effect of the autonomic nervous system (6). We used blockade of the autonomic ganglia with the NN-nicotinic receptor antagonist trimethaphan to eliminate the restraining effect of the baroreflex, thus allowing for the full expression of the effect of NOS blockade on BP. Furthermore, any BP effect NO may have through its modulation of sympathetic tone becomes irrelevant. Under these experimental conditions, the increase in BP induced by systemic NOS inhibition can be assigned to removal of the tonic modulatory effects of NO on basal vascular tone. In the present study, we have used this methodology to test the hypothesis that an intrinsic impairment of NO modulation of vascular tone plays a role in BP elevations early in the development of hypertension in human subjects. We compared the effect of systemic NOS inhibition with Nω-monomethyl-l-arginine (l-NMMA) on BP during autonomic blockade in three groups of patients: normotensives, prehypertensives, and young hypertensives. Under these conditions, impaired NO function would be reflected as a blunted pressor response to l-NMMA. A group of smokers was included as a “positive” control of impaired NO function.
MATERIALS AND METHODS
Subjects.
We studied a total of 66 subjects. Participants were recruited from the Vanderbilt University General Clinical Research Center volunteer database and Research Match. Subjects 18–60 yr old, both males and females with a body mass index (BMI) between 20 and 40 kg/m2, were considered eligible for the study. They were considered normotensives if seated BP at the screening visit was <120/80 mmHg. They were considered prehypertensives if seated BP was between 120 and 139 mmHg systolic or 80 and 89 mmHg diastolic. They were considered hypertensives if their seated BP, measured on three different occasions, was >140/90 mmHg or if they were taking antihypertensive medication. A group comprised of nine heavy smokers (≥2 packs/day) was included as positive controls because of their documented NO deficiency using the proposed method (6). All other subjects were not current smokers. All subjects were otherwise healthy (no concomitant cardiovascular or noncardiovascular diseases). All subjects were asked to avoid pregnancy for the duration of the study. Females taking oral contraceptives were allowed to continue using them.
Subjects abstained from caffeine for ≥72 h before testing. All medications known to affect the autonomic nervous system, including antihypertensive treatment, were withdrawn for at least 5 days or five half-lives before testing. During the screening visit, all of the subjects underwent a clinical examination, electrocardiogram (ECG), and admission urinalysis and routine laboratory test (CBC, glucose, lipid profile). Any antihypertensive medication was withheld before screening. Written informed consent was obtained before study entry. All studies were approved by the Vanderbilt University Institutional Review Board.
Study design.
Volunteers were asked to refrain from any food containing methylxanthines for 3 days before study. The volunteers were admitted to the Clinical Research Center at Vanderbilt University Medical Center the day that testing was performed.
The studies were conducted in the morning with the subject in the supine position ≥8 h after their last meal. BP was measured continuously through the volume clamp method (Finapres 2300; Ohmeda), and also every 2 min with an automated oscillometric brachial cuff (Vital-Guard 450C; Ivy Biomedical Systems). Heart rate was determined with continuous ECG monitoring. Bioelectrical body impedance (KIM4; Heinz Diefenbach, Frankfurt, Germany) was used in a subset of 39 subjects (10 of them normotensives, 9 prehypertensives, 10 hypertensives, and 7 smokers) to estimate cardiac output and calculate total peripheral resistance. One intravenous line was placed in the nondominant arm in a large antecubital vein. Three infusion ports were connected to this catheter, one for trimethaphan infusion, the second for infusion of phenylephrine, and the third for l-NMMA. A heparin lock was placed in the other arm to administer phenylephrine.
After a stable baseline was reached and recorded for 15 min, an infusion of 4 mg/min trimethaphan (Cambridge Pharmaceuticals) was started and continued throughout the study to assure complete autonomic blockade (4, 6). Briefly, after a stable BP recording was achieved, phenylephrine boluses were started at a dose of 2.5 μg, and the dose increased every 3 min until an increase in systolic blood pressure (SBP) of ≥20 mmHg was achieved. The absence of reflex bradycardia in response to the increase in BP was taken as evidence of effective autonomic blockade.
Intrinsic BP, in the absence of autonomic influences, was recorded, and then SBP was clamped at ∼110 mmHg, either by increasing it with individually titrated phenylephrine infusions starting with 0.05 μg·kg−1·min−1 or decreasing it with graded head-up tilt. This allowed us to study all subjects at similar clamped “adrenergic tone” and a comparable starting BP. Once BP was stable and no more adjustments in phenylephrine dose or tilt were needed, a new baseline period was recorded for 15 min. An infusion of 250 μg·kg−1·min−1 of l-NMMA was then added and maintained for 15 min. BP was measured every 2 min for the first 8 min and every 1 min for the last 7 min, when a stable recording was achieved. The last five measurements after stable recording were used for the analysis.
Data acquisition.
The surface ECG lead II was amplified, and no additional filters were applied. ECG and BP tracings were digitized with 14-bit resolution and 500-Hz sample frequency and recorded using the WINDAQ data acquisition system (DATAQ). Data were analyzed offline using a customized program for data analysis (DIANA, Dr. André Diedrich, Vanderbilt University, Nashville, TN) written in PV-Wave (VNI). Cardiac output was estimated from the body impedance data as previously described utilizing the method of Kubicek et al. (11).
Heart rate and BP variability.
A QRS detection algorithm, modified from Pan and Tompkins (16), was used to generate beat-to-beat values. The nonequidistant event time series of R-R intervals and BP values were interpolated, low-pass filtered (cutoff: 2 Hz), and resampled at 4 Hz. The estimation of the power spectral density was done by the Welch method, which is a fast-Fourier transform-based algorithm. Data segments of 300 s, recorded during stable resting conditions, were used for spectral analysis. Linear trends were removed, and power spectral density was estimated with the fast-Fourier transform-based Welch algorithm using three segments of 256 data points with 50% overlapping and Hanning window (14). The Hanning window was applied to previous estimation of the power spectral density. The power in the frequency ranges for very low frequencies (0.003 to <0.04 Hz), low frequencies (0.04 to <0.15 Hz), and high frequencies (0.15 to <0.40 Hz) were calculated for each interval according to task force recommendations (27). The estimation of heart rate and BP variability served a dual purpose, first to document autonomic blockade (by the abolishment of variability) and also to assess the sympathetic and parasympathetic status of subjects studied. It was performed at baseline and after autonomic blockade.
Statistical analysis.
Statistical analyses were performed using two complementary approaches. In the first analysis, baseline characteristics and preliminary comparisons of outcomes by a priori-determined groups (according to hypertensive status) were performed using Kruskal-Wallis H-test for more than two-groups comparison or Mann Whitney U-test for two-groups comparison. In the second approach of the analysis, multiple linear regression models were used to examine the association between the NO contribution to BP (increase in BP during NOS inhibition after autonomic blockade) and BP status (defined by seated BP at screening) among nonsmokers, after adjusting for age, gender, BMI, triglycerides, and high-density lipoprotein (HDL) cholesterol levels. The main outcome was the change in SBP in response to l-NMMA after the autonomic nervous system was blocked and then restored to baseline value, whereas the changes in diastolic BP or mean arterial pressure were secondary outcomes. The null hypothesis was that there would be no differences in the outcomes among nonsmokers categorized based on BP status (normotensives, prehypertensives, and hypertensives) in response to systemic infusion of l-NMMA after autonomic blockade. This secondary approach was performed only for nonsmokers, since smokers served as a positive control and we already confirmed in the primary analysis that smokers have a reduced response, and to exclude a possible bias due to the different characteristics of this particular group of subjects.
Unless otherwise noted, data are presented as means ± SE. All of the tests were two-tailed, and a P value of <0.05 was considered significant. Analyses were performed with the SPSS statistical software (SPSS version 19.0; SPSS) and Stata 11.2 (Stata).
RESULTS
Of the 66 subjects studied, at the screening visit, 20 were normotensives (9 males and 12 females), 19 were prehypertensives (BP between 120 and 139/80–89 mmHg), 18 subjects were hypertensives (BP ≥140/90 mmHg or on antihypertensive medication), and 9 subjects were heavy smokers (2 or more packs/day). We discontinued antihypertensive medications for at least 5 days or five half-lives. We were unable to comply with this requirement in one subject taking amlodipine, which has a half-life of 50 h. After medication was withdrawn for 5.6 ± 1.4 days, one subject did not meet BP criteria for hypertension and was then reclassified as prehypertensive. All analyses were performed considering 20 normotensives, 20 prehypertensives, 17 hypertensives, and 9 smokers. Table 1 shows the demographics and baseline characteristics for the different subgroups studied. Three hypertensive subjects were not taking antihypertensive medication at the time of enrollment (Table 2). Diuretics were the most common antihypertensive medication used (9 subjects), either alone or in combination. Angiotensin II receptor blockers (8 subjects) were the second most used agent.
Table 1.
Demographic, blood pressure, and resting vascular data
| NTs (<120/80 mmHg) | PreHTs (120–139/80–89) | HTs (≥140/90 mmHg) | SMKs | P1 (All Groups) | P2 (Nonsmokers) | |
|---|---|---|---|---|---|---|
| n | 20 | 20 | 17 | 9 | ||
| Age, yr | 40 ± 2.2 | 37 ± 2.7 | 45 ± 1.7† | 31 ± 2.2 | 0.004 | 0.065 |
| Gender, M/F | 9/12 | 13/5 | 8/10 | 6/3 | ||
| BMI | 25 ± 0.6 | 26 ± 0.8‡ | 31 ± 1.2* | 26 ± 1.1 | 0.001 | <0.001 |
| Screening visit (seated values) | ||||||
| SBP, mmHg | 107 ± 1.3 | 127 ± 1.2* | 148 ± 3.2*† | 113 ± 4.1 | <0.001 | <0.001 |
| DBP, mmHg | 70 ± 1.2 | 73 ± 1.9‡ | 98 ± 1.7*† | 78 ± 3.1 | <0.001 | <0.001 |
| HR, beats/min | 73 ± 2.7 | 77 ± 2.5 | 72 ± 2.7 | 76 ± 3.8 | 0.587 | 0.438 |
| Triglycerides, mg/dl | 90 ± 10.7 | 107 ± 11.6 | 140 ± 18.3 | 134 ± 25.0 | 0.083 | 0.050 |
| HDL-cholesterol, mg/dl | 61 ± 4.9 | 51 ± 3.7 | 52 ± 2.6 | 57 ± 3.4 | 0.241 | 0.228 |
| LDL-cholesterol, mg/dl | 101 ± 6.6 | 107 ± 6.6 | 117 ± 10.8 | 101 ± 13.9 | 0.754 | 0.601 |
| Study day (supine values) | ||||||
| SBPBaseline, mmHg | 101 ± 1.6 | 108 ± 2.1‡ | 126 ± 3.5*† | 106 ± 4.6 | <0.001 | <0.001 |
| SBPpost TrMT, mmHg | 91 ± 2.2 | 96 ± 3.5 | 106 ± 2.9* | 95 ± 4.8 | 0.009 | 0.004 |
| SBPrestored, mmHg | 100 ± 1.5 | 105 ± 1.6 | 108 ± 1.7* | 105 ± 3.9 | 0.009 | 0.003 |
| SBPpost L-NMMA, mmHg | 130 ± 3.1 | 137 ± 2.2† | 138 ± 3.7† | 120 ± 4.6 | 0.010 | 0.124 |
| Delta SBPTrMT, mmHg | −10 ± 2.4 | −12 ± 2.5 | −20 ± 3.1* | −10 ± 5.8 | 0.078 | 0.033 |
| Delta SBPL-NMMA, mmHg | 31 ± 2.4† | 32 ± 1.6† | 30 ± 2.7† | 16 ± 4.6 | 0.012 | 0.865 |
Values are means ± SE; n, no. of subjects. NTs, normotensive subjects; PreHT, prehypertensive subjects; HT, hypertensive subjects; Smk, smokers (2 packs/day); M, males; F, females; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBPBaseline, SBP at baseline; SBPpost TrMT, SBP during autonomic blockade with trimethaphan; SBPrestored, SBP after restoration with phenylephrine; SBPpost L-NMMA, SBP at the end of nitric oxide synthase (NOS) inhibition with Nω-monomethyl-l-arginine (l-NMMA); Delta SBPTrMT, change in SBP during autonomic blockade with trimethaphan; Delta SBPL-NMMA, change in SBP during NOS inhibition with l-NMMA; P1, P values among all 4 groups; P2, P values for nonsmokers. Statistical significance compared with normotensive subjects (*), compared with smokers (†), and compared with hypertensive subjects (‡).
Table 2.
Antihypertensive medications and blood pressure at the screening visit and blood pressure on the study day after discontinuation of medications
| Patient | Medications (Longest Half-Life, h; Time Withheld, days) | BP at Screening, mmHg (Seated-Supine) | BP at Study Day, mmHg (Seated-Supine) |
|---|---|---|---|
| LB | Atenolol (7,5) | 103/60–108/60 | 131/80–112/65 |
| PB | Hydrochlorothiazide (14,5) | 148/94–121/89 | 135/98–114/74 |
| AB | Amlodipine (50, 5), valsartan | 122/78–116/72 | 151/101–120/72 |
| LM | Fosinopril (12, 5) | 133/80–121/77 | 127/90–113/70 |
| MC | Hydrochlorothiazide (14,5), triamterene | 134/79–124/69 | 160/99–148/86 |
| GM | Hydrochlorothiazide (14,5), triamterene, lisinopril | 106/73–107/73 | 122/95–105/72 |
| CT | Hydrochlorothiazide (14,5), losartan | 127/72–109/65 | 160/109–143/82 |
| CC | None | 168/98–151/97 | 171/111–144/87 |
| CA | Diltiazem, olmesartan (13,5) | 145/74–123/71 | 162/89–136/77 |
| SK | Hydrochlorothiazide (14,6), valsartan | 136/81–116/75 | 152/90–132/79 |
| FH | Hydrochlorothiazide (14,5), lisinopril | 107/72–123/72 | 135/93–130/84 |
| WL | Losartan (11,5) | 154/99–132/89 | 146/103–125/78 |
| RB | Quinapril (25, 9) | 161/99–152/84 | −148/84 |
| MN | Hydrochlorothiazide (14,5), irbesartan (15) | 134/75–116/74 | 154/112–107/81 |
| RH | Hydrochlorothiazide (14,9), losartan | 117/81–113/79 | 150/97–108/71 |
| IP | None | 144/96–126/79 | −119/65 |
| WB | None | 149/92–135/86 | −124/81 |
| SK | Hydrochlorothiazide (14,5), losartan | 131/80–137/75 | 145/93–131/75 |
Three hypertensive subjects were not receiving any medication at the time of the screening or the study day. Only one hypertensive (LB) subject did not meet blood pressure criteria to be classified as hypertensive.
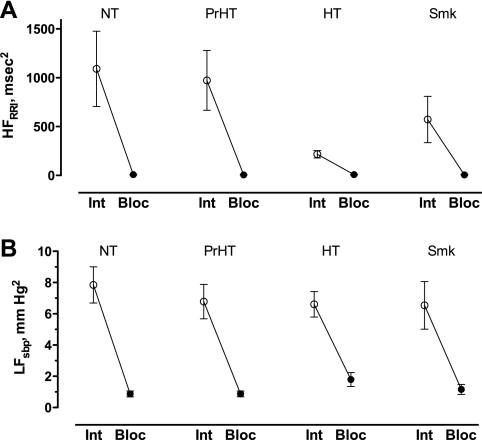
BP was significantly different between groups at baseline (Table 1), reflecting our a priori designation of subjects into normotensive, prehypertensive, and hypertensive groups. Among nonsmokers, baseline high-frequency variability of heart rate, which reflects parasympathetic modulation of heart rate, was significantly lower in the hypertensive group (P = 0.022 by Kruskal-Wallis test, Fig. 1A). We and others have previously reported that low-frequency variability of BP, which reflects sympathetic modulation of BP, is increased in hypertension. However, we were not able to reproduce those findings in this study, and low-frequency variability of BP was not different among groups (Fig. 1B). Trimethaphan virtually eliminated autonomic regulation of heart rate and BP, as evidenced by the virtual elimination heart rate and BP variability (P < 0.01 in all groups, Fig. 1). Trimethaphan produced a greater decrease in BP in the hypertensive group (P = 0.033 by Kruskal Wallis test, Fig. 2A), so that the differences in baseline BP between groups were virtually lost after autonomic blockade. As expected, less phenylephrine was needed to restore SBP among hypertensive subjects, but there was no difference between controls, prehypertensive subjects, or smokers (0.3 ± 0.07, 0.3 ± 0.06, 0.03 ± 0.02, and 0.24 ± 0.08 μg·kg−1·min−1 for controls, prehypertensives, hypertensives, and, smokers, respectively, P < 0.001 by Kruskal-Wallis test). In the subset of 36 subjects in whom total peripheral resistance was measured, we found that, as expected, at baseline, hypertensive subjects tended to have increased TPR (11.8 ± 1.2, 11.5 ± 1.0, 16.6 ± 1.8, and 10.6 ± 1.0 mmHg·l−1·min−1, for controls, prehypertensives, hypertensives, and smokers, respectively, P = 0.089 by Kruskal-Wallis test).
Fig. 1.
Spectral analysis of high-frequency variability of heart rate (R-R interval, HFRRI, A) and low-frequency variability of systolic blood pressure (LFsbp, B) before [○, intact (Int)] and after (●, Bloc) autonomic blockade with 4 mg/min trimethaphan in normotensive (NT), prehypertensive (PrHT), hypertensive (HT), and smoker (Smk) subjects. Autonomic blockade completely abolished autonomic modulation of heart rate and blood pressure.
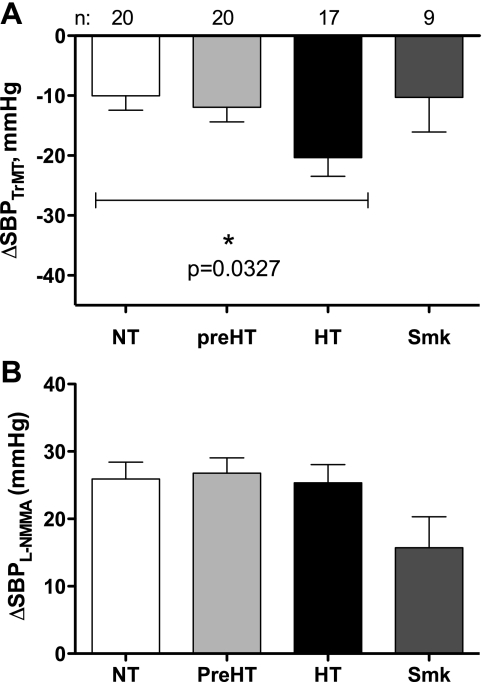
Fig. 2.
Change in systolic blood pressure in response to autonomic blockade with trimethaphan (ΔSBPTrMT, A) or nitric oxide synthase inhibition with Nω-monomethyl-l-arginine (l-NMMA) during autonomic blockade (ΔSBPL-NMMA, B) among NT (white bar), prehypertensive (preHT, light gray bar), HT (black bar), and Smk (dark gray bar) subjects.
In our primary analysis (unadjusted) for the main outcome, we did not find a difference in the response to NOS inhibition after autonomic blockade among normotensive, prehypertensive, or hypertensive subjects (P = 0.865 by Kruskal Wallis test, Fig. 2B) but only between normotensives and prehypertensives compared with smokers, who, as expected, had a smaller increase in SBP due to NOS inhibition during autonomic blockade (P = 0.035 and P = 0.022 between normotensive subjects and smokers and prehypertensive subjects and smokers, respectively, by Mann-Whitney U-test, Fig. 2B). When only nonsmoker subjects were analyzed, neither the absolute SBP after NOS inhibition nor the deltas in SBP were different among the three groups, and, in fact, the deltas were almost identical. Furthermore, we could not find any association in the increase in SBP due to NOS inhibition that could be attributed to a higher BMI, gender, older age, higher triglycerides, or HDL cholesterol levels. Likewise, we did not find any differences between groups in the change in TPR due to systemic NOS inhibition in the subset analysis (6.39 ± 2.2, 5.7 ± 1.3, 6.8 ± 1.8, and 5.1 ± 0.9 mmHg·l−1·min−1 for controls, prehypertensives, hypertensives, and smokers, respectively, P = 0.985 by Kruskal-Wallis test).
These initial analysis results were further confirmed among nonsmokers using multiple regression models, after adjusting for, age, gender, BMI, triglycerides, and HDL cholesterol. Our results showed that, after adjusting for all these variables, the NO contribution to SBP (increase in SBP due to NOS inhibition during autonomic blockade) was not significantly associated with BP status, nor any other covariates (all P > 0.05). Similarly, no association was observed between BP status and the NO contribution to diastolic BP or mean arterial pressure (all P > 0.05).
DISCUSSION
The main finding of this study is that, after removing autonomic influences, impaired NO dilation does not seem to contribute to hypertension early in the development of the disease.
We need to highlight that this conclusion has two important modifiers, as discussed below; these results were obtained in the absence of autonomic function, and we included only patients early in the hypertensive process. We do not argue against a role of impaired NO function in hypertension. Indeed, numerous studies have demonstrated that hypertensives have impaired acetylcholine-induced forearm vasodilatation, which is in part mediated by NO release (12, 17) and impaired vasoconstriction in response to intra-arterial administration of a NOS inhibitor (2). Flow-mediated dilation, which is thought to reflect NO function, is also reduced in hypertensive subjects.
The precise mechanism by which impaired NO function contributes to hypertension is less clear. Endothelial NO is released tonically into the underlying vascular tissues to induce vasodilation. Impairment of this process is thought to be an important component of the “endothelial dysfunction” described in hypertension. However, NO also modulates BP through interactions with the autonomic nervous system; NO can lower BP by reducing sympathetic tone and, conversely, sympathetic activation can impair NO-mediated dilation. Autonomic blockade with trimethaphan eliminates these NO-sympathetic interactions as well as the restraining effects of the baroreflex. In this setting, the increase in BP induced by the NOS inhibitor l-NMMA selectively reflects NO modulation of vascular tone. Using this approach, we found previously that l-NMMA increased SBP by 30 ± 3 mmHg in healthy individuals, indicating the importance of the direct vasodilatory effects of NO that tonically restrain BP. We used this same experimental approach to test the hypothesis that impairment of this mechanism contributes to the early development of hypertension. If true, then NO synthase blockade with l-NMMA, in the presence of ganglionic blockade, would produce less of an increase in BP in hypertensive individuals.
The main and unexpected finding of this study is that, after removing autonomic influences, impaired NO dilation does not seem to contribute to hypertension early in the development of the disease. Our “positive control” group, smokers, did show the expected blunted pressor response to l-NMMA, confirming the adequacy of our experimental approach. On the other hand, NOS inhibition with l-NMMA produced similar increases in BP in healthy controls, prehypertensives, and young hypertensive patients.
We do not believe that this conclusion is necessarily at odds with the substantial evidence that hypertension is associated with impaired NO-mediated vasodilation. Our results, however, do not support the notion that impaired modulation by NO of basal vasodilatory tone contributes to the development of hypertension early in the disease process. Rather, we suggest that NO contributes to hypertension through its suppression of sympathetic tone.
There is experimental evidence showing that NO can tonically inhibit sympathetic activity (20, 21, 29). It is not clear, however, how important this mechanism is in restraining BP in humans; studies in favor and against this possibility can be found in the literature. In some studies, the increase in BP produced by systemic administration of l-NMMA is accompanied by a similar reflex decrease in muscle sympathetic nerve activity to that produced by an equipressor dose of phenylephrine (3, 8), suggesting that NO does not tonically restrain central sympathetic outflow in humans. Other studies have reached the opposite conclusion; in one study, NOS inhibition increased BP but did not produce the expected baroreflex-mediated decrease in sympathetic nerve activity (15); in another, the increase in BP induced by NOS inhibition was partially reversed by the α-adrenergic antagonist phentolamine (21). These results suggest that the pressor response to systemic NOS inhibition is at least in part mediated by sympathetic activation, implying tonic inhibition of the sympathetic nervous system by NO.
Animal studies also show important interactions between the sympathetic nervous system and NO. Intracerebroventricular injection of l-NMMA, at doses that have no effect when given systemically, has been shown to increase sympathetic activity and BP (19, 24). The hypotensive effect of the central sympatholytic dexmedetomidine is absent in endothelial nitric oxide synthase (eNOS) knockout mice, indicating that an intact eNOS system within the central nervous system is required for autonomic BP regulation. It is possible; therefore, that NO normally restrains BP in part by inhibiting sympathetic tone. Because our experimental paradigm eliminated any interactions between NO and the autonomic nervous system, we cannot exclude the possibility that NO deficiency contributes to sympathetic activation and, through this mechanism, plays a role in the development of hypertension.
There is also increasing evidence that sympathetic activation contributes to many forms of hypertension and that sympathetic activation can contribute to impaired NO-mediated dilation. For example, acute sympathetic activation blunts flow-mediated dilation (9), mimicking an “endothelial dysfunction state,” and, conversely, the central sympatholytic moxonidine decreases sympathetic activity (30) and improves flow-mediated dilation (28). Thus, it is plausible that results from previous studies in hypertensive subjects do not reflect an intrinsic impairment of NO vasodilatation but a functional impairment secondary to increased sympathetic activity. It is particularly important to consider this caveat in diseases where both processes may coexist, as is the case of obesity-associated hypertension (7, 25).
This study has limitations inherent to clinical research. Our purpose in using ganglionic blockade was to remove autonomic modulation of BP. In the absence of baroreflex restrain, we found that partial NO synthase inhibition with l-NMMA resulted in an increase in BP of at least 30 mmHg, unmasking the true importance of the tonic modulation of NO to BP and confirming our previous results (6). Autonomic blockade also eliminated any effect NO may have on BP through its suppression of sympathetic activity. Conversely, sympathetic activation can suppress NO function, but we could not assess the importance of this mechanism directly because we used titrated doses of phenylephrine to ensure that all experimental groups start at a similar BP given that this was our primary outcome.
Modulation of vascular tone by NO has generally been attributed to NO generated by eNOS. However, recent studies using selective inhibitors of neural nitric oxide synthase (nNOS) have shown that NO derived from nNOS also contributes to the tonic modulation of vascular tone in humans. The precise site where nNOS generates NO to modulate vascular tone could include cells within the vessel wall or perivascular nerve cells because both express nNOS (23). In the present study, we cannot differentiate between NO derived from nNOS or eNOS because both enzymes are inhibited by l-NMMA.
As expected, a significant proportion of our hypertensive patients were obese, but no difference in the response to l-NMMA was found between obese and lean subjects, suggesting that obesity per se does not impair the tonic modulation by NO of basal vasodilator tone. We did not control when female subjects were studied in relation to their menstrual cycle because this was not the primary objective of the present study. We did not have a direct measure of NO production or redox status. Nonetheless, these would be areas of interest in future research.
It is important to emphasize that our conclusions are restricted to early stage disease. Our hypertensive patients were 45 ± 2 yr old, with no clinical evidence of vascular disease. It is possible, and even likely, that impaired NO-mediated dilation plays a greater role in sustaining hypertension once endothelial function is impaired by vascular disease. Because most of our hypertensive patients had previously received treatment, it could be argued that prior use of antihypertensive treatment could have produced a sustained improvement in NO-mediated dilation. Other studies, however, have documented impaired vasodilation by NO after short periods of medication washout (17, 26), and even during antihypertensive treatment (18).
In contrast to the lack of effect of NOS inhibition, we found that trimethaphan produced a greater decrease in BP in hypertensive subjects so that, after autonomic blockade, BP was no longer different between groups. These results suggest that sympathetic activation, rather than a primary impairment of NO dilation, accounts for most of the increase in BP observed in these patients.
Perspectives
Our results suggest that NO is one of the most potent metabolic determinants of BP in humans, tonically restraining it by at least 30 mmHg, but we found no evidence in favor of an intrinsic impairment of the vasodilatory effects of NO that tonically restrain BP early in the course of hypertension. If NO deficiency contributes to hypertension, it is likely to be due to loss of its tonic suppression of sympathetic activity, or seen later in the disease process in relation to vascular damage.
GRANTS
This work was supported, in part, by National Institutes of Health (NIH) Grants RO1 HL-67232, P01 HL-056693-080003, and M01 RR-000095-477450. A. Gamboa is supported by NIH Grant K23 HL-95905.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.G., A.D., D.R., and I.B. conception and design of research; A.G., L.E.O., G.F., and S.P. performed experiments; A.G. and L.C. analyzed data; A.G., L.E.O., A.D., L.C., D.R., G.F., and I.B. interpreted results of experiments; A.G. prepared figures; A.G. and I.B. drafted manuscript; A.G., L.E.O., A.D., L.C., S.P., and I.B. edited and revised manuscript; A.G., L.E.O., A.D., L.C., D.R., S.P., and I.B. approved final version of manuscript.
REFERENCES
- 1. Berry C, Brosnan MJ, Fennell J, Hamilton CA, Dominiczak AF. Oxidative stress and vascular damage in hypertension. Curr Opin Nephrol Hypertens 10: 247–255, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Calver A, Collier J, Moncada S, Vallance P. Effect of local intra-arterial NG-monomethyl-L-arginine in patients with hypertension: the nitric oxide dilator mechanism appears abnormal. J Hypertens 10: 1025–1031, 1992 [PubMed] [Google Scholar]
- 3. Charkoudian N, Joyner MJ, Barnes SA, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol 291: H1378–H1383, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D, Biaggioni I. The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens 21: 1677–1686, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Forte P, Copland M, Smith LM, Milne E, Sutherland J, Benjamin N. Basal nitric oxide synthesis in essential hypertension. Lancet 349: 837–842, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Gamboa A, Shibao C, Diedrich A, Choi L, Pohar B, Jordan J, Paranjape S, Farley G, Biaggioni I. Contribution of endothelial nitric oxide to blood pressure in humans. HTN 49: 170–177, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Grassi G, Seravalle G, Quarti-Trevano F, Mineo C, Lonati L, Facchetti R, Mancia G. Reinforcement of the adrenergic overdrive in the metabolic syndrome complicated by obstructive sleep apnea. J Hypertens 28: 1313–1320, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Hansen J, Jacobsen TN, Victor RG. Is nitric oxide involved in the tonic inhibition of central sympathetic outflow in humans? HTN 24: 439–444, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Hijmering ML, Stroes ESG, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Jacobi J, Schneider MP, John S, Schmieder RE. Impact of NO-synthase inhibition on renal hemodynamics in normotensive and hypertensive subjects. J Hypertens 20: 525–530, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Kubicek WG, From AH, Patterson RP, Witsoe DA, Castaneda A, Lillehei RC, Ersek R. Impedance cardiography as a noninvasive means to monitor cardiac function. J Assoc Adv Med Instrum 4: 79–84, 1970 [PubMed] [Google Scholar]
- 12. Linder L, Kiowski W, Buhler FR, Luscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation 81: 1762–1767, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Mayer BX, Mensik C, Krishnaswami S, Derendorf H, Eichler HG, Schmetterer L, Wolzt M. Pharmacokinetic-pharmacodynamic profile of systemic nitric oxide-synthase inhibition with L-NMMA in humans. Br J Clin Pharmacol 47: 539–544, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oppenheimer D. Neuropathology of autonomic failure. In: Autonomic Failure A Textbook of Clinical Disorders of the Autonomic Nervous System, edited by Bannister R. Oxford, UK: Oxford Medical Publications, 1988, p. 451–463 [Google Scholar]
- 15. Owlya R, Vollenweider L, Trueb L, Sartori C, Lepori M, Nicod P, Scherrer U. Cardiovascular and sympathetic effects of nitric oxide inhibition at rest and during static exercise in humans. Circulation 96: 3897–3903, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng 32: 230–236, 1985 [DOI] [PubMed] [Google Scholar]
- 17. Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22–27, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Panza JA, Quyyumi AA, Callahan TS, Epstein SE. Effect of antihypertensive treatment on endothelium-dependent vascular relaxation in patients with essential hypertension. J Am Coll Cardiol 21: 1145–1151, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Sakima A, Teruya H, Yamazato M, Matayoshi R, Muratani H, Fukiyama K. Prolonged NOS inhibition in the brain elevates blood pressure in normotensive rats. Am J Physiol Regul Integr Comp Physiol 275: R410–R417, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Sakuma I, Togashi H, Yoshioka M, Saito H, Yanagida M, Tamura M, Kobayashi T, Yasuda H, Gross SS, Levi R. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res 70: 607–611, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension 33: 937–942, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Sander M, Hansen PG, Victor RG. Sympathetically mediated hypertension caused by chronic inhibition of nitric oxide. Hypertension 26: 691–695, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation 117: 1991–1996, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Shankar R, Zhu JS, Ladd B, Henry D, Shen HQ, Baron AD. Central nervous system nitric oxide synthase activity regulates insulin secretion and insulin action. J Clin Invest 102: 1403–1412, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension 49: 27–33, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension 21: 929–933, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Task Force of the European Society of Cardiology, the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 28. Topal E, Cikim AS, Cikim K, Temel I, Ozdemir R. The effect of moxonidine on endothelial dysfunction in metabolic syndrome. Am J Cardiovasc Drugs 6: 343–348, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2: 997–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Wenzel RR, Spieker L, Qui S, Shaw S, Luscher TF, Noll G. I1-imidazoline agonist moxonidine decreases sympathetic nerve activity and blood pressure in hypertensives. Hypertension 32: 1022–1027, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Young CN, Fisher JP, Gallagher KM, Whaley-Connell A, Chaudhary K, Victor RG, Thomas GD, Fadel PJ. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J Physiol Lond 587: 4977–4986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]