Abstract
Mitochondrial electron transport chain (ETC) is the major source of reactive oxygen species during myocardial ischemia-reperfusion (I/R) injury. Ischemic defect and reperfusion-induced injury to ETC are critical in the disease pathogenesis of postischemic heart. The properties of ETC were investigated in an isolated heart model of global I/R. Rat hearts were subjected to ischemia for 30 min followed by reperfusion for 1 h. Studies of mitochondrial function indicated a biphasic modulation of electron transfer activity (ETA) and ETC protein expression during I/R. Analysis of ETAs in the isolated mitochondria indicated that complexes I, II, III, and IV activities were diminished after 30 min of ischemia but increased upon restoration of flow. Immunoblotting analysis and ultrastructural analysis with transmission electron microscopy further revealed marked downregulation of ETC in the ischemic heart and then upregulation of ETC upon reperfusion. No significant difference in the mRNA expression level of ETC was detected between ischemic and postischemic hearts. However, reperfusion-induced ETC biosynthesis in myocardium can be inhibited by cycloheximide, indicating the involvement of translational control. Immunoblotting analysis of tissue homogenates revealed a similar profile in peroxisome proliferator-activated receptor-γ coactivator-1α expression, suggesting its essential role as an upstream regulator in controlling ETC biosynthesis during I/R. Significant impairment caused by ischemic and postischemic injury was observed in the complexes I- III. Analysis of NADH ferricyanide reductase activity indicated that injury of flavoprotein subcomplex accounts for 50% decline of intact complex I activity from ischemic heart. Taken together, our findings provide a new insight into the molecular mechanism of I/R-induced mitochondrial dysfunction.
Keywords: reactive oxygen species, protein biosynthesis
mitochondrial dysfunction is the major contributor to myocardial ischemia/reperfusion injury. The investigation into the changes in ETC activities during ischemia and reperfusion has been extensively reviewed (44). Cardiac ischemia renders the myocardium in the physiological conditions of hypoxia, substrate deprivation, and acidosis. Mitochondria are thus situated in the highly reductive environment with low Po2 and low ADP. In the ischemic heart, oxygen delivery to the myocardium is not sufficient to meet the needs of mitochondrial oxidation. Reintroduction of oxygen with reperfusion greatly increases electron leakage from mitochondrial electron transport chain (ETC), leading to overproduction of superoxide (O2∙−) and O2∙−-derived oxidants in mitochondria (3, 21). Marked hyperoxygenation induced by reperfusion in the postischemic myocardium was detected by in vivo electron paramagnetic resonance (EPR) oxymetry (50).
The proteins of ETC are the major site for energy transduction and oxygen free radicals production in mitochondria. It was reported that cardiac ischemia resulted in damage to the mitochondrial ETC and this ischemic damage to the ETC contributed to myocardial injury during reperfusion (12, 36). Furthermore, mitochondrial ultrastructural injury occurred and progressed during myocardial ischemia (34). Despite the above alteration induced by ischemia in the ETC, recovery of mitochondrial function with restoration of contractility was detected upon reperfusion. However, it was observed that ETC-mediated oxygen consumption is disproportionately high relative to reduced contractility (23, 35). Therefore, defect of mitochondrial integrity in the postischemic heart was linked to the oxidative damage caused by reactive oxygen species (ROS) overproduction. Several groups (12, 13, 36, 50) have reported that the activities of the ETC from isolated mitochondria are decreased during reperfusion. The molecular mechanisms of ischemia-induced defects and reperfusion-induced damage to the ETC are still poorly understood due to a lack of deep insights into biochemical characterization of the ETC proteins in the ischemic and postischemic hearts. Recent studies have identified that alterations of ETC in the ischemic and postischemic myocardium are involved in posttranslational modifications, including increasing protein tyrosine nitration of complex I (30) and complex II (7), decreasing protein S-glutathionylation of complex II (13), and increasing hyperphosphorylation of complex IV (39). In vitro studies (7, 8, 13, 49) have also clarified that some of the above protein modifications contributed to the decrease in the enzymatic activity of specific ETC components.
Isolated mitochondria are commonly used to evaluate bioenergetics in hearts undergoing ischemia and reflow. The present study was therefore performed to assess the biochemical properties of the mitochondria isolated from ischemic and postischemic hearts, including 1) the enzymatic activities of ETC, 2) regulation of protein biosynthesis of ETC, and more specifically 3) impairment of complex I. Our findings indicate that the mitochondrial ETC is specifically undergoing a biphasic modulation of enzymatic catalysis and protein biosynthesis during the phase of ischemia and the phase of reperfusion. The detected dynamic behavior of the ETC, at least in part, contributes to the ischemic defect of mitochondria and reperfusion-induced mitochondrial dysfunction.
MATERIALS AND METHODS
Animals, Langendorff heart preparation, and measurement of left ventricular function and coronary flow.
Male Sprague-Dawley rats (3 to 4 mo, 350–400 g) were purchased from Harlan (Indianapolis, IN). All procedures were performed with the approval (protocol no. 10-003) of the Institutional Animal Care and Use Committee at Northeast Ohio Medical University (Rootstwon, OH) and conformed to the Guide for the Care and Use of Laboratory Animals. Langendorff-isolated heart was performed as described previously (51). All hearts were subjected to a 20-min baseline period under constant perfusion pressure. Hearts were randomly assigned to one of three study groups including control (perfusion for 60 min without ischemia), global ischemia (30 min), and reperfusion following global ischemia, and left ventricular (LV) developed pressure (LVDP) was continuously recorded using Powerlab 4/25 ADC (AD Instruments, Newton. NH). The following derived indexes of LV mechanical function were instantaneously recorded: peak systolic pressure (PSP), end diastolic pressure (LVEDP), developed pressure (LVDP = PSP − LVEDP), heart rate (HR), and rate pressure product (RPP = LVDP * HR).
Preparation of the mitochondria from the rat hearts.
Mitochondria were prepared by differential centrifugation (47). To increase recovery of the mitochondria from both viable and nonviable cardiomyocytes, Polytron/nagarse homogenate was subjected to centrifugation at 20,000 g for 10 min and resuspended in the buffer M containing 230 mM manitol, 70 mM sucrose, 1 mM EDTA, 5 mM Trizma/HCl buffer (pH 7.4), and protease/phosphatase inhibitors (1 tablet cOmplete and 1 tablet phosSTOP in 10 ml; Roche Applied Science, Indianapolis, IN) before oxygen consumption measurement. The mitochondria as prepared contain 410.2 nmol heme b/mg protein and 470.4 nmol aa3/mg protein and no detectable contamination of nuclear and endoplasmic reticulum. The recovery of mitochondria was determined by measuring percent recovery of citrate synthase activity in isolated mitochondria and tissue homogenate according to published methods (5, 16).
Assay of enzymatic activities of the mitochondrial ETC.
Mitochondrial preparations were subjected to analysis of electron transfer activities (ETAs) using a UV/VIS spectrophotometer. The ETA of complex I was determined by following the rotenone-sensitive ubiquinone-1 (Q1)-stimulated NADH oxidation (8). The ETA of complex II was assayed by thenoyltrifluoroacetone-sensitive ubiquinone-2 (Q2)-stimulated dichlorophenyl indophenol reduction (13). The ETA of complex III was assayed by ubiquinol-2 (Q2H2)-stimulated cytochrome c (cyt c) reduction and verified by inhibition with antimycin A (13). The ETA of complex IV was assayed by measuring ferrocytochrome c oxidation, and further confirmed by inhibition with potassium cyanide (15).
Immunoblotting analysis.
Western blotting with mitochondrial preparations was performed as described previously (13). Immunoblotting was carried out with anti-51-kDa antibody [against the flavin mononucleotide (FMN)-binding subunit of complex I, generated in-house], or anti-75-kDa polyclonal antibody (against 75-kDa subunit of complex I, generated in house), or anti-ND1 (hydrophobic protein of complex I), or anti-70-kDa antibody (against the FAD-binding subunit of complex II, and generated in-house), or anti-FeS antibody [monoclonal antibody against Rieske iron-sulfur protein (RISP) of complex III; Invitrogen, Carlsbad, CA], or anti-CoXI antibody (monoclonal antibody against the subunit 1 of complex IV; Invitrogen), or anti-MnSOD (polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, CA).
Transmission electron microscopy.
Blocks (not exceeding 1-mm cubed) of heart tissue were fixed in 3% glutaraldehyde prepared in phosphate buffer (100 mM, pH 7.4) containing sucrose (3.4%, wt/vol) for 2 h at room temperature. Specimens were washed in phosphate-buffered sucrose (osmolality = 425 mOsm) three times and postfixed for 1 h in 1% osmium tetraoxide in the same buffer. After three brief rinses in distilled water, the specimens were dehydrated in increasing concentrations (50–70-80–95-100%) of ethanol, embedded in Epon resin, and polymerized at 60°C for 16–24 h. Thin sections were examined in an electron microscope. The morphometric analysis of the mitochondria of the myocardium was done according to the published methods (26). Mitochondrial size was calculated based on micrograph at ×18,500, and volumetric density of mitochondria was computed based on micrograph at ×6,800.
Preparations of isolated NADH-cytochrome c reductase supercomplex from bovine heart.
Bovine heart mitochondrial NADH-cytochrome c reductase (NCR; supercomplex hosting complex I and complex III) was prepared from submitochondrial particles according to the published method (22). The NCR prepared contains 2.2 nmol heme b/mg protein with an electron transfer activity of 111.8 nmol cyt c reduced·min−1·nmol heme b−1 in the assay mixture containing 20 mM potassium phosphate buffer, 150 μM NADH, 50 μM ferricytochrome c, 180 μg/ml azolectin, 1 mM EDTA, and 2 mM NaN3 (22).
EPR spin-trapping experiment.
EPR spin trapping experiment with 5-diethoxylphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO) was used to detect O2∙−, which was performed on a Bruker EMX MICRO spectrometer (in Rootstown campus of NEOMED) operating at 9.86 GHz with 100 kHz of modulation frequency at room temperature. The reaction mixture was transferred to a 50-μl capillary, which was then positioned in the high-sensitivity cavity. The instrumental setting and the parameters of computer simulation was followed according to published method (14).
Statistical analysis.
All data were reported as group averages ± SE. Statistical analyses of LV function were performed at the end of the baseline period and at the end of 15, 30, or 60 min of reperfusion. Comparison between two groups was assessed by independent t-test and among multiple groups was assessed by one-way ANOVA followed by the least significant difference, Tukey's honestly significant difference or Games-Howell post hoc tests. Differences between two groups were determined with Student's t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
Myocardial functional recovery in the postischemic heart.
As compared with control preischemic baseline level, impairment of LV function was detected at the end of 15, 30, and 60 min of reperfusion. The functional recovery of LVDP was 20.0 ± 4.3% (15-min reperfusion, n = 6), 24.9 ± 4.9% (30-min reperfusion; n = 6), and 26.2 ± 3.8% (60-min reperfusion, n = 6). The RPP recovered to 18.3 ± 4.4% (15-min reperfusion), 22.2 ± 4.9% (30-min reperfusion), and 26.1 ± 5.8% (60-min reperfusion) compared with isolated hearts subjected to equal duration of reperfusion without ischemia. The parameters of hemodynamic performance are shown in the Table 1.
Table 1.
Hemodynamic values from isolated rat hearts subjected to ischemia and reperfusion and the effect of cycloheximide
| Measure | Equilibrium Baseline (20 min) | Ischemia (0 min) + RP (60 min) | End of Ischemia (30 min) | RP (15 min) | RP (30 min) | RP (60 min) | CHX + RP (30 min) |
|---|---|---|---|---|---|---|---|
| Heart rate, beats/s | 285 ± 15 | 293 ± 12 | ND | 262 ± 16 | 254 ± 11 | 269 ± 21 | 243 ± 19 |
| LVDP, mmHg | 96.7 ± 5.7 | 94.3 ± 3.6 | ND | 19.3 ± 1.8 | 24.1 ± 3.1 | 26.7 ± 4.1 | 10.1 ± 1.7 |
| LVEDP, mmHg | 4.7 ± 1.3 | 5.1 ± 1.7 | 31.1 ± 4.3 | 71.8 ± 9.4 | 58.6 ± 9.5 | 41.2 ± 6.0 | 69.0 ± 8.2 |
Values are means ± SE. RP, reperfusion; LVDP, left ventricular developed pressure; LVEDP, left-ventricular end-diastolic pressure; CHX, cycloheximide; ND, not determined.
State 3 ADP-stimulated respiration and mitochondrial integrity in the ischemic and postischemic heart.
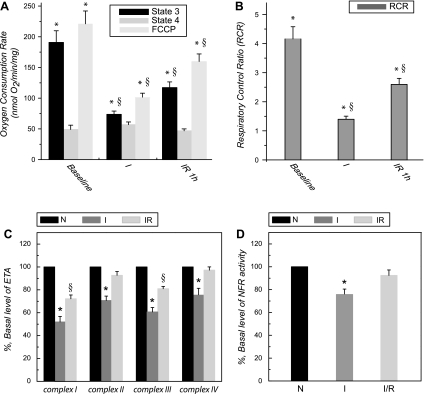
Overproduction of oxygen free radicals during myocardial ischemia/reperfusion can depolarize mitochondrial membrane potential and uncouple mitochondrial respiration (19, 20). Therefore, respiratory control ratio (RCR; defined as the ratio of state 3 to state 4) of mitochondrial preparation from hearts was evaluated at 30°C. As indicated in the Fig. 1, A and B, NADH-linked and ADP-stimulated respiration (state 3, driven by 5 mM malate/140 mM glutamate) of the mitochondria from ischemic hearts was decreased from 190.7 ± 18.0 to 73.6 ± 4.8 nmol O2·min−1·mg protein−1 (n = 4; P < 0.05), FCCP-induced uncoupling respiration was decreased from 219.9 ± 19.8 to 99.6 ± 6.6, and the RCR was decreased from 4.1 ± 0.4 to 1.4 ± 0.1 (n = 4; P < 0.05). In the postischemic heart, NADH-linked and ADP-stimulated/uncoupling respiration was decreased to 117.2 ± 9.1/160.3 ± 13.3 nmol O2·min−1·mg protein−1 (n = 4; P < 0.05), and the RCR was decreased to 2.6 ± 0.3 (n = 4; P < 0.05). These results indicate a significant defect in respiration and mitochondrial integrity in the ischemic and postischemic hearts. However, the functional recovery of LVDP during reperfusion was supported by partial restoration of mitochondrial RCR, ADP-stimulated, and uncoupling respirations. The results support the data directly showing recovery of high-energy phosphates including tissue ATP and phosphocreatine in the heart subjected to a similar protocol of ischemia and reperfusion as previously reported by Ambrosio et al. (1, 2).
Fig. 1.
A and B: state 3 and state 4 respiratory rates of mitochondrial preparations from control, ischemic, and postischemic hearts. Control hearts were obtained from 60-min reperfusion without ischemia. Ischemic and postischemic hearts were obtained through the conditions of global ischemia (30 min) and global ischemia (30 min)/reperfusion (60 min). Mitochondria were prepared and resuspended in the buffer M. Mitochondrial respirations were measured by the polarographic method at 30°C (7). Oxygen consumption by mitochondria (0.5 mg/ml) was induced by malate (5 mM)/glutamate (140 mM). State 3, oxygen consumption stimulated by ADP (0.5 mM); state 4, oxygen consumption after the addition of oligomycin (6 μg/mg mitochondria) followed by ADP addition. RCR is the ratio of state 3 and state 4 oxygen consumption rates; uncoupling respiration is oxygen consumption after the addition of FCCP (2.5 μM). *P < 0.05 vs. baseline control (N). §P < 0.05, ischemia (I) vs. iscehmia/reperfusion (IR). C and D: enzymatic activity of electron transport chain (ETC) in the mitochondria of ischemic and postischemic hearts (n = 5). ETA; electron transfer activity; NFR, NADH ferricyanide reductase.
Mitochondrial ETC activities and protein and mRNA expressions in the ischemic and postischemic hearts.
The recovery of mitochondria from myocardial tissue is 33.6 ± 6.5% (control heart; n = 5), 29.5 ± 5.2% (ischemic heart; n = 5), and 35.2 ± 5.2% (postischemic heart; n = 5) based on citrate synthase assay. There is no statistical difference in the mitochondrial recovery among control, ischemic, and postischemic hearts. Even though the amount of mitochondria recovered was roughly similar in the three conditions, the quality was not. It should be noted that the isolation procedures employed for “healthy” mitochondria may work differently for ischemic mitochondria, which could be ending up “enriching” selected populations of mitochondria, not necessarily representative of the whole mitochondrial population. Thus, the use of centrifugation at 20,000 g (rather than 5,000 g) should boost the representative of the whole mitochondrial population. Furthermore, two populations (subsarcolemmal mitochondria and interfibrillar mitochondria) of mitochondria with differential susceptibility to damage in ischemia should be mentioned as a potential limitation of current study.
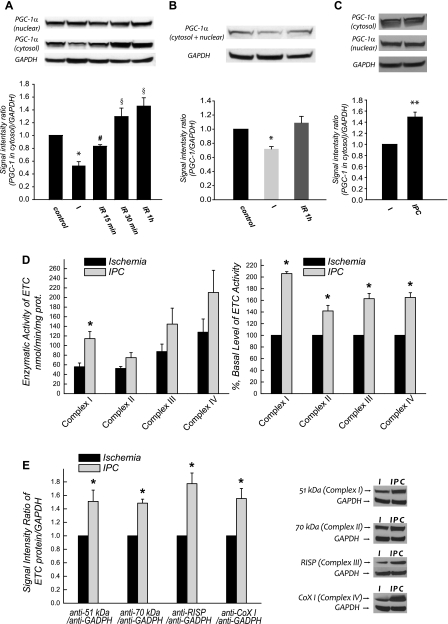
Mitochondria were then subjected to analysis of ETC enzymatic activities. The specific activities of complex I, complex II, complex III, and complex IV in the mitochondria of normal heart were 244.2 nmol NADH oxidized·min−1·mg protein−1, 386.5 nmol dichlorophenyl indophenol reduced·min−1·mg protein−1, 2255.6 nmol ferricytochrome c reduced·min−1·mg protein−1, and 2,008.0 nmol ferrocytochrome c oxidized·min−1·mg protein−1, respectively.
As indicated in Fig. 1C, a significant decrease of ETA was detected in the ischemic heart compared with control, including complex I (52.1 ± 4.6% of ETA remained; n = 5; P < 0.05), complex II (70.1 ± 5.1% remained; n = 5; P < 0.05), complex III (60.7 ± 3.9% remained; n = 5; P < 0.05), and complex IV (75.4 ± 5.8% remained; n = 5; P < 0.05). The concentrations of heme b and aa3 were diminished to 350.9 and 409.6 nmol/mg, respectively. These results clearly demonstrated an ischemia-induced defect of the mitochondrial ETC, and the defect was correlated to the impairments of ADP-stimulated respiration and mitochondrial integrity (Fig. 1B).
We then assessed the ETAs of 60 min postischemic heart. A significant decrease in the complex I activity (72.3 ± 3.3% remained; n = 5; P < 0.05) and complex III activity (80.9 ± 2.0% remained; n = 5; P < 0.05) was observed, suggesting the involvement of postischemic injury. However, we did not detect a significant impairment of absolute ETAs in complex II, complex IV (n = 5, not statistically significant), and the concentrations of heme b and aa3.
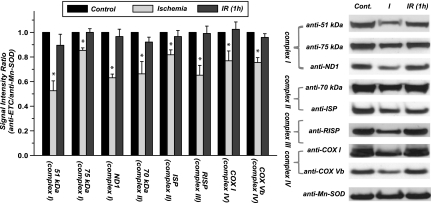
Protein expression in mitochondria was probed by Western blot with equal amounts (120 μg) of protein loading, and the SOD-2 was used as housekeeping protein or protein loading control. As shown in the Fig. 2, a significant reduction of ETC protein expression was detected in the mitochondria isolated from ischemic heart, but no reduction was observed in those isolated from the postischemic heart. Based on the densitometric analysis of blots using ImageJ Software, protein expression in ischemic heart was decreased by 47.4 ± 7.8% for complex I (probed with Ab against a 51-kDa subunit; n = 5; P < 0.05), by 33.7 ± 8.9% for complex II (probed with Ab against a 70-kDa subunit; n = 5; P < 0.05), by 34.8 ± 7.1% for complex III (probed with Ab against RISP; n = 5; P < 0.05), by 23.1 ± 3.3% for complex IV (probed with Ab against subunit I; n = 5; P < 0.05). Additional subunits of ETC proteins were probed by Western blotting (indicated by Fig. 2), including 75-kDa subunit of complex I (decreasing by 15.6 ± 1.6%; n = 5; P < 0.05), ND1 subunit of complex I (decreased by 37.2 ± 2.2%; n = 5; P < 0.05), iron sulfur protein of complex II (decreasing by 18.3 ± 3.6%; n = 5; P < 0.05), and subunit Vb of complex IV (decreasing by 24.5 ± 4.5%; n = 5; P < 0.05). These data suggested ischemic degradation of mitochondrial ETC and marginal elevation of ETC biosynthesis during reperfusion following ischemia.
Fig. 2.
Protein expression of ETC in the mitochondria of ischemic and postischemic rat hearts (n = 5). Mitochondrial preparations (120 μg) were immunoblotted using the specified antibodies. Complex I was probed with the antibody against 51-kDa flavin mononucleotide-binding subunit, anti-75 kDa (of iron-sulfur subcomplex) antibody, and anti-ND1 (of hydrophobic subcomplex) antibody. Complex II was probed with the antibody against 70-kDa FAD-binding subunit and the antibody against subunit II iron sulfur protein (ISP). Complex III was probed with the antibody against Rieske iron-sulfur protein (RISP). Complex IV was probed by the antibody against subunit I [subunit 1 of complex IV (COX 1)] and subunit Vb antibody. Signals were normalized to Mn-SOD (probed with anti-MnSOD antibody). *P < 0.05 vs. baseline control and IR 60 min.
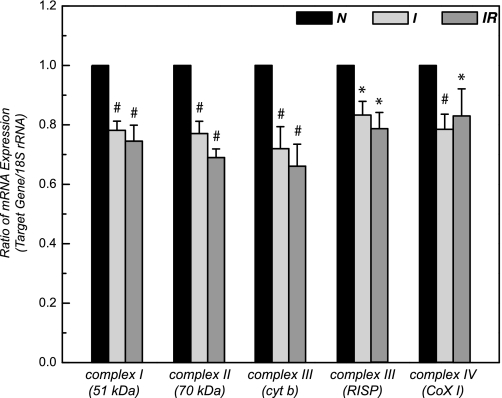
The mRNA level of ETC in the myocardium was measured by real-time PCR using 18S rRNA as a loading control. As shown in the Fig. 3, it was decreased by 18–29% (n = 5; P < 0.05) after 30 min of ischemia, likely due to a global increase of nuclease activity induced by ischemia. There was no significant difference in the mRNA level between ischemic and postischemic myocardium.
Fig. 3.
Primer sequences (forward/reverse) for real-time PCR were chosen from the Genbank mRNA sequence of rat by using Primer Express software (Applied Biosystems, Foster City, CA) were 51 kDa of complex I : 5′-GAAAACCTCATTTGGCTCGC-3′/5′-GCAGGATCTCCTTTG TCTTGT -3′; 70 kDa of complex II (structural gene, SdhA): 5′-ACGGCCAGGATCAGATTG TG -3′/5′-CGACTCTTCTCCAGCATTTGC-3′; cytochrome b of complex III: 5′-CCTATTATCTGCTATCCCTTAC-3′/5′-GTTATTTGATCCTGTTTCGTGG-3′; RISP of complex III: 5′-GTGAAGCGACCCTTCCTGTG-3′/5′-CGGGCACCTTGATGTCTGTG-3′; COX 1 of complex IV-AGTATTCGCCATCATAGCTGGCT-3′/5′- GCTTTTGCTCATGTGTCATTTAGG-3′; and 18S rRNA: 5′-GCTGGAATTACCGCGTGCT-3′/5′-CGGCTACCACATCCAAGGAA-3′. Real-time PCR was performed with the use of SYBR Green PCR master mix on an Applied Biosystem 7900HT System. Relative mRNA expression levels were calculated by comparing the target genes/18S rRNA. Reaction mixture of SYBR assay contained 6 μl of 2 × SYBR Green I master mix, 0.5 μM primers, and 20–120 ng cDNA (depending on the abundance of gene expression level; n = 10). Amplification was allowed to proceed for 40 cycles under the conditions of denatuation at 95°C for 10 s, annealing at 60°C for 15 s, and extension at 72°C for 30 s. To assess the specificity of the amplified PCR products, we performed a melting curve analysis after the last cycle. 18S rRNA was used as an endogenous control. All samples were tested in triplicate, and average values were used for quantification. Analysis was performed by using SDS version 2.2 software (Applied Biosystems) according to the manufacturer's instructions. The comparative cycle threshold (CT) method (ΔΔCT) was used for quantification of gene expression. *P < 0.05 vs. control (N). #P < 0.01 vs. control (N).
NADH-ferricyanide reductase activity in the ischemic and postischemic hearts.
Complex I (NADH-ubiquinol reductase) contains up to 45 different subunits with a total molecular mass near 1 MDa. With the use of chaotropic anions such as perchlorate, complex I can be resolved into three subcomplexes: a flavin subcomplex (Fp), an iron-sulfur subcomplex (Ip), and a hydrophobic subcomplex (Hp). Rotenone-insensitive NADH ferricyanide reductase (NFR) activity is the indicator of Fp from the intact complex I. As shown in the Fig. 1D, a marginal depression of NFR activity (75.3 ± 4.3% of NFR remained; n = 6; P < 0.05; Fig. 1D vs. 51.8 ± 4.7% of intact complex I remained in Fig. 1C) was detected in the mitochondria of ischemic heart, suggesting ∼ 50% of ischemic injury to complex I was caused by the impairment of Fp. There is no significant injury observed in the mitochondrial NFR activity from postischemic heart (92.4 ± 4.8% remained; n = 6 in Fig. 1D), indicating that Fp did not contribute to the postischemic injury of intact complex I.
Ultrastructural evidence of decreased mitochondrial ETC in the ischemic hearts.
The ultrastructure of normal or control myocardium from LV muscle is shown in Fig. 4, A and B. The mitochondria in normal or control myocytes were characterized by having electron dense matrices and tightly packed cristae, i.e., high density of cristae in the mitochondria (34).
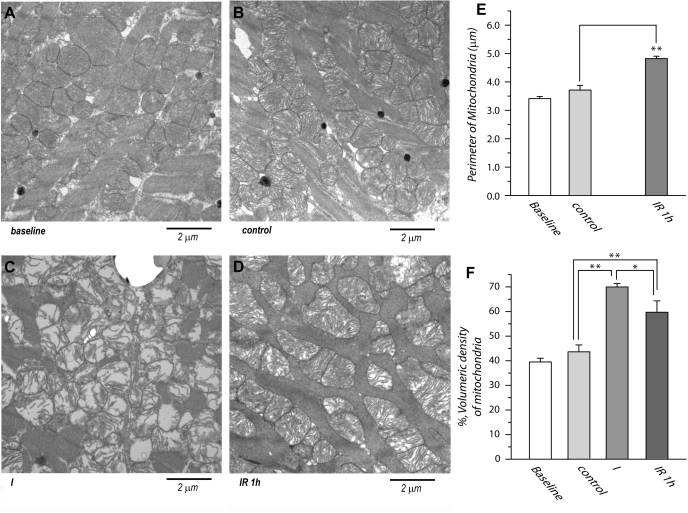
Fig. 4.
Transmission electron micrograph of ultrathin section from the left ventricle of rat hearts. A: normal myocardium. B: control myocardium (60-min perfusion), C: myocardium after 30 min of ischemia. D: postischemic myocardium after 30 min of ischemia and 60 min of reperfusion. Bar = 2 μm. Original magnification = ×11,000. E: mitochondrial size was calculated based on transmission electron microscopy (TEM) picture at ×18,500 (n = 25). Five sections were collected from each group, and 5 mitochondria for each section were measured. F: mitochondrial fractional area was calculated based on the TEM picture at ×6,800 (n = 5). Four sections were collected for each group of baseline control, ischemia, and ischemia/reperfusion and computed as the percentile of total myocardial fiber area. *P < 0.05. **P < 0.01.
Ultrastructural changes were occurred during progressive ischemia in the myocardium. After 30 min of ischemia, myofibrils were loosely packed, indicating severe swelling (34). Virtually all mitochondria were swollen (Fig. 4C), matrices were electron lucent, and cristae were degraded, severely disorganized, and dramatically decreased in density. The number of mitochondria in myocardium was not found appreciably reduced. This result was supported from the results from immunoblotting analysis of mitochondria isolated from ischemic heart (Fig. 2), confirming a dynamic process of ischemic degradation and subsequent reduction of ETC protein in the mitochondria.
The ultrastructure of myocardium after 60 min of postischemic reperfusion indicated increasing density of cristae in mitochondria, suggesting reduction of mitochondrial swelling. Electron dense matrices and highly packed cristae in most mitochondria were reverted. However, most mitochondria exhibited morphological changes with irregular shapes although they were clearly marginated (Fig. 4D).
Morphometric analysis indicated mitochondrial sizes from control myocardium and 1 h postischemic heart are 3.7 ± 0.2 μm (n = 25) and 4.9 ± 0.1 μm (n = 25; P < 0.001 vs. control) indicated in the Fig. 4E. Owning to severe swelling, rupture, and vague border of mitochondria from ischemic myocardium, calculation of the mitochondrial size is not feasible. Mitochondrial fractional area was calculated and computed as the percentile of total myocardial fiber area, indicating 43.6 ± 2.8% for control myocardium, 69.9 ± 1.4% for ischemic heart, and 53.6 ± 6.7% for 1 h postischemic heart (Fig. 4F).
Involvement of peroxisome proliferator-activated receptor-γ coactivator-1α in the postischemic biosynthesis of the mitochondrial ETC.
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a coactivator of the PPARγ (40). PGC-1α functions to coactivate several downstream transcriptional factors and plays a central integrative role in mitochondrial biogenesis (48). To provide further evidence regarding the role of PGC-1α in the ischemic deficiency and postischemic biosynthesis of the mitochondrial ETC, we analyzed the protein expression level of PGC-1α in myocardium by immunoblotting, and GAPDH was used as a protein loading control. We observed a dramatic decrease (by 46.9 ± 6.1%; P < 0.05; n = 6) in cytosolic PGC-1α expression after 30 min ischemia and a progressive increase during reperfusion at 15, 30, and 60 min (Fig. 5A). A significant change of PGC-1α expression due to myocardial ischemia and reperfusion was not observed in the nuclear fraction (Fig. 5A). Analysis of total PGC-1α expression combining the cytosolic and nuclear fractions indicated a 28.0 ± 3.3% (n = 6; P < 0.05) reduction after 30-min ischemia (Fig. 5B). Therefore, ETC protein expression in mitochondria was correlated with PGC-1α expression in the cytosol during ischemia and reperfusion.
Fig. 5.
Cardiac peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) expression in the ischemic and postischemic hearts (n = 6). Myocardial tissues were homogenized in 3 mM HEPES, 250 mM sucrose, 0.1% Triton X-100, and 2 mM DTT. Supernatant (cytosolic fraction) of tissue homogenate was collected by centrifugation at 8,000 g for 20 min. Pellet was resuspended in 25 mM Tris·Cl pH 7.6 containing 150 mM NaCl, 1% NP-40, 1% deoxycholate, 0.1% SDS, 2 mM EDTA, 5 mM DTT, and protease/phosphatase inhibitors cocktail (1×). Supernatant was collected by centrifugation at 16,000 g for 5 min (nuclear fraction) and subjected to analysis of PGC-1α expression in the nuclear fraction. A: cytosolic PGC-1α of issue homogenates (190 μg) and nuclear PGC-1α of pellets (190 μg) were immunoblotted with a polyclonal antibody against PGC-1. Protein expression level of GAPDH in tissue homogenates was used as a loading control. B: immunoblotting analysis using the sample mixture combined from cytosol and nuclear fractions. *P < 0.05 vs. baseline control, IR-15 min, IR-30 min, IR-1 h. #P < 0.05 vs. baseline control, IR-30 min, IR-1 h. §P < 0.05 vs. baseline control. C: immunoblotting analysis using the cytosolic fractions from ischemic heart (30-min I) and ischemic heart (30-min I) after precondition (IPC) using 2 episodes of 5-min ischemia separated by one 10-min and two 5-min reperfusion. Tissue homogenates were prepared for biochemical analysis after 30-min ischemia. D: same as C, except that enzymatic activities of mitochondrial ETC were measured. E: same as C, except that protein expression of ETC was probed with immunoblotting analysis.
The protein expression of cytosolic PGC-1α from preconditioned heart (2 episodes of 5-min ischemia separated by one 10-min and two 5-min reperfusion) was found significantly increased by 49.2 ± 8.8% (n = 3; P < 0.05) while compared with the level of ischemic heart (Fig. 5C), thus confirming the role of PGC-1α in cardioprotection (33). We further detected precondition-mediated upregulation of ETC activities and protein expression as shown in Fig. 5, D and E.
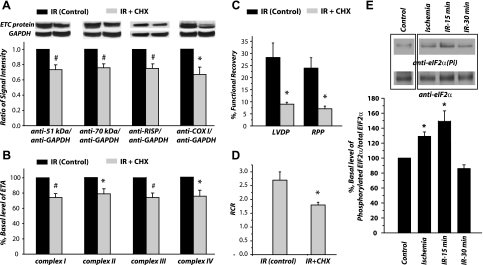
Inhibition of postischemic protein translation of mitochondrial ETC with cycloheximide.
Cycloheximide is an inhibitor of protein biosynthesis in eukaryotic organisms. Cycloheximide exerts its effect by interfering with the translocation step in protein synthesis, thus blocking translational elongation. To test whether translational control was involved, we treated rat heart with cycloheximide (1 mM) before 30 min of reperfusion. Tissue homogenates of cycloheximide-treated hearts were subjected to analysis. The protein expression (Fig. 6A) and ETA (Fig. 6B) in cycloheximide-treated heart were depressed to the level of ischemic heart. The expression of ETC proteins after cycloheximide treatment was decreased to 73.3 ± 6.3% for complex I (P < 0.05; n = 5), 75.7 ± 5.1% for complex II (P < 0.05; n = 5), 75.0 ± 6.0% for complex III (P < 0.05; n = 5), and 71.1 ± 6.5% for complex IV (P < 0.05; n = 5; Fig. 6A). The activities of ETC after cycloheximide treatment were decreased to 74.1 ± 5.2% for complex I (P < 0.05; n = 5), 78.7 ± 6.6 for complex II (P < 0.05; n = 5), 73.6 ± 5.9% for complex III (P < 0.05; n = 5), and 75.2 ± 7.8% for complex IV (P < 0.05, n = 5; Fig. 6B). Furthermore, we have detected that cycloheximide significantly inhibited reperfusion-mediated functional recoveries of LVDP (28.3 ± 5.9 vs. 9.0 ± 0.8%), RPP (23.9 ± 4.3 vs. 7.1 ± 1.1%), and RCR (2.7 ± 0.3 vs. 1.9 ± 0.5) as indicated in the Fig. 6, C and D, and Table 1. These results thus supported involvement of translational control in the rapid biosynthesis of mitochondrial ETC proteins during reperfusion. Note that cycloheximinde (1 mM) does not affect the respiration and the ETA of isolated mitochondria. Also, the ETA and ETC protein expression in mitochondria was not significantly affected in the normal heart perfused with Kreb buffer containing cycloheximide (data not shown).
Fig. 6.
Cycloheximide (CHX) partially blocks reperfusion-induced ETC protein increase (n = 5). Hearts were subjected to a 5-min baseline period under constant perfusion pressure. Hearts were randomly assigned to 1 of 2 study groups including 1 control and 1 CHX treatment group. Immediately 15 min before global ischemia, with the perfusion rate set at ∼0.25 ml/min, these groups received oxygenated Krebs buffer only (control group) or Krebs buffer containing CHX (1 mM). After 30 min of global ischemia, hearts were reperfused with oxygenated Krebs buffer (control) or Krebs buffer with CHX (1 mM) for additional 30 min. Tissue homogenates of left ventricles were subjected to immunoblotting analysis (A), ETA (B) including complexes I-IV, and functional recovery of left ventricular developed pressure and rate pressure product (C) and RCR (D). #P < 0.01 vs. IR-30 min control. *P < 0.05 vs. IR-30 min control. Note, 100% refers to the basal level or basal activity of IR-30 min control. E: cardiac eIF2α (eukaryotic initiation factor) expression in the ischemic and postischemic hearts (n = 3). Cytosolic fraction of tissue homogenates was immunoprecipitated with the polyclonal Ab against eIF2α, and then probed with the Ab against phosphorylated (Ser51) eIF2α and eIF2α. Note that blotting of control sample (lane 1 from left) was inserted due to a noncontiguous sample loading from ischemia and IR samples (lanes 2–4 from left).
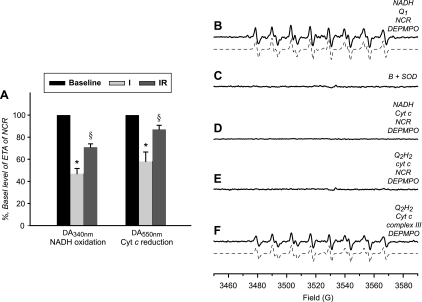
Mitochondrial NCR activity in the ischemic and postischemic hearts.
NCR is the supercompelx hosting complex I and complex III and mediates electron transport from NADH to ferricytochrome c. The NCR activity in the ischemic and postischemic hearts was decreased by 53.1 ± 4.8 and 29.1 ± 3.0% when assayed by NADH oxidation in the presence of ferricytochrome c (Fig. 7A). The NCR activity also can be assayed by measuring ferricytochrome c reduction (absorbance increase at 550 nm) in the presence of NADH; a decrease of 42.0 ± 8.6 and 13.0 ± 3.7% was observed (Fig. 7A). These data further supported that injury of ETC during reperfusion was specifically occurred in complex I and complex III (Fig. 1C). Presumably complex I and complex III are the major source of O2∙− production and sensitive to the oxidant stress induced by postischemic reperfusion. To provide direct evidence in supporting of complex I/complex III-mediated O2∙− generation, supercomplex of NCR was isolated from the mitochondria of bovine heart (22). Mediation of O2∙− by the complex I component from NCR supercomplex under the conditions of enzyme turnover was assessed with EPR spin trapping with DEPMPO (Fig. 7B). The detected DEPMPO/∙OOH adduct could be inhibited by SOD (Fig. 7C). No detectable DEPMPO/·OOH adduct was mediated by the NCR or the complex III component from NCR in the presence of NADH and cyt c (Fig. 7D) or ubiquinol-2 and cyt c (Fig. 7E), suggesting that O2∙− mediated by NCR or its complex III component was minimized in the supercomplex under the conditions of enzyme turnover. In contrast, when the complex III was isolated or detached from NCR supercomplex, significant O2∙− production can be mediated by complex III under the conditions of enzyme turnover (Fig. 7F).
Fig. 7.
Enzymatic activity of NADH-cytochrome c (cyt c) reductase (NCR) in the mitochondria from ischemic and postischemic hearts (A; n = 6) and O2∙− mediated by the supercomplex of NCR (B-F). A: NCR activity in the mitochondria was monitored by NADH oxidation or cyt c reduction described in materials and methods (*P < 0.05 vs. baseline and IR. §P < 0.05 vs. baseline). B: computer simulation (dashed line) superimposed on the experimental spectrum (solid line) obtained using NCR (4 μM, based on heme b), 5-diethoxylphosphoryl-5-methyl-1-pyrroline N-oxide (DEPMPO; 20 mM), NADH (1 mM), and Q1 (0.2 mM) in PBS. Experimental spectrum was recorded after signal averaging 4 scans at room temperature. C: same as B, except that SOD (1 U/μl) was added to the mixture before the reaction was initiated by NADH. D: same as B, except that Q1 was replaced with cyt c (25 μM). E: same as B, except that experiment was measured under the complex III turnover conditions in the presence of Q2H2 (1 mM) and cyt c (25 μM). F: experimental spectrum was obtained using complex III (4 μM, based on heme b), DEPMPO (20 mM), Q2H2 (1 mM), and cyt c (25 μM). Electron paramagnetic resonance measurements and computer simulation were performed according to the published procedure in the literature (14).
DISCUSSION
Main findings.
The main findings of this study elucidate biphasic change in the protein level of mitochondria during ischemia and reperfusion, which appears to be correlated to dynamic biosynthesis of PGC-1α and regulated by gene transcription and translational control. Furthermore, we have characterized the complex I of ischemic/postischemic hearts at the levels of Fp subcomplex and NCR supercomplex (Fig. 7).
Ischemic defects of mitochondrial ETC.
This study has demonstrated that a biphasic process occurred in the protein biosynthesis and enzymatic activity of the mitochondrial ETC during the transition from ischemia to reperfusion. The transition was likely due to the physiological alteration in which Po2 in the myocardium was overshooting upon flow restoration (50). We detected a marginal reduction of ETC protein expression in the mitochondria of ischemic heart, which should contribute to the ischemic defect. Ischemic defects in the respiration and ETC activity have been reported in the isolated heart mitochondria (12). It has been suggested that oxidative damage caused by ROS production has been attributed to ischemic defects due to higher ability to generate H2O2 by the mitochondria isolated from ischemic heart, which subsequently leads to the injury of ETC.
Two major molecular mechanisms are proposed to explain ischemia-induced downregulation of mitochondrial ETC protein. The first mechanism is likely caused by a downregulation of the mRNA level in the ischemic heart (∼20–30% downregulation compared with the normal control; Fig. 3). The second mechanism is related to reduction in the transcriptional factor PGC-1α in the ischemic heart (Fig. 5). PGC-1α deficiency in the ischemic hearts was presumably due to protein degradation triggered by low Po2, and then it downregulated ETC biosynthesis, augmenting the ischemic defect and impairment of the ETC activity. The additional mechanism involved was most likely regulated by the myocardial proteasome or increasing autophagy during ischemia. Powell et al. (38) has reported that myocardial 20S-proteosome activity was modestly enhanced during the early phase of global ischemia, which would actively remove ETC proteins during ischemia. Furthermore, in response to the physiological conditions of O2 and ATP depletion, autophagy can be induced to remove redundant ETC proteins during myocardial ischemia (32). Although ischemia-induced autophagy is driven by AMPK activation (32), which seems to contradict PGC-1α downregulation, AMPK activation mediated by ischemia likely sets a tone for the following upregulation of PCG-1α during early phase of reperfusion.
Biosynthesis of ETC during reperfusion.
Noteworthy upregulation (vs. ischemic defect) of mitochondrial ETC biosynthesis and enzymatic activity was detected during reperfusion. Discordance was observed between recovery of ETA and protein levels vs. poor recovery of cardiac contractile function. However, recovery of respiratory control index (or RCR in the Fig. 1A) was measured ∼35%, which is close to recovery of contractility. Therefore, modest injury of complexes I, II, and III during reperfusion should impair the protein-protein interaction of ETC (Fig. 1B), leading to poor recovery of RCR and contractility. The mechanisms contributing to reperfusion-induced ETC biosynthesis are discussed below:
1) We suggest that ETC protein expression is triggered in response to Po2 elevation, and the molecular mechanism that controls ETC biosynthesis likely serves as a sensor of Po2. Upregulation of cytosol PGC-1α and its mRNA level was consistently detected in the postischemic heart (Fig. 5A) and correlated with measures of ETC biosynthesis during reperfusion (Fig. 2). The above mechanism is further supported by the evidence showing that PGC-1 gene was activated during reperfusion via marginal increase of its mRNA level by ∼25.7% (data not shown). Cardiac PGC-1α that functions to coactivate several downstream transcriptional factors thus likely plays the role to coordinate rapid protein translation of ETC during reperfusion. It should be noted that experiments here were performed in crystalloid-perfused hearts, which are exposed to oxygen tension much higher than in vivo. Thus it is conceivable that the crucial role of oxygen tension seen in this study under review may be less important under different conditions. McLeod et al. (33) have previously noted a significant elevation of PGC-1α gene transcription induced in the preconditioned myocardium (vs. ischemia/reperfusion myocardium). In agreement of this result, PGC-1α protein expression from preconditioned heart significantly enhanced after ischemia (Fig. 5), thus supporting the role of PGC-1α in augmented resistance to ischemic injury.
2) Modest suppression of ETC translation and enzymatic activities by cycloheximide (CHX in Fig. 6) has significance since this observation implicated the involvement of translational control. The impact of translational control was detected on both nuclear (51 kDa, 70 kDa, RISP) and mitochondria (the subunit of complex IV)-encoded subunits of ETC (Fig. 6A). The magnitude of cycloheximide effect to inhibit protein biosynthesis was well correlated to the level of ETC recovery upon reperfusion (Fig. 2), but actinomycin D only executed a minor effect. Further evidence was provided by detecting the status of eIF2α phosphorylation showing marked upregulation during ischemia and modest downregulation during perfusion (Fig. 6E). However, a significant change of eIF4E phosphorylation was not observed, which was in agreement with the reports in the literatures (17, 18). It is likely that mitochondrial ribosome was similarly regulated to affect the translation of mitochondria and nuclear encoded subunits in concert.
Interestingly, real-time PCR analyses failed to show an increase of ETC mRNA level during reperfusion when compared with the mRNA level of ischemic heart (Fig. 3). Thus a lack of correlation was identified between ETC mRNA and protein levels. The findings supported that protein biosynthesis can occur within a short experimental duration (within 60 min) via translational control via internal ribosomal entry site (IRES) during reperfusion. Recent studies by Holcik and Sonenberg (24) indicate that some genes containing an IRES in the 5′-untranslated region of mRNA can escape the general translational control and undergo protein translation induced by special physiological conditions (24). These special physiological conditions include oxidant stress and apoptosis (24, 41). Specific protein translation mediated by IRES depends on specific physiological conditions. A well-documented example of IRES-mediated translational control is stress-induced Nrf-2 activation via enhancing internal initiation (29, 41). Therefore, rapid ETC translation may be mediated by a similar mechanism under the conditions of reperfusion-induced oxidant stress.
In addition, the role of myocardial proteosome in the postischemic injury is worth noting. Significant impairment of 20S/26S-proteosomal activities has been detected in the 60 min of postischemic rat heart (38). Bulteau et al. (6) have reported a similar result in an in vivo rat heart model of coronary occlusion/reperfusion. Therefore, diminished activity for protein degradation can facilitate the accumulation of excess ETC proteins and dysfunctional mitochondria in the postischemic heart.
Mitochondrial complex I in the ischemic and postischemic hearts.
Mitochondrial dysfunction following ischemia is mainly associated with impaired ETA activities. More severe impairment of ETA in mitochondria was observed in complex I in which 48% of intact complex I activity was reduced with 24% loss of NFR (Fp) activity after 30 min of ischemia. In addition, immunoblotting analysis indicated a 32% downregulation of the 51-kDa (FMN-binding subunit of complex I) expression in mitochondria (Fig. 2), matching the impairment of NFR activity. Since the injury of Fp represented by NFR activity accounts for 50% of the decline of intact complex I activity, there must be other factors contributing to the reduction of complex I activity during ischemia. These factors may include the loss of FMN (43), tissue acidosis and ATP depletion (42), ROS damage (36), and conformational change from active form to a “de-active′ form (31). It was reported that Fp can be dissociated from intact complex I in vitro under an acidic environment (14). Therefore, ischemic acidosis might facilitate Fp dissociation, which, in turn, hinders electron transfer and impairs complex I activity. Damage to the iron sulfur protein subcomplex (Ip) and/or hydrophobic protein subcomplex (Hp) could be another factor associated with the decline of intact complex I activity during ischemia. In the postischemic heart, 28% of intact complex I activity was decreased with no significant loss of NFR activity (Fig. 1D), suggesting the restoration of the Fp function. The functional recovery of Fp thus may play a role in the superoxide generation during reperfusion since a functional Fp is one of the sources for complex I-mediated O2∙−. In vitro S-glutathionylation of complex I has been reported to marginally enhance its ETA (8). Thereby, elevation in posttranslational modification with S-glutathionylation of complex I in the postischemic heart may also contribute to the functional recovery of mitochondria during reperfusion (9). No significant change compared with the control heart in the protein expression of complex I (probing of 51-kDa, 75-kDa, and ND1 subunits) was detected after 60 min of reperfusion (Fig. 2). These results indicated the occurrence of bioenergetic recovery due to reperfusion as seen in the parameters of hemodynamics and RCR (Fig. 1B). Furthermore, this physiological recovery was also supported by full or partial restoration of ETAs and protein expression in the complexes II, III, and IV (Fig. 2).
Mitochondrial complex III in the ischemic and postischemic hearts.
Thirty-nine and nineteen percent of complex III activity was decreased in the ischemic and postischemic hearts, respectively. However, a 26% downregulation of RISP expression was detected in the mitochondria of ischemic heart, indicating involvement of other factors in the ischemic defect of complex III. Ischemic damage to complex III, including to the iron-sulfur protein, has been previously reported (28). There is no alteration in the amount of RISP present in the mitochondria of postischemic heart, implicating oxidative injury of complex III due to reperfusion. Lipid peroxidation of cardiolipin in vivo may contribute to the above complex III defect (37).
Oxygen free radical production in the postischemic heart.
It was observed that reperfusion induced a marginal ETC biosynthesis that failed to trigger complete recovery of mitochondrial integrity based on analysis of the RCR (Fig. 1B) and transmission electron microscopy analysis of ultrastructure (Fig. 4). The detected mitochondrial defect was due to overproduction of oxygen free radicals during reperfusion, leading to oxidative damage of membrane integrity and impairment of ETC (complex I and complex III in this study). In support of the hypothesis of oxygen free radical-induced damage, preservation of cardiac function through SOD (52) or glutathione peroxidase mimetics (4, 25) during reperfusion has been established and well documented.
It should be acknowledged that most damage to the ETC occurs during ischemia, rather than reperfusion as previously reported by Hoppel and colleagues (10, 11, 27), the concept of which is substantiated in the present study. Despite increasing ETC activities caused by marginal ETC biosynthesis during reperfusion, relatively dramatic impairment in mitochondrial integrated respiration (state 3 O2 consumption in the Fig. 1A) persists. The present study substantiates the previous findings (12) in which dominant damage to ETC during ischemia sets the stage for an increase in ROS as a mechanism of persistent reperfusion injury. Equally viable based on the data in the current study is persistence of previous ischemic injury.
Ischemia/reperfusion-induced impairments of complex I and complex III in the isolated mitochondria were significant in this study (Figs. 1C and 7A). It has been documented that complexes I and III are the major sites for mitochondrial O2∙− production (45, 46). In vitro analysis of NCR supercomplex with EPR spin-trapping indicated that complex I has much higher catalytic activity to generate O2∙− (Fig. 7, B vs. E). No detectable O2∙− adduct of DEPMPO can be mediated by the intact NCR or its complex III component. However, O2∙− generation mediated by complex III was greatly enhanced once complex III was detached from the supercomplex (Figs. 7, E vs. F), presumably due to lack of protein-protein interaction between complex I and complex III. With the damage to mitochondrial integrity under high Po2, oxygen consumption by the ETC was not effectively coupled with oxidative phosphorylation during reperfusion. This resulted in electron leakage and subsequent O2∙− production by the ETC. Complexes I likely contributed to major oxygen free radical production in the early phase of reperfusion. However, the injury of complex I during ischemia/reperfusion can impair its protein-protein interaction with complex III, thus potentially enhancing complex III-mediated O2∙− production and augmenting overall oxidant stress in mitochondria during ischemia/reperfusion.
Conclusion.
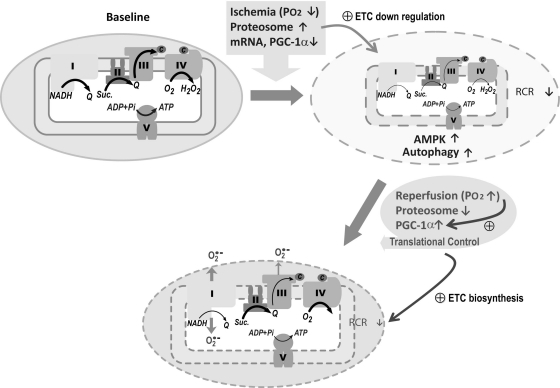
The proposed diagram in Fig. 8 illustrates a biphasic modulation of ETC activity in mitochondria during myocardial ischemia/reperfusion. Physiological conditions of hypoxia during ischemia trigger an upregulation of the proteolytic degradation, downregulating mRNA level in myocardium and PGC-1α expression, and subsequently decreasing the amount of ETC in the mitochondria, which results in impairment of mitochondrial integrity (due to decreasing oxidative phosphorylation and RCR). Ischemia further facilitates autophagy that is driven by AMPK activation (32). Overshooting Po2 upon reperfusion renders the cardiomyocytes under the conditions of hyperoxygenation, which triggers progressive upregulation of PGC-1α. Together with proteosome activity downregulated and translational control, increasing PGC-1α expression progressively enhances ETC biosynthesis in mitochondria during reperfusion. Failure to completely restore the mitochondrial integrity during reperfusion results in overproduction of O2∙− under the conditions of hyperoxygenation and low oxidative phosphorylation. This augments the oxidant stress in mitochondria and the consequent impairment of ETC activity. Specifically, reperfusion-induced injuries to complex I and complex III are detected in this study. The biological significance of this study was to elucidate the unique and dynamic properties of the mitochondrial ETC in response to the physiological changes of myocardial ischemia/reperfusion. Defining this molecular mechanism advances our understanding of the disease process of postischemic infarction.
Fig. 8.
Schematic delineation of biphasic modulation involved in Po2 proteosome activity, PGC-1α regulation, translational control, ETC biosynthesis, ETA, O2∙− production, membrane integrity, and oxidative phosphorylation in mitochondria during myocardial ischemia and reperfusion. Under normal physiological conditions (baseline), electron transfer was catalyzed by ETC and efficiently coupled with ATP synthesis (as denoted by bold arrow). Physiological conditions of ischemia trigger proteolytic degradation of ETC, which subsequently diminished its ETA (small and fine arrow) and impaired membrane integrity (dashed enclosed line). Ischemia also triggers autophagy driven by AMPK activation, which conditions favor subsequent PGC-1α activation. Reperfusion introduces the physiological conditions of hyperoxygenation. Together with downregulation of proteosome activity, upregulation of PGC-1α, and translational control, these alterations facilitate increasing ETC biosynthesis, enhancing O2∙− generation (bold arrow) due to impairment of oxidative phosphorylation (fine arrow) and augmenting oxidative stress. V, complex V or F1, F0ATPase.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-83237 (to Y.-R Chen) and HL-63744 (to J. L. Zweier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.-L.L., C.-L.C., and S.T.Y. performed experiments; H.-L.L., C.-L.C., and S.T.Y. analyzed data; H.-L.L. prepared figures; H.-L.L. drafted manuscript; J.L.Z. and Y.-R.C. interpreted results of experiments; J.L.Z. and Y.-R.C. edited and revised manuscript; Y.-R.C. conception and design of research; Y.-R.C. approved final version of manuscript.
REFERENCES
- 1. Ambrosio G, Weisfeldt M, Jacobus W, Flaherty J. Evidence for a reversible oxygen radical-mediated component of reperfusion injury: reduction by recombinant human superoxide dismutase administered at the time of reflow. Circulation 75: 282–291, 1987 [DOI] [PubMed] [Google Scholar]
- 2. Ambrosio G, Zweier J, Flaherty J. The relationship between oxygen radical generation and impairment of myocardial energy metabolism following post-ischemic reperfusion. J Mol Cell Cardiol 23: 1359–1374, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268: 18532–18541, 1993 [PubMed] [Google Scholar]
- 4. Baljinnyam E, Hasebe N, Morihira M, Sumitomo K, Matsusaka T, Fujino T, Fukuzawa J, Ushikubi F, Kikuchi K. Oral pretreatment with ebselen enhances heat shock protein 72 expression and reduces myocardial infarct size. Hypertens Res 29: 905–913, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Barrientos A. In vivo and in organello assessment of OXPHOS activities. Methods 26: 307–316, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem 276: 30057–30063, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chen CL, Chen J, Rawale S, Varadharaj S, Kaumaya PP, Zweier JL, Chen YR. Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J Biol Chem 283: 27991–28003, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, Chen YR. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry 46: 5754–5765, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Chen C, Rawale S, Chen C, Zweier J, Kaumaya P, Chen Y. Peptide-based antibodies against glutathione-binding domains suppress superoxide production mediated by mitochondrial complex I. J Biol Chem 285: 3168–3180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Q, Camara A, Stowe D, Hoppel C, Lesnefsky E. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol 292: C137–C147, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Chen Q, Hoppel C, Lesnefsky E. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther 316: 200–207, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294: C460–C466, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Chen YR, Chen CL, Pfeiffer DR, Zweier JL. Mitochondrial complex II in post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem 282: 32640–32654, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Chen YR, Chen CL, Zhang L, Green-Church KB, Zweier JL. Superoxide generation from mitochondrial NADH dehydrogenase induces self-inactivation with specific protein radical formation. J Biol Chem 280: 37339–37348, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Chen YR, Deterding LJ, Tomer KB, Mason RP. Nature of the inhibition of horseradish peroxidase and mitochondrial cytochrome c oxidase by cyanyl radical. Biochemistry 39: 4415–4422, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Crescenzo R, Lionetti L, Mollica MP, Ferraro M, D'Andrea E, Mainieri D, Dulloo AG, Liverini G, Iossa S. Altered skeletal muscle subsarcolemmal mitochondrial compartment during catch-up fat after caloric restriction. Diabetes 55: 2286–2293, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Crozier S, Vary T, Kimball S, Jefferson L. Cellular energy status modulates translational control mechanisms in ischemic-reperfused rat hearts. Am J Physiol Heart Circ Physiol 289: H1242–H1250, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Crozier S, Zhang X, Wang J, Cheung J, Kimball S, Jefferson L. Activation of signaling pathways and regulatory mechanisms of mRNA translation following myocardial ischemia-reperfusion. J Appl Physiol 101: 576–582, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doliba NM, Doliba NM, Chang Q, Babsky AM, Wroblewski K, Natelson BH, Osbakken MD. Mitochondrial oxidative phosphorylation in heart from stressed cardiomyopathic hamsters. J Mol Cell Cardiol 31: 543–553, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Ferrari R. The role of mitochondria in ischemic heart disease. J Cardiovasc Pharmacol 28: S1–10, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Ferrari R, Ceconi C, Curello S, Cargnoni A, Pasini E, De Giuli F, Albertini A. Role of oxygen free radicals in ischemic and reperfused myocardium. Am J Clin Nutr 53: 215S–222S, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Galante YM, Hatefi Y. Purification and molecular and enzymic properties of mitochondrial NADH dehydrogenase. Arch Biochem Biophys 192: 559–568, 1979 [DOI] [PubMed] [Google Scholar]
- 23. Gorge G, Chatelain P, Schaper J, Lerch R. Effect of increasing degrees of ischemic injury on myocardial oxidative metabolism early after reperfusion in isolated rat hearts. Circ Res 68: 1681–1692, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6: 318–327, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Hoshida S, Kuzuya T, Nishida M, Yamashita N, Hori M, Kamada T, Tada M. Ebselen protects against ischemia-reperfusion injury in a canine model of myocardial infarction. Am J Physiol Heart Circ Physiol 267: H2342–H2347, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Laguens R. Morphometric study of myocardial mitochondria in the rat. J Cell Biol 48: 673–676, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lesnefsky E, Chen Q, Slabe T, Stoll M, Minkler P, Hassan M, Tandler B, Hoppel C. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol 287: H258–H267, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Lesnefsky E, Gudz T, Migita C, Ikeda-Saito M, Hassan M, Turkaly P, Hoppel C. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys 385: 117–128, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Li W, Thakor N, Xu E, Huang Y, Chen C, Yu R, Holcik M, Kong A. An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res 38: 778–788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu B, Tewari AK, Zhang L, Green-Church KB, Zweier JL, Chen YR, He G. Proteomic analysis of protein tyrosine nitration after ischemia reperfusion injury: Mitochondria as the major target. Biochim Biophys Acta 1794: 476–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maklashina E, Sher Y, Zhou HZ, Gray MO, Karliner JS, Cecchini G. Effect of anoxia/reperfusion on the reversible active/de-active transition of NADH-ubiquinone oxidoreductase (complex I) in rat heart. Biochim Biophys Acta 1556: 6–12, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Matsui Y, Kyoi S, Takagi H, Hsu C, Hariharan N, Ago T, Vatner S, Sadoshima J. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy 4: 409–415, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLeod CJ, Jeyabalan AP, Minners JO, Clevenger R, Hoyt RF, Jr, Sack MN. Delayed ischemic preconditioning activates nuclear-encoded electron-transfer-chain gene expression in parallel with enhanced postanoxic mitochondrial respiratory recovery. Circulation 110: 534–539, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res 66: 913–931, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Neubauer S, Hamman BL, Perry SB, Bittl JA, Ingwall JS. Velocity of the creatine kinase reaction decreases in postischemic myocardium: a 31P-NMR magnetization transfer study of the isolated ferret heart. Circ Res 63: 1–15, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Federici A, Ruggiero FM. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ Res 94: 53–59, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Petrosillo G, Ruggiero FM, Di Venosa N, Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J 17: 714–716, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Powell SR, Wang P, Katzeff H, Shringarpure R, Teoh C, Khaliulin I, Das DK, Davies KJ, Schwalb H. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: essential role of the proteasome. Antioxid Redox Signal 7: 538–546, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem 281: 2061–2070, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Purdom-Dickinson SE, Sheveleva EV, Sun H, Chen QM. Translational control of nrf2 protein in activation of antioxidant response by oxidants. Mol Pharmacol 72: 1074–1081, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Rouslin W. Effects of acidosis and ATP depletion on cardiac muscle electron transfer complex I. J Mol Cell Cardiol 23: 1127–1135, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Rouslin W, Ranganathan S. Impaired function of mitochondrial electron transfer complex I in canine myocardial ischemia: loss of flavin mononucleotide. J Mol Cell Cardiol 15: 537–542, 1983 [DOI] [PubMed] [Google Scholar]
- 44. Sack MN. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc Res 72: 210–219, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237: 408–414, 1985 [DOI] [PubMed] [Google Scholar]
- 46. Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J 191: 421–427, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vercesi A, Reynafarje B, Lehninger AL. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem 253: 6379–6385, 1978 [PubMed] [Google Scholar]
- 48. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Zhang L, Chen C, Kang P, Garg V, Hu K, Green-Church K, Chen Y. Peroxynitrite-mediated oxidative modifications of complex II: relevance in myocardial infarction. Biochemistry 49: 2529–2539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL. Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation 111: 2966–2972, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Zuo L, Chen Y, Reyes L, Lee H, Chen C, Villamena F, Zweier J. The radical trap 5,5-dimethyl-1-pyrroline N-oxide exerts dose-dependent protection against myocardial ischemia-reperfusion injury through preservation of mitochondrial electron transport. J Pharmacol Exp Ther 329: 515–523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zweier JL, Rayburn BK, Flaherty JT, Weisfeldt ML. Recombinant superoxide dismutase reduces oxygen free radical concentrations in reperfused myocardium. J Clin Invest 80: 1728–1734, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]