Abstract
Calmodulin-dependent protein kinase II (CaMKII) has been proposed to be a therapeutic target for heart failure (HF). However, the cardiac effect of chronic CaMKII inhibition in HF has not been well understood. We have tested alterations of Ca2+ handling, excitation-contraction coupling, and in vivo β-adrenergic regulation in pressure-overload HF mice with CaMKIIδ knockout (KO). HF was produced in wild-type (WT) and KO mice 1 wk after severe thoracic aortic banding (sTAB) with a continuous left ventricle (LV) dilation and reduction of ejection fraction for up to 3 wk postbanding. Cardiac hypertrophy was similar between WT HF and KO HF mice. However, KO HF mice manifested exacerbation of diastolic function and reduction in cardiac reserve to β-adrenergic stimulation. Compared with WT HF, L-type calcium channel current (ICa) density in KO HF LV was decreased without changes in ICa activation and inactivation kinetics, whereas ICa recovery from inactivation was accelerated and Ca2+-dependent ICa facilitation, a positive staircase blunted in WT HF, was recovered. However, ICa response to isoproterenol was reduced. KO HF myocytes manifested dramatic decrease in sarcoplasmic reticulum (SR) Ca2+ leak and slowed cytostolic Ca2+ concentration decline. Sarcomere shortening was increased, but relaxation was slowed. In addition, an increase in myofilament sensitivity to Ca2+ and the slow skeletal muscle troponin I-to-cardiac troponin I ratio and interstitial fibrosis and a decrease in Na/Ca exchange function and myocyte apoptosis were observed in KO HF LV. CaMKIIδ KO cannot suppress severe pressure-overload-induced HF. Although cellular contractility is improved, it reduces in vivo cardiac reserve to β-adrenergic regulation and deteriorates diastolic function. Our findings challenge the strategy of CaMKII inhibition in HF.
Keywords: calmodulin-dependent protein kinase II, calcium channel, myocytes, excitation-contraction coupling, calcium handling, diastolic function
recent studies have demonstrated (18) that β1-adrenergic stimulation activates dual signaling pathways, cAMP/PKA and calmodulin-dependent protein kinase II (CaMKII), the former undergoing desensitization and the latter exhibiting sensitization with time. These findings suggest that the CaMKII signaling pathway may be important for the long-term changes of Ca2+ handling in heart failure (HF) where an increased sympathetic tone persists (17). Indeed, we found that in pressure-overload HF mouse left ventricle (LV), L-type calcium channel (LTCC) current (ICa) was altered with an increased density, slowed current decay and blunted Ca2+-dependent ICa facilitation (CDF) and that these changes are caused by the increased CaMKII activity (21). Although the increased ICa density and slowed inactivation may be compensatory to the reduced contractility in failing heart, the blunted CDF may reduce contraction reserve at fast heart rates (e.g., exercise).
In hearts, CaMKIIδ is the predominant CaMKII isoform, while CaMKIIγ is expressed at relatively low levels (24). Upregulation of CaMKII activity seems to be a general feature in ventricular myocytes of HF from diverse causes in patients and in animal models (27). CaMKII overexpression induces cardiac hypertrophy and failure as well as lethal ventricular arrhythmias ,whereas inhibition of CaMKII protects against cardiac remodeling and inhibits ventricular arrhythmias. Thus CaMKII has been proposed to be a therapeutic target for HF (3).
Although excessive CaMKII activation is linked to cardiomyopathy and arrhythmias, the cardiac effect of chronic CaMKII inhibition in HF has not been well understood. Pharmacological CaMKII inhibition in vivo requires cardiac-specific CaMKII inhibitors due to the ubiquitous distribution of CaMKII, which are, however, currently unavailable. To test the effect of chronic cardiac CaMKII inhibition, a cardiac predominant CaMKII isoform-δ KO mouse model was generated. The goal of this study is to decipher the alterations of Ca2+ handling and excitation-contraction (E-C) coupling in ventricular myocytes and the in vivo cardiac function and reserve to β-adrenergic regulation in the CaMKIIδ KO HF mouse model.
METHODS
Severe thoracic aortic banding.
Increased pressure in the proximal aorta was induced by severe thoracic aortic banding (sTAB) according to protocols approved by the Emory University Institutional Animal Care and Use Committee. Male WT and CaMKIIδ KO mice at 6 wk of age were anesthetized with ketamine (100 mg/kg ip) plus xylazine (5 mg/kg ip). A 3-mm left-sided thoracotomy was created at the second intercostal space. The transverse aortic arch was ligated (7–0 Prolene) between the innominate and left common carotid arteries with an overlying 28-gauge needle, and the needle was then removed, leaving a discrete region of stenosis. The chest was closed, and the left-sided pneumothorax was evacuated. This technique was reported in our recent publications (19–21).
Echocardiography in conscious mice.
Echocardiography was performed in conscious mice using a high-resolution murine echo machine (Visualsonics RMV707B with a 30-MHz transducer). A short-axis two-dimensional image-guided M-mode view of the LV was acquired at a sound speed of 1,540 m/s and stored digitally. The following parameters were measured digitally from the M-mode tracings, including LV dimensions at diastole and systole (LVDd, LVDs), anterior and posterior wall thickness at diastole and systole, and heart rate. Based on these measurements, left ventricular volume systole was calculated as [7.0/(2.4 + LVDs)] * LVDs3; left ventricular volume diastole was calculated as [7.0/(2.4 + LVDd)] * LVDd3; fractional shortening was calculated as [(LVDd − LVDs)/LVDd] × 100; ejection fraction (EF) was calculated as [(LV Vold − LV VolDs)/LV Vold] × 100%. All measurements were made as the average from five adjacent cardiac cycles. The observer who conducted the measurement was blind to the genotypes.
Isolation of ventricular myocytes.
Mouse LV myocytes were isolated enzymatically by a protocol described previously (21) with modifications. In brief, after retrograde perfusion with Krebs-Ringer solution at 2 ml/min (5 min), and the heart was then perfused with a fresh solution containing 0.8 mg/ml collagenase (Worthington type II) for ∼15 min. Next, the apex was removed and the LV wall was cut and put into a culture dish filled with “KB” solution. A small 90° curved forceps was used to carefully dissect a very thin layer of endomyocardium. The mid-region myocardium was dissected and discarded, leaving the remainder as epimyocardium. The endomyocardium and epimyocardium were minced into small pieces in KB solution, triturated, and studied within 4–6 h. All steps were performed at 36°C in solutions gassed with 95%O2-5%CO2. Only calcium-tolerant, quiescent, and rod-shaped cells showing clear cross striations were used for ICa recording.
ICa recording.
Isolated myocytes were placed in a continuously superfused (1.5 ml/min) recording chamber (volume of 1 ml) fixed to an inverted microscope. For ICa recording, we used the whole cell voltage-clamp technique with pipette resistances of 2–3 MΩ when filled with internal solution. The junctional potential was corrected by zeroing the potential just before pipette tip touched the cell membrane. After the cell membrane was broken by application of additional suction, cell capacitance and series resistance were electrically compensated. After access was gained in the whole cell voltage-clamp configuration, myocytes were allowed to equilibrate for 5 min with the internal solution before data were collected. The junction potential in this recording condition is 8.6 mV and not corrected during recording. ICa was recorded at room temperature. Cells were depolarized every 10 s from a holding potential of −50 mV to the test potentials from −40 to +60 mV (in 10-mV steps) for 300 ms. To determine the voltage dependence of ICa inactivation, we used a two-pulse protocol with a 2-s conditioning pulse ranging from −70 to +30 mV (from a holding potential of −50 mV), followed by a 300-ms test pulse to +10 mV. For the voltage dependence of ICa activation, we used a holding potential of −50 mV and steps of 300-ms duration test pulses from −40 to +60 mV in 10-mV steps. To evaluate the kinetics of ICa recovery from inactivation, we held cells at a holding potential of −50 mV and applied a 300-ms pulse to +10 mV, followed by a variable time period (ΔT) at −50 mV and a 300-ms test pulse to +10 mV. The time course of ICa recovery from inactivation was evaluated by fitting data for each cell with a monoexponential equation.
ICa run-down, when present, typically occurs within the first 5 min of recording. To minimize the ICa run-down, we added 5 mmol/l Mg-ATP to the pipette solution and commenced data acquisition after 5–10 min of equilibration between pipette solution and intracellular contents (22). Cells showing continuous current run-down (≈5%) were excluded from the analysis.
Na+/Ca2+ exchanger current recording.
The holding potential was set to −40 mV. The Na+/Ca2+ exchanger (INCX) current was elicited by 2-s descending voltage ramps applied from +80 to −120 mV with a 10-s interval. The NCX current density was defined as Ni2+-sensitive current (the difference of the currents before and after perfusion with 5 mM Ni2+). All experiments were conducted at ≈37°.
Solutions.
The Krebs-Ringer solution for cell isolation contained the following (in mmol/l): 35 NaCl, 4.75 KCl, 1.19 KH2PO4, 16 Na2HPO4, 134 sucrose, 25 NaCO3, 10 glucose, and 10 HEPES, pH 7.4 with NaOH. The KB solution for storage of cells contained the following (in mmol/l): 10 taurine, 70 glutamic acid, 25 KCl, 10 KH2PO4, 22 glucose, and 0.5 EGTA, pH adjusted to 7.2 with KOH. Test solution for ICa recording contained the following (in mmol/l): 135 TEA, 0.53 MgCl2, 1.8 CaCl2, 20 CsCl, and 5 HEPES, pH 7.4 with CsOH. Isoproterenol (ISO) was added in the test solution at different concentrations for the experiments where indicated. Pipette solution for ICa recording contained the following (in mmol/l): 110 CsOH, 90 aspartic acid, 20 CsCl, 10 tetraethylammonium Cl (TEA-Cl), 10 HEPES, 10 EGTA , 5 Mg-ATP, 5 Na2 creatine phosphate, 0.4 GTP (Tris), and 0.1 leupeptin, pH 7.2 with CsOH. The external solution for INCX recording contained the following (in mmol/l): 140 NaCl, 5 CsCl, 1 CaCl2, 1 MgCl2, 10 HEPES 10, and 5 glucose, pH 7.35 (NaOH). The internal solution for INCX recording contained the following (in mmol/l): 115 CsCl, 10 NaCl, 5 MgATP, 1 CaCl2, 20 TEA-Cl, 10 HEPES, and 10 EGTA, pH 7.25 (CsOH). Nifedipine (10 μM) and ouabain (50 μM) were added to the external solution to block the LTCC and Na+/K+-ATPase, respectively.
Western blot analysis.
To analyze the protein abundance, isolated ventricular myocytes were homogenized in 1% TX-100 buffer [50 mM Tris·HCl, pH 7.4, 4% glycerol, 1 mM DTT, 1% Triton X-100, 1 mM EDTA, Mini-Complete protease inhibitor cocktail (Roche), and phosphatase inhibitor cocktails I and II (Sigma-Aldrich)] and immunoblotted for target proteins using specific antibodies.
Primary antibodies used in this study include rabbit polyclonal anti-PKA antibody (Millipore); rabbit polyclonal anti-CaMKII antibody (M-176); polyclonal anti-phospho-CaMKII (Thr287) antibody (Cell Signaling Solutions, Lake Placid, NY); goat polyclonal anti-Cav1.2 antibody (sc-16230), rabbit polyclonal anti-β2-antibody (sc-569), rabbit polyclonal anti-β1 antibody (sc-568), mouse monoclonal anti-Bcl-2 antibody, and goat polyclonal anti–phospholamban (PLB) antibody (all from Santa Cruz Biotechnology); rabbit polyclonal anti-P-Ser16PLB antibodies (Badrilla, Leeds, UK); rabbit polyclonal anti-P-Thr17PLB antibodies (Badrilla, Leeds, UK); rabbit polyclonal anti-Giα3 antibody (371729; Calbiochem); rabbit polyclonal anti-NCX antibody (Swant); rabbit polyclonal anti-SERCA2a antibody (Badrilla); rabbit polyclonal anti-p-ERK1/2 antibody (Cell Signaling); and mouse monoclonal anti-β-actin antibody (Sigma). Secondary antibodies used for Western blot were donkey anti-goat IgG-horseradish peroxidase (HRP), goat anti-rabbit IgG-HRP, and goat anti-mouse IgG-HRP (all from Santa Cruz Biotechnology).
Cytosolic Ca2+ transient and sarcomere shortening measurements.
Myocytes were loaded with 2 μM fura-2 AM for 10 min, and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (IonOptix). After loading, cells were washed and resuspended in Tyrode solution and then placed in the cell chamber, stimulated at 1 and 3 Hz with a 4-ms duration and superfused at 37°C and imaged through a Fluor ×40 oil objective. Myocytes were then exposed to light emitted by a 75W Xenon lamp and passed though either a 340- or 380-nm wavelength filter. The emitted fluorescence is detected at 510 nm. The background fluorescence for each myocyte was determined by moving the myocyte out of the view and recording the fluorescence from the solution alone. Cell shortening was measured in myocytes that were loaded with fura-2 AM. This concentration of fura-2 AM did not affect contractility (compared with unloaded cells, data not shown). The Soft-edge software (IonOptix) was used to capture and analyze the changes in sarcomere length during cell shortening and relengthening.
Sarcoplasmic reticulum Ca2+ leak measurement.
Resting cytosolic Ca2+ concentration ([Ca2+]i) and sarcoplasmic reticulum (SR) Ca2+ ([Ca2+]SRT) were measured in isolated myocytes loaded 40 min with 2 μM fura-2 AM. Cells were stimulated 30 times at 1 Hz in normal Tyrode (NT; including 2 mmol/l Ca2+) to bring the [Ca]SRT to steady state. Na/Ca-exchange was then blocked in 0 Na+/0 Ca2+ NT so that little or no Ca2+ enters or leaves the cell following SR loading. In all conditions, [Ca2+]i was monitored while 0/0 NT solution with tetracaine (1 mM) was perfused for 30 s, followed by a rapid switch to 0/0 NT without tetracaine for 30 s. Tetracaine effectively blocks the ryanodine receptor, and the SR Ca2+-ATPase (SERCA) continues to function, pumping Ca2+ into SR from cytosol. The solution was then rapidly switched to 10 mmol/l caffeine NT solution for measurement of SR contents [Ca2+]SRT. The difference in [Ca2+]SRT (represented by the change in baseline) is indicative of SR Ca2+ leak.
Assessment of contributions of SERCA and NCX to diastolic Ca2+ removal.
Myocytes were perfused with Tyrode solution and stimulated at 1 Hz. Rapid application of 20 mM caffeine was employed to induce SR Ca2+ release and assess the contribution of NCX and slow transport systems (mitochondrial Ca2+ uniporter and sarcolemmmal Ca2+-ATPase). Decline of fluorescence (F340/380) during caffeine-induced Ca2+ transient was attributable to NCX and slow mechanisms. Because slow mechanisms remove only 0.5% of Ca2+ in mouse, the contribution of these mechanisms is negligible (13). The decline of fluorescence during stimulated twitches was attributable to both SERCA and NCX. SERCA contribution was therefore evaluated as the difference between decline of fluorescence during caffeine-induced Ca2+ transient and decline of fluorescence during electrical stimulated twitches.
Assessment of myofilament sensitivity to Ca2+.
Myocytes were perfused with Tyrode solution and stimulated with 1 Hz at 37°C. Myofilament sensitivity to Ca2+ was assessed from phase-plane diagrams of cell length vs. fura-2 ratio by measuring the gradient of the cell length-fura-2 trajectory during contraction and late relaxation. The position and shape of trajectory reflect myofilament response to the relative Ca2+ and, hence, can be used as a measurement of myofilament sensitivity to Ca2+ (9).
Terminal deoxynucleotide transferase-mediated dUTP nick-end labeling assay.
The hearts were first perfused with PBS and 10% formalin by injection through the right ventricle to wash out blood cells and then fixed in 10% formalin (for 24 h), embedded, and sectioned into serial 10-μm-thick tissue sections. The serial tissue sections from the LV were used for apoptosis measurement by using terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) assay. TUNEL assays were performed using in situ cell death detection kit (Roche), according to the manufacturer's instruction.
Statistical analysis.
Data are reported as means ± SE and analyzed by t-test or one-way ANOVA (using SigmaStat for Windows V3.5). Mann-Whitney rank sum tests were performed if tests for normality or equal variance failed. P < 0.05 is regarded as significant.
Use of vertebrate animals.
All approaches on live vertebrates were performed in accordance with protocols approved by Emory University Institutional Animal Care and Use Committee.
Statement of responsibility.
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Generation of pressure-overload HF in CaMKIIδ KO mice.
LV pressure overload was induced by sTAB, causing a change in blood flow velocities across the restriction site of 67.52 ± 3.99% for WT (n = 12) and 68.08 ± 3.97 for KO mice (n = 13; P > 0.05), indicating a similar dynamic change in these two genotypes of mice. HF was developed in WT and CaMKIIδ KO mice 1 wk after sTAB. All HF mice manifested clinical features of HF syndrome, including lethargy, impaired mobility, and edema. Hearts dissected from these mice were markedly enlarged (increased heart weight-to-body weight ratio) and dilated (increased LV end-diastolic volume) with decreased EF. Evidence of pulmonary venous congestion, a hallmark of HF, was noted with increased lung weight-to-body weight ratios (Table 1). No difference in the heart weight-to-body weight ratio, lung weight-to-body weight ratio, EF, and LV end-diastolic volume was observed between WT HF and KO HF mice at the first week after banding. However, compared with the WT HF mice, at post-sTAB week 3, the KO HF mice manifested a larger lung weight-to-body weight ratio with a smaller LV end-diastolic volume although both parameters were increased compared with the post-sTAB week 1 for each genotypes. The heart weight-to-body weight ratio was doubled at the first week after banding and maintained unchanged from post-sTAB week 1 to week 3, and there was no difference between WT HF and KO HF mice. These data suggest that CaMKIIδ KO did not blunt the process of hypertrophy and HF development but may impair diastolic function, which is manifested by the increase in lung edema and LV stiffness. Indeed, at post-sTAB week 3, the early diastolic transmitral inflow velocity-to-mitral annular flow velocity ratio (E/E' ratio) was significantly increased in KO HF (58.7 ± 6.3; n = 9) compared with WT HF (43.1 ± 1.1; n = 8; P < 0.05). An increase in the E/E' ratio suggests a high gradient to a low shift in volume, indicating increased LV filling pressure and wall stiffness (25). However, we did not observe difference in the survival rate between WT HF and KO HF mice for up to 6 wk after sTAB (≈50%). The significant reduction of LV contractility produced by sTAB in WT and CaMKIIδ KO mice is shown in Fig. 1, A and B. Although HF significantly increased CaMKII activity in WT and CaMKIIδ KO mouse LV, CaMKII activity (measured by the level of autophosphorylated CaMKII and by CaMKII activity assay kit; Ref. 24) in KO HF LV was ∼21% of the CaMKII activity in WT HF LV, even lower than that in the sham-operated WT mouse LV (i.e., lower than a physiological level; Fig. 1, C–E).
Table 1.
Morphometric and functional parameters for sham-operated and HF mice
| WT HF |
KO HF |
WT HF |
KO HF |
|||
|---|---|---|---|---|---|---|
| WT Sham | KO Sham | Post-sTAB week 1 | Post-sTAB week 1 | Post-sTAB week 3 | Post-sTAB week 3 | |
| HW/BW, mg/g | 4.6 ± 0.1 | 4.8 ± 0.1 | 9.96 ± 0.61* | 10.03 ± 0.95* | 10.5 ± 0.3* | 11.4 ± 0.6* |
| LW/BW, mg/g | 5.5 ± 0.2 | 6.0 ± 0.1 | 10.29 ± 0.87* | 11.51 ± 1.23* | 11.3 ± 0.7* | 14.4 ± 0.6*†‡ |
| LVDV, μl | 39.6 ± 0.8 | 36.2 ± 1.1 | 54.23 ± 7.48* | 54.62 ± 11.45* | 92.2 ± 2.8*‡ | 85.1 ± 5.6*†‡ |
| EF, % | 83.3 ± 0.7 | 88.2 ± 0.7 | 47.76 ± 4.62* | 47.04 ± 2.11* | 39.7 ± 2.7*‡ | 39.3 ± 2.1*‡ |
| Mice number | 40 | 38 | 15 | 12 | 32 | 24 |
Values are means ± SE.
WT, wild type; HF, heart failure; CaMKIIδ, calmodulin-dependent protein kinase IIδ knockout (KO); HW, heart weight; BW, body weight; LW, lung weight; LVDV, left ventricular diastolic volume; EF, ejection fraction.
P < 0.05, compared with sham.
P < 0.05, compared with WT HF;
P < 0.05, compared with postsevere thoracic aortic banding (sTAB) week 1.
Fig. 1.
Calmodulin-dependent protein kinase IIδ (CaMKIIδ) knockout (KO) heart failure (HF) mouse model. A: representative images of short-axis 2-dimensional M-mode echocardiography recorded from conscious wild-type (WT), CaMKIIδ KO, WT HF, and CaMKIIδ KO HF mice. B: mean values of left ventricular (LV) ejection fraction (EF) and fractional shortening (FS). C and D: HF-related increase in CaMKII autophosphorylation in WT and CaMKIIδ KO mouse LV. Discontinuous bands indicate separate experiments. E: mean values of CaMKII activity measured by CaMKII activity assay kit (Promega). Vertical bars represent SE.
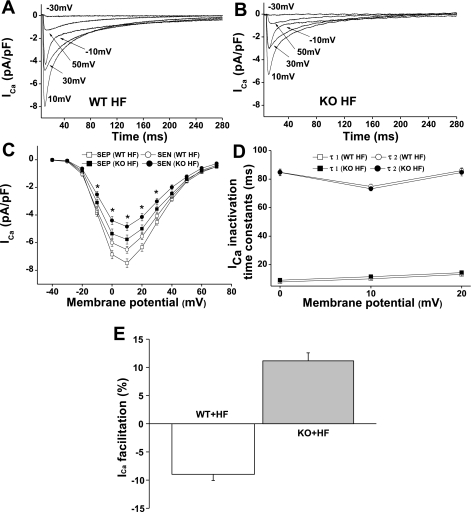
Altered ICa density in KO HF LV.
We (21) have recently reported a transmural gradient of ICa in the LV wall of sham-operated and pressure-overload HF mice. Here, we isolated myocytes from the subendocardial (SEN) and subepicardial (SEP) regions of KO HF mouse LV and recorded ICa in these myocytes. We found that ICa density was significantly larger in SEP than in SEN myocytes without differences in current decay kinetics or voltage-dependence of ICa activation between these two regions of LV (Fig. 2, A–D), suggesting that CaMKIIδ KO did not affect the spatial heterogeneity of ICa distribution in HF (Fig. 2C and Table 2). However, ICa density was significantly smaller in KO HF LV than in WT HF LV. For instance, the peak ICa in SEP and SEN myocytes from KO HF LV was 23 and 26% smaller than the ICa recorded in these 2 regions of myocytes from WT HF LV, respectively. Interestingly, ICa density in KO HF LV was similar to that recorded from WT sham LV but smaller than the ICa recorded from KO sham LV (24). In other words, HF increases ICa density in WT but decreases ICa density in KO. This is consistent with our recent report (21) that ICa remodeling in pressure-overload HF is a result of increased CaMKII-dependent LTCC phosphorylation. CaMKIIδ KO prevents HF-related ICa potentiation. In the setting of HF, the β-adrenergic receptor (AR)/PKA pathway is downregulated and the CaMKII activity is increased and becomes the main regulator of ICa (21). In KO HF, both PKA and CaMKII pathways are reduced, causing a significant ICa reduction.
Fig. 2.
L-type calcium channel current (ICa) recorded in WT HF and KO HF LV myocytes. A and B: representative ICa traces recorded from WT HF and KO HF ventricular myocytes (SEN) by 300-ms test pulses from a holding potential of −50 mV to potentials between −30 to +50 mV at pulse intervals of 10 s. C: ICa-voltage relationship in ventricular myocytes isolated from WT HF and KO HF LV. Current amplitudes were normalized to cell capacitance and plotted as mean values of density. *P < 0.05, KO HF compared with WT HF for both subepicardial myocytes (SEP) and SEN myocytes. D: mean values of ICa inactivation time constants. The τ1 and τ2 denote fast and slow inactivation time constants, respectively. E: Ca2+-dependent ICa facilitation results, showing negative ICa staircase in WT HF and positive ICa staircase in KO HF LV. Vertical bars represent SE.
Table 2.
ICa characteristics recorded in WT HF and CaMKIIδ KO HF LV
| WT HF (n = 18) |
KO HF (n = 16) |
||||
|---|---|---|---|---|---|
| ICa Kinetics | n | Means ± SE | n | Means ± SE | P Values (compared with WT HF) |
| Peak ICa, pA/pF | |||||
| SEP | 23 | −7.5 ± 0.3* | 20 | −5.8 ± 0.3* | <0.01 |
| SEN | 23 | −6.5 ± 0.3 | 17 | −4.8 ± 0.3 | <0.01 |
| ICa decay at +10 mV | |||||
| τ1, ms | 46 | 10.2 ± 0.4 | 36 | 11.7 ± 0.7 | >0.05 |
| τ2, ms | 46 | 74.9 ± 1.7 | 36 | 73.2 ± 1.5 | >0.05 |
| ICa activation | |||||
| V1/2, mV | 46 | −10.6 ± 0.5 | 37 | −10.2 ± 0.5 | >0.05 |
| K | 46 | 4.8 ± 0.1 | 37 | 4.8 ± 0.1 | >0.05 |
| ICa inactivation | |||||
| V1/2, mV | 33 | −21.2 ± 0.6 | 31 | −20.3 ± 0.3 | >0.05 |
| K | 33 | 6.0 ± 0.1 | 31 | 5.8 ± 0.1 | >0.05 |
| ICa recovery | |||||
| τ, ms | 25 | 221.9 ± 6.2 | 28 | 177.7 ± 6.1 | <0.01 |
| ICa facilitation (5th pulse vs. 1st pulse) | |||||
| Changes in ICa, % | 32 | −9.0 ± 1.1 | 31 | 11.2 ± 1.4 | <0.01 |
Values are means ± SE.
ICa, L-type calcium channel current; SEP, subepicardial myocytes; SEN, subendocardial myocytes; V1/2, half-maximal inactivation voltage; K, slope factor; τ, time constant;
P < 0.05, compared with SEN.
Recovery of CDF in KO HF LV.
Frequency-dependent CaMKII activation is the major mediator of CDF in ventricular myocytes (7, 26). In pressure-overload HF LV, CDF was abolished due to the saturated CaMKII-dependent LTCC phosphorylation at baseline (21). Again, we found that CDF was absent in WT HF myocytes. Instead, a negative ICa staircase was observed. However, CDF was detected in all tested myocytes (SEP and SEN) isolated from KO HF LV (Fig. 2E), although it was observed only in 76% of KO sham myocytes (24). These results indicate that a physiological CaMKII activity is required for CDF, and too high or too low levels of CaMKII activity will blunt CDF.
Voltage dependence of ICa activation and inactivation and ICa recovery time constants.
To determine the voltage dependence of ICa activation and inactivation, we used the pulse protocols shown in Fig. 3A. Using these approaches, we detected no significant differences in voltage-dependent ICa activation and inactivation between myocytes from WT HF and KO HF LV (Fig. 3A). The half-maximal activation and inactivation voltages and slope factors in WT HF and KO HF LV are summarized in Table 2. However, in KO HF LV, ICa recovery from inactivation was significantly accelerated compared with that recorded from WT HF LV (Fig. 3, B and C). The accelerated ICa recovery time course in KO HF ventricular myocytes may contribute to the restoration of CDF (10).
Fig. 3.
ICa kinetics. A: normalized voltage dependence of ICa activation and inactivation. B: representative ICa recovery traces recorded (in SEN myocytes) from WT HF and KO HF ventricular myocytes, respectively. C: mean values of normalized peak ICa (I/Imax) for ICa recovery from inactivation. Insets: recording protocol.
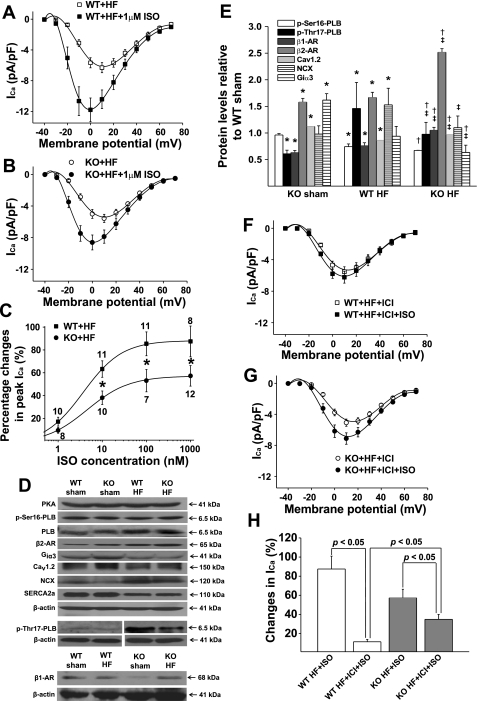
Reduced ICa response to β-adrenergic stimulation in KO HF LV.
ICa response to β-adrenergic stimulation was tested in WT HF and KO HF LV myocytes by exposure to various concentrations (1 nM, 10 nM, 100 nM, and 1μM) of the β-adrenergic agonist ISO. When ICa amplitude reached the steady-state, myocytes were then exposed to the next ISO concentration. We found that ICa response to ISO was significantly reduced in KO HF myocytes compared with WT HF myocytes (Fig. 4, A and B). As shown in the dose-response relationship (Fig. 4C), the EC50 was 3.7 ± 0.1 nM for WT HF and 4.6 ± 0.3 nM for KO HF myocytes (P < 0.05). The maximum increase in ICa by 1 μM ISO was 88.3 ± 0.3% for WT HF and 57.2 ± 0.5% for KO HF myocytes, respectively. These results suggest that inhibition of CaMKII activity significantly reduces β-adrenergic regulation of ICa in failing ventricle. The ICa-voltage relationship showed that ISO (1 μM) shifted the peak ICa potential from +10 to 0 mV in both WT HF and KO HF myocytes, a 10-mV shift to the left (Fig. 4, A and B). The shifting of the ICa-voltage relationship suggests a membrane potential-dependent potentiation effect of ISO on ICa. Similar findings were also observed in KO compared with WT LV myocytes as we reported recently (24). In line with the reduced ICa response to ISO, ISO-induced increase in SR Ca2+ transient and sarcomere shortening were smaller in KO HF (15 ± 5% for Ca2+ transient and 58 ± 2% for sarcomere shortening; n = 10) compared with WT HF myocytes (31 ± 3% for Ca2+ transient and 77 ± 9% for sarcomere shortening; n = 10; P < 0.05, compared with KO HF). By calculating the differences in actual values in the presence of ISO, in KO HF, SR Ca2+ transient became 16% smaller than that recorded in WT HF myocytes (there was no difference in baseline), and the difference in sarcomere shortening between KO HF and WT HF was also reduced, i.e., from 30% larger in KO HF at baseline to 16% larger in the presence of ISO. These results indicate a significant reduction of cardiac response to β-adrenergic stimulation in KO HF myocytes.
Fig. 4.
Protein expression and isoproterenol (ISO) effects on ICa. A and B: mean values of voltage-dependent ICa activation before and after ISO perfusion in ventricular myocytes isolated from WT HF and KO HF LV, respectively. C: dose-response relationship of peak ICa recorded in WT HF and KO HF LV in the presence of different concentrations of ISO. D: representative Western blot results for Ca2+ regulatory proteins in WT HF and KO HF LV. Discontinuous bands indicate separate experiments. Protein levels in KO HF LV relative to the levels in WT HF LV are statistically summarized in E (each value is a mean from 3 hearts). Vertical bars represent SE. *P < 0.05, compared with WT sham; †P < 0.05, compared with KO sham. ‡P < 0.05, compared with WT HF. F and G: ISO (1 μM) effect on ICa recorded in WT HF and KO HF ventricular myocytes in the presence of β2-adrenergic receptor (AR)-specific antagonist ICI118,551, respectively. H: ISO-induced percent increase in peak ICa in WT HF and KO HF ventricular myocytes with and without ICI118,551. Vertical bars represent SE.
We then measured the protein levels of PLB, p-Ser16-PLB, PKA, p-Thr17-PLB, β-ARs, LTCC α-subunits Cav1.2, and Giα3 in WT HF and KO HF LV and found no difference in the protein levels of p-Ser16-PLB, PLB, and PKA. However, an increase in β1-AR, β2-AR, and Cav1.2 protein levels and a decrease in p-Thr17-PLB and Giα3 levels were observed in KO HF LV (Fig. 4, D and E). The decrease in p-Ther17-PLB level is consistent with the reduced CaMKII activity in KO HF LV (Fig. 1). Importantly, it suggests a reduction of the localized CaMKII activity, which may explain the reduced ICa density observed in KO HF LV where the Cav1.2 protein level has actually been upregulated. Although β1- and β2-receptors were upregulated and Giα3 was reduced, ICa response to β-adrenergic stimulation was reduced in KO HF myocytes. These results suggest that in HF LV, ICa response to β-adrenergic stimulation is mainly mediated by CaMKII. CaMKII inhibition largely blunted β-adrenergic regulation on ICa. To determine the role of upregulated β2-receptor in β-adrenergic regulation on ICa, we tested ISO effects on ICa in myocytes from WT HF and KO HF LV in the presence of β2-AR antagonist ICI118,551 (10 nM; Ref. 24). We found that in the presence of ICI118,551, ISO induced an increase in the peak ICa by 11.4 ± 2.4% in WT HF LV and 35 ± 5.1% in KO HF LV, respectively (Fig. 4, F–G). The largely blunted ICa response to ISO in WT HF myocytes by β2-AR antagonist (from 88.3 ± 0.3 to 11.4 ± 2.4%) suggests that adrenergic regulation of ICa is predominantly mediated via β2-AR in the setting of HF, which is consistent with the known desensitization of β1-AR. In KO HF myocytes; however, β2-AR only plays a minor role in ICa regulation.
Altered SR Ca2+ leak, Ca2+-transient, sarcomere shortening and relaxation in KO HF ventricular myocytes.
To understand the alteration of Ca2+ handling and E-C coupling in HF ventricular myocytes when CaMKII activity is largely and chronically inhibited, we measured SR Ca2+ leak and recorded Ca2+ transient and sarcomere shortening in CaMKIIδ KO HF LV myocytes. It has been demonstrated that excessive activation of CaMKII but not PKA is the main mechanism for the diastolic SR Ca2+ leak in failing LV (1, 16). Consistent with these results, we found that SR Ca2+ leak (normalized to SR Ca2+ contents) was dramatically reduced in KO HF LV compared with WT HF LV, especially at higher SR content levels (Fig. 5, A and B). In addition, sarcomere shortening was increased in KO HF LV. The improvement of cellular contraction is, however, not a result of increased SR Ca2+ release because the peak of Ca2+ transient was similar between WT HF and KO HF LV (Table 3, possibly, the increased SR Ca2+ contents are offset by the reduced ICa in KO HF myocytes). To understand the mechanism for the increased contractility in KO HF myocytes, we assessed myofilament sensitivity to Ca2+ in WT HF and KO HF myocytes by measuring the gradient of cell length vs. fura-2 fluorescence during contraction and relaxation (9). As predicted, we found that in KO HF LV the gradient of trajectory was significantly increased for both systolic and diastolic phases compared with WT HF LV. The [Ca2+]i levels at which the maximum twitch was achieved were 1.25 ± 0.04 (F340/380) for WT HF (n = 28) and 1.16 ± 0.03 (F340/380) for KO HF (n = 21; P < 0.01) myocytes, respectively. The gradient of the trajectory during the relaxation was 0.94 ± 0.03 for WT HF and 1.53 ± 0.04 for KO HF myocytes at 300 ms (P < 0.01) and 1.01 ± 0.03 for WT HF and 1.62 ± 0.04 for KO HF myocytes at 600 ms (P < 0.01), respectively. A mean trajectory trace for WT HF and KO HF myocytes is shown in Fig. 5D. These results suggest that CaMKII KO in HF significantly increases myofilament sensitivity to Ca2+. It has been shown that troponin I (Tn-I) plays an important role in regulating myofilament sensitivity to Ca2+ and an isoform switch from slow skeletal muscle Tn-I (ssTn-I) to cardiac Tn-I (cTn-I) during development modifies cardiac contractility and relaxation(12). We have detected the Tn-I isoforms in LV myocytes and found that there was a significant increase in ssTn-I-to-cTn-I ratio in KO and KO HF compared with WT and WT HF mice, respectively (Fig. 5, E and F). The increased ssTn-I-to-cTn-I ratio may explain, at least in part, the increased myofilament sensitivity to Ca2+ observed in KO HF LV. This alteration may improve cellular contractility but also delay cardiac relaxation. However, the increase in myofilament sensitivity to Ca2+ may not be a major contributor to the significantly slowed sarcomere relaxation observed in KO HF myocytes because the Ca2+ transient decline was also significantly slowed (Fig. 5C and Table 3). To better understand the underlying molecular mechanisms, we measured the levels of SERCA2a expression and PLB phosphorylation at PKA-dependent and CaMKII-dependent phosphorylation sites. Our results revealed no change in SERCA2a expression but a dramatic reduction of CaMKII-dependent PLB phosphorylation at Ther17 without alteration of PKA dependent PLB phosphorylation at Ser16 (Fig. 4, D and E). The reduced CaMKII-dependent PLB phosphorylation may explain the slowed diastolic Ca2+ decline and also contribute to the slowed sarcomere relaxation. Moreover, a lower level of NCX proteins (Fig. 4, D and E) and a smaller INCX (Fig. 5, G and H) were observed in KO HF LV, which further deteriorated diastolic function.
Fig. 5.
Ca2+ handling and myofilament sensitivity to Ca2+. A and B: reduced SR Ca2+ leak in KO HF LV (n = 24) compared with WT HF LV (n = 21). C: representative traces of Ca2+ transient and sarcomere shortening recorded at 1 Hz pacing rate in myocytes isolated from WT HF and KO HF LV. D: mean trajectory of normalized sarcomere length vs. fluorescence (F340/380) recorded in WT HF (n = 28) and KO HF (n = 21) myocytes stimulated at 1 Hz. E: Representative Western blots of slow skeletal muscle troponin I (ssTn-I) and cardiac troponin I (cTn-I) detected in WT sham, KO sham, WT HF, and KO HF LV. F: changes of ssTn-I-to-cTn-I ratio relative to WT sham (n = 2 mice for each group). G: mean trace of INCX recorded in WT HF (n = 15) and KO HF (n = 13) ventricular myocytes. H: mean values of INCX recorded in WT sham, KO sham, WT HF, and KO HF LV myocytes at membrane potentials of +60 mV and −80 mV. Vertical bars represent SE.
Table 3.
Calcium transient and sarcomere shortening in HF ventricular myocytes
| Pacing Frequency of 1 Hz |
Pacing Frequency of 3 Hz |
|||
|---|---|---|---|---|
| WT HF (n = 16) | KO HF (n = 18) | WT HF(n = 10) | KO HF (n = 10) | |
| Calcium transients | ||||
| Baseline (F340/380) | 1.14 ± 0.04 | 1.10 ± 0.03 | 1.35 ± 0.09† | 1.36 ± 0.07† |
| Peak height (ΔF340/380) | 0.74 ± 0.04 | 0.76 ± 0.04 | 0.84 ± 0.07 | 0.88 ± 0.06 |
| Time to peak, ms | 25 ± 1 | 24 ± 1 | 29 ± 1 | 27 ± 2 |
| Time to 50% peak, ms | 12 ± 1 | 10 ± 1 | 12 ± 1 | 10 ± 1 |
| Time to 50% relaxation, ms | 52 ± 4 | 75 ± 3* | 50 ± 4 | 72 ± 4* |
| [Ca2+]i decay time constant, ms | 92 ± 4 | 121 ± 5* | 67 ± 4† | 113 ± 8* |
| Sarcomere shortening | ||||
| Fractional shortening, % | 3.46 ± 0.25 | 4.50 ± 0.36* | 6.46 ± 0.80† | 8.93 ± 0.71*† |
| Time to peak, ms | 67 ± 3 | 66 ± 4 | 75 ± 5 | 78 ± 3 |
| Time to 50% peak, ms | 32 ± 2 | 31 ± 3 | 33 ± 4 | 33 ± 4 |
| Time to 50% relaxation, ms | 49 ± 3 | 83 ± 8* | 39 ± 3† | 66 ± 6* |
| τ-Shortening, ms | 46 ± 3 | 73 ± 7* | 30 ± 3† | 50 ± 8* |
Values are means ± SE. [Ca2+]i, cytostolic Ca2+ concentration.
P < 0.05, compared with WT HF;
P < 0.05, compared with 1-Hz pacing.
Contributions of SERCA and NCX to diastolic Ca2+ removal.
The relative contributions of SERCA and NCX to the removal of diastolic Ca2+ were determined in WT HF and KO HF LV myocytes by measuring the decline of fluorescence (F340/380) during caffeine-induced Ca2+ transient and Ca2+ transient during stimulated twitches (at 1 Hz; Ref. 13). Our results showed that the contributions of SERCA and NCX to diastolic Ca2+ removal were 90.8 ± 2.3 and 9.2 ± 2.3% for WT HF myocytes (n = 10) and 95.3 ± 0.9 and 4.6 ± 0.9% for KO HF myocytes (n = 10), respectively. The reduced NCX fraction is consistent with the reduced NCX expression and function and contributes to the slowed Ca2+ transient decay and cellular relaxation in KO HF myocytes, on top of the reduced CaMKII-dependent PLB phosphorylation and increased myofilament sensitivity to Ca2+.
Reduced in vivo cardiac reserve to β-adrenergic regulation in CaMKIIδ KO HF mice.
In vivo heart function was measured in trained conscious mice by echocardiography as we recently reported (24). The β-adrenergic agonist ISO was injected in WT HF and KO HF mice (1.5 mg/kg ip), and heart function was measured at baseline and 10 min after ISO injection. KO HF mice manifested a reduced cardiac response to ISO compared with the WT HF mice. ISO increased LV EF by 29.91 ± 5.45% in KO HF (n = 10) and 51.67 ± 5.40% in WT HF mice (n = 23; P < 0.05), respectively, indicating a reduced cardiac reserve to β-adrenergic stimulation in KO HF mice (Table 4). Interestingly, ISO significantly increased heart rate in WT HF mice but not in KO HF mice. This is likely a result of impaired CaMKII-mediated regulation of SA nodal cell Ca2+ homeostasis in KO HF mice (23). The reduced cardiac response to ISO was similar to that observed in KO mice compared with WT mice (24), indicating changes associated with chronic CaMKII inhibition at baseline.
Table 4.
Changes in heart function in response to isoproterenol
| WT HF (n = 23) |
KO HF (n = 10) |
|||
|---|---|---|---|---|
| Control | ISO (1.5 mg/kg) | Control | ISO (1.5 mg/kg) | |
| Heart rate, beat/min | 644 ± 11 | 665 ± 6* | 645 ± 21 | 653 ± 15 |
| LVIDd, mm | 4.42 ± 0.07 | 3.93 ± 0.09* | 4.24 ± 0.15 | 4.05 ± 0.12* |
| LVIDs, mm | 3.58 ± 0.09 | 2.73 ± 0.12* | 3.37 ± 0.15 | 2.93 ± 0.15* |
| LV Vold, μl | 89.4 ± 3.5 | 68.54 ± 3.9* | 85.1 ± 5.6 | 72.9 ± 5.2* |
| LV Vols, μl | 55.0 ± 3.2 | 29.67 ± 3.0* | 50.3 ± 4.3 | 34.26 ± 4.35* |
| LV mass, mg | 152.2 ± 4.5 | 157.6 ± 4.8 | 159.0 ± 6.4 | 160.9 ± 10.1 |
| EF, % | 39.3 ± 1.5 | 58.8 ± 2.2* | 41.8 ± 1.5 | 54.4 ± 3.0*† |
| Changes of EF, % | 51.7 ± 5.4 | 29.9 ± 5.5† | ||
| FS, % | 19.1 ± 0.8 | 31.2 ± 1.5* | 20.4 ± 0.8 | 28.1 ± 1.9*† |
| Changes of FS, % | 65.6 ± 7.1 | 37.4 ± 7.0† | ||
Values are means ± SE.
LVIDd and LVIDs, left ventricular internal dimension of diastole and systole; LV Vold and LV Vols, left ventricular volume of diastole and systole; FS, fractional shortening.
P < 0.05, compared with control;
P < 0.05, compared with WT HF + isoproterenol (ISO).
Increased fibrosis and decreased apoptosis in CaMKIIδ KO HF mice.
Our cellular data showed an improved contractility in KO HF ventricular myocytes compared with WT HF myocytes. However, the in vivo EFs were similar in these mice. To understand this inconsistency, we detected the ventricular fibrosis and apoptosis in WT HF and KO HF mice. TUNEL staining was used to assess the number of apoptotic cells, and Masson-trichrome staining was used to assess development of cardiac fibrosis, as indicated by interstitial collagen deposition. We observed a more significant increase in LV fibrosis in KO HF LV compared with WT HF LV (Fig. 6A). The increase in ventricular fibrosis likely contributes to the in vivo LV dysfunction in KO HF mice by increasing ventricular wall stiffness and the diffusion distance of oxygen to myocytes. In line with this, the KO HF mice showed exacerbation of diastolic function, which is manifested by a reduced diastolic volume and an increase in the lung weight-to-body weight ratio (Table 1) and E/E' ratio compared with the WT HF mice. Interestingly, TUNEL staining showed that myocyte apoptosis was dramatically increased in the WT HF LV but much less increased in the KO HF LV compared with their sham-operated controls (Fig. 6B). Consistent with these changes, an increase in BcL-2 expression and ERK1/2 phosphorylation, the antiapoptotic signaling pathways in vivo (6, 11), was observed in KO HF LV (Fig. 6C).
Fig. 6.
Images and protein expressions of fibrosis and apoptosis. A: detection of fibrosis by Masson Trichrome staining. A more significant increase in fibrosis was observed in KO HF LV compared with WT HF LV. B: detection of myocyte apoptosis by terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) assay. Myocyte nuclei (blue) and the TUNEL-positive nuclei (green) are shown in the same picture (overlay). No obvious apoptosis was detected in WT sham and KO sham LV. However, myocyte apoptosis was markedly increased in WT HF LV but only slightly increased in KO HF LV. C: Western blot analysis of ventricular lysates shows dramatic increase in BcL-2 and phospho-ERK1/2 in KO HF LV compared with WT HF LV. Vertical bars represent SE. Data were from 3 mice in each group.
DISCUSSION
Recently, CaMKIIδ KO mice have been used to test the effect of chronic CaMKII inhibition on the development of cardiac hypertrophy using moderate TAB method (4, 14), but different results were reported. Backs et al. (4) reported that CaMKIIδ KO mice are resistant to pressure-overload hypertrophy, while Ling et al. (14) showed that cardiac hypertrophy was reproducible in CaMKIIδ KO mice, but the progressive transition to HF was blunted. This inconsistency is likely related to the mouse age at the time when TAB was applied (6-wk-old mice were used for TAB in the first study, while 8- to 12-wk-old mice were used for the second study), the genetic backgrounds [Backs et al. (4) targeted CaMKIIδ exons 1 and 2, which encode the ATP-binding domain, while Ling et al. (14) targeted exons 9–11, which encode the catalytic domain], or the actual extent of aortic restriction. However, neither group used sTAB to generate HF in the CaMKIIδ KO mice. This is the first time we report successful generation of pressure-overload HF model in (6-wk-old male) the CaMKIIδ KO mice by sTAB, a strategy commonly used to generate HF phenotype in mice(5, 21).
The moderate TAB has been used previously to generate compensated hypertrophy in mice. This method uses a constriction of 27-gauge stenosis in the transverse aorta between the innominate and left common carotid arteries and produces moderate hypertrophy (≈40% increases in heart mass) without significant reduction of ventricular contractility and clinical signs of HF (5, 8). In contrast, sTAB produces a constriction of 28-gauge stenosis in the transverse aorta. This slightly tighter (1/20 mm) constriction on the aorta consistently induces decompensated hypertrophy with LV dilation and dramatic reduction of EF with signs of circulatory failure, including lethargy, impaired mobility, and edema, i.e., HF. We have used the sTAB method to consistently produce HF model in the male C57BL6 mice (6 wk old; Refs. 19–21). This model may better reflect the acute pressure-overload HF phenotype caused by malignant hypertension and aortic stenosis.
ICa is an important current for E-C coupling in ventricular myocytes, and the CDF contributes to the increased contractile force at fast heart rates, which improves cardiac performance during exercise (15). However, this positive staircase is diminished in HF LV due to the saturated phosphorylation of LTCC by CaMKII (21). In addition, ICa is an important current contributing to action potential duration and, accordingly, the ventricular effective refractory period. ICa alteration in HF, especially the increased ICa density and changes of inactivation to mode 2 gating, contributes significantly to the enhanced ventricular arrhythmias (2). In this study, we found that CaMKIIδ KO in HF reduced ICa density to a physiological level (the level measured in the WT sham) and restored the HF-blunted CDF, suggesting a nice reversal of ICa that has been remodeled in failing LV(21). However, ICa response to β-adrenergic stimulation was reduced in KO HF LV even though both β1- and β2-receptors were upregulated. These results indicate that in failing heart, β-adrenergic regulation of ICa is mainly mediated by CaMKII. Inhibition of CaMKII largely blunted β-adrenergic effect on ICa. In addition, our study suggests that in failing LV β-adrenergic regulation of ICa is predominantly mediated by β2-receptor because β2-blocker largely blunted the ISO response on ICa (ISO effect on ICa was reduced by ≈77%), which is consistent with the desensitization of β1 receptors in the setting of HF(18). Interestingly, this HF-related β1-receptor desensitization was largely blunted in KO HF LV because β2-blocker only reduced the ISO effect on ICa by 38.6%.
Although ICa density was reduced, the sarcomere shortening was improved in KO HF LV myocytes, especially at higher pacing rate. The improved cellular contractility in KO HF LV is associated with the increased myofilament sensitivity to Ca2+ but may not correlate with the reduced SR Ca2+ leak because the actual Ca2+ transient did not increase in KO HF LV. It is likely that the effect of reduced SR Ca2+ leak on Ca2+ transient has been offset by ICa reduction and the decrease in CaMKII-dependent PLB phosphorylation and accordingly the decreased SR Ca2+ reuptake in KO HF LV.
Although myocyte contractility is improved, KO HF mice manifested significantly reduced efficiency of β-adrenergic regulation of cardiac contraction in vivo due to the reduced response of ICa, Ca2+ transient and sarcomere shortening to β-adrenergic stimulation, increase in ssTn-I isoform (which lacks PKA phosphorylation sites Ser 22/23; Ref. 12), and blunted heart rate adaptation. Also importantly, KO HF mice demonstrated exacerbation of diastolic dysfunction, which is associated with the reduced CaMKII-dependent PLB phosphorylation and NCX function and the increase in myofilament sensitivity to Ca2+ and interstitial fibrosis.
The mechanism for the increased fibrosis in KO HF LV is unclear. It is likely that this fibrotic response is reactive rather than reparative since it is developed shortly after sTAB. Indeed, the pressure-overload-induced myocyte apoptosis is suppressed in KO HF LV compared with WT HF LV, indicating less reparative fibrosis in long term. A recent study (14) showed that CaMKIIδ KO reduced fibrotic development in pressure-overload hypertrophy 6 wk after moderate TAB, which is consistent with the anticipated reduction in reparative fibrosis. Studies (28) in mice with CaMKIIδc overexpression have shown development of hypertrophy and progressive HF with only a mild LV fibrosis, suggesting that CaMKII may not be a powerful stimulator for in vivo fibrotic response. Instead, our results may indicate an antifibrotic role of CaMKII in acutely developed pressure-overload HF. Nevertheless, the increased fibrosis in KO HF mice can profoundly affect myocyte metabolism and performance and ultimately in vivo ventricular function by reducing the capillary density and increasing oxygen diffusion distance and ventricular wall stiffness, contributing to both systolic and diastolic dysfunction.
Limitations.
To understand the cardiac effect of chronic cardiac CaMKII inhibition in HF, we developed pressure-overload HF in CaMKIIδ KO mice by sTAB. However, as a limitation of this study, we cannot dissect the changes caused by CaMKII inhibition in steady-state HF from the CaMKII inhibition during HF development. Using the cardiac-specific CaMKII inhibitors in steady-state HF is ideal but currently unavailable. In addition, the actually role of CaMKII in regulating cardiac Ca2+ handling and E-C coupling in mice may differ from human. For instance, the percent contribution of SERCA and NCX to [Ca2+]i removal is different between mouse and humans (13). As a result, CaMKII inhibition may have different effects between these two species. Application of insights gleaned from the mouse model to HF patients needs to be cautious and further studies in human are obviously required.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R21-HL-088168 and R01-HL-083271 (to Y. Wang), American Health Assistant Foundation Grant H2007-019, and Emory University Pilot Grant 280249 and support from the Children's Healthcare of Atlanta.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.C., L.X., D.L., A.G., H.J.L., and T.K. performed experiments; J.C. and L.X. analyzed data; L.X. and Y.W. prepared figures; Y.W. conception and design of research; Y.W. interpreted results of experiments; Y.W. drafted manuscript; Y.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Eric N. Olson (University of Texas Southwestern Medical Center at Dallas) for sharing CaMKIIδ KO mice.
REFERENCES
- 1. Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97: 1314–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Anderson ME. Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc Med 14: 152–161, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Anderson ME. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther 106: 39–55, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA 106: 2342–2347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothermel BA, Berenji K, Tannous P, Kutschke W, Dey A, Nolan B, Yoo KD, Demetroulis E, Gimbel M, Cabuay B, Karimi M, Hill JA. Differential activation of stress-response signaling in load-induced cardiac hypertrophy and failure. Physiol Genetics 23: 18–27, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res 91: 776–781, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol 2: 173–177, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation 101: 2863–2869, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Howarth FC, Qureshi MA. Myofilament sensitivity to Ca2+ in ventricular myocytes from the Goto-Kakizaki diabetic rat. Mol Cell Biochem 315: 69–74, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol 171: 537–547, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirshenbaum LA, de Moissac D. The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation 96: 1580–1585, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Kruger M, Kohl T, Linke WA. Developmental changes in passive stiffness and myofilament Ca2+ sensitivity due to titin and troponin-I isoform switching are not critically triggered by birth. Am J Physiol Heart Circ Physiol 291: H496–H506, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol Heart Circ Physiol 274: H1335–H1347, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller BJ. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest 119: 1230–1240, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross J, Jr, Miura T, Kambayashi M, Eising GP, Ryu KH. Adrenergic control of the force-frequency relation. Circulation 92: 2327–2332, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 93: 592–594, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Verrier RL, Antzelevitch C. Autonomic aspects of arrhythmogenesis: the enduring and the new. Curr Opin Cardiol 19: 2–11, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res 95: 798–806, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Cheng J, Chen G, Rob F, Naseem RH, Nguyen L, Johnstone JL, Hill JA. Remodeling of outward K+ currents in pressure-overload heart failure. J Cardiovasc Electrophysiol 18: 869–875, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Cheng J, Joyner RW, Wagner MB, Hill JA. Remodeling of early-phase repolarization: a mechanism of abnormal impulse conduction in heart failure. Circulation 113: 1849–1856, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Tandan S, Cheng J, Yang C, Nguyen L, Sugianto J, Johnstone JL, Sun Y, Hill JA. Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure. J Biol Chem 283: 25524–25532, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang YG, Wagner MB, Joyner RW, Kumar R. cGMP-dependent protein kinase mediates stimulation of L-type calcium current by cGMP in rabbit atrial cells. Cardiovasc Res 48: 310–322, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, Wehrens XH, Mohler PJ, Song LS, Anderson ME. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci USA 106: 5972–5977, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu L, Lai D, Cheng J, Lim HJ, Keskanokwong T, Backs J, Olson EN, Wang Y. Alterations of L-type calcium current and cardiac function in CaMKIIδ knockout mice. Circ Res 107: 398–407, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol 49: 1903–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol Heart Circ Physiol 267: H982–H993, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res 63: 476–486, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 92: 912–919, 2003 [DOI] [PubMed] [Google Scholar]