Abstract
Transglutaminase (TG) function facilitates several vascular processes and diseases. Although many of these TG-dependent vascular processes have been ascribed to the function of TG2, TG2 knockout mice have a mild vascular phenotype. We hypothesized that TGs besides TG2 exist and function in the vasculature. Biotin-pentylamide incorporation, a measure of general TG activity, was similar in wild-type and TG2 knockout mouse aortae, and the general TG inhibitor cystamine reduced biotin-pentylamine incorporation to a greater extent than the TG2-specific inhibitor Z-DON, indicating the presence of other functional TGs. Additionally, 5-hydroxytryptamine-induced aortic contraction, a TG-activity-dependent process, was decreased to a greater extent by general TG inhibitors vs. Z-DON (maximum contraction: cystamine = abolished, monodansylcadaverine = 28.6 ± 14.9%, and Z-DON = 60.2 ± 15.2% vehicle), providing evidence for the importance of TG2-independent activity in the vasculature. TG1, TG2, TG4, and Factor XIII (FXIII) mRNA in rat aortae and vena cavae was detected by RT-PCR. Western analysis detected TG1 and TG4, but not FXIII, in rat aortae and vena cavae and in TG2 knockout and wild-type mouse aortae. Immunostaining confirmed the presence of TG1, TG2, and TG4 in rat aortae and vena cavae, notably in smooth muscle cells; FXIII was absent. K5 and T26, FITC-labeled peptide substrates specific for active TG1 and TG2, respectively, were incorporated into rat aortae and vena cavae and wild-type, but not TG2 knockout, mouse aortae. These studies demonstrate that TG2-independent TG activity exists in the vasculature and that TG1 and TG4 are expressed in vascular tissues.
Keywords: protein cross-linking, amidation, vascular disease
transglutaminases (tgs) are a family of enzymes that catalyze the formation of a covalent bond between a free amine group and the gamma-carboxamide group of a protein-bound glutamine (13). A variety of substrates can act as the amine-donor group, including protein- and peptide-bound lysines (25), polyamines such as spermidine (12), and monoamines such as 5-hydroxytryptamine (5-HT; Refs. 18, 37, 38) and norepinephrine (16). There are currently eight identified human TGs (20): TG1 through TG7 and Factor XIII (FXIII). To date, only TG1, TG2, and FXIII have been reported in the cardiovascular system. Information on cardiovascular TG1 is limited. TG1 has been found in myocardial endothelial cells (but not pulmonary endothelial cells; Ref. 5) where it plays a role in controlling barrier function of endothelial monolayers by stabilizing β-actin (6). In contrast, vascular TG2 and FXIII have been substantially more studied. TG2 is expressed in vascular endothelial and smooth muscle cells, as well as fibroblasts and monocytes-macrophages (34). TG2 is important to a number of physiological processes in the vasculature, including small artery remodeling (2) and fibroblast adhesion (32), and has been implicated in the development of hypertension and atherosclerosis (14). FXIII is expressed by platelets and cells of monocyte/macrophage origin (21). In addition to its classical role in the blood coagulation cascade (19), FXIII also promotes fibroblast adhesion (36), promotes healing of the myocardium after infarction (22), and contributes to angiogenesis (8). For a more comprehensive overview on the significance of vascular TGs, the reader is referred to the following reviews (4, 27).
Despite the ubiquitous expression and numerous physiological roles reported for TG2, TG2 knockout (KO) mice show a relatively mild phenotype (10, 23), with no change in blood pressure or heart rate compared with wild-type (WT) mice. FXIII derived from macrophages compensates for some of the vascular functions of TG2 lost in the TG2 KO mice, as evidenced by macrophage-derived FXIII contribution to the delayed flow-dependent small artery remodeling in these mice (3). However, macrophage recruitment does not explain why the contractile response to adrenergic agonists of the mesenteric artery in TG2 KO mice is not different from WT (3), as TG function is important for amine-stimulated vascular contraction (16, 38). Due to this evidence, we hypothesized that at least one other TG besides TG2 is present in vascular smooth muscle cells, where it plays a vital role in vascular function.
To address our hypothesis, aorta and vena cava tissues from normal, male Sprague-Dawley rats, as well as aorta tissues from TG2 KO and WT mice, were utilized. An artery and a vein were chosen for our model because both arteries and veins contribute to the regulation of blood pressure; arteries by affecting total peripheral resistance and vascular tone and veins through the regulation of vascular capacitance. In addition to this, previous studies (16) in our laboratory suggest that TG function may be more critical in veins than arteries. For example, norepinephrine-induced contraction of the rat vena cava is more sensitive to inhibition by cystamine, a general TG inhibitor, than norepinephrine-induced contraction of the rat aorta (16). This present study utilized a multidisciplinary approach that included genetic, pharmacologic, and biochemical methods.
METHODS
Animal use/ethics.
Two different colonies of male TG2 KO and WT mice [Melino (10), 25–35 g; and Graham (23), 25–27 g] and male Sprague-Dawley rats (250–300 g; Charles River Laboratories, Portage, MI) were used. The Melino TG2 KO mice are in a mixed Svj129-C57Bl/6 background and have not been crossed back into any background. The WT mice are on the same background and are bred separately; occasionally, the WT and KO mice are crossed and regenotyped to prevent genetic drift. Melino mice were deeply anesthetized with CO2 and euthanized by decapitation before removal of tissues. Procedures done with these mice were in accordance with the established University of Rochester guidelines on animal care and handling and approved by the University Committee for Animal Research. The Graham TG2 KO mice were backcrossed at least 10 times onto a C57Bl/6 background and maintained as a colony of homozygous TG2 KOs. WT C57Bl/6 nonlittermates were used as controls. Graham TG2 WT and KO mice were anesthetized with isoflurane and killed by cervical dislocation before tissue collection. Procedures done with these mice were in accordance with the Institutional Animal Use and Care Committee of the Academic Medical Center of Amsterdam. For both the Melino and Graham TG2 WT and KO mouse pairs, whole aortae were sent to Michigan State University for experimentation. Tissues were shipped overnight in cold physiological salt solution (103 mmol/l NaCl, 4.7 mmol/l KCl, 1.13 mmol/l KH2PO4, 1.17 mmol/l MgSO4-7H20, 1.6 mmol/l CaCl2-2H2O, 14.9 mmol/l NaHCO3, 5.5 mmol/l dextrose, and 0.03 mmol/l CaNa2 EDTA) on dry ice and immediately homogenized (Melino) or prepared for sectioning (Graham) upon arrival. Rats were anesthetized with pentobarbital (60 mg/kg ip) before the removal of tissues. All procedures that utilized animals were done in accordance with the guidelines put forth by Michigan State University and approved by the Institutional Animal Use and Care Committee. A total of 27 rats and 23 mice (6 Melino and 5 Graham TG2 KO and WT pairs) were used in these studies.
Western analysis.
Western analysis was performed as previously described (24). Briefly, 50 μg of protein from aorta and/or vena cava homogenates were separated on a 10% SDS gel and transferred to either nitrocellulose (for rat aorta and vena homogenates probed with an anti-FXIII antibody) or PVDF (for all other blots) membranes. After blocking for 3 h at 4°C in 4% wt/vol chick egg ovalbumin, blots were incubated with primary antibodies [mouse anti-TG1 (1:100; sc-166467; Santa Cruz Biotechnology, Santa Cruz, CA), rat anti-TG2 (1:2,000; Hybridoma Facility, University of Alabama at Birmingham, AL), mouse anti-TG4 (1:500; ATGA0140; ATGEN), rabbit anti-FXIII (1:1,000; ab83895; Abcam, Cambridge, MA), mouse anti-α-actin (1:1,000; Ab-2; EMD Biosciences, La Jolla, CA), and mouse anti-β-actin (1:1,000; A2228; Sigma, St. Louis, MO)] diluted in blocker overnight. As positive controls, 10 μl of rat skin lysate (cat. no. 1480; ProSci, Poway, CA), human prostate lysate (cat. no. 1312; ProSci Poway, CA), or HeLa cell lysate (sc-2222; Santa Cruz Biotechnology) were used for TG1, TG4, and FXIII, respectively. Densitometric analysis of the TGs was done using ImageJ (26), with protein levels of the TGs normalized to either β-actin (for rat aorta and vena cava homogenates) or α-actin (for KO and WT mouse aorta homogenates) protein.
TG activity assay.
TG activity assays on aortic homogenates from both normal rats and Melino TG2 KO and WT mice were performed using the amine donor biotin-pentylamide (BAP) incorporation (38). This assay has been used to measure FXIII (29), TG1 (6), TG2 (39), and TG4 (35) activity, supporting the idea that it is an assay for general TG activity. BAP incorporation is increased in high calcium environments and decreased with low calcium (30). This supports the idea that BAP incorporation is TG-activity dependent, as all TGs require calcium for full activity. Aortic protein homogenate was placed in reaction buffer (50 mmol/l Tris·HCl, pH 7.5, 150 mmol/l NaCl, 1 mmol/l PMSF, 2 mg/ml aprotinin and leupeptin, 1 mmol/l sodium orthovanadate, and 5 mmol/l CaCl2) containing 8 mmol/l BAP and either vehicle, 50 μmol/l Z-DON (a TG2-specific inhibitor; Zedira, Darmstadt, Germany), or 1 mmol/l cystamine (a general TG inhibitor; Sigma) for 1 h at 37°C, after which an equal volume of 2× SDS sample buffer was added to stop the reaction. Homogenates were separated by SDS-PAGE, transferred to PVDF, and blocked overnight in chick egg ovalbulmin [4% wt/vol in TBS (25 mmol/l Tris, 150 mmol/l NaCl, and 2 mmol/l KCl pH 7.4) with 0.1% Tween-20 and 0.025% NaN3]. The next day, blots were washed in TBS for 20 min, and then incubated with horseradish peroxidase-conjugated streptavidin (1:2,000; 1 h at 4°C; GE Healthcare, Piscataway NJ). BAP incorporation was visualized using ECL reagents (GE Healthcare). Quantification of bands in the BAP TG activity assay was done by gel densitometry with UN-SCAN-IT gel automatic digitizing system software (Silk Scientific, Orem, UT).
Isometric contraction in an isolated tissue bath.
Endothelial cell-intact rings of normal rat aorta were mounted in tissue baths for isometric tension as previously described (16). Tissues were treated with an initial concentration of 10 μmol/l phenylephrine (PE), an α1-adrenergic receptor agonist. An intact endothelial layer in the tissues was confirmed by a robust (>50%) relaxation to acetylcholine (1 μmol/l) in tissues contracted to half-maximum with PE. Tissues were incubated with either a TG inhibitor [50 μmol/l Z-DON, a TG2-specific inhibitor; 1 mmol/l cystamine, a general TG inhibitor; or 500 μmol/l monodansylcadavarine (MDC), a general TG inhibitor] or vehicle (DMSO for Z-DON and water for cystamine and MDC) for 30–60 min before cumulative addition of 5-HT (10−9 to 10−5 mol/l). Following assessment of 5-HT-induced contraction, tissues were washed and reincubated with vehicle or inhibitor for 30–60 min before the cumulative addition of KCl (6–100 mmol/l). Data are reported as a percentage of the initial contraction to PE.
RT-PCR.
Total RNA from ∼10 mg sections of aorta and vena cava was isolated using the MELT total RNA isolation system (Ambion/Applied Biosystems, Austin, TX). The concentration of the RNA isolated was determined using a Nanodrop spectrophotometer. One microgram of the DNase-treated total RNA was reverse transcribed using an oligo(dT)12–18 primer, dNTP mix, and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Primers for rat TG1 through TG5, TG7, FXIII, and β2-microglobulin (B2m) were obtained from www.realtimeprimers.com. To confirm the uniqueness of each of the primers, a BLAST search using each primer set biased toward picking “somewhat dissimilar sequences” was conducted by realtimeprimers.com. Each set was unique to the intended target using this approach. Table 1 lists the forward and reverse primer sequences used for each target. Expression of the mRNAs of each of the TGs was reported relative to B2m expression and reported as 2−ΔCt.
Table 1.
RT-PCR primer sequences
| Primer | Sequence |
|---|---|
| B2m | Forward: 5′-TGC TAC GTG TCT CAG TTC CA-3′ |
| Reverse: 5′-GCT CCT TCA GAG TGA CGT GT-3′ | |
| TG1 | Forward: 5′-GAC TAC TCT CGA GGC ACC AA-3′ |
| Reverse: 5′-CGT GTG CAG AGT TGA AGT TG-3′ | |
| TG2 | Forward: 5′-GGG AAT ACG TCC TCA CAC AG-3′ |
| Reverse: 5′-GTC ATC ATT GCA GTT GAC CA-3′ | |
| TG3 | Forward: 5′-GAA CCT GGA ACG GTA GTG TG-3′ |
| Reverse: 5′-GCT ATC ACT GCC TTT CTC CA-3′ | |
| TG4 | Forward: 5′-ATA GAA TGC ACC CCA GTG AA-3′ |
| Reverse: 5′-ACA TGC TTA CCA AGG CTC AG-3′ | |
| TG5 | Forward: 5′-TAT TTT CAA ACC CCC TCT CG-3′ |
| Reverse: 5′-TCT GCC TTT GTC CAC TCT TG-3′ | |
| TG7 | Forward: 5′-GGA CAG CCT GTG AAA TAT GG-3′ |
| Reverse: 5′-GGT GGA AGG TCT TTC CTG AT-3′ | |
| FXIII | Forward: 5′-AAA CTG CCC TGA TGT ATG GA-3′ |
| Reverse: 5′-CCC CAG TGT AGA AGG TGA TG-3′ |
Forward and reverse sequence of the primers used for RT-PCR. The uniqueness of each of the primers was confirmed by a BLAST search using each primer set biased toward picking “somewhat dissimilar sequences.” B2m, β2-microglobulin; TG, transglutaminase; FXIII, Factor XIII.
Immunohistochemistry.
In experiments utilizing sections from rat tissues, paraffin-embedded tissue sections of rat aorta and vena cava were dewaxed, unmasked, and treated as previously described (24). Fresh frozen sections of WT and KO mouse aortae from the Graham colony were equilibrated to room temperature before being fixed in cold (4°C) acetone for 10 min. To visualize TG1 in the mouse aortae, the Vector Mouse on Mouse basic kit (Vector Laboratories, Burlingame, CA) was utilized according to the manufacturer's instructions. Sections were incubated with primary antibodies [mouse anti-TG1 (1:100 for rat tissues, 1:50 for mouse aortae; sc-166467; Santa Cruz Biotechnology), mouse anti-TG4 (1:1,000; ATGA0140; ATGEN), and rabbit anti-FXIII (1:1,000; ab83895; Abcam)]. As controls, human skin (cat. no. 12–701-XA1; ProSci), human prostate (cat. no. 10–635-YA1; ProSci), and human placenta (AB4360; Abcam) were used for TG1, TG4, and FXIII, respectively. In all experiments, two tissue sections from the same animal were developed: one with primary antibody included and one with the primary antibody left out of the reaction. Visualization of TGs was done using the Vectastain Elite ABC kit (Vector Laboratories), used according to the manufacturer's instructions. TG1 and TG4 utilized goat-anti-mouse IgG as the secondary antibody (PK6101), while FXIII utilized horse-antirabbit IgG antibody (PK6102). All sections were developed using 3,3′-diaminobenzidine (Vector Laboratories) as the developing substrate and counterstained with either ImmPACT NovaRED substrate (for those stained using the Mouse on Mouse kit) or haematoxylin (all other sections).
Enzymatic tissue dissociation.
Rat thoracic aorta and vena cava tissues were isolated, dissected and cleaned before enzymatic dissociation as previously described (33). Cells were resuspended in PBS plus sodium nitroprusside (872 nmol/l). Cells were made to adhere to coverslips using the Shandon Cytospin 4 Centrifuge (Thermo Scientific, Waltham, WA).
Immunocytochemistry.
With the use of freshly dissociated vascular smooth muscle cells from rat aorta and vena cava tissues, immunocytochemistry was performed as previously described (16). Cells were incubated with primary antibodies [anti-α-actin (1:1,000; FITC conjugate; F3777; Sigma), rabbit anti-α-actin (1:100; ab5694; Abcam), mouse anti-TG1 (1:25; sc-166467; Santa Cruz Biotechnology), mouse anti-TG2 (1:100; TG100; LabVision, Fremont, CA), mouse anti-TG4 (1:25; ATGA0140; ATGEN), and rabbit anti-FXIII (1:250; ab83895; Abcam)] diluted in blocker. As a control, cells from the same animal were treated identical to the first set of cells but in the absence of primary antibodies. Secondary antibodies, diluted in PBS, were goat anti-mouse Alexa Fluor 568 (1:1,000; A11004; Invitrogen), goat anti-rabbit 568 (1:1,000; A11011; Invitrogen), and goat anti-rabbit 488 (1:1,000; A11008; Invitrogen).
Detection of in situ activity of TG using FITC-labeled peptides.
A recently described (15) method of detecting TG1- and TG2-specific TG activity in situ was used to differentiate TG1 and TG2 activity in normal rat aorta and vena cava tissues,and in TG2 KO and WT mouse aortae. Fresh, frozen tissue sections were blocked in PBS supplemented with 150 mmol/l NaCl and 1% BSA for 30 min at room temperature. Blocking solution was replaced with peptide solution (5 mmol/l CaCl2, 100 mmol/l Tris·HCl pH 8.0, and 1 mmol/l DTT) with FITC-labeled peptide (0.1 μmol/l K5 for TG1 activity and 1.0 μmol/l T26 for TG2 activity) or FITC-labeled peptide negative controls (K5QN or T26QN) consisting of the same amino acid sequence but with the reactive glutamine replaced with an asparagine. FITC-labeled peptides were developed in the lab of Kiyotaka Hitomi and shipped to Michigan State University for experimentation. After incubation (37°C, 90 min), peptide solution was removed, and stop solution (PBS with 25 mmol/l EDTA; 5 min, room temperature) was added. Sections were washed (PBS, 3 × 5 min, room temperature), and Prolong Gold with DAPI (Invitrogen) was placed on the sections and covered with a glass slip.
Image acquisition.
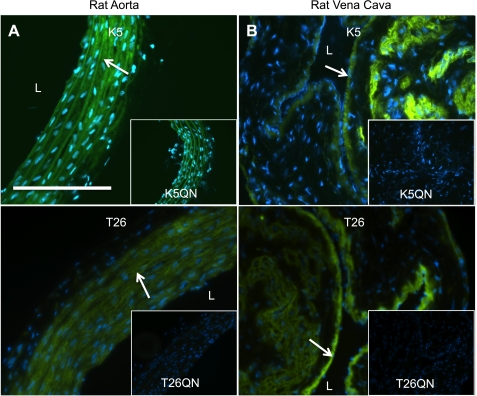
Images were visualized at ×10, ×20, and ×40 resolution using a Nikon Eclipse Ti-S microscope with NIS Elements BR 3.00 software for fluorescent images, and mmi Cell Tools software, Version 3.47 (MMI, Eching, Germany) was used for acquisition of immunohistochemistry images. A Nikon intensilight C-HGFI was used as the fluorescent light source. Images were captured with a Nikon Digital Sight DS-Qil camera and were unaltered when creating overlays. For all images, treatment and control slides were sequentially imaged using the same exposure time and LUT settings. [See Fig. 7 for the color levels of the rat aorta K5 and K5QN peptide images that were increased by the same amount in Photoshop (Adobe Systems, San Jose, CA) to assist the reader in viewing fluorescence.] All other images are published without modification.
Fig. 7.
In situ detection of TG activity in rat aorta and vena cava. Sections of fresh frozen rat aorta (A) and vena cava (B) were tested for their ability to incorporate FITC-labeled peptides that are specific for TG1 (0.1 μmol/l K5) or TG2 (1 μmol/l T26) activity, demonstrating the presence of active TGs in these tissues. Mutant peptides (0.1 μmol/l K5QN and 1 μmol/l T26QN) that consist of the same amino acids, except that the active glutamine has been replaced with an asparagine and thus does not act as a substrate for the active enzymes, were used as negative controls. Representative of tissue sections from 3 different animals. Arrows point to areas of TG activity. Scale bar = 100 μm. Nuclei are stained with DAPI (blue).
Statistical analysis.
All results were calculated as the means ± SE and analyzed using unpaired Student's t-test. Results were considered significant at P < 0.05.
RESULTS
Genetic and pharmacological evidence of the presence of additional TGs besides TG2 in the vasculature.
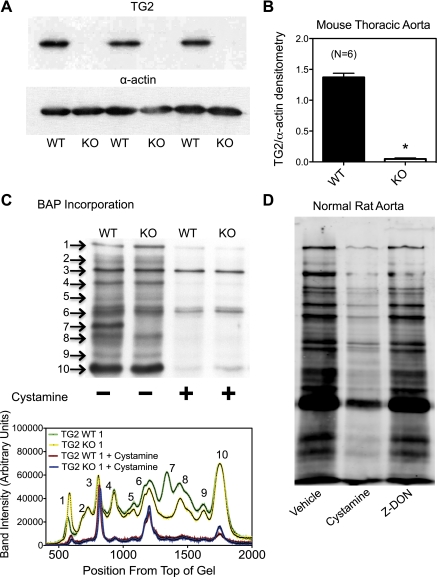
Western analysis (Fig. 1A) showed that the expression of TG2 protein (normalized to α-actin expression) in the Melino TG2 KO mouse aorta compared with WT was essentially abolished (Fig. 1B). Despite the lack of TG2, TG activity, assessed by BAP incorporation into proteins, remained in the KO tissues. The banding patterns of the incorporated BAP were similar in the TG2 KO and WT mouse aortae, (Fig. 1C), suggesting a source of TG activity besides TG2 is present in these tissues. While similar, distinct bands showed altered BAP incorporation in the KO vs. the WT, with bands from the KO generally lower in intensity than that of the WT (Fig. 1C, yellow and green lines). Some bands were completely lost (e.g., band 7), while others showed no difference between WT and KO (band 10). While this was the general trend, variance was observed from mouse to mouse (data not shown). Given this variance, it is unfair to make global statements about the banding patterns seen in these mice. Band intensity was generally reduced by the presence of 1 mmol/l cystamine (Fig. 1C, red and blue lines). BAP incorporation was also assessed for normal rat aorta homogenates in the presence of vehicle, cystamine (1 mmol/l), or Z-DON (50 μmol/l; Fig. 1D). The bands from aorta homogenates incubated with the TG2-specific inhibitor Z-DON showed slightly reduced intensity compared with vehicle, while homogenates incubated in the presence of cystamine produced bands of greatly reduced intensity. Taken together, these data demonstrate that TG activity independent of TG2 exists in rat and mouse aortae.
Fig. 1.
Transglutaminase (TG) activity in aortic tissues that lack TG2 or in the presence of TG inhibitors. A: Western analysis of TG2 protein shows that TG2 is essentially abolished in the TG2 knockout (KO) mouse vs. wild-type (WT) aorta. B: densitometry of TG2 in WT and KO mouse aortae. Bars represent the means ± SE of 6 different WT and KO mice. *P < 0.05. C: biotin-pentylamide (BAP) incorporation, a measure of TG activity, shows that the TG2 KO mouse still has significant levels of TG activity that are similar to those of WT. Blot represents aortae from 4 different TG2 WT and KO mouse pairs. Bottom: analysis of the blot in C using Un-Scan-It to determine the relative intensity of each BAP blot. Peaks represent different bands detected in the blot. Numbers next to the bands on the blots corresponds to the peak of the same number in the graph to the left. Green lines represent a WT animal and yellow a KO animal. Red lines represent the same WT animal in the presence of 1 mmol/l cystamine and blue lines a KO animal in the presence of cystamine. D: BAP incorporation into proteins of normal rat aorta homogenates in the presence of vehicle, 1 mmol/l cystamine, or 50 μmol/l Z-DON. BAP incorporation was reduced by both cystamine and Z-DON; however, BAP incorporation in the presence of cystamine was notably less than in the presence of Z-DON.
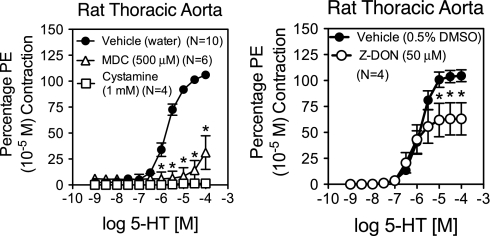
TG activity is necessary for aortic contraction to 5-HT (38). To differentiate the portion of this response that is due specifically to TG2 activity, 5-HT-induced contraction in the rat aorta was assessed in the presence of different TG inhibitors (Fig. 2). The general TG inhibitor cystamine (1 mmol/l) abolished 5-HT-induced contraction in the rat aorta. MDC (500 μmol/l), another general TG inhibitor, also significantly reduced contraction (maximum contraction = 28 ± 15% of vehicle). The TG2-specific inhibitor Z-DON (50 μmol/l) significantly reduced aortic contraction to 5-HT (maximum contraction = 60 ± 15% of vehicle), but the reduction compared with vehicle was not as large compared with the general TG inhibitors. However, maximum contraction to KCl, a receptor-independent, depolarization-dependent contractant, was significantly reduced in the presence of MDC (maximum contraction to vehicle = 85 ± 2% of PE contraction; MDC = 16 ± 2%; P ≤ 0.05) and modestly but significantly reduced by cystamine (vehicle = 88 ± 3% of PE contraction; cystamine = 60 ± 5%; P ≤ 0.05). Z-DON did not significantly reduce maximal aortic contraction to KCl (vehicle = 92 ± 9% of PE contraction; Z-DON = 77 ± 5%; P > 0.05).
Fig. 2.
Rat aorta contraction to 5-hydroxytryptamine (5-HT) in the presence of TG inhibitors. The ability of rat aortae to contract to 5-HT was tested in the presence of TG inhibitors or vehicle. All inhibitors were able to reduce contraction of the aorta compared with vehicle. Global TG inhibitor cystamine (1 mmol/l) abolished contraction to 5-HT, while monodansylcadavarine (MDC; 500 μmol/l) significantly reduced contraction (maximum contraction = 28.6 ± 14.9% of vehicle). The TG2-specific inhibitor Z-DON (50 μmol/l) only slightly reduced contraction to 5-HT (maximum contraction = 60.2 ± 15.2% of vehicle). Contraction is reported as a percentage of initial contraction to phenylephrine (PE). *P ≤ 0.05, significantly reduced from vehicle.
Because the above data suggest that TG activity besides that derived from TG2 is present in arterial tissues, RT-PCR was performed to determine global TG mRNA expression in rat aorta and vena cava tissues. Primers targeted to rat TG1 through TG5, TG7, and FXIII, were used (Table 1). mRNA for four different TGs (TG1, TG2, TG4, and FXIII) was detected in these tissues (Table 2). When expressed relative to B2m mRNA, the levels of TG2, TG4, and FXIII mRNA were all similar in the rat aorta. Relative expression of TG1 in the rat aorta was low but significant. In the vena cava, TG2 had the highest relative expression levels. Relative TG1 mRNA expression was significantly higher in the rat vena cava than in the aorta. TG4 and FXIII mRNA expression was not significantly different between the two tissues. These data suggest that rat aorta and vena cava tissues have the potential to synthesize TG1, TG2, TG4, and FXIII proteins.
Table 2.
TG mRNA expression
| ΔCt |
||
|---|---|---|
| Aorta | Vena Cava | |
| TG1 | 0.15 ± 0.03 | 2.0 ± 0.6* |
| TG2 | 2 ± 1 | 12 ± 3* |
| TG3 | ND | ND |
| TG4 | 2.0 ± 0.5 | 2.4 ± 0.7 |
| TG5 | ND | ND |
| TG7 | 0.02 ± 0.01 | 0.03 ± 0.02 |
| FXIII | 3.7 ± 0.2 | 4 ± 1 |
Values are means ± SE; n = 3. ΔCt, change in threshold cycle. For simplicity, values have been multiplied by 1,000. Primers targeted to rat TG1 through TG5, TG7, and FXIII were used to measure mRNA expression in ∼10 mg of aorta or vena cava tissues. mRNAs to 4 of the TGs were found in both tissues: TG1, TG2, TG4, and FXIII. Negligible amounts of mRNA were also found for TG7. Expression of the TGs is expressed relative to B2m. ND, no detection of mRNA by RT-PCR analysis.
P ≤ 0.05, significantly different expression from aorta.
TG1, TG2, and TG4 proteins are found in rat aorta and vena cava tissues.
To determine if the TG mRNA detected by RT-PCR is translated into proteins, we performed Western analysis for these TGs in rat aorta and vena cava tissues. Western analysis of rat aorta and vena cava homogenates for TG1, TG2, TG4, and FXIII showed bands immunoreactive to TG1, TG2, and TG4 antibodies but not FXIII (Fig. 3). The positive control for TG1 (rat skin lysate) migrated slightly lower than the bands detected in the rat aorta and vena cava tissues. We speculate that this may be due to alternate processing of this protein in the different tissue types. Consistent with the RT-PCR results, densitometric analysis of the TGs (normalized to β-actin protein expression) revealed that vena cava expression was significantly higher than aortic expression for TG1 and TG2 (TG1: aorta = 0.51 ± 0.05, vena cava = 0.84 ± 0.07; TG2: aorta = 0.80 ± 0.02, vena cava = 1.1 ± 0.1 arbitrary densitometry units, normalized to β-actin protein levels; P ≤ 0.05). A trend towards higher expression in the vena cava was seen for TG4; this was not significant (aorta = 0.25 ± 0.08 and vena cava = 0.18 ± 0.01 arbitrary densitometry units, normalized to β-actin protein levels; P > 0.05). All three of the triplicate bands were used in the densitometry of TG4, as these three bands also appeared in the positive control. As positive controls, rat skin (TG1), human prostate (TG4), and HeLa cell lysates (FXIII) were used.
Fig. 3.
Western analysis of normal rat aorta and vena cava tissues. A: immunoblotting with anti-TG antibodies detects TG1, TG2, and TG4 in aorta and vena cava tissue homogenates but not Factor XIII (FXIII). +, Positive control (rat skin lysate for TG1, human prostate lysate for TG4 and HeLa cell lysate for FXIII). Each lane represents an aorta or vena cava homogenate derived from a different animal for a total of 4 animals. B: densitometry of TG1, TG2, and TG4 protein found in blots in A. Normalization of the TGs to β-actin indicates significantly more TG1 and TG2, but not TG4, is present in vena cava compared with aorta tissues (means ± SE: aorta TG1 = 0.51 ± 0.05, TG2 = 0.80 ± 0.02, TG4 = 0.18 ± 0.01; vena cava TG1 = 0.842 ± 0.069, TG2 = 1.1 ± 0.1, TG4 = 0.48 ± 0.08). Arrows point to bands used for quantification. *P ≤ 0.05, significantly different from vena cava.
Immunohistochemical staining of rat aorta and vena cava tissues further confirmed the existence of TG1 and TG4 in the vasculature (Fig. 4, A and B). Similar to what has been reported for TG2 (16), these TGs were present in the smooth muscle cell layers of these tissues (arrows), although not exclusively. Significant immunohistochemical staining of FXIII in the rat aorta and vena cava was not observed (Fig. 4C). Positive controls used were human skin (TG1), human prostate (TG4), and human placenta (FXIII).
Fig. 4.
Immunohistochemical staining of TGs in normal rat aorta and vena cava. A: TG1 in rat aorta and vena cava tissues. Human skin was used as a positive control. B: TG4 in rat aorta and vena cava tissues. Human prostate was used as a positive control. C: FXIII in rat aorta and vena cava tissues. Human placenta was used as a positive control. Representative of tissues from four different animals. Arrows point to regions of staining. No primary denotes that primary antibody was left out of the reaction. L, lumen of the vessel. Scale bar = 100 μm.
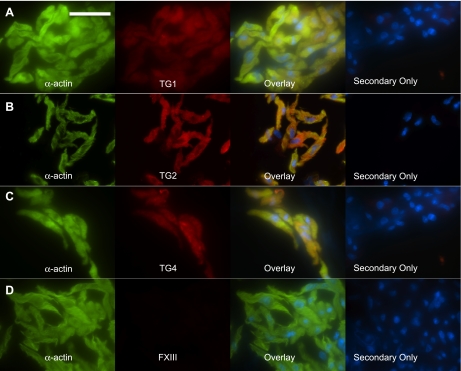
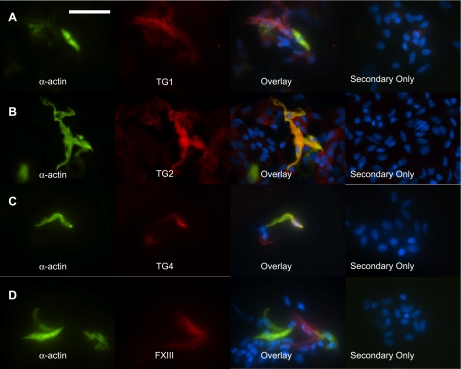
To confirm the presence of these TGs, including TG2, in smooth muscle cells, freshly isolated tissues were enzymatically dissociated and TGs were visualized by immunocytochemical staining. In these dissociations, significantly more cells were yielded from aorta tissues than from vena cava tissues, and a higher percentage of the cells was smooth muscle cells (verified by immunological staining of α-actin). This is consistent with the composition and size of the tissues, as the aorta is composed of mostly smooth muscle cells, while the vena cava only contains a single layer of smooth muscle cells. Freshly isolated aorta and vena cava vascular smooth muscle cells stained positive for TG1, TG2 and TG4 (Figs. 5 and 6). FXIII, in contrast, was not present in the smooth muscle cells from either tissue (Fig. 5D and 6D), although other cells in the dissociation would occasionally stain positive for FXIII (Fig. 6D). Taken together, these data suggest that aorta and vena cava tissues, and notably the vascular smooth muscle cells of these tissues, express TG1, TG2, and TG4.
Fig. 5.
TGs in freshly dissociated rat aorta smooth muscle cells. Rat thoracic aorta tissues were enzymatically dissociated to yield vascular smooth muscle cells. Confirmation that cells were smooth muscle cells was made by staining the cells with FITC-conjugated smooth muscle cell specific α-actin (green). Immunocytochemical detection of the TGs (red) demonstrated the presence of TG1 (A), TG2 (B), and TG4 (C) but not FXIII (D). Overlaying the red and green channels shows colocalization (yellow) of TG1, TG2, and TG4 with α-actin. Secondary only denotes cells from the same tissue dissociation that were incubated with secondary antibody but not primary antibodies. Representative of tissue dissociations from 4 different animals. Scale bar = 50 μm. Nuclei were stained with DAPI (blue).
Fig. 6.
TGs in freshly dissociated rat vena cava smooth muscle cells. Rat vena cava tissues were enzymatically dissociated to yield vascular smooth muscle cells. Confirmation that cells were smooth muscle cells was made by staining the cells with FITC-conjugated smooth muscle cell specific α-actin (green). Immunocytochemical detection of the TGs (red) demonstrated the presence of TG1 (A), TG2 (B), and TG4 (C) in smooth muscle cells. D: FXIII (red) was occasionally detected in the dissociation but was absent in smooth muscle cells (green). Overlaying the red and green channels shows colocalization (yellow) of TG1, TG2, and TG4 with α-actin. Secondary only denotes cells from the same tissue dissociation that were incubated with secondary antibody but not primary antibodies. Representative of tissue dissociations from 4 different animals. Scale bar = 50 μm. Nuclei are stained with DAPI (blue).
In situ assay specific for TG1 or TG2 activity demonstrates the functionality of these TGs in aorta and vena cava tissues.
A recently described (15) assay using fluorescent peptides that are specific substrates for TG1 (K5 peptide) and TG2 (T26 peptide) enzymes, enabling visualization of where the active TG enzymes are present, was performed on fresh frozen rat aorta and vena cava sections. TG4 was not included in these data, as a TG4-specific peptide substrate has yet to be developed. In both the aorta and vena cava, K5 and T26 incorporation, visualized by FITC fluorescence, was observed in these tissues (Fig. 7). Activity appeared primarily in the smooth muscle cell layers in both the aorta and vena cava (arrows), although activity was also seen in non-smooth muscle cell layers of the vena cava. Tissue sections incubated with the mutant peptide controls (K5QN and T26QN; FITC-conjugated peptides that contain the same amino acid composition but with the active glutamine replaced with an arginine residue, thus preventing incorporation by TG1 and TG2) did not show fluorescence. These data suggest that active TG1 and TG2 proteins are present in rat aorta and vena cava tissues.
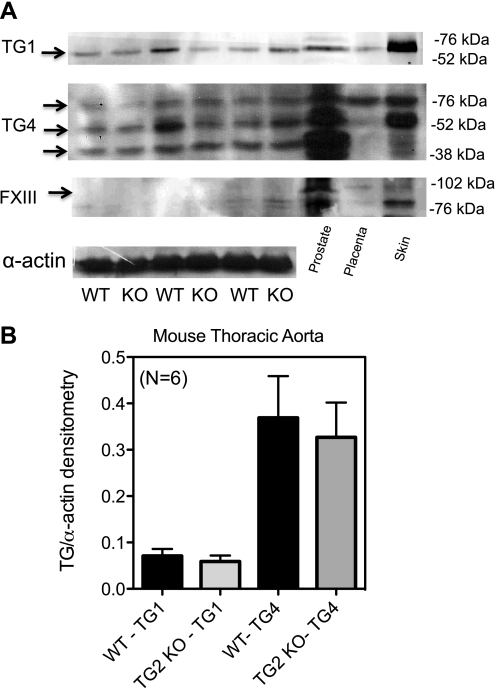
TG1, TG4, and FXIII are not upregulated in the TG2 KO mouse aorta.
To determine whether upregulation of other TGs present in the vessel could compensate for the loss of TG2 in the Melino KO mice, Western analysis was performed on these and WT aorta homogenates. Paralleling the findings in the rat, both TG1 and TG4 were detected in these tissues, while FXIII was absent (Fig. 8A). There was no significant difference between WT and TG2 KO aortic expression of TG1 and TG4 (Fig. 8B; densitometry of TG/α-actin: TG1 in TG2 WT = 0.071 ± 0.015, KO = 0.059 ± 0.013; TG4 in TG2 WT = 0.37 ± 0.09, KO = 0.33 ± 0.08 arbitrary densitometry units; all three of the triplicate bands were used in the quantification of TG4, as these three bands also appeared in the positive control).
Fig. 8.
TG isoform expression in the aorta of WT and TG2 KO mice. A: Western analysis of TG1, TG4, and FXIII in the TG2 KO and WT mouse. B: densitometric analysis of TG1 and TG4 in the TG2 KO mouse shows no statistical difference in the level of protein present between these 2 animals. Level of TG protein was compared with the amount of α-actin present. Arrows point to bands used for densitometry. Data represent aortae taken from 6 different WT and KO TG2 mice. P ≤ 0.05.
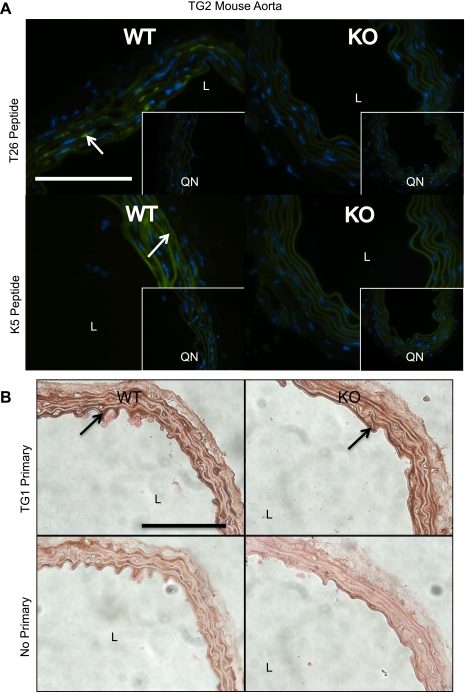
In the Graham TG2 KO mice, the abolition of TG2 activity was confirmed using the in situ assay for active TGs. Fresh frozen sections of the Graham TG2 KO mouse aorta incubated with T26 peptide showed decreased fluorescence compared with the WT (Fig. 9A, arrow points to area of TG2 activity), indicating a loss of TG2-specific TG activity in the KO. Surprisingly, while K5 incorporation, indicating TG1 activity, occurred in the TG2 WT mouse aortae, the KO mouse aortae did not show significant K5 incorporation (Fig. 9A; arrow points to area of TG1 activity), suggesting a loss of TG1-specific TG activity in these tissues. The difference in K5 incorporation between the TG2 WT and KO mouse aortae persisted when the concentration of K5 peptide was increased to 1 μmol/l (data not shown). For all sections treated with the K5QN and T26QN peptides, no fluorescence was detected outside of the elastic lamellae, which autofluoresced. The lack of TG1-specific activity in the TG2 KO aortae was not due to a lack of TG1 protein, as immunohistochemical staining for TG1 was detected in both the TG2 WT and KO mouse aortae (Fig. 9B).
Fig. 9.
In situ detection of TG activity and immunohistochemical detection of TG1 in WT and TG2 KO mouse aortae. A: sections of fresh frozen sections of aorta from WT and TG2 KO mice were assayed for their ability to incorporate K5 (TG1 activity) or T26 (TG2 activity). Representative of aortic sections from 5 WT and 5 KO mice. K5QN and T26QN are the mutant, negative control peptides for TG1 and TG2, respectively. Arrow points to areas of TG activity. Nuclei are stained with DAPI. B: immunohistochemical analysis shows that TG1 is present in aorta from both the WT (top) and TG2 KO (bottom) mice. Arrows point to darkened areas of TG1 staining. Representative of tissues from 5 different WT and TG2 KO mice. Scale bars = 100 μm. Nuclei are stained with DAPI (blue).
DISCUSSION
Scope and significance.
In the present study, we demonstrate the presence of TG2-independent TG activity in aorta and vena cava tissues. To elucidate the origin of this TG activity, a global analysis of the known TGs was performed in these tissues. We provide evidence that, in addition to TG2, these tissues also express two other TGs: TG1 and TG4. In the case of TG1, we provide evidence that this TG is indeed functional.
Expression of TGs in the vasculature.
To date, TG1, TG2, and FXIII have been the only TGs reported in the vasculature. Of these, only TG2 has been reported in vascular smooth muscle cells. In our studies, we detected TG1, TG2, and TG4 in aorta and vena cava tissues, specifically in smooth muscle cells from these tissues. The detection of TG4 in these tissues is especially notable, as it is in direct contrast to previous reports (7, 11) that have suggested that TG4 expression was restricted to the prostate. One of these studies specifically searched for TG4 mRNA in a number of different tissues, including the aorta. Possible explanations for this discrepancy include potential species differences, as our studies looked at rat tissues while human tissues were used in the other studies. Although the condition in human tissues is of greater importance than that of rat, knowing that species-specific differences exist is important when using results found in rats to extrapolate to the human condition. Additionally, it is possible that the primers used are specific for alternatively spliced forms of TG4 (7). Further investigation will be required to elucidate the cause for the discrepancy between our findings and previous studies.
While TG1, TG2, and TG4 were found both in aorta and vena cava tissues, the expression levels for these TGs were not equal in these two tissues. In all cases, the vena cava expressed mRNA that was either comparable (TG4 and FXIII) or significantly higher (TG1 and TG2) than aortic expression (Table 2). Similar levels of TG4 protein were found between aorta and vena cava tissues (Fig. 3). TG4 was found in all layers of the aorta and vena cava (Fig. 4B). Western analysis detected a triplicate-banding pattern for TG4 (Fig. 4). While TG4 has been reported to migrate to ∼77 kDa, the additional bands detected may indicate alternatively spliced forms of TG4 (7). Because the bands appeared in the positive control as well as the aorta and vena cava samples, they were included in the analysis of TG4.
In contrast to TG4, significantly more TG1 and TG2 protein was detected in the vena cava vs. the aorta (Fig. 3). The higher expression of TG1 and TG2 in vena cava tissues is consistent with both the RT-PCR data and the greater florescent intensity in the smooth muscle cell layers seen with the K5 and T26 peptides, indicating active TG1, in fresh frozen vena cava vs. aorta tissues (Fig. 7, top; note that the green levels have been increased in the rat aorta K5 and K5QN peptide control to better illustrate where TG1 activity is present; the other images have been unaltered). Also notable, the molecular mass of the bands detected by the anti-TG1 antibody was between 76 and 52 kDa (Figs. 4A and 8A), not the typically reported 90- to 106-kDa size of full-length TG1. However, the full-length TG1 has been reported to be an inactive zymogen that requires proteolytical processing for catalytic activity (31). The proteolytically processed human TG1 has been reported as a 66/33/10 kDa complex (31), which is consistent with the band observed for TG1 in Western analysis. A surprise finding in this study was the lack of K5 incorporation in the TG2 KO mouse (Fig. 9A), indicating a lack of TG1 activity in the aortae of these mice. TG1 was found to be present in these sections by immunohistochemical staining (Fig. 9B), so the loss of K5 incorporation was not due to a difference in TG1 expression between the KO and WT mice. These data may suggest that, in mouse aorta tissues, TG2 is necessary for the full activation of TG1, either by interacting with TG1 directly, or by acting indirectly to activate TG1.
Although mRNA for FXIII was found in rat aorta and vena cava tissues (Table 2), and immunocytochemical detection was seen in some cells of freshly dissociated vena cava tissues (Fig. 6D), no protein was detected by Western analysis (Fig. 3) or immunohistochemistry (Fig. 4) in rat aorta and vena cava homogenates nor was FXIII staining detected in freshly dissociated smooth muscle cells (Figs. 5 and 6). While it is possible that the mRNA detected in the rat aorta and vena cava is not translated into protein, another likely explanation for the mRNA detected may be that it is derived from macrophages or other FXIII-expressing cells that infiltrate the vasculature (21). This would explain why FXIII was detected in a limited number of cells in freshly dissociated vena cava tissues (Fig. 6D). Finally, our findings do not exclude the possibility that other TGs, or enzymes that carry out a TG-like reaction, are not present in the vasculature, as we did not perform in silico or functional searches for novel TGs. Together, these data suggest that, in addition to TG2, TG1 and TG4 are also present in the vasculature, specifically in aorta and vena cava smooth muscle cells.
Functional significance of the presence of TG1 and TG4 in the vasculature.
The presence of these other TGs in vascular smooth muscle cells helps explain why TG2 KO mice do not exhibit a drastic change in vascular function (23), despite the implication of TG activity in amine-mediated contraction of the rat aorta and vena cava (16, 38). This study found that the TG2-specific inhibitor Z-DON only partially reduces 5-HT-induced contraction in the aorta (Fig. 2, right), while the more general TG inhibitors MDC and cystamine reduce 5-HT-induced contraction to a greater extent (Fig. 2, left). However, a number of limitations to these data require mentioning. One limitation is the lack of membrane-permeable, TG2-specific inhibitors other than Z-DON. While the IC50 for Z-DON is 0.05 μmol/l, Z-DON only becomes cell permeable at 50 μmol/l, the concentration used in this study. Higher concentrations of Z-DON (≥80 μmol/l) have been reported to elicit cytotoxicity (28), and because Z-DON is a fairly new inhibitor, it is not clear whether at higher concentrations it will have off target effects or begin cross-reacting with other TGs. This limits us from using higher concentrations of this inhibitor. The extensive use of MDC and cystamine as general TG activity inhibitors despite having off-target effects, such as inhibition of caspase activity by cystamine (1, 17) and inhibition of receptor internalization by MDC (9), is due to the lack of more specific TG inhibitors. The contractile data of this study should be interpreted with these limitations taken into consideration. However, these data, and the observation that vascular tissues from TG2 KO mice have normal contractile phenotypes (3), support our hypothesis that more TGs besides TG2 contribute to vascular contractility.
The existence of TGs other than TG2 is consistent with the observation that TG2 KO mouse aortae can incorporate BAP into their proteins, resulting in a banding pattern similar to that of the WT mice (Fig. 1C). While the banding patterns between the KO and WT mice were similar in these two assays, distinct differences in banding intensity could be seen between the KO and WT (Fig. 1C) although a significant variance was observed between mice pairs (data not shown). The difference in BAP incorporation in rat aorta homogenates incubated with the general inhibitor cystamine compared with Z-DON (Fig. 1D) supports the idea that other TGs besides TG2 are functional in aortic tissues. Because the homogenates represent a cell-free environment, the concentration of Z-DON used in these experiments (50 μmol/l) should fully inhibit TG2 activity. This experiment demonstrates that in rat aortae that express TG2, further TG activity is present when TG2 activity is fully blocked.
The utilization of TG2 WT and KO mouse pairs from two different colonies.
In these studies, we made use of TG2 KO and WT mouse pairs from two different colonies, originally described by Nanda et al. (23) and De laurenzi and Melino (10). Both pairs of WT and KO mice have a C57BL/6 background: the Graham mice have been backcrossed onto this background, while the Melino mice were generated in a Svj 129-C57Bl/6 mixed background and have not been backcrossed. We performed Western analysis (Figs. 1A and 8A) and BAP TG assays (Fig. 1C) on aortae from the Melino TG2 mice. In contrast, our in situ TG-specific assay was performed on fresh frozen sections of aorta from the Graham KO and WT mice (Fig. 9A) and TG1 was visualized immunohistochemically in this background (Fig. 9B). While it may be convenient to extrapolate our findings between the two pairs of mice, caution should be taken in doing so because, while these mice originate from a similar genetic background, they are derived from different colonies. Although unlikely, it is possible that the expression of one or more of the TGs may vary between the colonies. An interesting study would investigate the possible variance of the multiple TGs found between these mice pairs. Although it is difficult to make quantitative comparisons between the two pairs of mice from our data, qualitatively we are able to show some similarities between them. TG2 protein detected in WT mouse aortae was lost in aortae from KO mice of the Melino background (Fig. 1B), while fresh frozen sections of the Graham KO mouse aortae showed a loss of TG2 activity compared with WT (Fig. 9A). Additionally, we demonstrate the presence of TG1 in both strains: by Western analysis in the Melino mice (Fig. 8A), and by immunohistochemically in the Graham mice (Fig. 9B). Note that these data only demonstrate that TG1 is present in both mouse pairs; direct comparisons between the amount of TG1 expressed and its functionality in the aortae of the two mice pairs cannot be made from our data.
Conclusions and significance.
In conclusion, the present study demonstrates the presence of three TGs in the vasculature: TG1, TG2, and TG4. The novelty of our detection of TG1 and TG4 is highlighted in that only a few studies have suggested that TG1 is present in cardiovascular tissues and TG4 has been thought to have prostate-specific expression. All three of these TGs were present in vascular smooth muscle cells, where their redundant activity may contribute to the mild cardiovascular phenotype of the TG2 KO mice. Although the individual importance of each of the TGs to vascular function was beyond the scope of this study, our data showed that BAP incorporation into WT and KO aorta proteins (Fig. 1C) was similar but did show specific differences between WT and KO. These data suggest that while redundancy in protein targets for each of the TGs occurs, some proteins may be preferential targets for certain TGs. At this time, more information is needed to determine what function each TG is playing the vasculature. This would include identification of protein substrates targeted by the specific TGs, identification of the subcellular localization of the TGs, and whether the expression and function of the individual TGs are regulated under certain physiological conditions. Specifically, elucidating the individual importance of these TGs in vascular functions dependent on TG activity, such as angiogenesis (8), fibroblast adhesion (14), and small artery remodeling (2), as well as their contribution to disease states such as hypertension and athrosclerosis (14), is an exciting prospect for future research.
GRANTS
The work was supported by American Heart Association Award No. 11PRE7290038 and Program Project Grant PO1-HL-70687.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.B.J., K.H., M.I., G.V.J., G.C., and S.W.W. conception and design of research; K.B.J., H.P.-J., J.M.T., and S.W.W. performed experiments; K.B.J., H.P.-J., J.M.T., and S.W.W. analyzed data; K.B.J., H.P.-J., J.M.T., K.H., E.N.B., G.V.J., G.C., and S.W.W. interpreted results of experiments; K.B.J. and S.W.W. prepared figures; K.B.J. drafted manuscript; K.B.J., J.M.T., K.H., M.I., E.N.B., G.V.J., and S.W.W. edited and revised manuscript; K.B.J., H.P.-J., J.M.T., K.H., M.I., E.N.B., G.V.J., G.C., and S.W.W. approved final version of manuscript.
REFERENCES
- 1. Bailey CD, Johnson GV. The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging 27: 871–879, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res 96: 119–126, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Bakker EN, Pistea A, Spaan JA, Rolf T, de Vries CJ, van Rooijen N, Candi E, VanBavel E. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res 99: 86–92, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res 45: 271–278, 2008. [DOI] [PubMed] [Google Scholar]
- 5. Baumgartner W, Golenhofen N, Weth A, Hiiragi T, Saint R, Griffin M, Drenckhahn D. Role of transglutaminase 1 in stabilisation of intercellular junctions of the vascular endothelium. Histochem Cell Biol 122: 17–25, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Baumgartner W, Weth A. Transglutaminase 1 stabilizes beta-actin in endothelial cells correlating with a stabilization of intercellular junctions. J Vasc Res 44: 234–240, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Cho SY, Choi K, Jeon JH, Kim CW, Shin DM, Lee JB, Lee SE, Kim CS, Park JS, Jeong EM, Jang GY, Song KY, Kim IG. Differential alternative splicing of human transglutaminase 4 in benign prostate hyperplasia and prostate cancer. Exp Mol Med 42: 310–318, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dardik R, Loscalzo J, Inbal A. Factor XIII (FXIII) and angiogenesis. J Thromb Haemost 4: 19–25, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Davies PJ, Cornwell MM, Johnson JD, Reggianni A, Myers M, Murtaugh MP. Studies on the effects of dansylcadaverine and related compounds on receptor-mediated endocytosis in cultured cells. Diabetes Care 7, Suppl 1: 35–41, 1984. [PubMed] [Google Scholar]
- 10. De Laurenzi V, Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol 21: 148–155, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubbink HJ, Verkaik NS, Faber PW, Trapman J, Schroder FH, Romijn JC. Tissue specific and androgen-regulated expression of human prostate-specific transglutaminase. Biochem J 315: 901–908, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Folk JE, Park MH, Chung SI, Schrode J, Lester EP, Cooper HL. Polyamines as physiological substrates for transglutaminases. J Biol Chem 255: 3695–3700, 1980. [PubMed] [Google Scholar]
- 13. Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J 368: 377–396, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haroon ZA, Wannenburg T, Gupta M, Greenberg CS, Wallin R, Sane DC. Localization of tissue transglutaminase in human carotid and coronary artery atherosclerosis: implications for plaque stability and progression. Lab Invest 81: 83–93, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Itoh M, Kawamoto T, Tatsukawa H, Kojima S, Yamanishi K, Hitomi K. In situ detection of active transglutaminases for keratinocyte type (TGase 1) and tissue type (TGase 2) using fluorescence-labeled highly reactive substrate peptides. J Histochem Cytochem 59: 180–187, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson KB, Thompson JM, Watts SW. Modification of proteins by norepinephrine is important for vascular contraction. Front Physiol 1: 12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem 278: 3825–3830, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Wei L, Laskin DL, Fanburg BL. Role of protein transamidation in serotonin-induced proliferation and migration of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 44: 548–555, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorand L. Factor XIII and the clotting of fibrinogen: from basic research to medicine. J Thromb Haemost 3: 1337–1348, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4: 140–156, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Muszbek L, Adany R, Mikkola H. Novel aspects of blood coagulation factor XIII. I. Structure, distribution, activation, and function. Crit Rev Clin Lab Sci 33: 357–421, 1996. [DOI] [PubMed] [Google Scholar]
- 22. Nahrendorf M, Hu K, Frantz S, Jaffer FA, Tung CH, Hiller KH, Voll S, Nordbeck P, Sosnovik D, Gattenlohner S, Novikov M, Dickneite G, Reed GL, Jakob P, Rosenzweig A, Bauer WR, Weissleder R, Ertl G. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation 113: 1196–1202, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem 276: 20673–20678, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Ni W, Geddes TJ, Priestley JR, Szasz T, Kuhn DM, Watts SW. The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol 154: 663–674, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pisano JJ, Finlayson JS, Peyton MP. [Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine]. Science 160: 892–893, 1968. [DOI] [PubMed] [Google Scholar]
- 26. Rasband WS. ImageJ (Online). National Insitutes of Health, Bethesda, MD: http://rsbinfo.nih.gov/ij/ [1997–2011] [Google Scholar]
- 27. Sane DC, Kontos JL, Greenberg CS. Roles of transglutaminases in cardiac and vascular diseases. Front Biosci 12: 2530–2545, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaertl S, Prime M, Wityak J, Dominguez C, Munoz-Sanjuan I, Pacifici RE, Courtney S, Scheel A, Macdonald D. A profiling platform for the characterization of transglutaminase 2 (TG2) inhibitors. J Biomol Screen 15: 478–487, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Slaughter TF, Achyuthan KE, Lai TS, Greenberg CS. A microtiter plate transglutaminase assay utilizing 5-(biotinamido)pentylamine as substrate. Anal Biochem 205: 166–171, 1992. [DOI] [PubMed] [Google Scholar]
- 30. Smethurst PA, Griffin M. Measurement of tissue transglutaminase activity in a permeabilized cell system: its regulation by Ca2+ and nucleotides. Biochem J 313: 803–808, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steinert PM, Chung SI, Kim SY. Inactive zymogen and highly active proteolytically processed membrane-bound forms of the transglutaminase 1 enzyme in human epidermal keratinocytes. Biochem Biophys Res Commun 221: 101–106, 1996. [DOI] [PubMed] [Google Scholar]
- 32. Stephens P, Grenard P, Aeschlimann P, Langley M, Blain E, Errington R, Kipling D, Thomas D, Aeschlimann D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J Cell Sci 117: 3389–3403, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Thakali K, Galligan JJ, Fink GD, Gariepy CE, Watts SW. Pharmacological endothelin receptor interaction does not occur in veins from ET(B) receptor deficient rats. Vascul Pharmacol 49: 6–13, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomazy V, Fesus L. Differential expression of tissue transglutaminase in human cells. An immunohistochemical study. Cell Tissue Res 255: 215–224, 1989. [DOI] [PubMed] [Google Scholar]
- 35. Tseng HC, Lin HJ, Tang JB, Gandhi PS, Chang WC, Chen YH. Identification of the major TG4 cross-linking sites in the androgen-dependent SVS I exclusively expressed in mouse seminal vesicle. J Cell Biochem 107: 899–907, 2009. [DOI] [PubMed] [Google Scholar]
- 36. Ueki S, Takagi J, Saito Y. Dual functions of transglutaminase in novel cell adhesion. J Cell Sci 109: 2727–2735, 1996. [DOI] [PubMed] [Google Scholar]
- 37. Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115: 851–862, 2003. [DOI] [PubMed] [Google Scholar]
- 38. Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLos One 4: e5682, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang J, Lesort M, Guttmann RP, Johnson GV. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J Biol Chem 273: 2288–2295, 1998. [DOI] [PubMed] [Google Scholar]