Abstract
During moderate actual or simulated hemorrhage, as cardiac output decreases, reductions in systemic vascular conductance (SVC) maintain mean arterial pressure (MAP). Heat stress, however, compromises the control of MAP during simulated hemorrhage, and it remains unknown whether this response is due to a persistently high SVC and/or a low cardiac output. This study tested the hypothesis that an inadequate decrease in SVC is the primary contributing mechanism by which heat stress compromises blood pressure control during simulated hemorrhage. Simulated hemorrhage was imposed via lower body negative pressure (LBNP) to presyncope in 11 passively heat-stressed subjects (increase core temperature: 1.2 ± 0.2°C; means ± SD). Cardiac output was measured via thermodilution, and SVC was calculated while subjects were normothermic, heat stressed, and throughout subsequent LBNP. MAP was not changed by heat stress but was reduced to 45 ± 12 mmHg at the termination of LBNP. Heat stress increased cardiac output from 7.1 ± 1.1 to 11.7 ± 2.2 l/min (P < 0.001) and increased SVC from 0.094 ± 0.018 to 0.163 ± 0.032 l·min−1·mmHg−1 (P < 0.001). Although cardiac output at the onset of syncopal symptoms was 37 ± 16% lower relative to pre-LBNP, presyncope cardiac output (7.3 ± 2.0 l/min) was not different than normothermic values (P = 0.46). SVC did not change throughout LBNP (P > 0.05) and at presyncope was 0.168 ± 0.044 l·min−1·mmHg−1. These data indicate that in humans a cardiac output adequate to maintain MAP while normothermic is no longer adequate during a heat-stressed-simulated hemorrhage. The absence of a decrease in SVC at a time of profound reductions in MAP suggests that inadequate control of vascular conductance is a primary mechanism compromising blood pressure control during these conditions.
Keywords: hyperthermia, lower body negative pressure, blood pressure, vascular resistance
avoidance of syncope depends on the ability to maintain brain blood flow in the face of falling arterial blood pressure. Brain blood flow is compromised during profound drops in arterial blood pressure, the latter of which is governed by cardiac output and systemic vascular conductance (SVC). Blood pressure control, and thus the ability to avoid syncope, are severely impaired in the heat-stressed human (15, 35), but it remains unknown whether this is due to an inadequate cardiac output and/or inadequate control of SVC.
Passive heat stress leads to cutaneous vasodilation and thus increased skin blood flow. Although vascular conductance in noncutaneous beds (i.e., splanchnic, renal, and muscle) decreases in response to heat stress, that decrease is not sufficient to counter cutaneous vasodilation, resulting in large increases in SVC (4, 24, 25). In mildly heat-stressed individuals, head-up tilt in the absence of syncopal symptoms decreases SVC, but this decrease is lower relative to when individuals are normothermic (20, 35, 36). Despite those observations, SVC responses leading up to and just before syncope (i.e., presyncope) remain unknown. Identifying SVC responses leading up to and at presyncope may provide insight into the mechanisms contributing to compromised blood pressure control while in this thermal condition.
We (4) recently observed relatively small decreases in cutaneous vascular conductance during simulated hemorrhage to presyncope in heat-stressed subjects. Given that upwards to 50% of cardiac output is directed towards the skin during heat stress (24), coupled with very little cutaneous vasoconstriction at presyncope in heat-stressed subjects, it is possible that insufficient reductions in vascular conductance is a primary mechanism for compromised blood pressure control during a simulated hemorrhagic challenge. However, SVC includes cutaneous and noncutaneous beds, and it remains unknown what changes occur in noncutaneous beds, which potentially has a large influence on SVC during a heat-stressed-simulated hemorrhagic challenge to presyncope.
Large increases in cardiac output during heat stress are necessary for arterial blood pressure to be maintained during pronounced increases in SVC. The magnitude of the increase in cardiac output during heat stress has implications for the ability to withstand a simulated hemorrhage, likely by allowing for a greater “reserve” from which cardiac output can decrease before the onset of presyncopal symptoms (2, 15). However, the magnitude of the reduction in cardiac output leading up to and at the point of syncope in the heat-stressed human remains unknown.
This study evaluated cardiac output and SVC responses during profound passive heat stress (elevation in core temperature of ∼1.2°C), as well as throughout a simulated hemorrhagic challenge via lower body negative pressure (LBNP) until signs of ensuing syncope were evident. It was hypothesized that leading up to and at presyncope, cardiac output would progressively decrease relative to pre-LBNP heat-stressed values. This decrease in cardiac output would be accompanied by minimal changes in SVC, thereby implicating inadequate decreases in SVC as a major determinant for heat-stressed-induced compromised blood pressure control.
METHODS
Subjects.
Eleven males with a means ± SD age, mass, and height of 24 ± 3 years, 77.5 ± 8.1 kg, and 181 ± 7 cm, respectively, participated in this study. Subjects were nonsmokers, not taking medications, and were free of any known cardiovascular, metabolic, or neurological diseases. Written informed consent was obtained from all subjects before participating in this study. Study procedures were approved by the Ethics Committee of Copenhagen (H-1–2010-040) and were performed in accordance with the Declaration of Helsinki.
Instrumentation and measurements.
Each subject was dressed in a water-perfused, tube-lined suit (Med-Eng, Ottawa, Canada) that covered the entire body, except the head, face, hands, feet, and one forearm. The suit permitted the control of skin and core temperatures by changing the temperature of the perfusing water. Each subject was then placed into an LBNP chamber, sealed at the iliac crest, while in the supine position.
A catheter was placed in the brachial artery of the nondominant arm for the measurement of arterial pressure, a flow-directed pulmonary arterial catheter (93A-831H-7.5F; Baxter Healthcare, Irvine, CA) was introduced through the basilic vein, and pulmonary temperature was measured from a thermistor at the tip of the catheter. Mean skin temperature was measured via the weighted average of six thermocouples attached to the skin (31). Cardiac output was measured via thermodilution through the pulmonary artery catheter, repeated two to four times, at baseline and each level of heat stress. Cardiac output was also obtained intermittently throughout subsequent LBNP, but as presyncope became imminent, measures were repetitively obtained until the end of LBNP. Vascular pressures were referenced to atmospheric pressure via uniflow pressure transducers (Baxter Healthcare) zeroed 5 cm below the sternal angle and connected to a pressure monitoring system (Dialogue 2000; IBC-Danica, Copenhagen, Denmark). Heart rate was obtained from an electrocardiogram. Stroke volume was calculated by dividing cardiac output by heart rate. SVC was calculated by dividing cardiac output by mean arterial pressure (MAP).
After instrumentation, subjects were supine for ≥30 min before normothermic measures, during which time 34°C water was perfused through the tube-lined suit. Subjects were then exposed to a passive heat stress by perfusing ∼49°C water through the suit until core temperature increased ∼1.2°C. While remaining heat stressed, subjects underwent an LBNP tolerance test until the onset of presyncopal symptoms. For this evaluation, LBNP began at 15 mmHg below atmospheric pressure and after ∼4 min LBNP was increased to 40 mmHg below atmospheric pressure and remained at that level until presyncopal symptoms developed, defined by the following criteria: continued self-reporting by the subject of feeling faint or nauseous, continued systolic blood pressure of <80 mmHg, and/or relative bradycardia accompanied by narrowing of pulse pressure (30, 34, 35).
Plasma catecholamine concentrations were measured in blood samples obtained at normothermia, after core temperature increased ∼1.2°C, and immediately upon LBNP termination. Arterial blood was drawn into a tube containing EDTA (Vacutainer system) and immediately suspended into ice-water and spun (2,000 g for 10 min) within 60 min and then stored at −80°C until analysis. Analysis was performed using a commercially available enzyme-linked immunosorbent assay kit (2-CAT ELISA; Labor Diagnostika Nord; lower level of detection norepinephrine: 44 pg/ml, epinephrine: 11 pg/ml).
Data analysis.
Blood pressure, heart rate, and skin temperature were sampled at 200 Hz via data acquisition (Biopac System, Santa Barbara, CA). Data were analyzed at normothermia, after an ∼0.7 and 1.2°C core temperature increase, and throughout LBNP. Cardiac output and related cardiovascular measures during LBNP, independent of LBNP level, were analyzed post hoc at the time points closest to 25, 50, 75, and 100% of duration of the LBNP exposure, with the exception of one subject in which a cardiac output was not obtained at 50%. These measures were obtained at time points corresponding to 25 ± 7, 51 ± 7, 76 ± 5, and 97 ± 3% of LBNP duration. Changes in each variable over the course of the protocol (i.e., baseline, heating, and LBNP) were examined with a one-way repeated-measures ANOVA. In the event of a significant main effect, a Bonferroni post hoc test was used to examine differences between each time point and normothermia along with difference from peak heat stress (i.e., 1.2°C). Catecholamine data failed the Shapiro-Wilkinson test for normality; thus Friedman nonparametric, repeated measures test was used to test for variance, followed by a Student-Newman-Keuls signed rank post hoc test. Data were analyzed using SigmaPlot 11.2 (Systat Software, San Jose, CA) with significance set at P < 0.05 and reported as means ± SD.
RESULTS
Passive heat stress elevated pulmonary artery blood temperature from 36.8 ± 0.3 to 37.4 ± 0.3 and then to 38.0 ± 0.2°C for normothermia, mid, and final heat stress levels (P < 0.001 between all measures). At these time points, mean skin temperature increased from 35.0 ± 0.6 to 38.4 ± 0.5°C (P < 0.001) but then plateaued at 38.7 ± 0.6°C (P > 0.99) when internal temperature was elevated 1.2°C.
Plasma epinephrine concentrations increased from normothermia (39.3 ± 24.4 pg/ml) to peak heat stress (51.1 ± 27.3 pg/ml) and was greatest immediately post-LBNP (128.2 ± 104.9 pg/ml; all comparisons P < 0.05). Plasma norepinephrine concentrations followed the same trend; there was an increase from normothermia (72.4 ± 25.3 pg/ml) to peak heat stress (150.6 ± 54.6 pg/ml) to post-LBNP (279.8 ± 231.7 pg/ml; all comparisons P < 0.05).
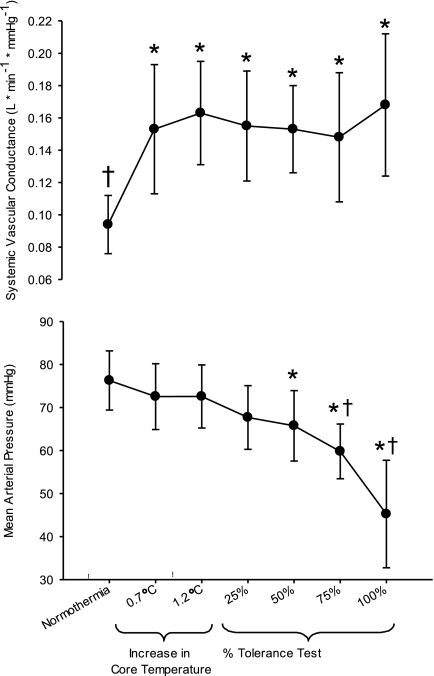
MAP remained stable throughout heating (P > 0.05 compared with normothermia). At 50, 75, and 100% of the LBNP test, MAP was lower than both normothermic and heat stress levels (all P ≤ 0.011). From 75 to 100% of LBNP duration (i.e., termination of the test), MAP precipitously dropped such that final MAP was 45 ± 12 mmHg (Fig. 2). Time to presyncope was 469 ± 179 s.
Fig. 2.
Systemic vascular conductance and mean arterial pressure at normothermia, after core temperature was increased ∼0.7°C and 1.2°C, and during the simulated hemorrhage to presyncope. *Significantly different from normothermia (P < 0.05). †Significantly different from heat stress (i.e., 1.2°C) just before the onset of lower body negative pressure (P < 0.05).
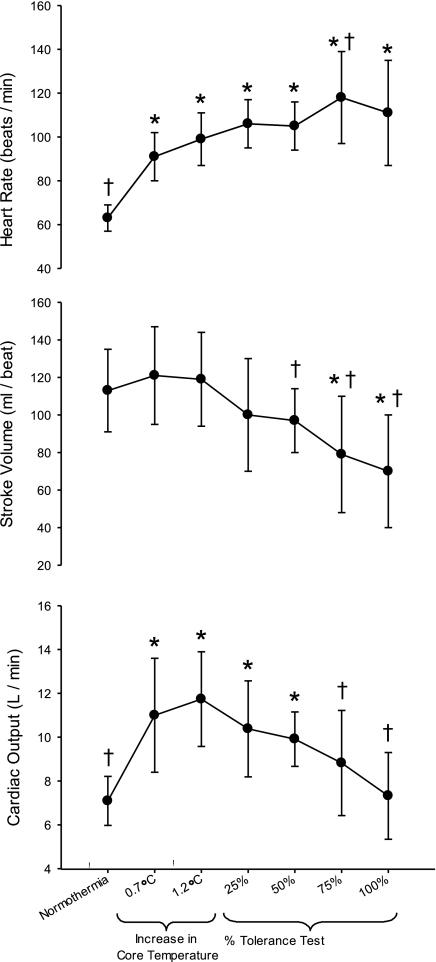
Heart rate was elevated above baseline normothermia at all levels of heat stress and LBNP (all P < 0.001; Fig. 1). Stroke volume remained similar to normothermia at 0.7 and 1.2°C elevations in internal temperature (P > 0.99). However, at 75 and 100% of the LBNP test, stroke volume was lower than at normothermia (P < 0.001). Compared with peak heat stress (i.e., 1.2°C), stroke volume was lower at 50, 75, and 100% of the LBNP test (P ≤ 0.007; Fig. 1). Heat stress elevated cardiac output above normothermic baseline (P < 0.001) and remained elevated at 25 and 50% of the completion of the LBNP test (P < 0.001). However, at 75 and 100% of the LBNP duration, cardiac output was lower relative to that at 1.2°C (P < 0.001). When LBNP was terminated, cardiac output was not different relative to normothermic baseline (P > 0.99).
Fig. 1.
Heart rate, stroke volume, and cardiac output at normothermia, after core temperature was increased ∼0.7°C and 1.2°C, and during simulated hemorrhage to presyncope. *Significantly different from normothermia (P < 0.05). †Significantly different from heat stress (i.e., 1.2°C) just before the onset of lower body negative pressure (P < 0.05).
SVC increased at both levels of heating relative to normothermia (P < 0.001). However, despite LBNP reducing MAP to 45 ± 12 mmHg, there were no differences in SVC at any level of LBNP relative to corresponding measures obtained at 1.2°C elevation in internal temperature (all P > 0.99; Fig. 2).
DISCUSSION
The two primary findings from this study are as follows: 1) cardiac output at the onset of syncopal symptoms in heat-stressed humans is similar to baseline normothermic cardiac output; and 2) SVC did not decrease during simulated hemorrhage sufficient to provoke syncopal symptoms despite a progressive and profound reduction in MAP. These data demonstrate that a cardiac output sufficient to maintain MAP in normothermic conditions is no longer adequate during a heat-stressed simulated hemorrhagic challenge and that impaired blood pressure control during heat stress is primarily due to an absence of a decrease in SVC in response to decreasing cardiac output during a hypotensive challenge.
Just before the LBNP challenge when the subjects were heat stressed, cardiac output was ∼5 l/min greater than normothermic values. This elevated cardiac output potentially allows for a greater range for cardiac output to decrease during a simulated hemorrhagic challenge before the onset of syncopal symptoms, relative to when individuals are normothermic (11, 13). At the end of the LBNP challenge, cardiac output was similar relative to when these individuals were normothermic. This is in contrast to observations at presyncope under normothermic conditions when cardiac output is always below pre-LBNP levels (11, 13, 21, 37). The reason that a cardiac output that is adequate to maintain MAP in normothermic conditions is no longer sufficient during a heat-stressed simulated hemorrhagic challenge (Fig. 1) is due to the pronounced increase in SVC with heat stress that is unchanged during the simulated hemorrhagic challenge. Put another way, during heat stress a cardiac output of ∼7 l/min is not sufficient to maintain MAP while SVC remains approximately double its normothermic value (Fig. 2).
Central blood volume, ventricular filling pressures, and end-diastolic left ventricular volume are reduced with passive heat stress (1, 2, 5, 15, 22). However, when central blood volume and ventricular filling pressure are preserved during heat stress via volume infusion, resulting in a ∼3 l/min elevation in cardiac output compared with heat stress alone (2), LBNP tolerance is similar to that established during normothermia (15). Those observations, together with the present findings, suggest that acute increases in cardiac output during heat stress (via volume infusion) compensates for attenuated reductions in SVC such that MAP is better maintained during a simulated hemorrhagic challenge. In other words, elevating cardiac output in heat-stressed individuals by volume infusion attenuates the magnitude of the reduction in arterial blood pressure during a simulated hemorrhagic challenge, likely through a greater reserve by which cardiac output could be reduced before MAP being so low to provoke syncopal symptoms (15). It also possible that just before syncopal symptoms when left ventricular filling is greatly diminished, there is a stimulation of ventricular receptors leading to bradycardia and vasodilation [the Bezold-Jarisch reflex (18)]. If this occurred, any signals leading to systemic vasodilation were offset by baroreceptor unloading-induced vasoconstriction, resulting in no net change in SVC (Fig. 2). Regardless, increasing left ventricular end-diastolic volume with volume loading may attenuate the onset of bradycardia and vasodilation through ventricular receptors (18) and thereby partly explain the mechanism by which volume loading improves orthostatic tolerance in heat-stressed subjects (15).
It is well established that during an orthostatic challenge in normothermic conditions, SVC decreases at the onset of the challenge and then continues to decrease either throughout the orthostatic challenge or until just before syncopal symptoms (11, 17, 21, 37). Three classic studies exposed normothermic individuals, while instrumented in a similar manner as the present study, to graded LBNP until symptoms of syncope (11, 21, 37). SVC consistently decreased throughout LBNP, such that at presyncope SVC was reduced 27–43% from pre-LBNP values. This is contrary to the present data in which SVC did not change from baseline heat stress (1.2°C: 0.163 l·min−1·mmHg−1) to presyncope (0.168 l·min−1·mmHg−1). Thus it is clear the decreases in SVC that occur during LBNP to presyncope in normothermic individuals (11, 21, 37) fail to occur while heat stressed (Fig. 2). Given these differences, it is not surprising that heat stress can reduce one's tolerance to a simulated hemorrhage by ∼80% compared with normothermic conditions (15).
The mechanism(s) for the lack of decrease in SVC during the simulated hemorrhage challenge during heat stress is not well understood. SVC is the sum of the vascular conductances in all vascular beds. Thus an absence of a decrease in SVC during LBNP does not necessarily indicate an absence of a change in vascular conductance of a particular vascular bed. Although the present study was not designed to investigate vascular responses in individual beds, cutaneous vascular conductance when measured in an uncovered forearm (i.e., not locally heated) slightly decreases (e.g., ∼15%) at presyncope in heat-stressed individuals (4). In the cited and present studies, the majority of the skin is exposed to a higher skin temperature (i.e., under the water-perfused suit) relative to skin temperature of the uncovered forearm. It remains unknown whether skin under the water-perfused suit constricts to the same, or perhaps more likely to a lesser extent, relative to the skin outside the water-perfused suit. Thus the extent of cutaneous vasoconstriction of the uncovered arm may be greater relative to that which occurs under the water-perfused suit. That said if in the present study whole body cutaneous vascular conductance decreased ∼15%, given the observation of a lack of change in SVC at presyncope (Fig. 2), vascular conductance of the noncutaneous beds must have slightly increased leading up to and at presyncope. Observations supporting this conclusion are consistent with reductions in muscle sympathetic nerve activity, coupled with limb vasodilation, in some individuals leading up to presyncope while normothermic (10). Nevertheless, the important finding of the present study is that leading up to and at presyncope there is an absence of a measureable decrease in SVC when individuals are heat stressed.
Contrary to the present findings, others have observed decreases in SVC during a heat-stressed orthostatic challenge (20, 35). There a few possibilities for this apparent discrepancy. First, the present study invoked a profound heat stress (∼25% greater core temperature increase vs. previous studies) that lead to a SVC ∼40% greater than previous studies (20, 35), presumably via greater increases in cutaneous vasodilation. It is possible that the observed findings are, therefore, a result of this more profound heat stress and associated greater increase in SVC before the onset of the hemorrhagic challenge. Second, the method used to cause the hypotensive challenge may influence SVC responses to that challenge; given volume shifts with LBNP are different relative to head-up tilt (30). Importantly, LBNP is the preferred approach to simulate a hemorrhagic challenge (3). Changes in SVC due to the venoarteriolar response may be different between LBNP and head-up tilt conditions, with a greater nonautonomic increase in SVC during head-up tilt relative to LBNP due to splanchnic pooling during heat-up tilt but not LBNP (30), possibly contributing to the observed discrepancies.
A lack of a significant decrease in SVC at presyncope is due to either 1) insufficient/absent sympathetic activation and/or 2) an inadequate response to the sympathetic activation. Regarding point 1, increases in muscle sympathetic nerve activity (MSNA) during head-up tilt are preserved in heat-stressed subjects (6). The elevation in MSNA for a given reduction in central blood volume is greater when individuals are heat stressed (7), and in supine individuals, the number of MSNA bursts for a given diastolic blood pressure “bin” is greater when subjects are heat stressed (14). Together, these observations imply that the sympathetic nervous system (at least to muscle beds) remains intact and has the capacity to increase nerve firing during the conditions of the present protocol. In contrast to muscle, however, skin sympathetic nerve activity is unchanged during a variety of hypotensive challenges while stressed (8, 33). However, it is unknown if sympathetic firing remains consistent at presyncope or if there is a reduction in MSNA just before test termination, as observed in normothermic individuals (10). It also remains unknown whether sympathetic neural activity to other regions, such as renal and splanchnic vascular beds, in response to a hypotensive challenge remains functional in heat-stressed individuals.
Despite normal or perhaps elevated MSNA responses to hypotension in heat-stressed individuals, impaired pre/postjunctional adrenergic responses for a given level of sympathetic activation could explain impaired decreases in SVC during heat stress (point 2). We observed elevated plasma norepinephrine concentrations (relative to when the subjects were normothermic) at peak heat stress (i.e., 1.2°C elevation in internal temperature), which further increased upon cessation of the hemorrhagic challenge. This observation suggests that the hemorrhagic challenge was sufficient to evoke increases in sympathetic activity, a conclusion consistent with muscle sympathetic nerve recordings during similar conditions (7), and that during the simulated hemorrhagic challenge the release of norepinephrine from sympathetic nerves is likely normal. Thus it may be that postjunctional effects of these adrenergic agents are either ineffective or insufficient to decrease SVC during heat stress. This hypothesis is supported by observations of attenuated decreases in SVC to systemic infusions of α-adrenergic agonists during heat stress (9). In contrast, local α-adrenergic responsiveness remains intact in heated skeletal muscle (16). Taken together, impairments in postsynaptic vascular responses to systemically administered α-adrenergic agonists are likely due to impaired responsiveness of nonmuscle beds such as the cutaneous vasculature, splanchnic, and/or renal regions. In support of that hypothesis, heat stress attenuates vasoconstrictor responsiveness to exogenous adrenergic agents in mesenteric arteries of rats (19) and isolated skeletal muscle feed arteries in humans (12). Likewise cutaneous vasoconstrictor responsiveness to intradermal administration of norepinephrine is attenuated during whole body and local heating (32), likely through a nitric oxide sympatholytic-type response that accompanies cutaneous vasodilation during whole body and local heating (27, 28). Given that observation, it is not surprising that during a heat-stressed-simulated hemorrhagic challenge there is relatively little cutaneous vasoconstriction (4). This lack of pronounced cutaneous vasoconstriction, despite large reductions in blood pressure, is particularly detrimental to blood pressure control in the heat-stressed human when upwards to 50% of cardiac output is going to the skin (24, 26). Thus any change (or lack thereof) in cutaneous vascular conductance has a large potential to dictate changes in SVC. This is supported by findings that rapid skin surface cooling of heat-stressed individuals causes abrupt and large decreases in SVC secondary to cutaneous vasoconstriction (35). Therefore, persistently elevated SVC during a simulated hemorrhagic challenge during heat stress is likely due, in part, to inadequate reductions in cutaneous vascular conductance.
Limitations in the interpretation of the findings.
For concerns of subject safety, LBNP was terminated at the onset of syncopal symptoms (i.e., presyncope) and thus no subject actually fainted. However, the present study was not designed to identify responses in the fainted human but rather to assess the factors that contribute to the early onset of symptoms of ensuing syncope when heat stressed. Additionally, because each cardiac output measurement takes ∼30 s to obtain, it was impossible to time the last cardiac output measurement at the exact point of test termination. The final cardiac output was obtained at 97 ± 3% of the LBNP tolerance test. Regardless, the present data provide insight into the cardiovascular responses leading up to the point of syncope.
The magnitude of the effect for a given change in SVC on arterial pressure can be dependent on baseline conductance, resulting in a controversy regarding whether it is preferred to report such data as vascular conductance or its inverse, vascular resistance (23). We chose to present vascular conductance; however, identical findings were observed when these data were analyzed as vascular resistance (i.e., an absence of a significant increase in vascular resistance during LBNP relative to heat stress alone; data not shown). Therefore, the interpretation of the present data remains the same regardless of whether expressed as vascular conductance or resistance.
A possible limitation is that each thermodilution measurement required injection of 10 ml of saline. Cardiac output measures were obtained throughout LBNP, so individuals with prolonged tolerance received greater amounts of saline. It may be that the amount of saline administered increased central blood volume and ventricular filling pressures and, in turn, increased cardiac output and possibly LBNP tolerance (15). However, the amount of saline administered during the LBNP component of the challenge averaged 135 ± 80 ml (range: 70–340 ml), while we (15) previously showed upwards of 2 liters of saline was necessary to preserve LBNP tolerance during heat stress, perhaps due to the rate of which saline leaves the vascular space. Therefore, it is unlikely the small volume of saline administered to acquire cardiac output measures influenced the findings, particularly the primary observation of a lack of decrease in SVC during the LBNP challenge.
The present work was only conducted on heat-stressed subjects. There are two reasons why these procedures were not also obtained while subjects were normothermic. 1) The subjects only needed to be heat stressed to address the proposed hypothesis (i.e., an absence of a decrease in SVC leading up to and at presyncope during simulated hemorrhage). 2) Inclusion of a normothermic trial would require recatheterization of the subjects, and associated risks of these procedures, given the need to conduct normothermic and heat stress trials on differing days. Because of these added risks, we did not feel it was ethically appropriate to conduct a normothermic trial given multitudes of studies showing clear increases in systemic vascular resistance (or decreases in SVC) leading up to and at presyncope in normothermic conditions (11, 17, 21, 29, 37).
In conclusion, profound cutaneous vasodilation associated with heat stress combined with a simulated hemorrhagic challenge sufficient to cause syncopal symptoms results in a cardiac output that, although sufficient while normothermic, is no longer adequate during a heat-stressed-simulated hemorrhagic challenge. Further, SVC did not decrease despite profound reductions in arterial pressure during the simulated hemorrhagic challenge. These data indicate that an absence of an appropriate decreases in SVC to a simulated hemorrhagic challenge is the primary mechanism by which heat stress compromises the control of arterial pressure to such a challenge. These data have important implications for the treatment of hemorrhaging heat-stressed individuals (e.g., soldiers, firefighters, and mine workers). Given inadequate decreases in SVC in the face of falling cardiac output, the maintenance of blood pressure in such individuals will require decreases in vascular conductance (perhaps via skin cooling) and/or increases in cardiac output (via rapid infusion of large volumes of fluid).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-61388 and HL-84072, a grant from Jakob Ehrenreich, and the Grethe Ehrenreichs Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S.G., M.O., T.S., N.H.S., P.I.J., M.A.M., and C.G.C. performed experiments; M.S.G., P.I.J., and M.A.M. analyzed data; M.S.G., M.O., T.S., N.H.S., P.I.J., M.A.M., and C.G.C. interpreted results of experiments; M.S.G. prepared figures; M.S.G. drafted manuscript; M.S.G., M.O., T.S., N.H.S., P.I.J., M.A.M., and C.G.C. edited and revised manuscript; M.S.G., M.O., T.S., N.H.S., P.I.J., M.A.M., and C.G.C. approved final version of manuscript; C.G.C. conception and design of research.
ACKNOWLEDGMENTS
We thank the volunteers for participation.
REFERENCES
- 1.Brothers RM, Keller DM, Wingo JE, Ganio MS, Crandall CG. Heat-stress induced changes in central venous pressure do not explain inter-individual differences in orthostatic tolerance during heat stress. J Appl Physiol 1283–1289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bundgaard-Nielsen M, Wilson TE, Seifert T, Secher NH, Crandall CG. Effect of volume loading on the Frank-Starling relation during reductions in central blood volume in heat-stressed humans. J Physiol 588: 3333–3339, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol 96: 1249–1261, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Crandall CG, Shibasaki M, Wilson TE. Insufficient cutaneous vasoconstriction leading up to and during syncopal symptoms in the heat stressed human. Am J Physiol Heart Circ Physiol 299: H1168–H1173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol 586: 293–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui J, Shibasaki M, Low DA, Keller DM, Davis SL, Crandall CG. Muscle sympathetic responses during orthostasis in heat-stressed individuals. Clin Auton Res 21: 381–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol 96: 2103–2108, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci 116: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cui J, Wilson TE, Crandall CG. Phenylephrine-induced elevations in arterial blood pressure are attenuated in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol 283: R1221–R1226, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Dietz NM, Halliwill JR, Spielmann JM, Lawler LA, Papouchado BG, Eickhoff TJ, Joyner MJ. Sympathetic withdrawal and forearm vasodilation during vasovagal syncope in humans. J Appl Physiol 82: 1785–1793, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Fu Q, Arbab-Zadeh A, Perhonen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol 286: H449–H457, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ives SJ, Andtbacka RH, Noyes RD, McDaniel J, Amann M, Witman MA, Symons JD, Wray DW, Richardson RS. Human skeletal muscle feed arteries studied in vitro: the effect of temperature on alpha(1)-adrenergic responsiveness. Exp Physiol 96: 907–918, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jardine DL, Melton IC, Crozier IG, English S, Bennett SI, Frampton CM, Ikram H. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol 282: H1804–H1809, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol 587: 1131–1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller DM, Sander M, Stallknecht B, Crandall CG. alpha-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol 588: 3799–3808, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lollgen H, Dirschedl P, Koppenhagen K, Klein KE. Cardiac factors in orthostatic hypotension. Acta Astronaut 27: 93–95, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol 1: 90–102, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Massett MP, Lewis SJ, Kregel KC. Effect of heating on the hemodynamic responses to vasoactive agents. Am J Physiol Regul Integr Comp Physiol 275: R844–R853, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol 276: R203–R212, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Murray RH, Thompson LJ, Bowers JA, Albright CD. Hemodynamic effects of graded hypovolemia and vasodepressor syncope induced by lower body negative pressure. Am Heart J 76: 799–811, 1968 [DOI] [PubMed] [Google Scholar]
- 22.Nelson MD, Altamirano-Diaz LA, Petersen SR, DeLorey DS, Stickland MK, Thompson RB, Haykowsky MJ. Left ventricular systolic and diastolic function during tilt-table positioning and passive heat stress in humans. Am J Physiol Heart Circ Physiol 301: H599–H608, 2011 [DOI] [PubMed] [Google Scholar]
- 23.O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Rowell LB. Thermal stress. In: Human Circulation: Regulation During Physical Stress. Oxford, UK: Oxford University Press, 1986, p. 174–212 [Google Scholar]
- 25.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974 [DOI] [PubMed] [Google Scholar]
- 26.Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969 [DOI] [PubMed] [Google Scholar]
- 27.Shibasaki M, Davis SL, Cui J, Low DA, Keller DM, Durand S, Crandall CG. Neurally mediated vasoconstriction is capable of decreasing skin blood flow during orthostasis in the heat-stressed human. J Physiol 575: 953–959, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibasaki M, Durand S, Davis SL, Cui J, Low DA, Keller DM, Crandall CG. Endogenous nitric oxide attenuates neutrally mediated cutaneous vasoconstriction. J Physiol 585: 627–34, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens PM, Lamb LE. Effects of lower body negative pressure on the cardiovascular system. Am J Cardiol 16: 506–515, 1965 [DOI] [PubMed] [Google Scholar]
- 30.Taneja I, Moran C, Medow MS, Glover JL, Montgomery LD, Stewart JM. Differential effects of lower body negative pressure and upright tilt on splanchnic blood volume. Am J Physiol Heart Circ Physiol 292: H1420–H1426, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol 66: 1586–1592, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci 97: 122–128, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol 536: 615–23, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol 291: R1443–R1448, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol 93: 85–91, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki F, Monji K, Sogabe Y, Sone R. Cardiac and peripheral vascular responses to head-up tilt during whole body thermal stress. J UOEH 22: 147–158, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effects of head-down-tilt bed rest on cerebral hemodynamics during orthostatic stress. J Appl Physiol 83: 2139–2145, 1997 [DOI] [PubMed] [Google Scholar]