Abstract
Previous studies have indicated that there is increased activation of the paraventricular nucleus (PVN) in rats with chronic heart failure (CHF); however, it is not clear if the preautonomic neurons within the PVN are specifically overactive. Also, it is not known if these neurons have altered responses to baroreceptor or osmotic challenges. Experiments were conducted in rats with CHF (6–8 wk after coronary artery ligation). Spontaneously active neurons were recorded in the PVN, of which 36% were antidromically activated from the rostral ventrolateral medulla (RVLM). The baseline discharge rate in RVLM-projecting PVN (PVN-RVLM) neurons from CHF rats was significantly greater than in sham-operated (sham) rats (6.0 ± 0.6 vs. 2.6 ± 0.3 spikes/s, P < 0.05). Picoinjection of the N-methyl-d-aspartate (NMDA) receptor antagonist d,l-2-amino-5-phosphonovaleric acid significantly decreased the basal discharge of PVN-RVLM neurons by 80% in CHF rats compared with 37% in sham rats. Fifty-two percent of spontaneously active PVN-RVLM neurons responded to changes in the mean arterial pressure (MAP). The changes in discharge rate in PVN-RVLM neurons after a reduction in MAP (+52 ± 7% vs. +184 ± 61%) or an increase in MAP (−42 ± 8% vs. −71 ± 6%) were significantly attenuated in rats with CHF compared with sham rats. Most PVN-RVLM neurons (63%), including all barosensitive PVN-RVLM neurons, were excited by an internal carotid artery injection of hypertonic NaCl (2.1 osmol/l), whereas a smaller number (7%) were inhibited. The increase in discharge rate in PVN-RVLM neurons to hypertonic stimulation was significantly enhanced in rats with CHF compared with sham rats (134 ± 15% vs. 92 ± 13%). Taken together, these data suggest that PVN-RVLM neurons are more active under basal conditions and this overactivation is mediated by an enhanced glutamatergic tone in rats with CHF. Furthermore, this enhanced activation of PVN-RVLM neurons may contribute to the altered responses to baroreceptor and osmotic challenges observed during CHF.
Keywords: paraventricular nucleus, rostral ventrolateral medulla, sympathetic activity, baroreflex, osmotic challenge
it is well known that sympathetic outflow is enhanced in patients with chronic systolic heart failure (CHF) and various animal models of experimental CHF (13, 27). Recent studies (12, 21) have focused on how neurochemical changes in the central nervous system contribute to the augmented sympathetic drive in CHF. The paraventricular nucleus (PVN) of the hypothalamus is an important site that integrates and responds to a variety of neural and humoral signals regulating sympathetic drive and extracellular fluid volume. Previous studies have indicated that there is an increased activation of the PVN in rats with CHF, as measured by an increase in hexokinase activity (30) or Fos activation (31). Zhang et al. (52) observed that the spontaneous activity of neurons in general (unidentified as to their projection) within the PVN was also enhanced in rats with CHF. The PVN includes neuroendocrine-related functional neurons that project to the median eminence, posterior pituitary, and preautonomic neurons that send long descending projections to the brain stem and spinal cord regions that are important in dictating autonomic outflow (3, 44). However, it was not clear if the preautonomic neurons within the PVN were specifically overactive during CHF.
The PVN projects heavily to the rostral ventrolateral medulla (RVLM). The number of PVN neurons projecting to the RVLM (PVN-RVLM neurons) is an average of sevenfold greater than the number of PVN neurons projecting to the spinal cord (40), and this pathway contributes to the changes in sympathetic nerve activity (SNA) observed after activation of the PVN. Consistent with these observations, Chen et al. (7) showed that the activity of PVN-RVLM neurons was temporally correlated with renal SNA (RSNA) and so might contribute to overall sympathetic activation. Neurotransmitters that can influence the activity of those PVN-RVLM neurons have the potential to influence basal resting sympathetic tone. Thus, our present study focused on PVN-RVLM neurons.
Glutamatergic tone within the PVN is known to drive neurons within the PVN (25). Our previous study (24) indicated that the expression of the N-methyl-d-aspartate (NMDA) receptor subunit NR1 was increased within the PVN of rats with CHF. Additionally, the increase in RSNA in response to NMDA microinjected into the PVN is higher in rats with CHF compared with that in sham-operated rats (24). These data suggest that enhanced glutamatergic tone in the PVN may contribute to sympathetic activation in rats with CHF. However, the specific mechanism for the activation of the preautonomic neurons within the PVN is less well understood. In addition, there is no electrophysiological in vivo evidence to indicate an altered activation in PVN-RVLM neurons in response to NMDA.
Baroreflex abnormalities are associated with increased sympathetic activation in both patients with CHF and experimental animal models of CHF (38). It is also known that the PVN regulates baroreflex-mediated sympathetic outflow (29, 50). Neurons within the PVN are activated by baroreflex challenges, as measured by an increased discharge rate using the method of extracellular single-unit recording (6) and c-Fos expression (33). It is possible that altered activation of neurons within the PVN during baroreflex activation may contribute to the altered SNA responses during CHF. However, it is not known if the activation of PVN-RVLM neurons is specifically altered in CHF.
Recent studies have shown that body fluid hyperosmolality can stimulate SNA (45) and that the PVN neurons may play an important role in the osmotically induced increase in SNA (46). It has been reported that blockade of local ionotropic glutamate (2) or ANG II type 1 receptors (5) in the PVN significantly attenuates hyperosmolality-induced acute increases of SNA. Additionally, inhibition of the RVLM prevents SNA responses to an intracarotid injection of hypertonic NaCl (42). Taken together, these data suggest that PVN-RVLM glutamatergic neurons mediate hyperposmolalty-induced overactivation of SNA. However, it is not known whether the responses of PVN-RVLM neurons to osmotic challenges are altered in rats with CHF.
In the present study, we first determined if PVN-RVLM neurons have an increased basal firing rate in rats with CHF. Second, we sought to determine if the responses of these PVN-RVLM neurons to endogenous and exogenous glutamatergic (NMDA) stimulation are altered in rats with CHF. Finally, we examined whether PVN-RVLM neurons have an altered response to baroreceptor or osmotic challenges and thus contribute to the altered baroreceptor or osmotic reflex function in rats with CHF.
METHODS
Male Sprague-Dawley rats weighing between 200 and 220 g were obtained from SASCO Breeding Laboratories (Omaha, NE) and assigned to two groups: a sham-operated (sham) control group and a coronary artery ligated CHF group. Male Sprague-Dawley rats weighing between 250 and 300 g were assigned to a normal group of control rats. This study was approved by the Institutional Animal Care and Use Committee of the University of Nebraska and was carried out under the guidelines of the American Physiological Society and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Model of CHF in rats.
CHF was produced by left coronary artery ligation in rats. Briefly, a left thoracotomy was performed through the fifth intercostal space, the pericardium was opened, the heart was exteriorized, and the left anterior descending coronary artery was ligated. Sham rats were treated the same as CHF rats except that their coronary arteries were not ligated, as previously described (51). Experiments were carried out 6–8 wk after coronary artery ligation or sham surgery. The degree of left ventricular (LV) dysfunction and CHF was determined using both hemodynamic and anatomic criteria. LV end-diastolic pressure (LVEDP) was measured with a Mikro-Tip catheter (Millar Instruments, Houston, TX) at the time of the terminal experiment. To measure infarct size, the heart was dissected, and the atria and right ventricle were removed. A digital image of the LV was captured with a digital camera (Kodak, Rochester, NY), and the percentage of the infarcted area to the total LV area was quantified with SigmaScan Pro (Aspire Software, Ashburn, VA). Infarct size (in %) was found by dividing the size of the infarcted area by the total size of the LV. Rats with an infarct size > 30% of the total LV wall and elevated LVEDP ≥ 15 mmHg were considered to be in CHF, as previously documented (19, 20, 24, 51).
General surgical procedures.
Each rat was anesthetized by an intraperitoneal injection of urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip). Adequate depth of anesthesia was assessed by the absence of corneal reflexes and paw withdrawal response to a noxious pinch. Supplemental doses of anesthesia were administered to maintain an adequate depth of anesthesia during the experiment. The right femoral artery and femoral vein were cannulated for the recording of arterial blood pressure and administration of chemicals, respectively. Mean arterial pressure (MAP) and heart rate (HR) were simultaneously recorded on a PowerLab data-acquisition system (8SP, AD Instruments). Baroreceptor challenges were induced by an intravenous injection of phenylephrine (5–30 μg/kg) or sodium nitroprusside (4–20 μg/kg) to increase or decrease arterial blood pressure, respectively. A catheter was inserted into the right internal carotid artery (ICA) about 1.0 cm via the occipital artery for the infusion of osmotic solutions. This allowed blood flow to be maintained through the carotid arteries while delivering solutions directly to the forebrain. Body temperature was maintained at ∼37°C with an animal temperature controller [ATC 1000, World Precision Instruments (WPI)].
Extracellular single-unit recordings in the PVN.
Rats were placed in a stereotaxic apparatus (model 620, Stoelting, Chicago, IL). The stereotaxic coordinates for the PVN were determined according to Paxinos and Watson's atlas (32). Typically, three tracks were explored for extracellular recording in each rat, from −1.4 to −2.1 mm caudal to the bregma, 0.4 mm lateral (right side) to the midline, and with a depth of 7.4–8.6 mm ventral to the dorsal surface. Extracellular single-unit recording was carried out using a single micropipette (resistance: 5–15 MΩ) filled with 0.5 M sodium acetate containing 2% pontamine sky blue. The glass micropipettes were advanced using a microdrive controller (Type 860, Hugo Sachs Elektronik) into the PVN. The spontaneous activity of neurons was amplified (gain: 1,000) with an alternating current/direct current differential amplifier (model IX1, Dagan) with a low-frequency cutoff at 30 Hz and a high-frequency cutoff at 3 kHz. Neuronal discharge was recorded on a PowerLab data-acquisition system (8/30, AD Instruments). The frequency of the neuronal discharge was analyzed with special software (SpikeHistogram, AD Instruments). The pontamine sky blue was iontophoresed (−15 μA, 10 min) to mark the site of the last recorded neuron, and other recording sites were extrapolated from the marked point according to Paxinos and Watson's atlas (32).
Pressure picoinjection with extracellular unit recordings in vivo.
To combine PVN single-unit recording with pressure ejection, multibarrel glass micropipettes (three barrels) were used. Single micropipettes were pulled for recording. Two ejection pipettes were pulled (Multi-Pipette Puller PMP-107, MicroData Instruments) from thick-walled glass capillary tubing with a calibrated narrow inner diameter (outer diameter: 1.0 mm, thick wall: 0.5 mm, Micropipette Glass 50610, Stoelting), which was redefined to a final outer diameter of ∼30 μm (Microprocessor-controlled Microforge DMF1000, WPI). Recording and ejection pipettes were then assembled in a specially designed electrode holder (HMD-2, Narishige) to permit the tips and as long a part of the tapering ends as possible to touch one another (1). The tip of the recording pipette was advanced 30 μm beyond the ejection tip. About 5 nl volume of fluid was ejected by air pressure ejection (20 psi, pulse duration: 10–50 ms, Picospritzer, General Valve), which was measured by a microscope with a calibrated graticule. These parameters for picoinjection were based on those used in previous studies (39, 49). A vehicle (5 nl of artificial cerebrospinal fluid) picoinjection to control for the volume effect were also performed.
Identification of PVN-RVLM neurons.
Antidromic stimulation was used to identify PVN-RVLM neurons in vivo. The RVLM (−12.5 mm caudal to the bregma, 2.1 mm right of the midline, and 9.2 mm ventral to the surface of cerebellum) was functionally identified by a 15- to 20-mmHg blood pressure response to a microinjection of NMDA (100 pmol in 50 nl) during a 10-s period (26). RVLM stimulation was ipsilateral to PVN recording. Subsequently, a concentric bipolar electrode (outer diameter: 500 μm, tip tapered at 60°, FHC, WPI) was placed at the same location in the RVLM to allow for antidromic stimulation with a stimulator (model A310, WPI).
When a spontaneously active neuron was recorded, standard tests were performed to ensure its antidromic nature (16). First, we examined if the neurons responded to RVLM stimulation (pulse width: 3–10 ms, current intensity: 0.1–1.0 mA, and stimulation frequency: 0.5 Hz) with consistent onset latency spikes at a discrete stimulus threshold. Second, we examined if the neurons responded to each pulse in a high-frequency 300-Hz stimulus train. In many cases, the collision test was performed to examine whether the stimulus-evoked spikes could be canceled by spontaneous action potentials. In some cases, RVLM stimulus intensity was increased gradually above threshold to determine if a discontinuous decrease (>2 ms) in the antidromic onset latency could be detected. Such antidromic latency “jumps” were interpreted to indicate the presence of a terminal arborization in the vicinity of the RVLM-stimulating electrode. A window discriminator (model 74-60-1C, FHC) and oscilloscope (model OS-5100RA, LG) was used to discriminate the PVN spontaneous action potential, and the signal was translated to the TTL pulse as a trigger with an adjustable latency to evoke the RVLM stimulation. At the end of the experiment, stimulation sites in the RVLM were marked by passing 1 mA of anodal direct current for 20 s.
Histology of recording sites within the PVN.
At the end of the experiment, the rat was euthanized with an overdose of anesthesia. Rat brains were then removed, frozen, and sectioned as previously described (57). The dye spots for recording sites in the PVN and the sites of electrolytic lesion in the RVLM were identified with a light microscope. Rats whose recording sites were within the boundaries of the PVN were used for data analysis. The location of the center of the dye spot was transferred to a histological map based on the rat atlas (45).
Statistical analysis.
Due to the large variability of baseline neuronal discharge rates, the percentage of change was used for comparison before and after treatments. The discharge rates of PVN neurons were averaged during three time periods: 1) 60 s for baseline discharge, 2) 20 s immediately after the ICA injection of osmotic solutions or NMDA/N-monomethyl-l-arginine (l-NMMA) picoinjections, and 3) 10 s for the maximum or minimum bin after the onset of changes in MAP by phenylephrine or sodium nitroprusside. The baroreflex response sensitivity of PVN-RVLM neurons defined as the maximal change in discharge for a corresponding change in MAP in sham and CHF rats. The baroreflex response sensitivity of HR was defined as the maximal change in HR for a given corresponding change in MAP in sham and CHF rats. PVN neurons were considered to be responsive if their peak discharge frequency after treatment changed >30% from baseline. Baseline pulse pressure, systolic arterial pressure, MAP, and HR were averaged during 2 min before treatment. Data are presented as means ± SE. Differences between groups were determined by two-way ANOVA followed by a Newman-Keuls test for post hoc analysis of significance (StatView II, Berkeley, CA). P values of <0.05 were considered statistically significant.
RESULTS
Baseline hemodynamic parameters after coronary artery ligation.
Baseline hemodynamic parameters, heart weight, and infarct size were measured 6–8 wk after coronary ligation or sham surgery (Table 1). Eleven rats that displayed myocardial infarcts of <30% (of the LV wall) or LVEDP < 15 mmHg were excluded from the study. A total of 36 rats with coronary artery ligation surgery were used in this study. Coronary artery-ligated rats showed an average infarct size of 40.7 ± 1.7% and LVEDP of 18.9 ± 1.3 mmHg. CHF rats also had decreased pulse pressure, systolic arterial pressure, and maximum dP/dt compared with sham rats. There were no statistically significant differences in baseline pulse pressure, MAP, or HR between sham and CHF rats. These histological and hemodynamic data suggest decreased contractile function in CHF rats, consistent with previous data of our laboratory (19, 20, 24, 51).
Table 1.
Baseline values of morphological and hemodynamic parameters in sham and CHF rats
| Sham Rats | CHF Rats | |
|---|---|---|
| Number of rats/group | 26 | 36 |
| Body weight, g | 398 ± 7 | 406 ± 6 |
| Heart weight, mg | 1,063 ± 19 | 1,501 ± 24* |
| Heart weight/body weight, mg/g | 2.7 ± 0.1 | 3.7 ± 0.1* |
| Infarct size, %LV | 0 | 40.7 ± 1.7* |
| LV end-diastolic pressure, mmHg | 2 ± 1 | 18.9 ± 1.3* |
| Maximum dP/dt, mmHg/s | 7,525 ± 486 | 5,903 ± 414* |
| Basal mean arterial pressure, mmHg | 93 ± 3 | 88 ± 2 |
| Systolic arterial pressure, mmHg | 132.9 ± 4.5 | 118.9 ± 2.8* |
| Diastolic arterial pressure, mmHg | 73.5 ± 2.4 | 72.6 ± 1.5 |
| Pulse pressure, mmHg | 59.5 ± 3.7 | 46.2 ± 2.4* |
| Basal heart rate, beats/min | 317 ± 14 | 323 ± 12 |
Values are means ± SE.
Sham, sham operated; CHF, chronic heart failure; LV, left ventricle.
P < 0.05 vs. sham rats.
Identification of PVN-RVLM neurons.
In 179 spontaneously active neurons recorded in the PVN, 66 units were antidromically activated from the RVLM in 26 sham rats and 36 CHF rats. In 6 normal rats, 17 spontaneously active neurons were recorded in the PVN, of which 5 units were antidromically activated from the RVLM.
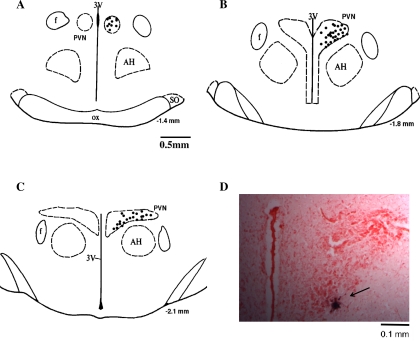
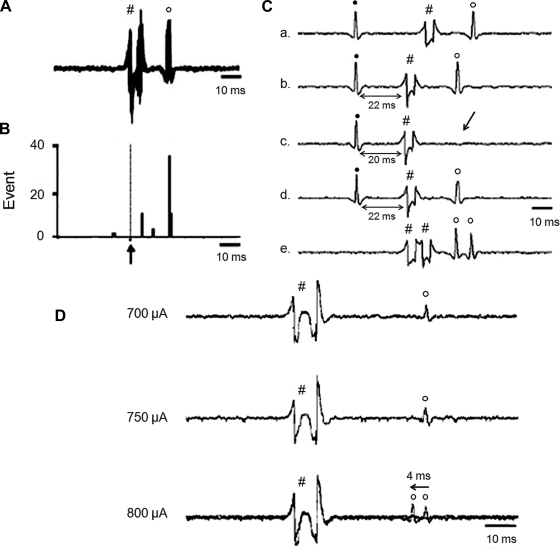
Figure 1 shows a schematic distribution of recorded neurons within the PVN. We first examined if the neurons responded to RVLM stimulation with consistent onset latency. We then performed a collision test in a portion of the recorded neurons (16 of 66 neurons, 24%). Furthermore, we observed that gradually increasing the stimulation of the RVLM produced an abrupt decrease in the antidromic onset latency in all the neurons tested (13 of 13 neurons, 100%). Three examples of antidromic identification of a single PVN-RVLM neuron are shown in Fig. 2. Figure 2, A–C, shows one neuron that had antidromic spikes evoked by stimulation of the RVLM with constant latency (22 ms), and the antidromic spike was canceled when the interval between the spontaneous action potential and the stimulation was reduced to <21 ms. A different single PVN-RVLM neuron indentified with a latency jump test is shown in Fig. 2D. Gradually increasing RVLM stimulation from 700 to 800 μA produced an abrupt decrease (≈4 ms) of the antidromic onset latency. This latency jump indicates that the action potential shifted to a nearby branch of the axon. Overall, there was a fair amount of variability in the latency, ranging from the lowest of 15 ms to the highest of 110 ms, with the average latency of 54.0 ± 4.5 ms and and average axonal conduction velocity of 0.5 ± 0.1 m/s.
Fig. 1.
A–C: approximate locations of neurons that were antidromically activated from the rostral ventrolateral medulla (RVLM). The distance (in mm) posterior to the bregma is shown for each section. AH, anterior hypothalamic nucleus; f, fornix; 3V, third ventricle; OX, optic tract; SO, supraoptic nucleus. D: histological photo showing the recording site in the paraventricular nucleus (PVN) of one rat. Arrowhead shows a marked recording location.
Fig. 2.
Two neurons indentified as RVLM-projecting PVN (PVN-RVLM) neurons. One neuron was indentified as a PVN-RVLM neuron with consistent onset latency (B) and a collision test (C). A “latency jumping” test was performed in another neuron (D). A: five superimposed sweeps of neuron activity with constant latency (22 ms) antidromic spikes evoked by stimulation of the RVLM. B: peristimulus histogram of spike occurrence triggered by electric stimulation of the RVLM with 40 sweeps. C: antidromic stimulation-evoked action potential with constant latency (22 ms; a, b, and d) for the same neuron as in C. RVLM stimulation evoked an antidromic spike that was cancelled when the interval between the spontaneous action potential and stimulation was reduced to <21 ms and high frequency (333 Hz, 3 ms) after the test (e). All segments (a–e) represent two superimposed sweeps. D: latency jumping in the PVN, a gradual increase in RVLM stimulation from 700 to 800 μA, produced an abrupt decrease (≈4 ms) of the antidromic onset latency. This latency jump indicates that the action potential shifted to a nearby branch of the axon site on the axon. ↑, electric stimulation; ●, spontaneous action potentials; #, stimulus artifacts; ○, antidromic spikes.
Baseline discharge of PVN-RVLM neurons.
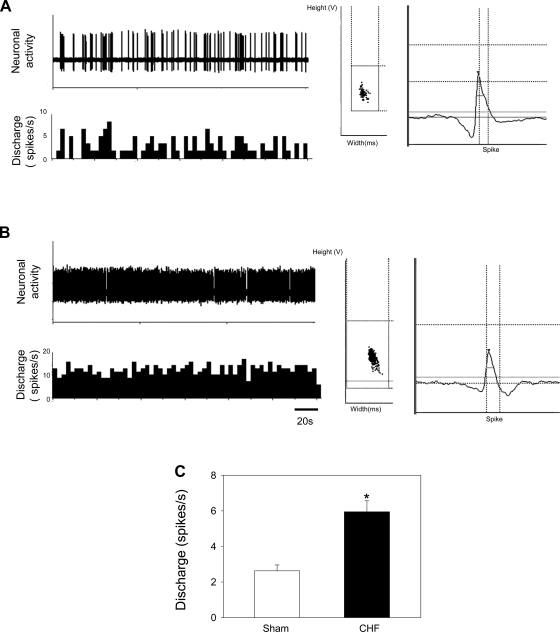
The baseline discharge rate in PVN-RVLM neurons in CHF rats was significantly greater than in sham rats (6.0 ± 0.6 spikes/s, n = 37, vs. 2.6 ± 0.3 spikes/s, n = 29, P < 0.05), as shown in Fig. 3. There were, however, no statistical differences in baseline discharge rates in PVN neurons that could not be evoked by RVLM stimulation between sham and CHF rats (2.5 ± 0.3 spikes/s, n = 68, vs. 3.4 ± 0.4 spikes/s, n = 45, P > 0.05). Interestingly, although there was a tendency for more spontaneously active neurons that were antidromically identified in CHF rats compared with sham rats, this was not statistically significant (37 of 82 neurons, 45%, vs. 29 of 97 neurons, 30%, P = 0.18 by χ2-test).
Fig. 3.
A and B: segments of original recordings (left) and spike discriminator output demonstrating a single-unit discharge (right) from an individual sham-operated (sham) rat (A) and a rat with chronic heart failure (CHF; B). C: composite data of the baseline discharge rate of PVN-RVLM neurons in rats with CHF and in sham rats. Values are presented as means ± SE. *P < 0.05 vs. sham rats.
Effect of NMDA and d,l-2-amino-5-phosphonovaleric acid on PVN-RVLM neurons.
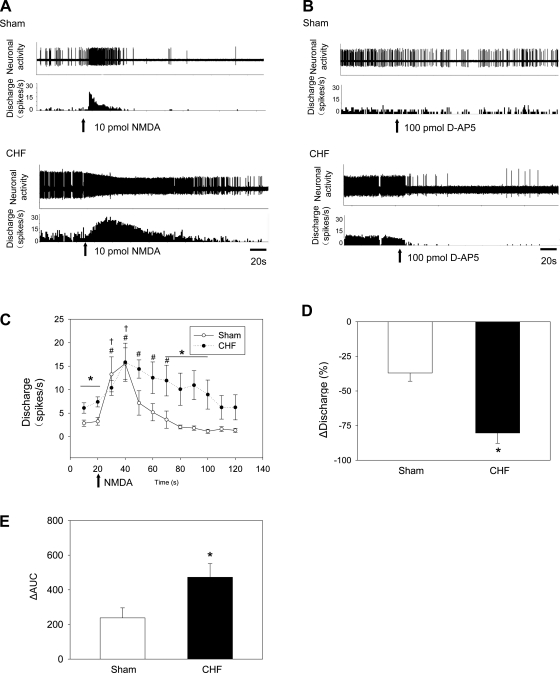
The original representative recording in Fig. 4A shows the response of PVN-RVLM neurons to picoinjection of NMDA (10 pmol) in sham and CHF rats. Application of NMDA significantly increased the activity of PVN-RVLM neurons in sham and CHF rats. Although there was no significant difference between the maximal increase in firing of PVN-RVLM neurons between sham and CHF groups, interestingly, the increase in response to NMDA was sustained longer in CHF rats (50 s) compared with sham rats (20 s; Fig. 4C). There was a significant difference between the sham and CHF groups 70–100 s after the application of NMDA. The change in the area under the curve was also increased in CHF rats compared with sham rats, suggesting a greater overall response (Fig. 4E). To confirm the repeatability of the response, NMDA was picoinjected twice. There were no significant differences between the first and second applications of NMDA in sham and CHF rats (sham rats: 12.9 ± 4.0 vs. 10.3 ± 2.8 spikes/s, P > 0.05; CHF rats: 11.4 ± 2.2 vs. 10.4 ± 2.4 spikes/s, P > 0.05).
Fig. 4.
A and B: tracings of changes in the discharge of PVN-RVLM neurons after picoinjection of N-methyl-d-aspartate (NMDA; A) or the NMDA receptor antagonist d,l-2-amino-5-phosphonovaleric acid (d-AP5; B) in sham and CHF rats. C and D: mean data of changes in the discharge of PVN-RVLM neurons after picoinjection of NMDA (C) or d-AP5 (D) in sham and CHF rats. E: integral from 10 to 120 s of the difference between each NMDA treatment and the baseline discharge of PVN-RVLM neurons in sham or CHF rats separately [change in area under the curve (ΔAUC)]. Values are presented as means ± SE. †P < 0.05 after treatment in sham rats vs. the first 10 s before treatment in sham rats (n = 5); #P < 0.05 after treatment in CHF rats vs. the first 10 s before treatment in CHF rats (n = 5); *P < 0.05, CHF rats vs. sham rats (n = 5 rats/group).
The original representative recording in Fig. 4B shows that the response of a PVN-RVLM neuron to picoinjection of the NMDA receptor antagonist d,l-2-amino-5-phosphonovaleric acid (d-AP5; 100 pmol) in sham and CHF rats. In rats with CHF, the decrease in firing after d-AP5 was larger than in sham rats (Fig. 4D). In addition, responses to NMDA were blocked by pretreatment with d-AP5 in both sham (10.3 ± 1.3 vs. 2.6 ± 1.9 spikes/s, P < 0.05) and CHF (10.6 ± 2.0 vs. 1.1 ± 0.6 spikes/s, P < 0.05) rats. There were no significant changes in MAP and HR after picoinjection of NMDA or d-AP5 treatment in sham and CHF rats.
There were no significantly effects of picoinjection of vehicle (5 nl of artificial cerebrospinal fluid) on the discharge rates of PVN neurons in normal rats (baseline: 2.5 ± 0.5 spikes/s vs. after picoinjection: 2.4 ± 0.5 spikes/s, n = 5, P > 0.05).
Effect of NMDA and l-NMMA on PVN-RVLM neurons.
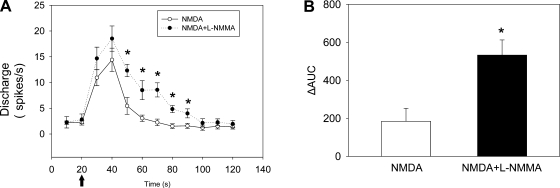
To test the interaction between nitric oxide (NO) and NMDA receptors on PVN-RVLM neurons, five PVN-RVLM neurons were recorded in normal rats. The responses to picoinjections of NMDA (10 pmol) with or without prior l-NMMA (10 pmol), an inhibitor of NO synthase, in the PVN were examined. The time course of the change in the discharge of PVN-RVLM neurons is shown in Fig. 5. There was no significant difference between the maximal increase in firing of PVN-RVLM neurons between the picoinjection of NMDA with prior l-NMMA and picoinjection of NMDA alone. However, the increase in the discharge to NMDA was sustained for a longer duration after l-NMMA. Consistent with this, the change in area under the curve was increased by NMDA and l-NMMA treatement compared with NMDA treatment alone. There were no significant changes in MAP and HR after picoinjection of NMDA or l-NMMA treatment.
Fig. 5.
A: time course of changes in the discharge of PVN-RVLM neurons after picoinjection of NMDA (10 pmol) only and NMDA (10 pmol) + N-monomethyl-l-arginine (l-NMMA; 10 pmol). n = 5 rats/group. B: integral from 10 to 120 s of difference between each NMDA treatment and the baseline discharge of PVN-RVLM neurons (ΔAUC). Values are presented as means ± SE. *P < 0.05 vs. NMDA-only treatment.
Effect of baroreceptor challenge on PVN-RVLM neurons.
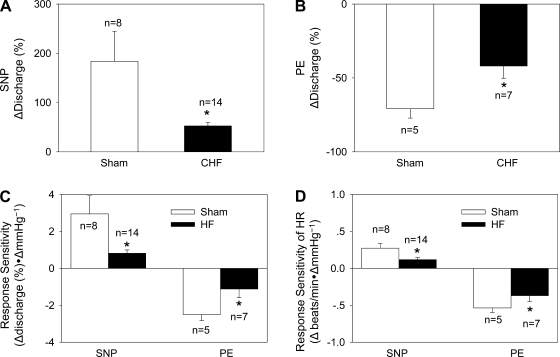
Fifty-two percent of PVN-RVLM neurons (24 of 46 neurons) responded to an increase or decrease in arterial blood pressure (Table 2 and Fig. 6). The changes in discharge rates of PVN-RVLM neurons to decrease (+52 ± 7%, n = 14, vs. +183 ± 61%, n = 8, P < 0.05) or increase (−42 ± 8%, n = 7, vs. −71 ± 6%, n = 5, P < 0.05) in MAP were significantly attenuated in the CHF group compared with the sham group, respectively (Fig. 7). Since the baseline firing rates were different between the groups, we compared the responses using the “percent change” from baseline. Similarly, the change in HR after an increase or decrease in MAP was attenuated in CHF rats compared with sham rats (Fig. 7).
Table 2.
Summary of discharge characteristics for rostral ventrolateral medulla-projecting paraventricular nucleus neurons responsive to baroreceptor or osmotic challenges
| Neuronal type | Discharge response | Number of Neurons | Baseline Discharge, spikes/s | Response to Internal Carotid Artery Injection of 2.1 osmol/l NaCl, % | Baroreceptor Input | Response to Intravenous Injection of SNP, % | Response to Intravenous Injection of PE, % |
|---|---|---|---|---|---|---|---|
| Increased response to osmotic stimulation | Increased | 35 total | |||||
| 19 CHF | 6.7 ± 0.8* | +133.6 ± 15.4* | 15/18 | +52.2 ± 6.7* | −41.9 ± 8.3* | ||
| n = 14 | n = 7 | ||||||
| 16 sham | 2.6 ± 0.5 | +92.1 ± 13.3 | 9/13 | +183.8 ± 60.9 (n = 8) | −70.9 ± 6.3 (n = 5) | ||
| Decreased response to osmotic stimulation | Increased | 5 total | |||||
| 2 CHF | 8.9 ± 4.7* | −53.0 ± 11.5 | 0/2 | ||||
| 3 sham | 2.6 ± 0.6 | −58.2 ± 1.8 | 0/2 | ||||
| Unresponsive to osmotic stimulation | − | 16 total | |||||
| 11 CHF | 4.0 ± 0.6 | −4.2 ± 4.1 | 0/8 | ||||
| 5 sham | 2.2 ± 0.5 | 0.6 ± 4.0 | 0/3 |
Values are presented as means ± SE; n = 56 neurons total.
SNP, sodium nitroprusside (5–10 μg); PE, phenylephrine (10–20 μg).
P < 0.05 vs. sham rats.
Fig. 6.
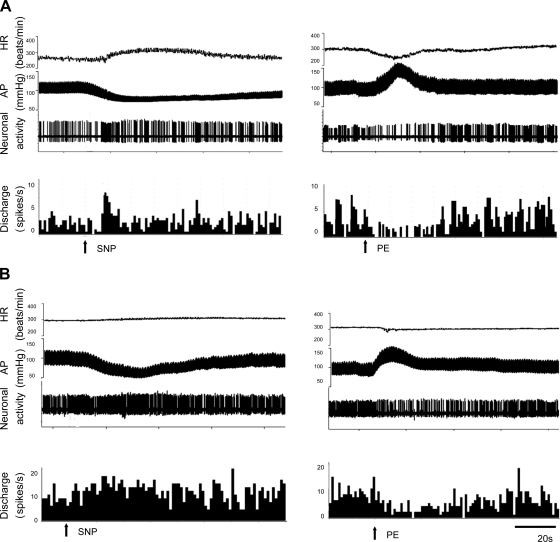
Segments of original recordings from an individual sham rat (A) and a rat with CHF (B), demonstrating changes in heart rate (HR; in beats/min), arterial pressure (AP; in mmHg), and PVN-RVLM neuronal activity and discharge rate (in spikes/s) to intravenous injections of sodium nitroprusside (SNP; left) or phenylephrine (PE; right).
Fig. 7.
A and B: percent changes in discharge after intravenous injections of SNP to lower mean AP (MAP; A; sham rats: n = 8 and CHF rats: n = 14) or PE to increase MAP (B; sham rats: n = 5 and CHF rats: n = 7) in PVN-RVLM neurons. C: baroreflex response sensitivity of PVN-RVLM neurons defined as the change in discharge for a given change in MAP in sham and CHF rats. D: baroreflex response sensitivity of HR defined as the change in HR for a given change in MAP in sham and HF rats. Values are presented as means ± SE. *P < 0.05 vs. sham rats.
Effect of osmotic challenge on PVN-RVLM neurons.
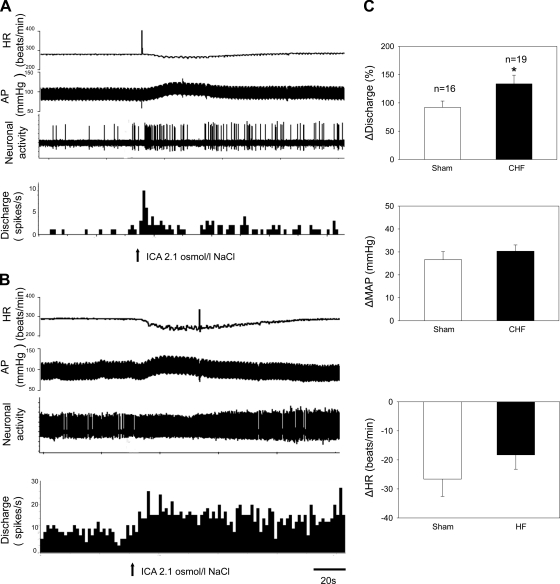
Examples of the responses in PVN-RVLM neuronal discharge, MAP, and HR after ICA injection of 2.1 osmol/l NaCl in a sham rat and in a CHF rat are shown in Fig. 8, A and B. ICA injection of 2.1 osmol/l NaCl elicited an increase in MAP and neuronal activity but a decrease in HR. Compared with sham rats, the changes in MAP and HR were not significantly different in CHF rats (Fig. 8C).
Fig. 8.
A and B: segments of original recordings from an individual sham rat and a rat with CHF demonstrating HR (in beats/min), AP (in mmHg), PVN-RVLM neuronal activity, and nerve discharge (spikes/s) responses to an internal carotid artery (ICA) injection of 2.1 osmol/l NaCl. C: mean data of changes in discharge, MAP, and HR after an ICA injection of 2.1 osmol/l NaCl in sham and CHF rats. Values are presented as means ± SE. *P < 0.05 vs. sham rats.
The majority of PVN-RVLM neurons (63%) were excited by ICA injection of 2.1 osmol/l NaCl, whereas 7% were inhibited (Table 2). The changes in neuronal discharge of PVN-RVLM neurons to hypertonic stimulation were enhanced (Fig. 8C) in rats with CHF compared with sham rats (+134 ± 15%, n = 19, vs. +92 ± 13%, n = 16, P < 0.05). The data analyzed as absolute changes in firing rate after hypertonic stimulation also demonstrated enhanced responses in the CHF group compared with the sham group (+7.5 ± 1.0 spikes/s, n = 19, vs. +2.3 ± 0.4 spikes/s, n = 16, P < 0.05). The response to isotonic saline was conducted on the same identified PVN-RVLM neurons. ICA injection of 0.3 osmol/l NaCl, as a control, had no significant effects on the discharge rates of PVN-RVLM neurons (sham rats: +10 ± 4% vs. CHF rats: +3 ± 6%, P > 0.05), MAP (sham rats: −2.4 ± 2.5 mmHg vs. CHF rats: −0.7 ± 1.8 mmHg, P > 0.05), or HR (sham rats: +1.2 ± 1.2 beats/min vs. CHF rats: +1.9 ± 0.5 beats/min, P > 0.05) in sham (n = 7) or CHF (n = 6) rats.
Interestingly, all of the PVN-RVLM neurons (24 of 24 neurons, 100%) that responded to baroreceptor challenges were also excited with the osmotic stimulus. Another four neurons, which were inhibited by the osmotic stimulus, did not respond to the baroreflex challenges.
DISCUSSION
The PVN and RVLM both play an important contributing role in the increased sympathetic outflow observed during CHF (12, 28, 53). The present study found that PVN-RVLM neurons are more active under basal conditions and are endogenously driven by an enhanced glutamatergic mechanism in CHF (50). Consistent with these observations, previous work from our laboratory (24) has shown that NMDA receptors are upregulated in the PVN and appear to contribute to the tonic sympathoexcitation observed during CHF (20). The responses of PVN-RVLM neurons to baroreflex challenge were attenuated, whereas the responses to hypertonic osmotic stimulation were enhanced in rats with CHF compared with sham rats. These results suggest that enhanced activation and sensitivity of PVN-RVLM neurons may contribute to the elevated basal level of SNA and altered autonomic reflexes observed in CHF.
The PVN is an important integrative (24) site in the control of sympathetic outflow and cardiovascular function, particularly via its projections to principal centers of sympathetic drive, the RVLM, and the intermediolateral cell column of the spinal cord (4, 8, 11, 14). It is well known that baseline sympathetic outflow mediated by the RVLM is mainly dependent on the spontaneous activity of preautonomic neurons (9, 10). Previous studies (34, 36) have demonstrated that PVN axons have terminal sites closely associated with spinally projecting RVLM neurons, many of which are likely to terminate on sympathetic preganglionic neurons. Recently, Chen et al. (7) demonstrated that the activity of PVN-RVLM neurons is temporally correlated with RSNA and so may contribute to basal SNA.
A previous study (41) has indicated that the PVN preautonomic neurons constitute a heterogeneous population. We (30) have previously demonstrated that hexokinase activity, a marker of neuronal activation, was increased in the PVN of rats with CHF. Subsequently, it was demonstrated that Fra-like immunoreactivity, an indicator of long-term neuronal activation (47), and c-Fos (31) are also increased in the PVN of rats with CHF. However, it was not clear if the “identified” preautonomic neurons within the PVN were specifically overactive. In the present study, we identified that PVN-RVLM neurons were specifically overactive in rats with CHF by recording extracellular single units in vivo, which resembles a more integrative condition compared with slice recording in the in vitro condition. Although one limitation of this technique is that antidromic stimulation from the RVLM will not distinguish between neurons projecting to or through this region, it is of interest to note that the latency jitter at high stimulus intensities observed in some of the recordings suggests that we were stimulating at a branch point in the axon indicative of terminal arborization in the vicinity of the RVLM-stimulating electrode. In other words, these PVN-RVLM neurons were mostly terminating within the vicinity of the RVLM. Furthermore, Han et al. (15), using patch-clamp techniques in PVN slice preparation combined with the retrograde labeling technique, found that myocardial infarction induces an elevation of the firing activity in PVN-RVLM neurons but not in intermediolateral cell column-projecting PVN neurons. These results in a slice preparation suggest that neuronal excitability is elevated in PVN-RVLM neurons in rats with CHF and are consistent with the observations in this study. The overactivation of PVN-RVLM neurons may partially contribute to the overactivation of the sympathetic system in CHF.
A reduced peripheral baroreceptor afferent signal (due to the lower systolic pressure) might contribute to the higher baseline firing rate and the blunted response to baroreceptor withdrawal in CHF rats (37). One speculative explanation for this can be that perhaps this is due to a reduced withdrawal of inhibitory GABA/NO inputs in CHF. There is a tendency for an increase in the baseline discharge rate of PVN neurons that could not be evoked by RVLM stimulation in CHF rats compared with sham rats, but this did not reach statistical significance, perhaps due to the heterogeneity of this neuronal population.
We have previously shown that NMDA-mediated sympathoexcitation in the PVN is enhanced in rats with CHF (2). In the present study, we observed that compared with the sham group, the duration of increased firing to NMDA of PVN-RVLM neurons was longer in CHF rats. Perhaps the increased peak response in RSNA observed previously may be due to more NMDA receptors on a greater number of PVN-RVLM neurons since the peak responses in firing of PVN-RVLM neurons were not significantly different. We hypothesize that attenuated inhibitory mechanisms, probably involving NO, may contribute to the altered responses to NMDA. In the present study, the increase in the discharge to NMDA was sustained for a longer duration after l-NMMA, an inhibitor of NO synthase. Consistent with this, the change in the area under the curve was increased with NMDA and l-NMMA treatment compared with NMDA treatment alone in normal rats (Fig. 5). These results are similar in a qualitative sense to those observed with picoinjection of NMDA in rats with CHF compared with sham rats. We have previously observed that prior treatment of NO blockade in the PVN prolongs RSNA responses to NMDA (25), consistent with in vitro PVN slice recordings showing that NO blockade prolongs the firing of neurons in the PVN (23). Taken together, these data suggest that reduced NO-mediated inhibition during NMDA stimulation maybe one contributing factor in the exaggerated responses to NMDA in rats with CHF.
It has been well established that the baroreflex is markedly depressed in CHF (35, 48). It has also been reported that a portion of preautonomic neurons in the PVN respond to changes in MAP (6). Our study shows that the changes in discharge rates of PVN-RVLM neurons after an increase or a decrease in arterial pressure were significantly attenuated in CHF rats compared with sham rats. All of the PVN-RVLM neurons that responded to the baroreflex challenge also responded to the osmotic challenge. It is well known that the PVN is a pivotal integrative nucleus that regulates body fluid equilibrium. The present finding implies that PVN-RVLM neurons may play an important role in processing the interaction between the baroreflex and osmotic regulation. These results suggest that the altered responses in PVN-RVLM neurons to various autonomic challenges may be integrated at this level and contribute to the elevated sympathoexcitation that is observed during CHF. Taken together, the PVN appears to be an important site that is involved in the integration of various autonomic reflexes and thus dictates increased overall sympathetic activity in disease states such as CHF.
In a recent review, Toney and Stocker (46) suggest that neurons of the forebrain organum vasculosum laminae terminalis play a pivotal role in triggering hyperosmotic activation of SNA by recruiting neurons in specific regions of the hypothalamus, brain stem, and spinal cord. In the present study, we demonstrated that the discharge rate of PVN-RVLM neurons is altered by hypertonic stimulation and that the responses were enhanced in rats with CHF compared with sham rats. It is known that glutamate is involved in the osmotic response in the PVN (2, 43). In addition, increased glutamatergic activity on sympathetic regulation, due to the upregulation of NMDA NR1 receptor subunits within the PVN, has been suggested to contribute to the elevated sympathoexcitation during CHF (24). Thus, the upregulation of NMDA receptors and the subsequent increase in glutamatergic activity within the PVN may contribute to the altered osmotic response in CHF. Altogether, these results imply that altered PVN-RVLM neuronal activity in response to hyperosmolality might also contribute to the elevation of SNA in CHF; however, the precise stimulus and the detailed mechanism that may be involved remain to be elucidated.
Several mechanisms may contribute to the activation of the PVN in CHF. The renin-angiotensin-aldosterone system is activated in CHF (22). Both ANG II and aldosterone may activate central nervous system pathways leading to sympathetic hyperactivity. In addition, increased circulating cytokines cause the induction of cyclooxygenase-2 expression in the microvasculature of the PVN, resulting in enhanced proinflammatory cytokines in the PVN and sympathoexcitation in CHF (18). The effects of proinflammatory cytokines on the exaggerated sympathetic activity in CHF may occur via modulation of neurotransmitters in the PVN (17).
In summary, PVN-RVLM neurons are more active under basal conditions in CHF rats compared with sham rats, and the potentiated glutamatergic NMDA receptor-activated responses by these neurons may contribute to this tonic endogenous overactive state. These PVN-RVLM neurons may also be part of the central nervous system circuitry that critically contributes to the altered autonomic reflexes commonly observed in the CHF state. These results suggest that changes in the activation of PVN-RVLM neurons may contribute to the resultant elevated sympathoexcitation that is commonly observed during CHF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-62222.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.X. and K.P.P. conception and design of research; B.X. performed experiments; B.X. analyzed data; B.X., H.Z., and K.P.P. interpreted results of experiments; B.X. prepared figures; B.X., H.Z., and K.P.P. drafted manuscript; B.X., H.Z., and K.P.P. edited and revised manuscript; B.X., H.Z., and K.P.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kurtis Cornish for generating the rats with heart failure.
REFERENCES
- 1.Akaoka H, Saunier CF, Chergui K, Charlety P, Buda M, Chouvet G. Combining in vivo volume-controlled pressure microejection with extracellular unit recording. J Neurosci Methods 42: 119–128, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JF. A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol 576: 569–583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience 5: 1931–1958, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28: 95–99, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chen QH, Toney GM. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 281: R1844–R1853, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103: 4–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90: 169–173, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Dampney RA. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol 42: 197–227, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32: 419–425, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. Am J Physiol Regul Integr Comp Physiol 284: R259–R276, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Feng QP, Carlsson S, Thoren P, Hedner T. Characteristics of RSNA in experimental congestive heart failure in the rat. Acta Physiol Scand 150: 259–266, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus–a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12: 717–727, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han TH, Lee K, Park JB, Ahn D, Park JH, Kim DY, Stern JE, Lee SY, Ryu PD. Reduction in synaptic GABA release contributes to target-selective elevation of PVN neuronal activity in rats with myocardial infarction. Am J Physiol Regul Integr Comp Physiol 299: R129–R139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1–32, 1981 [DOI] [PubMed] [Google Scholar]
- 17.Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res 83: 737–746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res 99: 758–766, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic AT1 receptor blockade normalizes NMDA-mediated changes in RSNA and NR1 expression within the PVN in rats with heart failure. Am J Physiol Heart Circ Physiol 298: H1546–H1555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leenen FH. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res 101: 221–223, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Leenen FH, Skarda V, Yuan B, White R. Changes in cardiac ANG II postmyocardial infarction in rats: effects of nephrectomy and ACE inhibitors. Am J Physiol Heart Circ Physiol 276: H317–H325, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience 118: 585–601, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Li YF, Mayhan WG, Patel KP. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2328–H2336, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Matsumura K, Kagiyama S, Fukuhara M, Fujii K, Iida M. Chronic administration of olmesartan attenuates the exaggerated pressor response to glutamate in the rostral ventrolateral medulla of SHR. Brain Res 1058: 161–166, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Packer M. Role of the sympathetic nervous system in chronic heart failure–a historical and philosophical perspective. Circulation 82, Suppl I: I1–I6, 1990 [PubMed] [Google Scholar]
- 28.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev 5: 73–86, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst 22: 211–219, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Patel KP, Zhang K. Neurohumoral activation in heart failure: role of paraventricular nucleus. Clin Exp Pharmacol Physiol 23: 722–726, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Patel KP, Zhang K, Kenney MJ, Weiss M, Mayhan WG. Neuronal expression of Fos protein in the hypothalamus of rats with heart failure. Brain Res 865: 27–34, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (5th ed.). San Diego, CA: Academic, 2005 [Google Scholar]
- 33.Polson JW, Mrljak S, Potts PD, Dampney RA. Fos expression in spinally projecting neurons after hypotension in the conscious rabbit. Auton Neurosci 100: 10–20, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience 88: 949–957, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Rabelo E, De Angelis K, Bock P, Fernandes TG, Cervo F, Klein AB, Clausell N, Irigoyen MC. Baroreflex sensitivity and oxidative stress in adriamycin-induced heart failure. Hypertension 38: 576–580, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res 120: 164–172, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Schultz HD, Li YL. Carotid body function in heart failure. Respir Physiol Neurobiol 157: 171–185, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev 16: 101–107, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Seagard JL, Dean C, Hopp FA. Role of glutamate receptors in transmission of vagal cardiac input to neurones in the nucleus tractus solitarii in dogs. J Physiol 520: 243–253, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Stern JE. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537: 161–177, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stocker SD, Osborn JL, Carmichael SP. Forebrain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol 35: 695–700, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980 [DOI] [PubMed] [Google Scholar]
- 45.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand 177: 43–55, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Toney GM, Stocker SD. Hyperosmotic activation of CNS sympathetic drive: implications for cardiovascular disease. J Physiol 588: 3375–3384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vahid-Ansari F, Leenen FH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol 275: H2140–H2146, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Wang W, Brandle M, Zucker IH. Influence of vagotomy on the baroreflex sensitivity in anesthetized dogs with experimental heart failure. Am J Physiol Heart Circ Physiol 265: H1310–H1317, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Tonic glutamatergic input in the rostral ventrolateral medulla is increased in rats with chronic heart failure. Hypertension 53: 370–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Z, Coote JH. The influence of the paraventricular nucleus on baroreceptor dependent caudal ventrolateral medullary neurones of the rat. Pflügers Arch 438: 47–52, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol 283: H423–H433, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol 84: 217–232, 2004 [DOI] [PubMed] [Google Scholar]