Abstract
The pulmonary artery (PA) wall, which has much higher hydraulic conductivity and albumin void space and approximately one-sixth the normal transmural pressure of systemic arteries (e.g, aorta, carotid arteries), is rarely atherosclerotic, except under pulmonary hypertension. This study constructs a detailed, two-dimensional, wall-structure-based filtration and macromolecular transport model for the PA to investigate differences in prelesion transport processes between the disease-susceptible aorta and the relatively resistant PA. The PA and aorta models are similar in wall structure, but very different in parameter values, many of which have been measured (and therefore modified) since the original aorta model of Huang et al. (23). Both PA and aortic model simulations fit experimental data on transwall LDL concentration profiles and on the growth of isolated endothelial (horseradish peroxidase) tracer spots with circulation time very well. They reveal that lipid entering the aorta attains a much higher intima than media concentration but distributes better between these regions in the PA than aorta and that tracer in both regions contributes to observed tracer spots. Solutions show why both the overall transmural water flow and spot growth rates are similar in these vessels despite very different material transport parameters. Since early lipid accumulation occurs in the subendothelial intima and since (matrix binding) reaction kinetics depend on reactant concentrations, the lower intima lipid concentrations in the PA vs. aorta likely lead to slower accumulation of bound lipid in the PA. These findings may be relevant to understanding the different atherosusceptibilities of these vessels.
Keywords: hydraulic conductivity, low-density lipoprotein cholesterol
in humans, different vessels have very different proclivities to develop atherosclerotic lesions. Arteries with thick walls and high lumen pressures, such as the aorta, coronary, and carotid arteries, are by far the most likely to develop atherosclerosis (39, 69). In contrast, the pulmonary artery (PA), a lower pressure, intermediate thickness artery, is normally lesion-free, except under pulmonary hypertension (PH) (13, 19). Compelling evidence argues that atherogenic, plasma-derived low-density lipoprotein (LDL) cholesterol transport and accumulation in the artery wall, particularly in its subendothelial intima (SI), are fundamental initiating events of early atherosclerotic lesions (13, 50). High SI LDL concentrations appear to lead to LDL binding to the SI extracellular matrix (ECM) to form lipid packets, called liposomes, containing lipid from one or many LDL particles. This LDL binding and modification precede monocyte diapedesis and foam cell formation in lesion-prone aortic areas (41, 47). This study asks, does the PA have less LDL entry, transport, and concentration, leading to slower retention than the aorta wall? Such differences and differences in LDL modification may underlie their different atherosclerotic susceptibilities.
Tracer Entry and Accumulation in the Aorta
Experiments (7, 9, 21, 36–38, 51) and theory (23, 66, 73) have investigated how macromolecules cross the aortic endothelium to enter the vessel wall. Early calculations of Weinbaum et al. (66) for their leaky junction-cell turn over hypothesis/model showed that the presence of only one cell every few thousand whose junctions with its neighboring endothelial cells are leaky could account for the locally enhanced endothelial macromolecular permeability that has been observed. This holds even when the en face area of the leak is only a millionth of the total endothelial surface. Stemerman (52) found that macromolecules cross the aortic endothelium in focal spots, not uniformly. Aortic endothelial cells (ECs) in the M phase of mitosis (37, 38), ECs in the process of dying and/or sloughing of neighboring dying cells (36), or those containing stigmata (9) temporarily allow easily detectable macromolecules of different sizes, e.g., Evans blue albumin conjugate, horseradish peroxidase (HRP), and Lucifer yellow-low density lipoprotein, through their junctions (7). In rat aorta, the average HRP leak frequency is ∼1/(2,000–6,000) cells (7, 36–38) and leak duration is ∼1 h (9). Chuang et al. (9) injected rats with HRP, killed them at various circulation times up to 4 min, examined aortic spots en face, and observed very rapid spot grown, with spot diameters ∼30 μm to >200 μm in 4 min. Tompkins (61) injected 125I-LDL into four squirrel monkeys, killed them 30 min later, harvested numerous vessels, sectioned the vessels extensively, and placed the sections on photographic film. From these films they determined the LDL concentration profile through the walls of various vessels.
Yuan et al. (73) postulated a two-dimensional (2-D), convection-diffusion model that explicitly includes the endothelium, SI, internal elastic lamina (IEL), and media layers of the artery wall. Although they found a large convective flow through the leaky endothelial cleft that advected tracer, their model failed to explain the HRP spot growth seen in Chuang et al. (9). By examining the ultrarapid freezing/rotary shadow etchings of the rabbit SI of Frank and Fogelman (14), Huang et al. (23) found the SI to be far sparser than the media. Their heterogeneous fiber matrix theory calculated SI Darcy permeability and tracer diffusivity to be 10–100 times the measured media values. With these and other measured and guesstimated parameters, they extended the model of Yuan et al. (73) to reveal a previously unanticipated flow pattern in the wall. The mismatches between SI and media flow resistance and between leaky and normal junction hydraulic conductivity caused strong advective macromolecular transport parallel to the endothelium in the SI in a ∼200-μm diameter region around the leaky junction, consistent with observed rapid HRP spot growth (9).
Schwenke and Carew (43) found that aortic SI LDL concentration increased within 2 wk of lipid feeding. Simionescu and colleagues (41, 47) and later Frank and colleagues (14, 42) used freeze fracture to image what they called extracellular lipid liposomes, comprised of the lipid from one to hundreds of LDLs, bound to aortic SI ECM. Yin et al. (72) developed a hierarchy of nucleation-polymerization models for the formation and growth of extracellular lipid liposomes in the aortic SI that predicted the observed liposome size distributions in chronically hypercholesteremic and short-term cholesterol-fed rabbits (14). It is tempting to presume that, since these lipid-tissue interactions are local, their kinetics would be location independent, given the not radically dissimilar ECM. Heart valve leaflets also accumulate lipid at high blood cholesterol and calcify, thereby loosing their capacity to prevent blood backflow (5). Valves are an ideal test case. Although leaflet and artery wall ultrastructures differ (leaflets have 2 endothelia, no IEL, and a central ECM layer with interstitial cells), Zeng et al. (76) found focal, rather than uniform, endothelial HRP leaks, HRP spot growth similar to the aorta, and thin, sparse SIs beneath each endothelium. We (78) proposed a 2-D convection-diffusion model that included these intimae, fit (only) three parameters (to a single experiment), and measured or took from the aorta all other parameters. The model explained the spot data (78) and a multitude of previously perplexing data on 125I-LDL valve penetration (60). Combining this LDL transport with the liposome kinetics (all parameters fixed) of Yin et al. (72) predicted lipid accumulation and extracellular lipid liposome size distributions in rabbits fed a high lipid diet for 1 mo in excellent agreement with the experimental liposome size distribution (75).
Transport and accumulation in the PA.
Few transport studies have been reported for the PA. Lever and Jay (34) measured the steady-state uptake of labeled albumin by various rabbit blood vessels. They found the fractional albumin volume much greater in the PA than in the aorta wall. Shou et al. (46) showed that, at physiological lumen pressures ΔP*, the rat PA wall has a much higher hydraulic conductivity L*p than its aortic wall, both with intact and denuded endothelium. Their data indicate similar endothelial L*p (computed at the lowest ΔP*). Thus the PA is more permeable to both fluid and macromolecules and its medial tissue is likely much sparser than the aorta. Given their different ΔP*, it is not a priori obvious which experiences the higher fluid flux. Shou et al. (45) found this flux, wall L*p × ΔP*, was the same in the aorta, PA and even the inferior vena cava, to within experimental error. Shou et al. (46) found the rat PA endothelium also leaks HRP focally, not uniformly, over a 4-min circulation and PA and aorta focal HRP spot growth with HRP circulation time are both rapid and surprisingly similar to each other and to the aorta study of Chuang et al. (9). No such PA LDL spot growth data are available, but Lever et al. (35) measured fibrinogen and 125I-LDL uptake in numerous vessels after 5- to 15-min circulation in vivo. They report similar values for total fibrinogen uptake in the descending thoracic aorta and in the PA, both approximately one-half of that in the inferior vena cava and in the ascending aorta. They do not report LDL values, but their prose indicates that the LDL and fibrinogen, molecules of similar size, values were similar. This study asks, in view of the large PA-aorta differences of ΔP*, L*p, and fractional macromolecular volumes, how can their macromolecular transport turn out to be so similar? We hypothesize that both vessels' mismatches in SI-media density and in leaky junction/normal endothelium Lp lead to similar SI spreading flows that advect tracers radially to form similarly (due to similar overall L*pΔP*) rapidly growing tracer spots.
Since the PA and aortic walls have similar basic layered structures, we adapted the geometry of Huang et al. (23) mathematical transport model in the aortic wall to the PA. The far less dense PA wall has very different transport parameters than the aortic wall. Since one studies transport into vessel walls to see if early lipid accumulation rates correlate with vessel disease susceptibility, we hypothesize that the normotensive PA has a lower SI LDL concentration than the aorta; with any reasonable lipid accumulation kinetics [e.g., the liposome kinetics of Yin et al. (72)], this should lead to slower lipid accumulation.
MATHEMATICAL MODELS
Model Description
The transport problem for comparison with the experiments of interest (9, 61) is an initial value problem that starts with zero tracer concentration everywhere, followed by a step jump to a time-independent lumen concentration.1 Since electron micrographs (45, 46) show the PA and aorta have the same basic structural layers: endothelium, SI, IEL, media, and adventitia, Fig. 1, A and B, applies to both vessels the same basic 2-D convection-diffusion model (similar, but not identical, to that in Refs. 23, 73). We only describe it in words, leaving the equations to appendix a. The model excludes the adventitia, since its loose connective tissue plays a minor role in transport, and the vaso vasorum, since advection transports outwardly from the vessel lumen. Water crosses the entire endothelium; large macromolecules only cross the junctions of rare leaky ECs (Fig. 1C). We arrange these leaky cells (taken as round) in a periodic surface array, each surrounded by a uniform endothelium of normal permeability. The models below are 2-D (r*, z*) axisymmetric on a periodic (by abuse of geometry) cylindrical unit, with its origin at the center of the leaky cell. Let R1* be the average effective EC radius. The (area or number) fraction, R*2/ξ*2, of cells that leak defines the radius, ξ*, of the cylindrical unit, roughly half the average distance between leaky ECs, where periodic boundary conditions apply.
Fig. 1.
Schematic of the periodic wall unit in terms of a cartoon of the physiological wall structure with nondimensional variables (A) and in terms of an idealized mathematical diagram with dimensional variables (B) for the filtration and macromolecular transport models in the arterial wall. C: finer scale model of Ref. 63 of the leaky cleft for our equations (Eq. A17 and Eq. A18); Eq. A18 enters our problem as a boundary condition at the endothelial surface. EC, endothelial cell; SI, subendothelial intima; IEL, internal elastic lamina; SMC, smooth muscle cell; EL, elastic lamellae. For all figures, see Table 2 for definitions of parameters.
The local average superficial water velocities in the radial U* and normal W* directions are volumetric flow rates divided by the total normal cross-sectional area. Velocities satisfy Darcy's Law and incompressibility, which combine to give Laplace's equation, and concentrations (per unit total volume) satisfy an advective-diffusion equation in the SI and in the media.2 Lumen and extravascular pressures are prescribed, and W* is continuous at the IEL; hydraulic conductivity boundary conditions apply at the normal endothelium, in the leaky junction and at the IEL. Tracer fluxes and concentrations (per unit void volume) are either prescribed or continuous at these surfaces. Axisymmetry and periodicity imply zero fluid or mass flux at r*=0, ξ* (see appendix a for nondimensionalizations and for mathematical details). The transmural pressure drop drives plasma (here called water) through normal and (wider, thus higher Lp*) leaky EC junctions into the SI. The pressure drop is thus much smaller across leaky than normal junctions. This creates a large SI pressure gradient that spreads water and advected tracer in the SI parallel to the endothelium away from the leaky cleft. Water and advected tracer seep through the IEL fenestrae into the media. Any reasonable (i.e., monotonic: higher local concentration yields higher local rate of lipid accumulation) liposome kinetics [here, Yin et al. (72)] can compare lipid accumulation in the aorta and PA.
Solution Methods
Transport models.
Given the complex, two-layered, 2-D system geometry, we have chosen a direct discretization, finite difference on a nonuniform mesh approach to solve for the pressure and concentration distributions.3 The mesh is dense near the leak and sparse far from it. The discretized filtration model becomes a (large) number (equal to the total number of mesh points) of simultaneous linear algebraic equations, which MATLAB (The MathWorks) easily solves. For the macromolecular transport model, we choose the Hopscotch Method, a fast second order partial differential equation solver. This method, a type of finite difference technique described by Gourlay (15), is fast, efficient, and unconditionally stable.
Due to significantly less computer power available, earlier studies (23, 73) made a number of simplifications to solve such 2-D, two-region, advective-diffusion theories for transport in the aorta. As it turns out, these simplifications strongly impact the accuracy of the calculated solutions, particularly in the critical region near the leaky junction. appendix b details these simplifications and how we have solved our models without them.
Constants and Parameters in the Models
Table 1 summarizes baseline values (and sources) of our aorta and PA model constants/parameters. Table 2 summarizes the definitions of all abbreviations, symbols, and subscripts. All aorta parameter values are from the literatures. Based on the similarities Shou et al. (45, 46) found, we assume the PA and aorta share geometric, EC (R*1, γl, fl, L*1), SI (L*1, γ1, f1,), and IEL (L*I, ϕI, af, γI, fI), but not media, parameters. Parameters where they differ appear either in parenthesis in Table 1 or in appendix c. Examples are the aortic SI's Darcy permeability, K*p1, and tracer diffusivity, D*1. The heterogeneous fiber matrix theory of Huang et al. (23) based on the ultrastructural ultrarapid freezing/rotary shadow etchings of Frank and Fogelman (14) of (only the) aortic SI ECM calculated averages and upper bounds for K*p1 and D*1. Since Fig. 4 in Shou et al. (45) indicate the PA SI appears to compress at a lower pressure (∼40 vs. ∼80 mmHg) and the PA wall is generally sparser/more permeable than the aorta, we choose the upper bounds of Huang et al. (23) for the PA and average values for the aorta. The effective hydraulic conductivity of normal endothelium, L*pnj, and of the media, L*p2, follow from intact (L*p1) and denuded (L*pd) hydraulic conductivity data measured on the same vessels by
| (1,2) |
where L*p1 and Lp1 × L*p2 and Lp1 is the nondimensional theoretical value from Eq. A12. The right side of Eq. 1 neglects the thin, sparse SI layer because its Lp is much greater than those of the other layers. [SI compaction theory (24) provides a more refined view of the SI contribution, but at a finer level of resolution than this model. Here we lump these effects into the calculated L*pnj.] Note Kp2* = L*p2μL2*. Curry (11) calculates the retardation coefficient, fi, in region i for the leaky junction (i = l) (including glycocalyx), SI (i = 1), IEL fenestrae (i = I), and media (i = 2) by
| (3) |
Table 1.
Baseline values of the constants/parameters in the rat aorta and PA models
| Constants/Parameters | Baseline Values | Reference No. |
|---|---|---|
| L*1, μm | 0.2 | (23) |
| L*l, μm | 2.0 | (67) |
| L*I, μm | 1.0 | (73) |
| L*2, μm | 100 (50) | (34) |
| R*1, μm | 15 | (67) |
| ΔR*, nm | 20 | (67) |
| ΔP*, mmHg | 100 (16) | (45) |
| L*pt, cm·s−1·mmHg−1 | 2.79 × 10−8 (1.90 × 10−7) | (45) |
| L*pd, cm·s−1·mmHg−1 | 4.89 × 10−8 (2.99 × 10−7) | (45) |
| ϕI | 0.043 | (22) |
| af, μm | 1.0 | (22) |
| γ1 | 0.5 (LDL) 1.0 (HRP) | (23) |
| K*p1, cm2 | 1.08 (2.8) × 10−12 | (23) |
| γ2 | 0.08 (0.234) (albumin) | (34, 55) |
| fl | 1.0 | (73) |
| f1 | 0.75 (LDL)1.0 (HRP) | (23) |
| f2 | 0.3 (HRP) | (23) |
| D*1, cm2/s | 6.02 × 10−8 (LDL); 4.12 (5.0) × 10−7 (HRP) | (23) |
| D2*, cm2/s | 8.4 (0.54) × 10−9 HRP (LDL); for PA in text | (23, 62) |
| D*2r/D*2z | 3.0 | (73) |
| μ, g·cm−1·s−1 | 7.2 × 10−3 | (20) |
Note: the pulmonary artery (PA) is assumed to have the same parameter values as the aorta unless a different value is specified in parenthesis or in results and discussion. HRP, horseradish peroxidase. Definitions of parameters/constants are listed in Table 2.
Table 2.
Symbols and abbreviations
| Symbol | Definition | Sub/Superscript | Definition |
|---|---|---|---|
| Bi | Biot number kL2/D*2z | a | Adventitia |
| C | Concentration | d | With ECs denuded |
| D | Diffusivity | f | Fenestra |
| F | SMC volume fraction | i | Dummy subscript |
| G0 | Eq. A12 | l | Leak |
| J0, J1 | Bessel functions order 1.2 | lj | Leaky junction |
| Kp | Darcy permeability | ls | At leaky junction's SI exit |
| L | Thickness or height | nj | Normal tight junction |
| Lp | Hydraulic conductivity | r | Radial component |
| P | Pressure | t | Total intact wall |
| Pe | Peclet number | ω | Water |
| R1, R2 | EC radius, R1+ ΔR | z | Axial component |
| U | Radial velocity | I | IEL |
| V | Velocity vector | Im | Media side of IEL |
| W | Axial velocity | L | Lumen |
| af | Fenestra radius | SI | Subendothelial Intima |
| f | Retardation coefficient | 1 | Subendothelial Intima |
| k | Endothelial mass transfer coefficient | 2 | Media |
| q | Mass flux vector | * | Superscript: dimensional quantity |
| r | Radial coordinate | ||
| rf | Average fiber radius | ||
| t | Time (dimensional) | ||
| z | Axial coordinate | ||
| ε | Void fraction | ||
| γ | Solute void fraction | ||
| μ | Viscosity or micron | ||
| ξ | Radius of periodic unit | ||
| ξI | Mean half-distance between adjacent fenestrae | ||
| σf | Solvent drag reflection coefficient | ||
| τ | Nondimensional time | ||
| ϕI | IEL fenestra fractional area | ||
| ψ | Solute partition coefficient | ||
| ΔP | Transmural pressure | ||
| ΔR | EC junction thickness | ||
| ∇ | Gradient | ||
| ∇· | Divergence |
Fig. 4.
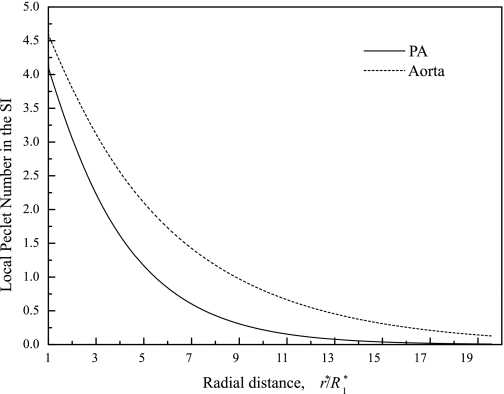
Local Peclet number in the radial direction in the SI for the PA (solid line) and the aorta (dash line).
σfi the solvent drag reflection coefficient in region i. The matrix structures in these regions account for solute filtration; thus σfi = (1 − Ψi)2, Ψi the solvent partition coefficient in region i. For the solutes and matrices here, Huang et al. (23) found Ψi ≈ γi/γwi, the ratio of the fractional volumes available to macromolecules (γi) and water (γwi,). Due to similar matrix structures (33), we equate the IEL fenestrae to SI transport parameters, γI = γ1, γwI = γwl, DI* = D1*.
Due to the paucity of experimental data for the PA (or for a PA fiber matrix theory; Refs. 12, 23), we adopted a fitting scheme based on the transwall LDL profiles of Tompkins et al. (61) similar to those described in detail in our theoretical study of transport in heart valve leaflets (78) to get two media macromolecular transport parameters: the mass transfer coefficient, k, through the normal endothelium and the media diffusion coefficient, D*2z, for LDL. Again we choose the Tompkins' profile with the lowest concentrations that likely derives from tissue sections with only normal junctions, i.e., far from an LDL leak, where our 2-D model looses its r* dependence.4 Fitting the one-dimensional (1-D) convection-diffusion model (setting ∂/∂r* ≡ 0) to these data gives k and D*2z, both for LDL. Since no value for the PA D*2z(HRP) is known, we use the Stokes-Einstein (11, 18) size-scaling Diz* ∼ rs*−1, rs* = solute radius {rs*(LDL) [rs*(HRP)] = 11 (3) nm (23)}, to calculate the free solution diffusivity of LDL as 11/3 that of HRP. Since the fiber matrix diffusivity is the free solution value × exp[−(1 − ε)1/2(1 + rs*/rf*)] (11), for the PA (void fraction ε = 0.46 and fiber radius rf* ∼ 5.9 nm: see appendix c), this increases the HRP matrix diffusivity by an additional factor of 2.72; thus D*2z(HRP) ∼ 9.97 D*2z(LDL) from Table 1. We set the media distribution volume/void space for albumin (γ2) equal to that for HRP, since albumin and HRP are similar in size, i.e., rs*(albumin) ∼ 3.5–4 nm (10).
RESULTS AND DISCUSSION
Derived Parameter Values
Table 1 lists the measured values of L*p1 (intact) and L*pd (denuded) at physiological pressures for the aorta (PA) of 100 (20) mmHg (45), not averaged over the measurement range, as in Shou et al. (45). With these values, Eqs. 1–2 give L*pnj = 6.50 (5.21) × 10−7 cm·s−1·mmHg−1; L*p2 = 5.77 (4.07) × 10−7 cm·s−1·mmHg−1 for the aorta (PA). appendix c discuses these derived Lp values and compares them with each other and with published values. appendix c details parameters that our group measured (22) after publication of our earlier model (23). It discusses that study's estimation methods for some parameters and how we have significantly improved upon them.
The fitting scheme described in Constants and Parameters in the Models gives the two optimized PA transport parameters, the medial z-diffusivity D*2z = 6.57 × 10−9 cm2/s (LDL) and endothelial mass transfer coefficient k = 5.18 × 10−9 cm/s (LDL). Figure 2 shows the best fitted result (continuous curve) with the experimental data (discrete points). This k is nearly identical to Tompkins' PA value [which is 3–5 times their aorta value of 0.83 ∼ 1.5 × 10−9 cm/s (59)], and this D*2z is about four times Tompkins' D. Tompkins' model has mass transfer coefficients for the lumen and for the adventitial boundaries and a single diffusivity for the interior of the vessel wall. Tompkins notes that his PA fit is not satisfactory: in retrospect, since his model did not include an SI with a much higher LDL space, it needed to keep D small to achieve a sharp concentration drop near the endothelium. Unfortunately, this also resulted in an underestimate of the LDL concentration far from the boundaries. Our inclusion of an SI naturally reflects this drop and our higher fit value of D (Fig. 2) models the media concentration far better.
Fig. 2.
Nondimensional tissue low-density lipoprotein (LDL) concentration profile for the pulmonary artery. Normal distance is in the direction perpendicular to the endothelial surface. Discrete points are experimental data from Ref. 61. Continuous curve is the best-fit result with the one-dimensional model, where the segment with high values near z = 0 corresponds to the subendothelial intima.
Our PA D*2z is also about an order of magnitude greater than those in the studies of Tompkins (59), Truskey et al. (62), and Dabagh et al. (12) that had similar effective lumped aortic wall diffusivities 0.50, 0.54, 0.43 × 10−9 cm2/s and our k is about half the k = 1.1 and 1.9 × 10−8 cm/s that Tompkins (59) calculated from his squirrel monkey descending aorta and Truskey et al. (62) from their rabbit aorta data. Again these are parameters calculated with different transport models for different experiments on different vessels in different species. Both groups inferred their values from a 1-D model with neither a sparse SI nor advection (mainly parallel to the endothelium), facts discovered later, but rather a finite or semi-infinite, uniform medium with a permeable boundary. Tompkins (59) estimated LDL diffusivities by comparing the predicted and measured fraction of the 125I-LDL in the wall. Truskey et al. (62) examined the LDL in Häutchen preparation, roughly endothelium and SI, after 10 min 125I-LDL circulation. Because the SI is in fact far more porous than the media, most of the 125I-LDL in the wall resides there at a 10-min circulation. Tompkins's (59) aortic profiles also had very high LDL concentrations adjacent to the endothelium and nearly zero beyond it. To explain a high SI LDL concentration/fraction and nearly zero beyond (far lower than found in the PA), a model assuming uniform wall porosity would need to raise k and lower LDL diffusivity to get so much LDL into the SI and to keep it from spreading. Tompkins's medial LDL concentrations were too close to zero for us to use them to find a precise improvement on their aorta D*2z. Moreover, from the calculated numbers below, the media Peclet number, W*2L*2/D*2z ∼ 5 × 102, meaning medial z-diffusion is negligible relative to advection. Thus we simply took the largest of these three literature D*2z values, although the true aorta value could easily be two to three times that number.
Our PA D*2z(LDL) exceeds our aortic D*2z(LDL) by a factor of 12; as noted, the real ratio is likely ∼4. Tompkins simplified geometric model yielded a factor of 3: that the PA media diffusivity far exceeds that of the aorta is consistent with a variety of evidence that the PA is far sparser and more permeable to both water and macromolecules than the aorta: the PA has a far higher L*pt than the aorta (45). The in vitro experiments of Lever and Jay (34) showed that the PA wall has a greater distribution volume albumin than systemic arteries. Tompkins et al. (59, 61) found 30 min medial LDL concentrations ∼10 times greater in the PA than in the aorta. appendix c uses fiber matrix theory to find a theoretical range for the ratio of PA to aorta D*2z.
Pressure Distribution
Figure 3 plots nondimensional PA (solid lines) and aorta (dash lines) variables: 1) the pressure, PSI, in the SI at z = 0; 2) the pressure, PIm, at the IEL-media surface; and 3) the normal velocity, W*I, across the IEL. All of these curves attain maximum values at the leaky cleft (r*/R*1 = 1) and decay to lower, otherwise uniform values far from the leak (r*/R*1 > 10). The pressure just beneath the leak is the highest in the wall because the drop across the much wider leaky junction is far smaller than across the normal junctions and this propagates into the wall. It also sets up a large SI radial pressure gradient that drives an SI radial velocity. Far from the leak, the leak's effect decays; the curves in Fig. 3 loose their r* dependence, i.e., the model reduces to 1-D. Although, aortic PSI and RI* are similar in shapes to those in Fig. 6 of the earlier aortic study of Huang et al. (23), our larger L*pt leads to higher pressures and velocities, and our more accurate calculation near the leak leads to significantly higher W*I there. More critically, PIm(r) at the IEL-media surface is quite different. As appendix b details, Huang et al. (23) initially simplified the 2-D media model by assuming its pressure depends only on z*, i.e., is 1-D. Even with their small a postiori 2-D correction, their PIm curve is nearly horizontal, i.e., r* independent. In contrast, our fully 2-D convection-diffusion model results in a clear rise/maximum in PIm(r*) z = 0 (and in W*I) near r*/R*1 = 1. This confirms, as one would expect, that the leaky cleft's impact on P extends beyond the SI. Since the media Darcy permeability, Kp2*, is small, this effect damps quickly with z in the media. This effect is a bit more pronounced in the PA due to its looser media. Concentration plots below show it impacts media mass transport, especially in the PA, due to its media's higher macromolecular distribution volume.
Fig. 3.
Nondimensional pressure in the subendothelial intima (PSI) at z = 0 and at the interface of the IEL and media (PIm), and the normal velocity (W*I) across the IEL for the pulmonary artery (PA, solid lines) and the aorta (dash lines) as functions of radial distance r from the center of the leaky cell. Radial distance r* in this and subsequent plots is normalized by the radius of an endothelial cell (R*1; thus the leaky junction is at r*/R*1 = 1), as opposed to r: = r*/L*p2 (Eq. A1), to focus on the junction region. Pressure is normalized by the transmural pressure drop (ΔP*), 100 mmHg for the aorta and 16 mmHg for the PA (45).
Fig. 6.
Theoretical predictions from current theory vs. the data of Ref. 61 for LDL vs. z for curves from normal (A and B) and enhanced (C) permeability regions. C: assumed endothelial denudation and blood in vessel during early fixation to obtain such high LDL concentration at lumen boundary. Calculation scenarios described in text.
The nondimensional filtration model allows comparison of PA and aorta pressure distributions with their large transmural pressure difference ΔP* factored out. The PA intima pressure PSI lies above the aorta PSI. This indicates that the flow resistance due to the endothelium plus SI, including effects of SI compression and IEL fenestral blockage (22, 24), contribute a smaller fraction of the total wall resistance in the PA than the aorta. [The statement of Shou et al. (45) to the contrary was based on intact L*pt and denuded L*pd values corresponding to ΔP* where the SIs of both vessels were fully compressed, i.e., PA: 40–100 mmHg; aorta: >80 mmHg. The present conclusions use PA values at physiological ΔP* ∼ 20 mmHg, where its SI is not fully compressed.] It is not surprising that the dimensional PA and aorta normal velocity W*I curves are similar far from the leak (large r*) since, as Shou et al. (45) note, the vessels' transmural water fluxes [L*ptΔP*] are nearly identical: 2.79 × 10−6 (PA) vs. 3.04 × 10−6 cm/s (aorta). These fluxes reflect the uniform parts (r*/R*1 >> 1) of W*I curves of Fig. 3, where the leak's impact is negligible and the 2-D model reduces to 1-D in z. In 1-D, the continuity equation, Eq. A4, implies W*I = W2* ≠ W2*(z), i.e., W2* is uniform and equals L*ptΔP*. More strikingly, the dimensional PA and aorta W*I are remarkably similar near the cleft, despite the vessels' very different ΔP* and total wall L*pt.
The local SI radial Peclet number, PeSI = f1r*U1*/γ1D*1, the ratio of the SI radial advection to molecular diffusion, is a way to represent the radial flow rate nondimensionally. Figure 4 plots PeSI for HRP vs. radial position for the PA (solid) and aorta (dash). Despite the larger PA than aorta leaky junction Lplj*, the PA PeSI is still smaller than the aorta PeSI. Since the aorta has the far larger transmural pressure, ΔP* (100 vs. 16 mmHg), which normalizes pressures, this does not contradict the similar aorta/PA PSI curves in Fig. 3. Figure 4 shows PeSI ∼4.0 at the leak (r*/R*1 = 1). The regime where advection dominates diffusion (PeSI > 1.0) extends to r*/R*1 ∼ 5.5 (PA) and ∼8.5 (aorta). Huang et al. (23) guesstimated fractional IEL fenestral areas ϕI = 0.002 (0.01), which our group later measured to be far larger ϕI = 0.043 (22), for rat aorta, and this led to an overestimate of the advection-dominated region's extent as r*/R*1 ∼ 11.0 (∼9.0). There are at least three reasons their value turned out so high. 1) Since the IEL barrier forces fluid in the SI to flow radially, a smaller IEL fenestrae fractional area, ϕI, means higher IEL resistance and more radial spread. 2) Their nearly r*-independent W*I (see appendix b; contrast with Fig. 3) understated fluid drainage from the SI near the leak, forcing additional radial flow in the SI. 3) Their severely underestimated normal junction L*pnj (see appendix b) exaggerated the leaky/normal cleft Lp mismatch, which led to a too high radial SI pressure gradient, and thus radial velocity, UI*.
Concentration Distribution
The velocity field (Eq. A5) advects tracer in Eq. A15. Figure 5 plots the HRP concentration, at 4-min HRP circulation, in the SI [CSI(z = 0)] and at the IEL-media interface (CIm) for the PA and the aorta5 Since for at least 4-min studies, HRP that penetrates into the SI through leaky clefts is easily distinguishable from uniform leakage, Fig. 5 neglects HRP transport across the normal endothelium (it only calculates the nonuniform part), i.e., sets k (Eq. A19b) = 0.
Fig. 5.
Concentration distributions of horseradish peroxidase (HRP) at 4-min circulation in the SI (CSI) at z = 0 and at the IEL-media interface (CIm) for the PA and the aorta. The radial distance (r*) is normalized by the radius (R*1) of an endothelial cell, and thus the leaky junction is at r*/R*1 = 1.
Both concentrations, CIm and CSI, adjacent to the IEL are higher near the leak (r*/R*1 ∼ 1) and decay to very low values away from it (r*/R*1 >> 1). Aortic CIm is higher than in Huang et al. (23) and nonuniform, due to the higher ϕI noted above and the improved solution method in appendix b, leading to larger, nonuniform W*I. There is a concentration drop across the IEL (CIm ≪ CSI) in both vessels due to the media's much smaller macromolecular distribution volume. Near the leak, where concentrations are large, aorta CSI is higher than PA CSI, but the opposite is true for CIm because the SI/media void space mismatch in the aorta is far starker than in the PA. In fact, γ2 = 0.08 for HRP in the aorta (34), about one-third (0.234) in the PA (55). Macromolecules in the aortic SI have far more difficulty entering its media than in the PA and therefore have a higher CSI but lower CIm there. Phrased differently, the PA IEL and media's lower transport resistance extend the leaky cleft's impact further into the media. Far from the leak, CSI is still higher in the aorta because, as Fig. 4 illustrates, its radial SI advection is larger than in the PA. The concentration curves become more 1-D, i.e., the leaky junction's impact wanes, as z increases into the media and the pressure decays to the uniform (in r) zero boundary condition Eq. A25.
Theory vs. Experimental LDL Concentration Profiles
Figures 8C and 6D of Tompkins et al. (61) present transwall profiles of various magnitudes. Zeng et al. (78) were very successful at plausibly explaining the huge variation in transvalve LDL profiles of Tompkins et al. (60) by showing that sections of different distances from localized leaks could reproduce all of their profiles. Similarly, our model attributes the high concentrations of Fig. 6D of Tompkins et al. to the sections in question being close to a focal leak; our Fig. 2 used their lowest concentration curve 2, no leak, to fit k and D*2(LDL). Figure 6A shows a very good comparison of the theory for a slice (an optimized) 178 μm from the center of a leak, averaged over the slice thickness and length vs. the data from their (similar) curves 3 and 4. Figure 6B does the same for their curve 1, which has a wider region of high LDL values, indicative of a thicker region of the SI, here taken as 5 µ, for the section 252 μ from a leak. Finally, Fig. 8 of Tompkins et al. contains a PA curve from what they call an “enhanced permeability” region, found in only the right branch of one of four monkey PAs, where LDL values are approximately five times the earlier values (indicating likely endothelial damage/denudation) and where the LDL curve does not flatten out until ∼40% of the wall depth. This unique shape (relative to the other PA curves) suggests something unusual may have happened. Speculative scenarios with a uniformly damaged endothelium can also account for this profile (Fig. 6C).
Fig. 8.
HRP spot growth at 4 min circulation in the PA and aorta. Experimental results: discrete points from Refs. 9 and 46; theoretical predictions: continuous curves. Curves 1 and 2: aorta; curves 3, 4, and 5: PA. Curves 1 and 3 from SI HRP concentrations; curves 2, 4, and 5 from integration of HRP concentrations across vessel wall. Curves 2 and 4 use Intth = 1 × 10−5 cm; curve 5 uses Intth = 1.2 × 10−5 cm. Curve 1 uses Cth = 0.05; curve 3 uses Cth = 0.02.
Growth of HRP Leakage Spots
Figure 3 exhibits large radial PSI gradients, i.e., U1, that advect macromolecules away from the leak in the SI, rapidly growing HRP spots. Chuang et al. (9) and Shou et al. (46) both observed this rapid HRP spot growth en face in rat aorta. HRP spots grow extremely rapidly in the first minute after HRP injection and more slowly thereafter. Measurements stop after 4 min, since the cumulative penetration of HRP through normal EC junctions after 4 min darkens the background, making it difficult to distinguish leakage spot from background.
The threshold (Cth) for the HRP DAB reaction product visibility can vary slightly with different HRP batches, with precise staining reaction conditions and with photographic exposure. Naturally, one would prefer Cth not be adjustable. As in Huang et al. (23), we require a single Cth for a set of four HRP circulation times for a given vessel type. Figure 7 plots model solutions for SI HRP concentration C1(r*/R*1), averaged dz across the SI [due to the SI separation of scales (1), its z-variation is very small], and equates Cth to C(30-s circulation) at r*/R*1 = average experimental 30-s spot radius. Thirty seconds are the sum of an unknown (i.e., not measured in Refs. 9 or 46) time lag t0 for the tracer to reach the lumen adjacent to the vessel of interest from its femoral vein injection point plus 30-t0-seconds model time where tracer enters the wall. We draw a horizontal line at Cth on Fig. 7 and read off the abscissa values at which it crosses the curves for different circulation times to predict the HRP spot edge. We vary t0 to get a best fit. Curve 1 (aorta) in Fig. 8 shows good agreement for Cth = 0.05 and t0 = 25 s. Our t0 = 25 s agrees with Huang et al. (23), but our Cth, 0.05 is smaller than their Cth = 0.13 (23). This is expected since, as detailed in the discussion of Fig. 4, Huang et al. overestimated U1* and consequent SI advection, leading to higher HRP C1(r*/R*1, t) at all t than the aorta curves of Fig. 7. Their matching at 30 s thus leads to a higher Cth. Zeng et al. (78) used a 2-D advection-diffusion model similar to ours, but with two SIs for the valve leaflet aspects to predict HRP leakage spot growth in valve. They found Cth = 0.015, closer to our aortic value than Huang et al. (23).
Fig. 7.
Evolution with HRP circulation time of SI tissue-to-plasma HRP concentration distribution for the PA (solid lines) and aorta (dash lines). Four curves for the each vessel represent (left to right) 30-, 60-, 120-, and 240-s circulation. Best data fits obtain for time lags t0=15 sec (PA) and 25 s (aorta) for HRP to travel from the femoral vein to the vessel. Visibility threshold HRP concentrations, chosen as in the text, are 0.02 (PA) and 0.05 (aorta).
Since the PA U*1 (or PeSI; Fig. 4) is lower and its media's resistance is smaller than the aorta's, its SI HRP concentration curves (Fig. 7) are also lower, thus yielding a smaller Cth = 0.02. Figure 8, curve 3, plots the PA theoretical spot growth, comparable to the aorta's for ∼1 min but slightly smaller thereafter. The t0 = 15 s is less than the aortic value, since tracer injected in the femoral vein reaches the PA before the lungs, heart, and aorta.
It is unsatisfactory that the aorta, PA and valve leaflet use different Cth, even with the improved aorta value (23). Despite the PA slower radial SI tracer advection, Shou et al. (46) found that both have remarkably identical HRP spot growths, further suggesting the above fitting procedure is missing something important. All of these calculations compare spots with SI HRP spread, presuming media HRP is negligible. Since the normal fluid fluxes across both vessel walls are the same, but the denuded L*pd of the PA (45) is much larger than of the aorta, this flow in the PA spreads fluid and macromolecules not only radially in the SI but also into the media. Even low media concentrations can represent significant HRP in a thick media. One measures spot radii by taking light micrographs en face from above a vessel lit from below. This view through the off-white, adventitia-free vessel (9, 46) in effect integrates the HRP (the only brown) absorbance (modulo scattering) across the wall. The integrated media contribution may not even be negligible in the aorta [γ2(HRP) ∼ 0.08 (55)] and, a fortiori, not in the PA [γ2(HRP) ∼ 0.234 (34)]: the theoretical media HRP concentration (CIm curves in Fig. 5) is higher in the PA than aorta. Figures 3 and 6D of Tompkins et al. (61) show experimental media PA LDL concentration more than 10 times their aorta value (61).
Let A(λ) be the HRP reaction product's absorbance, the logarithm of the ratio I/I0 of the intensities of the transmitted and incident beams, at the interrogating wavelength λ, ε(λ) the HRP absorptivity, z the light path normal to the endothelium, L = L1 + L2, and C(r, z) the nondimensional absorber (tracer) concentration. Beer-Lambert's Law of optics states
| (4) |
This is the simplest log-linear assumption for tracer absorbance, e.g., for photographic film sensitivity. Its use ignores tissue scattering for simplicity (unknown material scattering parameters) and tissue thinness (∼100 μm). Figure 9 plots the radial distribution of A/ε = ∫cdz (using white light, i.e., average ε), and not simply of SI HRP concentration, for the PA (solid) and the aorta (dash) at different HRP circulation times. Viewed this way (compare Figs. 7 and 9) HRP spot growth in the PA and aorta are remarkably similar. More critically, a single threshold value (Intth) of A/ε = of 1×10−5 cm intersects all of these curves to fit the data well (Fig. 8, curves 2 and 4) for all circulation times for both vessels and for t0 as before, 15 s (25 s) for the PA (aorta). Slight adjustments in the PA threshold value, e.g., curve 5 for Intth = 1.2×10−5, can lower the PA curve, but the data error bars do no warrant it. The aorta whole wall shape of curve 2 improves on the too-rapid early growth of its SI HRP curve 1. This indicates that even the aorta, and a fortiori, the PA (permeable) media have nonnegligible HRP content. appendix d analyzes the sensitivity of these results to the precise values of the model parameters.
Fig. 9.
Radial distribution of A(r*/R*1;λ)/ε(λ) = ∫0LC(r*/R*1;z)dz, the integral of the HRP concentration dz across the SI and the media. Curves (solid line, PA; dashed line, aorta) from left to right are at HRP circulation times 30, 60, 120, and 240 s. Horizontal threshold, Intth, = 1 × 10−5 cm of A/ε intersects the concentration profiles to give the HRP spot edge vs. circulation time.
SI LDL Concentrations Their Impact on Lipid Accumulation
The arterial SI ECM glycosaminoglycans (GAG) and proteoglycans trap and bind a portion of the plasma LDL that crosses the endothelium to form lipid packets, labeled extracellular lipid liposomes, each of which contains the lipid from one or many LDL particles (2, 49, 68). It is a critical prelesion atherogenesis event. The accumulation of a high concentration of liposomes is believed to trigger the recruitment of blood-borne monocytes that mature to macrophages and devour lodged lipid. When overloaded, lipid-laden macrophages turn into foam cells (26). In large arteries like the aorta, liposomes have been widely studied in early atherogenesis. In contrast, no reports have thus far looked for, much less evinced, their presence in the PA. One suspects that, under normal conditions where the PA is atherosclerosis-resistant, it would contain far fewer, if any, liposomes than the aorta. Given that liposome formation and growth are kinetic processes, their rates depend on local tissue concentration. Our transport theory predicts LDL entry into and concentration distribution in the aorta and PA SIs under normal and hypertensive conditions. One can then speculate whether such differences correlate with vessel disease susceptibility.
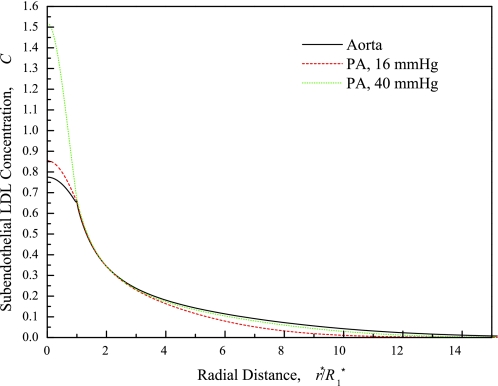
Figure 10 shows model-simulated SI LDL (not HRP) concentration profiles near the leak in the PA and aorta with measured and guesstimated parameter values given in the caption. It shows that, although the PA LDL concentration just below the leak is higher than the aorta's, the aorta's LDL concentration is higher than either the normotensive or the hypertensive PA's in the region r* ≳ 2R*1. This outer region's area is more than 100 times that of the leaky cell (where the PA LDL concentration is larger) and thus more significant for total lipid accumulation. Moreover, the aorta's LDL curve extends further radially. Finally, Sun (53) report that macromolecular leakage spot frequencies in rat of 7.6 ± 1.6/104 cells (aorta) and approximately half that, 3.4 ± 1.2/104 cells (PA). Simply put, the aorta has more, bigger, and darker SI spots than the PA. This is consistent with the aorta accumulating lipid far faster than the PA. The investigation of Cardoso and Mourao (4) of GAG fractions from normal human arteries obtained at necropsy further explains why the aorta entrains lipid faster than the PA. They found that the thoracic aorta has similar dermatan sulfate but a 30% higher chondroitin 4/6-sulfate content, both GAGs known to interact with LDL, than the PA. Of its isomeric forms, the 6-sulftaed form constituted 50% (43%) of the chondroitin 4/6-sulfated proteoglycan in the thoracic aorta (PA). The 6-sulfated form binds strongly to LDL, whereas the 4-sulfated isomer shows little or no interaction with LDL (8). Therefore, the aorta has at least 1.6 times more effective content that efficiently binds LDL than the PA. This difference may actually be larger, since the PA has essentially no 4/6-sulfate + dermatan sulfate fraction of the highest molecular weight, which binds LDL most efficiently (4).
Fig. 10.
Distribution of LDL concentration (C) in the subendothelial intima, normalized by the plasma LDL concentration, as a function of r at 10 min LDL circulation time for the normotensive aorta (solid line) and the PA with normal (16 mmHg, dashed line) and elevated (40 mmHg, dotted line) transmural pressure. Parameters: L*1 = 1 μm (23); media LDL distribution volumes γ2 estimated from HRP values and molecular radii: 0.025 (aorta), 0.074 (PA); PA parameters at 16 and 40 mmHg same except for L*pt(L*pnj) = 1.60 × 10−7 (3.35 × 10−7) cm·s−1·mmHg−1 at ΔP* = 40 mmHg (45). L*pnj is 41% lower than at 16 mmHg (24, 45).
Unlike when it is normotensive, under PH, the PA becomes noticeably atherosclerotic. Figure 10 shows that elevation of ΔP* increases the PA LDL concentration substantially beneath the leak and nearly to aortic levels far from the leak. What is needed for a quantitative study, but is not known, is what chronic PH and its associated remodeling does to LDL-ECM interactions and to leakage spot frequency. [Sun (53) showed that acute changes in ΔP* do not seem to affect leakage frequency.] Clearly, this uniform rise in PA SI LDL concentration with PH suggests that lipid entrainment kinetics will speed up relative to the normotensive state.
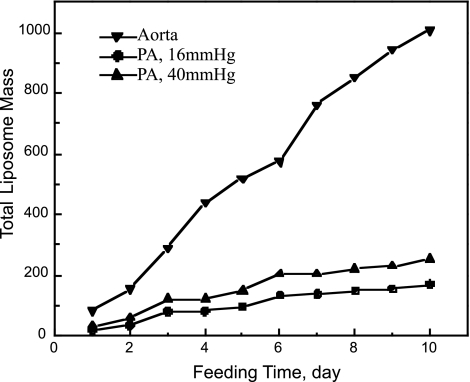
To make quantitative estimates of how in Fig. 10 the SI LDL concentration profiles might affect lipid accumulation without remodeling in these cases, one could input these profiles to the extracellular lipid liposome formation and growth models/parameters developed by Yin et al. (72) for rabbit aortic accumulation data. With the use of these aorta parameters and the 1.6 higher aorta vs. PA lipid binding constant, this procedure quantitatively correctly predicts liposome size distributions for rat aortic valves, despite the different tissue type (75). Due to the absence of liposome data in either normo- or chronically hypertensive PAs and due to the unknown leakage frequency of chronically hypertensive PAs we do not belabor this calculation here, but Fig. 11 just presents sample model results. It reflects the trends anticipated in the last two paragraphs, with more and darker aorta tracer spots leading to faster SI lipid accumulation and PH increasing PA lipid accumulation. Since extracellular lipid liposomes typically contain lipid from one to several hundred LDL particles, their growth kinetics are highly nonlinear and thus dramatically amplify even the apparently modest differences in SI LDL concentration in Fig. 10 into large differences in entrainment rates. More detailed calculations (not shown) suggest a shift from smaller to larger liposomes as ΔP* increases in the PA, a size distribution more similar to that in the aorta.
Fig. 11.
Short-term growth of SI total liposome mass, normalized by the ratio of the rate constant for lipid binding to extracellular matrix to that for the (assumed j-independent) growth of a liposome comprised of j LDL particles by the appending of a single unbound particle. Model includes these 2 kinetic processes plus the formation of liposomes comprised of j LDL particles by the merging of particles of size i (i < j) with those of size j-i (for details of the equations and calculation methods, see Refs. 70, 72, and 75.
Once sufficient lipid has been entrained, blood-borne monocytes and medial SMCs enter the SI to scavenge entrained lipid and can necrose there. PA SMCs have less actin and are less compactly organized in human vessels (32) and are more susceptible to the antimyogenic effects of heparin in cultured lines (58) than aortic SMCs, all of which might effect different availabilities. Cholesterol feeding increases different intermediate filament proteins in these two SMC types (40). Yet, both the PA and the thoracic aorta in apolipoprotein-deficient mice are amongst the first vessels to develop foam cell lesions that quickly progress to develop multiple layers of lipid-filled macrophages and SMCs and both SMC types are similar in their caveolar bands and in their response to sterol-binding agent filipin (44). Thus it is unclear if SMC differences play an important role in the two vessels' distinct lesion-forming proclivities.
Conclusions
This study seeks to gain insight into why the aorta is far more susceptible to atherosclerotic lesion formation than the PA. It models and compares water and macromolecular transport from the lumen into the aorta and PA walls to see if there are stark differences in LDL delivery to the tissue. In the process, it reworks the established transport model for the aorta to incorporate parameters values that were later measured and found to be quite different from the values initially assumed/guesstimated. With these parameters, the aorta and PA models explain a range of available independent tracer transport data into these vessels' walls. Although the models have numerous parameters, the results are sensitive to only a small subset of them, and those parameters are well characterized. They show that the similar whole-wall tracer spot growth in the high-pressure aorta and the low-pressure PA is a consequence of the similar mismatch in leaky-to-normal endothelial cell junction hydraulic conductivities. The models find that, whereas the overall transmural water flow (45) and total tracer advection are similar in both vessels, leading to this similar whole-wall (SI + media) tracer spot growth, the PA's lower SI/media density mismatch (the PA has a far less dense media than the aorta) results in a higher fraction of the tracer in the PA's wall residing in its media and less in its SI. Since there is strong evidence that early lesions occur only in the SI, this may partially account for the slower, so slow that it has not yet been observed, lipid accumulation in the PA compared with the aorta. The likely nonlinearity of lipid accumulation kinetics can magnify these SI concentration differences into large differences in lipid entrainment rates, as Fig. 11 shows. The model shows that simply increasing PA transmural pressure, as in PH, increases PA SI concentration and the predicted rate of lipid accumulation in the PA modestly. It is unknown whether chronic pulmonary hypertension increases spot frequency (acute PH does not) or LDL-PA ECM affinity. Such changes, together with the modest predicted increase in the lipid accumulation rate due to elevated pressure may be relevant to PH's higher PA atherogenic susceptibility.
APPENDIX A: MATHEMATICAL MODELS: FILTRATION, MASS TRANSFER, LIPOSOME FORMATION, AND GROWTH
The mathematical model consists of Laplace's equation for the pressure distribution, Darcy's Law requiring the velocity be proportional to the pressure gradient, the advection-diffusion equation for the tracer concentration profile and its time evolution, and initial and boundary conditions. Below we define all variables, dimensional and nondimensional, detail these equations, in dimensional form in parentheses followed by their nondimensional form, and solve the entire system of equations by finite difference techniques on a highly nonuniform mesh as described in the text. We invoke the results of prior simplified subscale models of transport through holes and slits to determine transport parameters for leaky endothelial junctions and IEL fenestrae.
Filtration
A variable (without) with superscript asterisk indicates a (non)dimensional quantity. L*p and K*p are hydraulic conductivity and permeability, each carrying a subscript denoting the region the parameter represents. The nj denotes normal and lj leaky EC junctions. The μ is the fluid viscosity. The transmural pressure drop, ΔP*, is the difference between lumen, P*L, and adventitia pressures, P*a. The media (∼total wall) thickness, L*2, normalizes all lengths, e.g., spatial coordinates:
| (A1) |
We normalize water velocities, Vi* = (UI*, W*I); pressures, Pi*; conductivities, L*pnj and Lpij*; and Darcy permeabilities, Kpi*, with the media conductivity, L*p2 and permeability, Kp2*, and ΔP*:
| (A2) |
| (A3) |
where and i = 1, 2 denotes the SI and media layers, respectively, throughout.
Since we model the SI and media as porous media, the filtration flow in each satisfies the equation of continuity with the assumption of constant fluid mass density
| (A4) |
and Darcy's Law, whose the dimensional form is Vi* = −(Kpi*/μ)∇*Pi* and nondimensional form is
| (A5) |
This approach contrasts with detailed triphasic theories of solvent and ion transport through single-layer articular cartilage matrix that has a fixed charge distribution (16, 17, 31). Luckily, studies (23, 24, 27–29, 64, 78) show the Darcy approach explains and predicts data well for transport on the scale of complex layered structures with different ECMs at different layers. Substituting Eqs. A5 into A4 gives the governing equation for the filtration model,
| (A6) |
Axisymmetry at r = 0 and no radial flux at r = ξ (periodicity) give r-boundary conditions:
| (A7a) |
| (A7b) |
Since the osmotic pressure is generally assumed uniform across large vessel walls, we relate the water velocities, W1, across the endothelium, z = 0, to the local pressure drop by
| (A8a1) |
| (A8b1) |
| (A8c1) |
| (A8a2) |
| (A8b2) |
| (A8c2) |
R1 (R2) is the average inner (outer) leak radius and ΔR = R2 − R1 its width. Equation A8c assumes the EC is impenetrable to water, but relaxing this assumption is of little consequence (78). Lpnj is an area-averaged value for normal junctions and ECs.
Since the IEL fenestrae are located randomly relative to the leak, we model the flow across the IEL (thickness LI ) with an effective hydraulic conductivity, LpI, boundary condition:
| (A9a) |
| (A9b) |
The reference adventitia (z = 1) pressure is:
| (A10) |
Subscale models for Lplj* and LpI*.
Let L*l be the leaky junction's length. Yuan et al. (73) modeled leaky junction hydraulic conductivity, normalized by Lp2*, as steady 1-D slit flow, giving:
| (A11) |
The simplified periodic unit fenestral pore interaction model of Yuan et al. (23, 73) calculates IEL Lp1*. This model considered a cylindrical slab with a circular fenestral pore in the center of an otherwise impermeable top IEL surface of finite thickness and a media below. They solved analytically for the filtration flow through this geometry and calculated a fenestral Lp equal to the flow rate through the fenestra divided by the fenestra area and the difference between the given, assumed uniform pressure above the feneestra and the average pressure on the surface just below the IEL. Let af be the mean fenestral radius, 2ξI the mean distance between neighboring fenestrae and ΦI = af2/ξI2, the IEL fractional fenestral area. The result is:
| (A12) |
The water velocity, Wf, through the fenestra is
| (A13) |
where J0(x) and J1(x) are the zeroth and the first order Bessel functions, and μn (n = 1, 2, 3, . . .) are the roots of the eigenvalue equation J1(μnξI) = 0.
One solves for the pressure distribution from the governing equations, Eq. A6, and boundary conditions, Eqs. A7–A10. Darcy's Law, Eq. A5, yields the velocity distribution.
Macromolecular Transport Model
Let us begin with the new variables and parameters that enter the mass transfer problem. C*i is the dimensional solute concentration, locally averaged over all phases, tissue and fluid, present, for both the SI and the media (i = 1, 2). Cp* is the initial plasma solute concentration; q*i: = (q*ir,q*iz) is the solute flux vector; τ, the nondimensional time; Di*, the diffusion coefficient; fi, the retardation coefficient representing the ratio of solute to water velocities; and γi, the volume available for macromolecules per unit total tissue + fluid volume. Peir and Peiz are the velocity variables, nondimensionalized in a manner appropriate for mass transfer studies, in the form of r and z Peclet numbers. The nondimensional variables are:
| (A14a) |
| (A14b) |
Macromolecular transport in the artery wall satisfies the equation of continuity with the assumption of constant mass density:
| (A15) |
The constitutive equation for the nondimensional solute flux vector qi = (qir,qiz) has contributions from convection and diffusion in both the r and z directions defined by
| (A16a) |
| (A16b) |
Huang et al. (23) explain the correct placement of γi and fi in (Eqs. A15–A16); their c* equals our C*/γ.
Subscale model for ql.
Following Tzeghai et al. (63) (Fig. 1B), define
| (A17) |
Ll*, Pel, fl, and Dl* are the leaky junction height or length, Peclet number, retardation coefficient, and diffusion coefficient. Wl* in the leaky junction is uniform, yielding a junction Peclet parameter, Pel, unlike variables in Eq. A14b. Tzeghai et al. describe the local macromolecular flux ql through a fenestra by a 1-D, quasisteady, advection-diffusion model through a slit that integrates to
| (A18) |
Note that the normalization of ql in Eq. A17 is different from that of qi in Eq. A14b.
Outside the leaky junction, a mass transfer coefficient k describe how macromolecules cross the endothelium. Given the local concentration definitions, the z = 0 boundary conditions are:
| (A19a1) |
| (A19b1) |
| (A19c1) |
| (A19a2) |
| (A19b2) |
| (A19c2) |
where the Biot number, Bi: = kL2*/D*2z, the ratio of transendothelial mass transfer to SI diffusion.
Let subscript I indicate the IEL. Axisymmetry at r = 0 and periodicity at r = ξ give
| (A20) |
The conservation boundary conditions at the IEL are
| (A21) |
where qI is the local average solute flux across the IEL. Since ϕI ≪ 1, interaction among IEL fenestrae should be negligible and qI should be proportional to the fractional fenestral area, ϕI:
| (A22) |
where qf is the fenestral solute flux. The study of Yuan et al. (73) used the same subscale model to estimate qf as Tzeghai et al. (63) did for the fenestae above. giving the analogous results of
| (A23a) |
| (A23b) |
where CIs and CIm are the entrance (SI) and exit (media) solute concentrations in the IEL fenestra. The SI and media concentration matching conditions are
| (A24) |
again since our local solute concentration is a local average over all phases, not just the fluid.
The concentration in the adventitia is assumed to be zero (see footnote 5); thus
| (A25) |
The initial condition introduces a tracer at t = 0 into the lumen, for an initially tracer-free tissue:
| (A26) |
APPENDIX B: IMPROVEMENTS TO APPROXIMATIONS MADE AND TO CALCULATIONAL METHODS AND PARAMETERS USED IN PREVIOUS MODEL SIMULATIONS OF TRANSPORT IN THE AORTA
Approximations
As noted, our transport models are similar, but not identical to those in Huang et al. (23) and Yuan et al. (73), but we solve them with improved parameters and without assumptions that compromise simulation accuracy. Neither of these earlier studies solves the equations of continuity, Eq. A4, and of solute conservation, Eq. A15, in the SI directly. Rather, since the ratio of SI thickness to mean distance ξ* between leaky cells is much less than one [L*1 = 0.2 ∼ 1 μm (23); ξ* = 670 μm (23)], they replaced these equations by their integrals dz* (normal to the endothelium) across the SI. This implicitly assumes that, in analogy to boundary layer problems (1), the radial fluid velocity (U*1) and the radial solute flux (q*1r) are independent of z*. This integration simplifies the model by transforming the boundary value problem in the SI simply into boundary conditions for the media problem. This assumption is indeed reasonable in the regions far from the leaky site, where this separation of scales justifies a boundary layer-type treatment. However, it does not hold near the leak itself, since the length scale over which the dynamic variables change in the radial direction in that region is of the order of the thickness of the leak itself, i.e., ∼20–100 nm. In this region, L*l is not small compared with the length scale of radial variation, but is large compared with it. Thus the radial velocity in the SI is a strong function of z* near the leak. This region is not small, since the hydraulic conductivity of the leaky junction is ∼5,000 L*pnj (nj = normal junctions). Zeng (74) developed separation of scales to all orders and uses accurate numerical solutions (also used, but not belabored, here) to examine its repercussions in detail. Suffice it here to say that the leaky region is a particularly sensitive region in which to sacrifice accuracy, since all tracer that enters the wall in this model at short circulation times enters via the leaky junction. Thus any errors in advection and concentration here will propagate throughout the larger solution.
Huang et al. (23) also initially assumed the filtration model in the media was 1-D (in z* only) when they solved the simplified SI filtration problem analytically and the media problem numerically. They then corrected the media problem by replacing the uniform pressure boundary condition at the IEL surface with one that states that W*l is the product of L*pl and the difference of SI P*1(r*, L*1) and the r*-independent media pressure at z* = L*1 + L*I. This simplification was based on Yuan et al. (73) result that the pressure distribution just beneath the IEL was nearly uniform. However, as noted above, the results in Yuan et al. were based on the assumption that the SI, the IEL fenestral opening and the media had the same transport properties, e.g., permeability and diffusion coefficient. As it turns out, the SI and IEL fenestrae have very different ECMs (and thus transport properties) from the media and monoclonal antibody studies indicate (33) that the IEL fenestrae's proteoglycan matrix is a continuation of that in the SI. The present calculation directly solves the SI and media problems with the appropriate parameters and without these simplifications. In results and discussion, these changes showed that the impact of the aorta's leaky EC junction extends into the media, causing nonuniformities in the pressure, velocity, and macromolecular concentration near the IEL. The PA wall's similar layered ultrastructure (45) and looser media suggest, and Figs. 3 and 5 find, even more significant pressure and concentration nonuniformities in the PA than in the aorta.
Parameters
Since the publication of Huang et al. (23), our group has measured (22) some of the parameters that we needed to guesstimate in that work. For example, we had used 20 μm as the half-distance between fenestrae (ξI) and 0.002 as the fractional area of the fenestra (ϕI = af2/ξI2, so fenestral radius af = 0.9 μm) in the IEL, both measured for sheep (48). Huang et al. (22) subsequently found the diameter of the fenestra (2af) was 1.95 ∼ 2.51 μm, but ϕI was 0.039 ∼ 0.056 and thus ξI ∼ 4.1–6.4 μm at different lumen pressures from 0 to ∼150 mmHg. The earlier values overestimated IEL resistance to water flow and macromolecular transport. Peter Weinberg (personal communication) noted that his experiments of water flow across elastin sheets also suggested far less resistance than we had earlier calculated.
Rather than using our Eqs. 1 and 2 to find L*pnj and L*p2, Huang et al. (23) first fixed all other parameters and constants in the filtration model, then fit these two parameters by requiring the average pressure (P*SI,ave) in the SI to be 47% of the lumen pressure (P*L) and requiring Peclet number Pe2z: = f2W*2L*2/(γ2D*2z) = 4.4. Both of these criteria derived from measurements of rabbit aorta. The first requirement came from Vargas et al. (65)'s measurement of Lpt*:L*pnj = 4.07 × 10−8: 8.6 × 10−8 cm·s−1·mmH2O−1. Since Lpt* × P*L = L*pnj × (P*L − PSI,ave*), the criterion should have found PSI,ave* = 0.53P*L. Tedgui and Lever (57) measured the transport of 131I-albumin in the rabbit aortic media and calculated Pe2z with a 1-D model to be ∼4.4, the second criterion. However, Ref. (57)'s Peclet number Pe2z: = f2W*2L*2/D*2z = 4.4 did not include the media tracer space γ2. Since γ2 ∼ 0.08 for the aorta, including γ2 in this criterion leads to a severe underestimate of the water velocity across the vessel wall in the z direction and, therefore, also of Lpt* fitted this way.
Consider some concrete numbers. Huang et al. (23) used the above criteria to calculate L*pnj= 1.13 × 10−8 cm·s−1·mmHg−1, one-fifth our rat aorta value (45). Using parameters of Huang et al. that fit the HRP leakage spot data of Chuang et al. (9) best, the inclusion of γ2 ∼ 0.08 in the Peclet number of their parameter estimation criterion gives a value for the only unknown, W2, so small that it corresponds to L*pt ∼ 0.63 × 10−8 cm·s−1·mmHg−1. This value is, not surprisingly, far smaller than Tedgui and Lever's (56) measured 4.00 (2.44) × 10−8 cm·s−1·mmHg−1, at 70 (180) mmHg (rabbit aorta), and Ref. (45)'s 2.79 × 10−8 cm·s−1·mmHg−1 average over 80–140 mmHg (rat aorta; Table 1). Instead of the above two criteria that derive from very different sources to fit the critical conductivities, we have consistently employed the direct rat aorta of Shou et al. (45) (which is quite close to the rabbit values of Ref. 56) and rat PA measurements (Table 1) and Eqs. 1–2.
APPENDIX C: DERIVED PARAMETER VALUES AND THEIR SIGNIFICANCE
Recall Eqs. 1–2 give L*pnj = 6.50 (5.21) × 10−7 cm·s−1·mmHg−1; L*p2 = 5.77 (4.07)×10−7 cm·s−1·mmHg−1 for the aorta (PA). We compared L*pnj for the aorta with previous measurements, both for rabbit, that used similar or different techniques. Tedgui and Lever (56) found 4.58 × 10−8 cm·s−1·mmHg−1 at 180 mmHg, slightly lower than our value since the SI may be (only) slightly more compressed(24) at the higher ΔP*. Using a more primitive measurement technique, Vargas et al. (65) found 11.9 × 10−8 cm·s−1·mmHg−1 at 74 mmHg, higher than our value but closer to Tedgui and Lever's 70 mmHg value. At 70 mmHg, the SI is much less compressed, leading to a higher apparent endothelial L*p, than at 100 mmHg(22, 24). Given the species difference and the different ΔP* and SI compression level, these and our values are quite consistent.
We note that the PA L*pnj value is about eight times that for the aorta. The likely reason for this is that L*pnj is an effective parameter (cf., Eq. 1) that includes the effects of the SI, in particular, its compression that leads to IEL fenestral blockage. Since at physiological pressures, the aorta's SI is nearly fully compressed, while the PA SI is quite relaxed (see Refs. 22, 24 and Fig. 5A of Ref. 45), fenestral blocking lowers the aorta's effective parameter far more than in the PA. Thus the true endothelial monolayer Lp for these vessels are likely far more similar than their effective values, but at the level of modeling of this study, it is the effective values that enter. Shou et al. (45) discuss more technical points arguing this conclusion as well. Note that the denuded PA LpI* is approximately five times the aorta value. This is consistent with a thinner, more porous PA media (45). The effective IEL hydraulic conductivities, LpI* = 3.21 × 10−7 (aorta) and 1.13 × 10−6 cm·s−1·mmHg−1 (PA), are consistent with the PA's thinner elastic layers (45).
We now give two arguments why PA EC leaky junction Lplj* must exceed the aorta value and a delicate discussion of the likely factor. 1) In the work of Lever et al. (35), 5- to 15-min circulation time studies found similar PA and aortic endothelial permeabilities to fibrinogen (similar size LDL), yet far higher to Evans blue albumin conjugate (∼7- to 8-nm diameter).6 At 5- to 15-min circulation, fibrinogen likely traverses mainly leaky, and albumin all, endothelial junctions. SI LDL Concentrations Their Impact on Lipid Accumulation (Sun Y, Jan KM, Rumschitzki D, unpublished observations; and Ref. 53) state that the PA has approximately half the aorta leakage frequency. Thus to maintain similar overall fibrinogen transport, the PA must have wider leaky junctions than the aorta. 2) If the both vessels had similar leaky junctions, the smaller difference between leaky and normal junction Lp would yield smaller radial pressure gradients and slower radial advection in the PA than in the aortic SI. Since advection appears to be the major mechanism by which large molecules enter and spread in the SI, the vessels' similar short-time fibrinogen entry and the PA lower leakage frequency would require the ratio of PA: aorta (effective) Lplj* to exceed their ratio of L*pnj. Since leaky cleft Lp ∼ (ΔR)2 (Eq. A9; Ref. 73) and the PA: aorta ratio of L*pnj [Ref. 45 calls it Lp(e + i)] is ∼8, their EC junction width (ΔR*) ratio should be >2.9. In rat aorta, mitotic and dead or dying cells account for approximately more than one-half of the EC leaks. Electron micrographs of HRP-stained transverse sections of rat aorta show aortic ΔR* ∼ 80–1,330 nm (mitotic) and ∼15–1,000 nm (dying or dead cells) (6). Yet, Huang et al. (23) set aortic ΔR* = 20 nm, even smaller than the mean LDL diameter (∼23 nm), for the aorta's leaky junction constriction to correctly predict HRP spot growth data. Since SI compaction yields a lower effective than actual normal endothelial conductivity L*pnj, it must also lower the aorta's effective leaky junction conductivity Lplj*, i.e., ΔR* to ∼20 nm, to maintain realistic SI radial flow. In contrast, in the PA at physiological pressures, the SI is uncompressed; so effective and real L*p values should be more similar and realistic. As such, we retain the aorta's effective ΔR* ∼ 20 nm but multiply by 4, i.e., ΔR* ∼ 80 nm, for the PA. We test if this PA Lplj* explains both LDL profiles across the wall and the similar PA/aorta radial advective HRP spread of Shou et al. (45).
Let us now account for our large aorta: PA D*2z ratio. From fiber matrix theory (11), one can combine expressions for ε and γ to show that the ratio of diffusion through a fiber matrix vs. through a free solution is exp{−[(1 − ε) − ln γ]1/2}. Lever and Jay (34) report ε for the aorta (PA) as 0.43 (0.46), and Lever et al. (35) find media fibrinogen spaces (from their Carotid plots, very similar to LDL space) as γ ∼ 0.005 (∼0.02), which gives a PA/aorta fiber matrix diffusivity ratio of 1.37. [These numbers yield (different) average fiber radius r*f in the media of the two vessels of 5.0 (5.9) nm, both larger than the 3.22-nm aorta value of Dabagh et al (12); we know of no data on these sizes.] This, of course, is not the whole story. Dabagh et al. (12) used the theory of Huang and Tarbell (25) for the factor that reduces diffusivity due to the SMC obstruction. This fraction is in terms of the SMC volume fraction F, whose aorta value they state is 20–40%, and use 40% to find a factor of 0.797. Since the PA media has far fewer SMCs than the aorta (30), taking it as 10% in the PA gives a factor of 0.971 (0% gives 1). Finally, the detailed ultrastructures of Shou et al (45) of the media of these two vessels show ∼9 (5) [aorta (PA)] repeated layers of elastic [of average thickness 5.7 (3.4) nm] separated by tissue [average thickness 11.4 (14.9) nm], believed to be comprised of ECM and SMCs. The aorta thus has more layers with thicker elastin between them; thus the PA elastins likely reduce z-diffusion less than aorta elastins. We now consider the medial z-diffusivity as the effective diffusivities of slabs in series (3), which is the sum of the ith slab's fraction of the entire thickness divided by its diffusivity. Let the diffusivity of an elastin layer approximately equal to its fenestral area fraction (ϕI = 0.043 in the aorta; likely higher in the thinner, looser PA elastins) times the fiber matrix diffusivity (since no SMCs are in its fenestrae) and the diffusivity of the intervening tissue include both SMCs and fiber effects. One then finds a PA: aorta ratio of z-diffusivities of 2.1–4.2, for PA ϕI = 0.43–0.10. Combining this factor with the likely factor of 2–3 underestimate of our aorta diffusivity (see Derived Parameter Values) yields the factor of ∼10–12 between the PA and aorta D*2z used. Finally, fiber matrix theory of Curry (11) gives K*p = 3r*fε/[4(1 − ε)] and the theory of Huang and Tarbell (25) derives a SMC correction to the fiber matrix K*p of (1 − F)/(1 + F). Since neither accounts for the media's elastic layers, we only use these relations to find an L*pd ratio. Recall L*pd = Kdi*μL2. With the F and r*f numbers above, these equations predict a (far too good) ratio = 6.0 of PA:aorta L*pd, nearly identical to the ratio 6.1 of the experimental values in Table 1.
APPENDIX D: PARAMETER SENSITIVITY
Even though our model has many parameters, only two are adjustable, and those are fixed by the experiment in Fig. 2 and remain unchanged. Table 3 tests model sensitivity to precise parameter values. It increases each parameter (except f1 and γ1, which are decreased) 10% with all others fixed and reports the percent change in predicted 4 min SI HRP spot size, normalized by R*1. The results are most sensitive to (directly measured) R*1 (raising R*1 lengthens junctions but divides spot sizes by a larger value) and to γ1 [lowering γ1 makes it harder to reach Cth (or Intth) and lowers the SI/ media void mismatch; this lowers radial SI tracer spread relative to its media entry]. Spot size is far less sensitive to the other values, except, trivially, to Cth. Minor effects: Increasing L*pnj lowers SI ∂P/∂r, and thus SI spot growth. Raising L*p2 or ϕI lowers the pressure drop across the media + IEL and thus raises it across the endothelium (ΔP is fixed), leading to a larger SI ∂P/∂r and faster spot growth. Raising L*p2 increases SI thickness over which one integrates the tracer concentration, so a lower tracer concentration achieves Cth, i.e., bigger spots. Increasing D*2z carries tracer away from the IEL into the media, thus increasing tracer transport from the SI to the media. This lowers SI values and thereby slightly decreases the SI spot size.
Table 3.
Parameter sensitivity analysis
| Parameter | Sensitivity |
|---|---|
| L1 | 1.224% |
| Ll | −0.785% |
| L2 | 1.309% |
| R1 | −5.109% |
| ΔR | 1.108% |
| ΔP* | 1.128% |
| Lpnj* | −1.432% |
| L*p2 | 0.695% |
| ϕI | 0.048% |
| L*p1 | 0.320% |
| K*p1 | 0.928% |
| γ1(−) | −3.729% |
| γ2 | −1.824% |
| f1(−) | 0.516% |
| f2 | −1.249% |
| D*1 | 0.600% |
| D*2z | −0.715% |
| Cth | −3.138% |
| μ | −1.566% |
| t0 | −0.083% |
The (−) after γ1 and f1 means they are decreased by 10%, all others are increased by 10%, e.g., increased L1 by 10%, the spot size, normalized by R1, at 4 min is increased by 1.224%.
GRANTS
Supported for this work was provided by National Heart, Lung, and Blood Institutes Grant 5-RO1-HL067383 and National Science Foundation Grant IOS-0922051.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.Z. performed the analysis; Z.Z., K.-M.J., and D.S.R. analyzed results; Z.Z. and D.S.R. interpreted results; Z.Z. prepared figures; Z.Z. and D.S.R. drafted manuscript; K.-M.J. and D.S.R. conception and design of research; D.S.R. edited and revised manuscript; D.S.R. approved final version of manuscript.
Footnotes
In these experiments, tracer was introduced in sufficient excess and the experimental time was sufficiently short to maintain time-independent lumen concentrations.
Huang et al. (23) found that use of the more complicated Brinkman equation here was just equivalent to using Darcy's law with a corrected permeability. Moreover, it is hard to argue that one should explicitly include no-slip on region boundaries (Brinkman) when one has lumped no-slip on the far more abundant ECM fibers and on the medial elastic into region permeabilities.
Equation A6, the 2-D Laplace equation, has a straightforward analytical solution as a sum of Bessel functions that we do not use for three reasons. 1) The IEL matching conditions makes its implementation cumbersome. 2) Since the ratio of cleft thickness ΔR* to ξ* is ∼10−4, one must retain [O(104)] terms to resolve the scales over which the dynamic variables change near the leaky junction. 3) Since this complex velocity distribution requires a direct discretization for the 2-D advection-diffusion equation, we simply use this approach for both equations.
As in Zeng et al. (78), only monkey data are available. Our transport model with similar monkey data-derived parameters, describes both monkey and rat valve leaflet data well.
Figure 6, A and B, indicates a non-zero concentration (due to the vaso vasorum) at z = 1 that Tompkins et al. (59) model by a mass transfer coefficient km2 there. Since 1) we do not know how to find km2 independently of the figure; 2) assuming C(z = 1) = 0 only introduces a very small error into, e.g., Figs. 6; and 3) even in the worst case, the fraction of the walls' tracer holdup, needed to calculate spot growth, is negligible at z = 1; we simply assume C(z = 1) = 0.
Fibrinogen is longer and narrower (a ∼7 nm × 50- to 70-nm trimer) than LDL At 5- to 15-min circulation, it likely traverses only the leaky junctions; at longer times, it likely reptates through the normal junctions as well.
REFERENCES
- 1. Batchelor GK. An Introduction to Fluid Mechanics. Cambridge, MA: Cambridge University Press, 1967 [Google Scholar]
- 2. Berenson GS, Radhakrishnamurthy B, Srinivasan SR, Vijayagopal P, Dalferes ER. Proteoglycans and potential mechanisms related to atherosclerosis. Ann NY Acad Sci 454: 69–78, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Bird RBB, Stewart WE, Lightfoot EN. Transport Phenomena. New York: John Wiley & Sons, 2002 [Google Scholar]
- 4. Cardoso LE, Mourao PA. Glycosaminoglycan fractions from human arteries presenting diverse susceptibilities to atherosclerosis have different binding affinities to plasma LDL. Arterioscler Thromb 14: 115–124, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Chan KL. Is aortic stenosis a preventable disease? J Am Coll Cardiol 42: 593–599, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chen YL, Jan KM, Lin HS, Chien S. Ultrastructural studies on macromolecular permeability in relation to endothelial cell turnover. Atherosclerosis 118: 89–104, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Chien S, Lin SJ, Weinbaum S, Lee MM, Jan KM. The role of arterial endothelial cell mitosis in macromolecular permeability. Adv Exp Med Biol 242: 59–73, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Christner JE, Baker JR. A competitive assay of lipoprotein: proteoglycan interaction using a 96-well microtitration plate. Anal Biochem 184: 388–394, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Chuang PT, Cheng HJ, Lin SJ, Jan KM, Lee MM, Chien S. Macromolecular transport across arterial and venous endothelium in rats. Studies with Evans blue-albumin and horseradish peroxidase. Arteriosclerosis 10: 188–197, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Curmi PA, Juan L, Tedgui A. Effect of transmural pressure on low density lipoprotein and albumin transport and distribution across the intact arterial wall. Circ Res 66: 1692–1702, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Curry FE. Mechanics and thermodynamics of transcapillary exchange. In: Handbook of Physiology. The Cardiovascular System. Microcirculation. Bethesda, MD: American Physiology Society, 1984, sect 2, vol IV, p. 309–374 [Google Scholar]
- 12. Dabagh M, Jalali P, Tarbell JM. The transport of LDL across the deformable arterial wall: the effect of endothelial cell turnover and intimal deformation under hypertension. Am J Physiol Heart Circ Physiol 297: H983–H996, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duff GL, Mc MG. Pathology of atherosclerosis. Am J Med 11: 92–108, 1951 [DOI] [PubMed] [Google Scholar]
- 14. Frank JS, Fogelman AM. Ultrastructure of the intima in WHHL and cholesterol-fed rabbit aortas prepared by ultra-rapid freezing and freeze-etching. J Lipid Res 30: 967–978, 1989 [PubMed] [Google Scholar]
- 15. Gourlay AR. Hopscotch: a fast second order partial differential equation solver. J Inst Math Appl 6: 375–390, 1970 [Google Scholar]
- 16. Gu WY, Lai WM, Mow VC. A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. J Biomech Eng 120: 169–180, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Gu WY, Sun DN, Lai WM, Mow VC. Analysis of the dynamic permeation experiment with implication to cartilaginous tissue engineering. J Biomech Eng 126: 485–491, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Happel J, Brenner H. Low Reynolds Number Hydrodynamics. Englewood Cliffs, NJ: Prentice-Hall, 1965 [Google Scholar]
- 19. Heath D, Wood EH, Dushane JW, Edwards JE. The relation of age and blood pressure to atheroma in the pulmonary arteries and thoracic aorta in congenital heart disease. Lab Invest 9: 259–272, 1960 [PubMed] [Google Scholar]
- 20. Holman JP. Heat Transfer. New York: McGraw-Hill, 1986, p. 647 [Google Scholar]
- 21. Huang AL, Jan KM, Chien S. Role of intercellular junctions in the passage of horseradish peroxidase across aortic endothelium. Lab Invest 67: 201–209, 1992 [PubMed] [Google Scholar]
- 22. Huang Y, Jan KM, Rumschitzki D, Weinbaum S. Structural changes in rat aortic intima due to transmural pressure. J Biomech Eng 120: 476–483, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Huang Y, Rumschitzki D, Chien S, Weinbaum S. A fiber matrix model for the growth of macromolecular leakage spots in the arterial intima. J Biomech Eng 116: 430–445, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Huang Y, Rumschitzki D, Chien S, Weinbaum S. A fiber matrix model for the filtration through fenestral pores in a compressible arterial intima. Am J Physiol Heart Circ Physiol 272: H2023–H2039, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Huang ZJ, Tarbell JM. Numerical simulation of mass transfer in porous media of blood vessel walls. Am J Physiol Heart Circ Physiol 273: H464–H477, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Hurt E, Bondjers G, Camejo G. Interaction of LDL with human arterial proteoglycans stimulates its uptake by human monocyte-derived macrophages. J Lipid Res 31: 443–454, 1990 [PubMed] [Google Scholar]
- 27. Huyghe JM, Arts T, van Campen DH, Reneman RS. Porous medium finite element model of the beating left ventricle. Am J Physiol Heart Circ Physiol 262: H1256–H1267, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Huyghe JM, Vancampen DH. Finite deformation-theory of hierarchically arranged porous solids 2. Const Behav Int J Eng Sci 33: 1873–1886, 1995 [Google Scholar]
- 29. Khakpour M, Vafai K. Critical assessment of arterial transport models. Int J Heat Mass Transfer 51: 807–822, 2008 [Google Scholar]
- 30. Kierszenbaum AL. Histology and Cell Biology. An Introduction to Pathology. Philadelphia, PA: Mosby Elsevier, 2007, p. 671 [Google Scholar]
- 31. Lai WM, Mow VC, Sun DD, Ateshian GA. On the electric potentials inside a charged soft hydrated biological tissue: streaming potential versus diffusion potential. J Biomech Eng 122: 336–346, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Lalezari S, Hazekamp MG, Bartelings MM, Schoof PH, Gittenberger-De Groot AC. Pulmonary artery remodeling in transposition of the great arteries: relevance for neoaortic root dilatation. J Thorac Cardiovasc Surg 126: 1053–1060, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Lark MW, Yeo TK, Mar H, Lara S, Hellstrom I, Hellstrom KE, Wight TN. Arterial chondroitin sulfate proteoglycan: localization with a monoclonal antibody. J Histochem Cytochem 36: 1211–1221, 1988 [DOI] [PubMed] [Google Scholar]
- 34. Lever MJ, Jay MT. Albumin and Cr-EDTA uptake by systemic arteries, veins, and pulmonary artery of rabbit. Arteriosclerosis 10: 551–558, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Lever MJ, Jay MT, Coleman PJ. Plasma protein entry and retention in the vascular wall: possible factors in atherogenesis. Can J Physiol Pharmacol 74: 818–823, 1996 [PubMed] [Google Scholar]
- 36. Lin SJ, Jan KM, Chien S. Role of dying endothelial cells in transendothelial macromolecular transport. Arteriosclerosis 10: 703–709, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Lin SJ, Jan KM, Schuessler G, Weinbaum S, Chien S. Enhanced macromolecular permeability of aortic endothelial cells in association with mitosis. Atherosclerosis 73: 223–232, 1988 [DOI] [PubMed] [Google Scholar]
- 38. Lin SJ, Jan KM, Weinbaum S, Chien S. Transendothelial transport of low density lipoprotein in association with cell mitosis in rat aorta. Arteriosclerosis 9: 230–236, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Mitchell JRA, Schwartz CJ. Arterial Disease. Oxford, UK: Blackwell, 1965, p. 50–65 [Google Scholar]
- 40. Molony L, Hagen PO, Schachat FH. Intermediate filament heterogeneity in normal and hypercholesterolemic rabbit vascular smooth muscle cells. Exp Cell Res 163: 78–86, 1986 [DOI] [PubMed] [Google Scholar]
- 41. Mora R, Lupu F, Simionescu N. Prelesional events in atherogenesis. Colocalization of apolipoprotein B, unesterified cholesterol and extracellular phospholipid liposomes in the aorta of hyperlipidemic rabbit. Atherosclerosis 67: 143–154, 1987 [DOI] [PubMed] [Google Scholar]
- 42. Nievelstein PF, Fogelman AM, Mottino G, Frank JS. Lipid accumulation in rabbit aortic intima 2 hours after bolus infusion of low density lipoprotein. A deep-etch and immunolocalization study of ultrarapidly frozen tissue. Arterioscler Thromb 11: 1795–1805, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis 9: 908–918, 1989 [DOI] [PubMed] [Google Scholar]
- 44. Severs NJ, Simons HL. Caveolar bands and the effects of sterol-binding agents in vascular smooth muscle plasma membrane. Single and double labeling with filipin and tomatin in the aorta, pulmonary artery and vena cava. Lab Invest 55: 295–307, 1986 [PubMed] [Google Scholar]
- 45. Shou Y, Jan KM, Rumschitzki DS. Transport in rat vessel walls. I. Hydraulic conductivities of the aorta, pulmonary artery, and inferior vena cava with intact and denuded endothelia. Am J Physiol Heart Circ Physiol 291: H2758–H2771, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Shou Y, Jan KM, Rumschitzki DS. Transport in rat vessel walls. II. Macromolecular leakage and focal spot size growth in rat arteries and veins. Am J Physiol Heart Circ Physiol 292: H2881–H2890, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Simionescu N, Vasile E, Lupu F, Popescu G, Simionescu M. Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am J Pathol 123: 109–125, 1986 [PMC free article] [PubMed] [Google Scholar]
- 48. Song SH, Roach MR. Quantitative changes in the size of fenestrations of the elastic laminae of sheep thoracic aorta studied with SEM1. Blood Vessels 20: 145–153, 1983 [DOI] [PubMed] [Google Scholar]
- 49. Srinivasan SR, Radhakrishnamurthy B, Pargaonkar PS, Berenson GS, Dolan P. Lipoprotein-acid mucopolysaccharide complexes of human atherosclerotic lesions. Biochim Biophys Acta 388: 58–70, 1975 [DOI] [PubMed] [Google Scholar]
- 50. Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W, Jr, Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 85: 391–405, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis. American Heart Association. Circulation 89: 2462–2478, 1994 [DOI] [PubMed] [Google Scholar]
- 52. Stemerman MB, Morrel EM, Burke KR, Colton CK, Smith KA, Lees RS. Local variation in arterial wall permeability to low density lipoprotein in normal rabbit aorta. Arteriosclerosis 6: 64–69, 1986 [DOI] [PubMed] [Google Scholar]
- 53. Sun Y. The focal spread of macromolecular tracers in vessel walls: frequency and effecct of intima compaction and blood pressure. In: Chemical Engineering. New York: City University of New York, 2008, p. 142 [Google Scholar]
- 55. Tedgui A, Lever MJ. Effect of pressure and intimal damage on 131I-albumin and [14C]sucrose spaces in aorta. Am J Physiol Heart Circ Physiol 253: H1530–H1539, 1987 [DOI] [PubMed] [Google Scholar]
- 56. Tedgui A, Lever MJ. Filtration through damaged and undamaged rabbit thoracic aorta. Am J Physiol Heart Circ Physiol 247: H784–H791, 1984 [DOI] [PubMed] [Google Scholar]
- 57. Tedgui A, Lever MJ. The interaction of convection and diffusion in the transport of 131I-albumin within the media of the rabbit thoracic aorta. Circ Res 57: 856–863, 1985 [DOI] [PubMed] [Google Scholar]
- 58. Templeton DM, Zhao Y, Fan MY. Heterogeneity in the response of vascular smooth muscle to heparin: altered signaling in heparin-resistant cells. Cardiovasc Res 45: 503–512, 2000 [DOI] [PubMed] [Google Scholar]
- 59. Tompkins RG. Quantitative analysis of blood vessel permeability of squirrel monkeys. Am J Physiol Heart Circ Physiol 260: H1194–H1204, 1991 [DOI] [PubMed] [Google Scholar]
- 60. Tompkins RG, Schnitzer JJ, Yarmush ML. Macromolecular transport within heart valves. Circ Res 64: 1213–1223, 1989 [DOI] [PubMed] [Google Scholar]
- 61. Tompkins RG, Yarmush ML, Schnitzer JJ, Colton CK, Smith KA, Stemerman MB. Low-density lipoprotein transport in blood vessel walls of squirrel monkeys. Am J Physiol Heart Circ Physiol 257: H452–H464, 1989 [DOI] [PubMed] [Google Scholar]
- 62. Truskey GA, Roberts WL, Herrmann RA, Malinauskas RA. Measurement of endothelial permeability to 125I-low density lipoproteins in rabbit arteries by use of en face preparations. Circ Res 71: 883–897, 1992 [DOI] [PubMed] [Google Scholar]
- 63. Tzeghai G, Ganatos P, Pfeffer R, Weinbaum S, Nir A. A theoretical model to study the effect of convection and leaky junctions on macromolecule transport in artery walls. J Theor Biol 121: 141–162, 1986 [DOI] [PubMed] [Google Scholar]
- 64. Vankan WJ, Huyghe JM, Drost MR, Janssen JD, Huson A. A finite element mixture model for hierarchical porous media. Int J Num Methods Eng 40: 193–210, 1997 [Google Scholar]
- 65. Vargas CB, Vargas FF, Pribyl JG, Blackshear PL. Hydraulic conductivity of the endothelial and outer layers of the rabbit aorta. Am J Physiol Heart Circ Physiol 236: H53–H60, 1979 [DOI] [PubMed] [Google Scholar]
- 66. Weinbaum S, Tzeghai G, Ganatos P, Pfeffer R, Chien S. Effect of cell turnover and leaky junctions on arterial macromolecular transport. Am J Physiol Heart Circ Physiol 248: H945–H960, 1985 [DOI] [PubMed] [Google Scholar]
- 67. Wen GB, Weinbaum S, Ganatos P, Pfeffer R, Chien S. On the time dependent diffusion of macromolecules through transient open junctions and their subendothelial spread. 2. Long time model for interaction between leakage sites. J Theor Biol 135: 219–253, 1988 [DOI] [PubMed] [Google Scholar]
- 68. Wight TN. Proteoglycans in pathological conditions: atherosclerosis. Fed Proc 44: 381–385, 1985 [PubMed] [Google Scholar]
- 69. Woolf N. Pathology of Atherosclerosis. London: Butterworth, 1982, p. 189–199 [Google Scholar]
- 70. Yin Y. The initiation and growth of extracellular lipid liposomes in arteries and valves. In: Department of Mechanical Engineering. New York: City College of the City University of New York, 1999 [Google Scholar]
- 72. Yin Y, Lim KH, Weinbaum S, Chien S, Rumschitzki DS. A model for the initiation and growth of extracellular lipid liposomes in arterial intima. Am J Physiol Heart Circ Physiol 272: H1033–H1046, 1997 [DOI] [PubMed] [Google Scholar]
- 73. Yuan F, Chien S, Weinbaum S. A new view of convective-diffusive transport processes in the arterial intima. J Biomech Eng 113: 314–329, 1991 [DOI] [PubMed] [Google Scholar]
- 74. Zeng Z. Macromolecular transport in heart valves and blood vessel walls. In: Chemical Engineering. New York: City University of New York, 2006, p. 235 [Google Scholar]
- 75. Zeng Z, Nievelstein-Post P, Yin Y, Jan KM, Frank JS, Rumschitzki DS. Macromolecular transport in heart valves. III. Experiment and theory for the size distribution of extracellular liposomes in hyperlipidemic rabbits. Am J Physiol Heart Circ Physiol 292: H2687–H2697, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Zeng Z, Yin Y, Huang AL, Jan KM, Rumschitzki DS. Macromolecular transport in heart valves. I. Studies of rat valves with horseradish peroxidase. Am J Physiol Heart Circ Physiol 292: H2664–H2670, 2007 [DOI] [PubMed] [Google Scholar]
- 78. Zeng Z, Yin Y, Jan KM, Rumschitzki DS. Macromolecular transport in heart valves. II. Theoretical models. Am J Physiol Heart Circ Physiol 292: H2671–H2686, 2007 [DOI] [PubMed] [Google Scholar]