Abstract
The vestibules of adult guinea pigs were lesioned with gentamicin and then treated with perilymphatic infusion of either of two growth factor mixtures (i.e., GF I or GF II). GF I contained transforming growth factor α (TGFα), insulin-like growth factor type one (IGF-1), and retinoic acid (RA), whereas GF II contained those three factors and brain-derived neurotrophic factor. Treatment with GF I significantly enhanced vestibular hair cell renewal in ototoxin-damaged utricles and the maturation of stereociliary bundle morphology. The addition of brain-derived neurotrophic factor to the GF II infusion mixture resulted in the return of type 1 vestibular hair cells in ototoxin-damaged cristae, and improved vestibular function. These results suggest that growth factor therapy may be an effective treatment for balance disorders that are the result of hair cell dysfunction and/or loss.
Avian vestibular receptors have been shown to continuously produce new hair cells (HCs), and to spontaneously renew HCs following injury (1–4). In birds, renewal of vestibular HCs results in a return of function (5–7). Mammals were once thought to possess a full complement of vestibular HCs at birth that once lost were not replaceable (8). However, the vestibular sensory epithelium of mammals has been shown to possess a limited restorative capacity (9, 10). An in vivo study (11) has attributed partial renewal (i.e., 50%) of vestibular hair cells (HCs) to direct transdifferentiation of support cells into HCs. In contrast, the results of an in vitro ototoxin study with maculae explants (12) has suggested that a substantial number of the vestibular HCs were sublethally damaged and that most of the HC renewal was due to self-repair. It is possible that both of these studies (11, 12) are correct, and that both mechanisms (i.e., transdifferentiation and self-repair) contribute to vestibular HC renewal in mammals (13).
Growth factors can effect vestibular HC renewal in birds and mammals following an injury to the vestibule (13). Transforming growth factor α (TGFα) has been shown to increase cell proliferation in adult murine macula and crista explants following neomycin damage (14). Insulin and insulin-like growth factor type one (IGF-1) have been shown to be mitogenic in cultures of the avian utricular macula (15, 16). Insulin was found to enhance proliferation in undamaged macula (17) by interacting with either TGFα or epithelial growth factor (EGF). Retinoic acid (RA) is a powerful morphogen for the inner ear (18). A change in RA concentration can initiate precocious differentiation of chick otocyst HCs (19) and formation of supernumerary HCs in developing mouse organ of Corti (20). Perilymphatic infusion of TGFα with insulin induced cell proliferation in both utricular sensory (21) and extrasensory epithelia (22) of adult rats. However, neither TGFα nor insulin alone could stimulate cell proliferation (21). Brain-derived neurotrophic factor (BDNF) is essential for the survival of vestibular ganglion neurons (23). BDNF has been shown to protect vestibular neurons from ototoxins (24) and also been suggested to play a role in the development of type 1 hair cells (25).
We have investigated whether the enhancement of vestibular HC renewal by growth factor infusion therapy can result in an improvement in vestibular function. Adult guinea pig inner ears were lesioned with gentamicin, and then treated with the infusion of either of two different growth factor mixtures (i.e., GF I and GF II). Utricles from the vestibules of animals treated with GF I were assayed for HC renewal and the cristae of the animals treated with GF II were assayed for both the type of HCs (type 1 vs. type 2) and the recovery of horizontal vestibulo-ocular reflexes (HVOR). Our observations show that treatment of gentamicin-damaged vestibules with GF I resulted in a highly significant enhancement of HC renewal, and that the addition of BDNF to the GF II therapy mixture resulted in the additional benefit of the return of type 1 vestibular HCs and improved vestibular function.
Methods
The care and use of animals was approved by the Animal Care and Use Committees of the Albert Einstein College of Medicine and Naval Medical Center at San Diego.
Aminoglycoside Exposure.
Adult Dunkin-Harley (nonpigmented) guinea pigs (Harlan–Sprague–Dawley) weighing 400 g were anesthetized with ketamine (35 mg/kg, i.m.) and xylazine (15 mg/kg, i.m.). Postauricular incisions exposed both tympanic bullae where 200 μl of a 20 mg/ml solution of gentamicin sulfate was injected into each middle ear cavity. Bullae were sealed with dental cement and incisions sutured.
Infusion of the Perilymphatic Fluid Space.
Only the right vestibule of each animal was infused in the treated guinea pigs, and the contralateral ear acted as a gentamicin-exposed, untreated control. Alzet Model 2002 osmotic minipumps (0.5 μl/h) were loaded with either TGFα (2 μg/ml), IGF-1 (200 ng/ml), or RA (10−8 M) in PBS (pH 7.4) and 0.1% BSA. Pumps with this mixture were implanted s.c. in nine guinea pigs, which were designated as the Growth Factor One (GF I) group. Growth Factor Two (GF II) animals received the same mixture as GF I, but were supplemented with human recombinant BDNF at 1 mg/ml (Regeneron; n = 3). There were also untreated [i.e., no gentamicin, no surgery (n = 3)] bilateral gentamicin-exposed animals (n = 3) and bilateral gentamicin-exposed animals infused with carrier solution (n = 3). A custom microcatheter was attached to the pump (26). Seven days after gentamicin, each guinea pig was implanted with an osmotic pump containing either GF I, GF II, or carrier solution. Each pump was placed between the scapulae and a catheter brought through a tunnel to the epitympanic bulla. The catheter tip was inserted into a 0.2-mm hole that was 2 mm away from the posterior edge of the stapedial footplate to allow for direct infusion of the vestibule. A silastic bead on the catheter sealed the vestibule. To extend the period of infusion to 1 month, pumps were exchanged after 2 weeks.
Preparation of Vestibular Specimen for Anatomic Evaluation.
Treated and untreated guinea pigs were killed at 5, 9, or 16 weeks post-gentamicin exposure. For evaluation of maculae, temporal bones were placed in fixative (2.5% glutaraldehyde, 0.1% cacodylate), and postfixed for 2 h in 1% osmium tetroxide. Utricles were dissected and otoconial membranes removed. Maculae were critical point dried, sputter coated, and viewed by using a JEOL 6400 scanning electron microscope.
All horizontal canal cristae were excised and fixed in 2% glutaraldehyde in 0.1 M phosphate buffer, postfixed in 1% osmium tetroxide, and embedded in Araldite. Serial (1 μm) sections were stained with 0.5% toluidine blue. Specimens for ultrastructural evaluation were selected from unstained 1-μm sections and remounted, and ultrathin sections cut, stained, and placed on slot grids. Eight grids from each specimen were examined on a JEOL 200 electron microscope.
Quantification of Hair Cell Renewal.
GF I (macula utriculus).
Scanning electron micrographs (SEMs) were taken at ×1,500 from the striola, peristriola, nonstriola, and edge areas of each macula. This yielded 16 to18 micrographs of 4,600 μm2 fields for HC counts. All cells bearing stereocilia bundles on their surfaces were counted. The criteria for inclusion as an immature renewing hair cell were that their stereocilia bundles had to be <6 μm in height and lack a stair-step configuration. Stereocilia bundle maturation was determined from 100 randomly selected hair cells.
GF II (crista ampullaris).
Sections (1 μm) of horizontal canal cristae were examined 50 μm ± 10 μm from the planum semilunatum. The stereologic quantification method of nuclear volume assessment was used and volume fractions of cell nuclei of cristae were measured by point counting (27). Four nonoverlapping fields of two sections, each 6 μm apart, were used for evaluation (eight fields/specimen) of 18 specimens (28). Type 1 HC nuclei (diameter = 2.03 ± 0.47) in the middle third of the long axis of the epithelium were identified by round nuclei, diffuse nucleoli with the hair cell soma enveloped by a nerve calyx. Type 2 HC nuclei (diameter = 2.04 ± 0.44) were located in a position similar to the type 1 HCs, but with button-type nerve endings. Support cells had dense, irregularly shaped nuclei with prominent stained nucleoli in the basal third of the epithelium (29). Points superimposed on nuclei of type 1and 2 HCs, support cells, and nuclei-free tissue were recorded separately, and each cell nuclear volume fraction relative to neuroepithelial tissue was calculated. The standard error of the estimates was calculated (30), and our error estimates were acceptably low (i.e., 18–35% error vs. 70–800% difference between groups). Cell count means are presented ± SEM.
Functional Vestibular Testing.
At the time of gentamicin exposure or 14 weeks after exposure, guinea pigs were implanted with a scleral search coil and a skull cap (31, 32). At weeks 2 and 16 after gentamicin exposure, animals were subjected to vestibular testing on a computerized rotational table (Neurokinetics, Pittsburgh) in a dark room. Animals were restrained in a container that rigidly fixed the head maintaining the horizontal semicircular canal in an earth-horizontal yaw axis of rotation (32, 33). HVOR measures were completed with sinusoidal horizontal acceleration at a peak angular velocity of 40°/sec. Eye position relative to head and table position was measured by using a scleral search coil in a magnetic field generated by two field coils (CNC Engineering, Seattle, WA) oriented 90° to each other. Sensitivity was calibrated to 200 mV/degree with a linear response range of ±10°. Horizontal eye and table position were recorded at 200 samples/sec. Eye signals were digitized and desaccaded deriving slow wave eye velocity, which was compared with peak head velocity to calculate gain and phase.
Sinusoids were fitted to the eye velocity's raw tracing for one cycle. Several cycles were averaged together to obtain gain and phase for one trial. HVOR gain was defined as the ratio of the peak of the eye velocity's fitted sinusoid to the head velocity sinusoid's peak. Phase was defined as the temporal difference between peak eye velocity and peak stimulus velocity, measured in degrees. If the eye and head velocity peaks were opposed, a phase was given a value of zero degrees. A positive number indicated a phase lead.
The directional asymmetry was also tested by using least-squares methods to estimate the leftward (Gleft) and rightward (Gright) gains of the slow phase eye velocity responses to the measured head velocity. For the estimation procedure, the relative phase shifts of the horizontal slow phase eye velocity and head velocity data (with polarity reversed) were determined by cross-correlation analysis and the head velocity data were shifted to eliminate the phase difference. A Marquardt–Levenberg optimization algorithm (“leastsq.m” routine for matlab, Mathworks, Natick, MA) was then used to estimate the directional gains for the slow phase eye velocity with respect to the reversed-polarity head velocity by minimizing the least-squares error between the slow phase eye data and the function: GRL = directional gain either right or left; ER = rightward eye velocity; EL = leftward eye velocity; Ḣ = slow phase head velocity (with polarity reversed relative to the slow phase eye velocity),
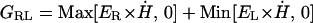 |
Statistical Analysis.
ANOVA was chosen as the method of statistical analysis to contrast the means of three groups after verifying that the assumptions of normalcy of the distribution of residuals and equality of the variances were met. Individual group physiologic data were compared with repeated-measures ANOVA (between groups factor = treatment; within groups factor = stimulus frequency) and post hoc paired comparisons made with Newman–Keuls multiple range test. Because there were no differences between rightward and leftward gains, the analyses were performed separately for each direction of rotation. One-way ANOVA was used to test differences in mean nuclear volume fractions. A Newman–Keuls multiple comparison post hoc test was used when significant differences were found by ANOVA. An unpaired Student's t test was used to make statistical comparisons between two groups with small means. A level of P < 0.05 was considered significant.
Results
Treatment with GF I Accelerates Vestibular Hair Cell Renewal in the Macula of the Utricle.
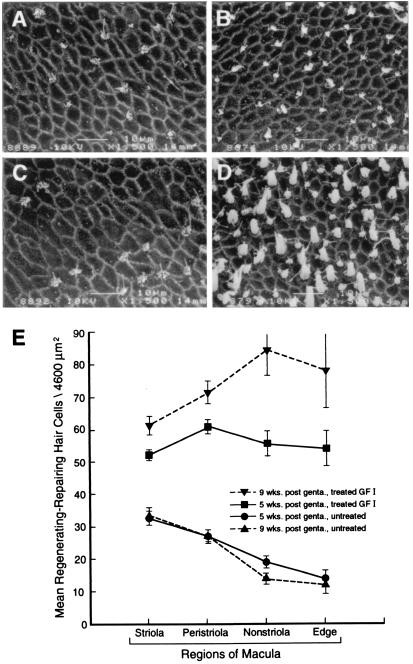
Low-power scanning electron micrographs (SEMs) of gentamicin-exposed, untreated (Fig. 1A) and GF I-treated (Fig. 1B) utricular maculae from the same guinea pig illustrate a robust HC renewal response activated by the infusion of the GF I mixture into the perilymph. Examination of low-power SEMs of the cochlear ducts from this and the other animals in this study showed extensive loss of basal turn HCs in both the untreated and GF I-treated ears. There was no auditory HC renewal in response to GF I infusion. The differences in the HC density counts taken from the utricles at 5 and 9 weeks post-gentamicin exposure show that the HC renewal response continued even after the infusion of GF I had ended. There is a significant increase in the HC density from >2-fold at 5 weeks to >3-fold (P < 0.001) at 9 weeks in the GF I-treated utricles compared with gentamicin-damaged, untreated specimens (Fig. 1C). There was no increase in HC density in inner ears infused with carrier vehicle (data not shown). This increase in HC renewal in the GF I-treated utricular maculae is even more evident when evaluated on a site-specific basis (see Fig. 2). The density of renewing HCs in the striola region of the macula (Fig. 2 A, B, and E) shows a significant (i.e., 2-fold; P < 0.001) increase in response to GF I infusion in 9-week animals. However, this difference between GF I-treated and untreated ototoxin-damaged utricles increased to >6-fold in the non-striola and edge regions (Fig. 2 C, D, and E). Another important component in the treatment response of the gentamicin lesioned utricles with GF I is the maturation of the stereociliary bundles of the renewed hair cells (Fig. 3). Treatment with GF I initiated maturation of the stereociliary bundles (Fig. 3C, and compare Fig. 3 B and A).
Figure 1.
Treatment of ototoxin-damaged vestibules with GF I results in a significant increase in the renewal of vestibular hair cells. (A and B) Scanning electron micrographs of utricular maculae at 9 weeks post-gentamicin exposure. The vestibule in A received no further treatment after gentamicin exposure, whereas the vestibule in B received infusion of GF I for 1 month (i.e., weeks 1–5). When A is compared with B it is evident that treatment with GF I resulted in increased density of vestibular HCs in the macula of the utricle. (C) The quantification of HC renewal in ototoxin-damaged, untreated, and GF I-treated vestibules. [Bars denote ±SEM; asterisks, P < 0.001; 5 weeks (n = 6), 9 weeks (n = 3); scale bars = 100 μm.]
Figure 2.
Treatment with the GF I had the greatest effect on increasing hair cell renewal in the nonstriola and edge areas of the macula. (A–D) Scanning electron micrographs of utricular maculae at 9 weeks postgentamicin exposure. The striola region is represented in A and B—A being ototoxin-damaged, untreated and B receiving 1 month of GF I infusion. The edge region is depicted in C and D—C being a gentamicin-damaged, untreated macula and D a macula receiving 1 month of GF I therapy. A comparison of these micrographs shows that GF I treatment greatly increased the number of renewing vestibular HCs in the edge region. (E) Hair cell counts from the four regions of the macula at 5 and 9 weeks post-gentamicin exposure. [Bars denote ±SEM; 5 weeks (n = 6), 9 weeks (n = 3); scale bars = 10 μm.]
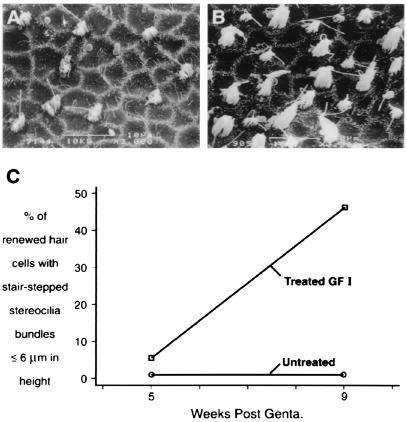
Figure 3.
Treatment of ototoxin-damaged utricles with GF I stimulated the maturation of stereociliary bundles. (A and B) Scanning electron micrographs of the peristriolar region of utricular maculae at 9 weeks post-gentamicin exposure. The vestibule in A remained untreated after gentamicin exposure, whereas the vestibule in B received 1 month of GF I infusion. A comparison of these micrographs shows that only the stereociliary bundles in B have progressed to a stair-stepped arrangement of their stereocilia. Quantification of HC stereociliary bundle maturation is presented in C. (n = 100 hair cells/condition; scale bars = 10 μm.)
Addition of BDNF to GF II Restores Type 1 Hair Cells to Gentamicin-Damaged Cristae and HVOR.
Histological evidence: Light microscopy.
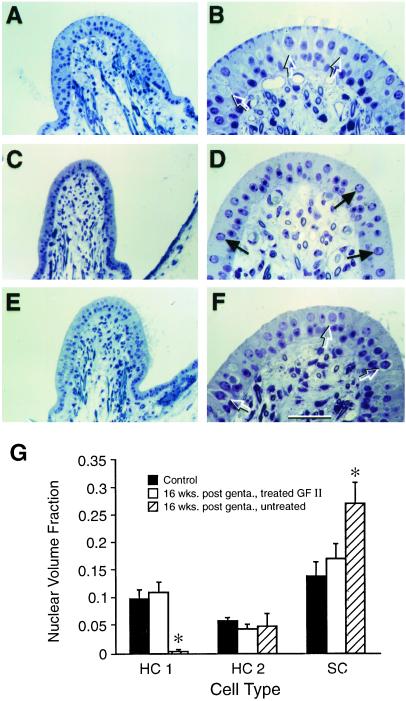
A series of photomicrographs of 1 μm toluidine blue-stained cross-sections through the horizontal crista ampullaris of the different treatment groups are depicted in Fig. 4. These include untreated (Fig. 4 A and B), gentamicin-exposed, untreated (Fig. 4 C and D), and gentamicin-exposed, GF II-treated (Fig. 4 E and F) specimens. Only the GF II-treated (Fig. 4 E and F) cristae regained type 1 vestibular hair cells. Microscopic analysis of cell types present in the cristae from horizontal semicircular duct specimens is presented in Fig. 4G. Analysis of nuclear volume fractions of these specimens was used to quantify type 1 and 2 HCs (HC1 and HC2) as well as supporting cells (SC) in the cristae from 16 week post-gentamicin (Fig. 4 C–F) and control (Fig. 4 A and B) guinea pig horizontal canals.
Figure 4.
Treatment of gentamicin-damaged inner ears with GF II containing BDNF results in the return of type 1 hair cells in the cristae. (A–F) Photomicrographs of toluidine blue-stained, 1-μm sections of horizontal cristae in the plane of the planum semilunatum at 16 weeks post-gentamicin exposure. (A and B) The normal anatomy of the horizontal crista from a control animal. (C and D) The abnormal anatomy of a gentamicin-damaged horizontal crista that received no additional treatment. (E and F) Normal-appearing anatomy of a horizontal crista from a gentamicin-damaged vestibule after treatment with GF II. Control and ototoxin-damaged GF II-treated cristae show a similar histology with many type 1 HCs present (open arrows) in both A, B, E, and F, whereas the untreated crista in C and D shows a very different histology with few HCs (solid arrows) and no detectable type 1 hair cells. (G) The quantification of cell types in cristae from control; gentamicin-exposed, untreated; and gentamicin-exposed, GF II-treated animals. For each group (n = 3), asterisks = P < 0.01. (Bars denote ±SEM; scale bar = 50 μm in B, D, and F and 100 μm in A, C, and E.)
The results of this analysis (Fig. 4G) demonstrate that many type 1 HCs were present in the cristae of both control and gentamicin-exposed, GF II-treated guinea pig vestibules (Fig. 4 A, B, E, and F), but not from gentamicin-exposed, untreated animals (Fig. 4 C and D). Gentamicin-damaged, untreated cristae had very few type 1 HCs and a greater percentage of HC nuclei that were classified as type 2 HC phenotype as well as an excess of nuclei that possessed the characteristics of supporting cell nuclei (Fig. 4G). Control values for cell nuclear volume fraction were: 0.0975 ± 0.016 for type 1 HCs; 0.058 ± 0.005 for type 2 HCs; and 0.138 ± 0.025 for support cells. Gentamicin-exposed (without trophic factor) ears had a nuclear volume fraction of type 2 HCs (0.048 ± 0.022 not significant vs. control) and almost no type 1 HCs [0.0025 ± 0.0025; F(2,4) = 10.81873; P < 0.01]. Support cell nuclear volume fraction was also increased [F(2,4) = 21.04558; P < 0.01] in this group. Animals exposed to gentamicin followed by growth factor infusion into one ear showed a type 1 (0.110 ± 0.017) HC and type 2 (0.043 ± 0.007) HC nuclear volume fraction, which was not different from control values (P > 0.05). Type 1 HC nuclear volume fraction, however, was greater than that of gentamicin-exposed ears [F(2,4) = 11.87406; P < 0.05]. Contralateral gentamicin-exposed, untreated ears from GF II-treated animals had HC nuclear volume fraction ratios similar to other gentamicin-exposed ears (data not shown).
Ultrastructural evidence: Transmission electron microscopy.
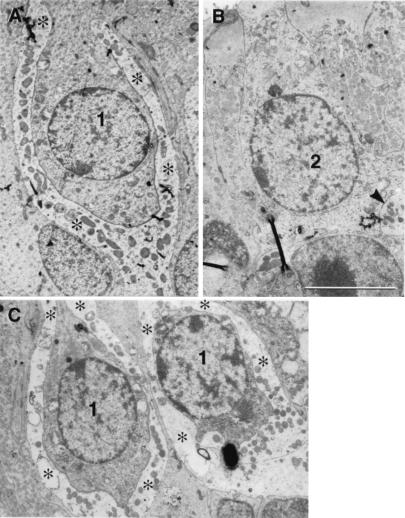
Additional evidence for the presence of type 1 HCs in the 16-week specimens of GF II-treated cristae is presented in the electron micrographs of Fig. 5. The type 1 HCs with their prominent nerve calyx endings are present in both control crista (Fig. 5A) and ototoxin-exposed, GF II-treated crista (Fig. 5C), but not in the crista of gentamicin-exposed, untreated animals (Fig. 5B).
Figure 5.
Treatment of gentamicin-damaged vestibules with GF II resulted in the return of type 1 hair cells. (A–C) Transmission electron micrographs presenting regions of HC renewal from the horizontal cristae of control (A), gentamicin-exposed, untreated (B), and gentamicin-exposed, GF II-treated (C) guinea pigs. Type 1 HCs (1) and their calyx type nerve and endings (asterisks) are evident in both control (A) and GF II-treated (C) cristae, but not in the gentamicin-exposed, untreated (B) crista where only two type 2 HCs with button-type nerve endings (arrowhead) were identifiable. (Scale bars = 5 μm.)
Functional evidence: Vestibulo-ocular response-gain and phase measurements.
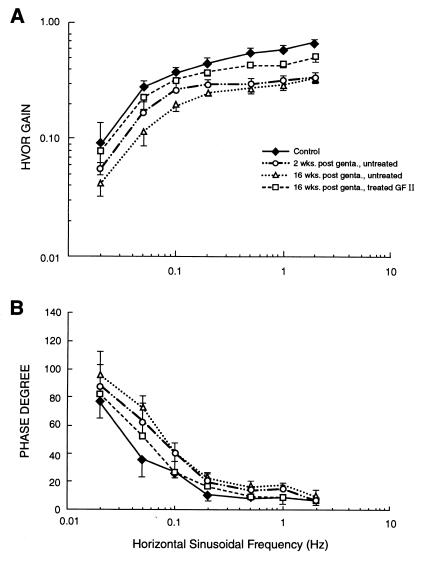
Treatment of gentamicin-instilled animals with GF II also produced a significant improvement of HVOR performance. Repeated-measures ANOVA of directional gains from the control, gentamicin-exposed, and gentamicin-exposed + GF II-treated groups (within groups factors: stimulus frequency and direction) revealed that there was no significant difference between the gain of responses during leftward versus rightward rotation. The gain and phase of this symmetric VOR response is shown for the control and bilateral treatment groups in Fig. 6. Repeated-measures ANOVA (between groups factor: treatment group; within groups factor: stimulus frequency) indicated a significant main effect of treatment on the HVOR gain [F(2,6) = 95.7; P < 0.001]. Newman–Keuls comparisons indicated that the gains in the gentamicin-exposed, GF II-treated group were significantly greater at 0.1, 0.2, 0.5, 1, and 2 Hz than the respective gains in the group with only gentamicin exposure (P < 0.05), but that the gains of the GF II-treated group did not differ significantly from the control group at the respective stimulus frequencies. The analyses of the phase data (ANOVA and Newman–Keuls tests on data displayed in Fig. 6B) also showed a parallel recovery of HVOR phase in the GF II-treated animals at 0.1, 0.5, and 1 Hz. The gain recovery was also apparent in Newman–Keuls comparisons of the gains during leftward and rightward half-cycles of rotation. Thus, GF II treatment produced a functional recovery of HVOR gain and phase across a range of stimulus frequencies.
Figure 6.
Treatment of gentamicin-damaged vestibules with GF II results in a return of vestibular function. HVOR gain (A) and phase degree (B) measurements are reported in response to horizontal stimulus of a frequency range of from 0.02–2 Hz for control; 2 and 16 weeks post-gentamicin, untreated; and 16 weeks post-gentamicin, GF II-treated guinea pigs. The GF II-treated animals approach control values, whereas the performance of untreated animals worsened from 2 to 16 weeks post-gentamicin. (Bars denote ±SEM.)
Discussion
The results demonstrate that infusion of growth factors (i.e., GF I and GF II) into vestibular ototoxin-damaged inner ears affects HC renewal, maturation of stereociliary bundles, type of HCs present, and the recovery of horizontal vestibulo-ocular reflex performance. Several possibilities exist to explain the observed vestibular HC renewal in response to growth factor therapy. Several growth factors have been demonstrated both in vitro (24, 34–36) and in vivo (37, 38) to protect auditory sensory cells from aminoglycoside toxicity. However, in all of these studies the protectant molecule had to be present before ototoxin exposure. In the present study, growth factors were infused 1 week after exposure to gentamicin, and there was no observed protective effect of growth factor infusion on auditory hair cells. Therefore, growth factor protection of vestibular HCs from gentamicin damage is not a likely mechanism to explain our results. Another possibility is that gentamicin may have induced sublethal damage to the HCs (12, 13, 39) rather than their loss via either apoptosis or necrosis (11, 13, 40). The possibility of HC self-repair cannot be ruled out by our results. However, it seems likely that there would have been a higher level of self-repair of damaged HCs in the gentamicin-exposed, untreated vestibules than was observed. Therefore, it is unlikely that self-repair of damaged HCs can account for the majority of our observed HC renewal. The replacement of lost HCs in the avian vestibule is thought to involve a proliferative response (2–4). Although the results of an in vitro study (41) analyzing mammalian vestibular HC regeneration do not support a proliferation-mediated mechanism, the results of an in vivo study (42) do suggest cell proliferation. There is in vivo evidence that existing vestibular support cells can transdifferentiate into replacement hair cells (11, 43). Avian and amphibian HC regeneration studies also support transdifferentiation (44–46). It is quite possible that both a regenerative cell proliferative response to treatment with growth factors (21, 47) and transdifferentiation of existing support cells (11) contribute to our observed HC renewal results. Previous studies without trophic factor infusion therapy (11, 29, 42) have reported a recovery of type 2 vestibular HCs (>50%), but almost no recovery of type 1 HCs (≤5%). In the present study, the observed recovery of HVOR function after GF II therapy is associated with the recovery of type 1 HCs in the damaged cristae. The GF II mixture contained BDNF, which is known to be an essential trophic molecule for vestibular ganglion neurons (23) and has been suggested to function in the differentiation of calyceal afferents on type 1 vestibular hair cells (25). A recent study has also shown that transotic administration of BDNF resulted in an increase in type 1 HCs in the gentamicin-damaged vestibules of chinchillas (47). Therefore, these studies (23, 25, 47) and our results suggest that exogenous BDNF supports neuronal survival, facilitates the formation of new calyceal endings on HCs, and leads to the reappearance of type 1 HCs in ototoxin-damaged crista. Neuronal survival and hair cell-primary afferent interactions may be essential factors in the improved vestibular function initiated by GF II therapy. The results reported in this study represent the first report of both a return of type 1 HCs and an improvement in vestibular function after ototoxic damage in the vestibule of a mammal. These findings and those of recent growth factor therapy studies in ototoxin-damaged adult rat and chinchilla vestibules (21, 47) suggest that the infusion of a mixture of exogenous growth factors (e.g., GF II) may be clinically applicable to the treatment of some balance disorders.
Acknowledgments
We thank Drs. Carey Balaban and Bradley Goldstein for their reviews and helpful comments and Dr. David Tomko for help in establishing vestibular testing and for analysis software. We also express gratitude to the late Jan Parmentier of Neurokinetics, and to Chuck Chase, CNC Engineering, for invaluable assistance in establishing testing apparatus. Supported by grants from the Office of Naval Research (to R.D.K.) and the National Organization for Hearing Research (to T.R.V.).
Abbreviations
- HC(s)
hair cell(s)
- GF I
growth factor mixture 1
- GF II
growth factor mixture 2
- HVOR
horizontal vestibulo-ocular reflexes
- BDNF
brain-derived neurotrophic factor
- TGFα
transforming growth factor α
References
- 1.Jorgensen J M, Mathiesen C. Naturwissenschaften. 1988;75:319–320. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- 2.Roberson D F, Weisleder P, Bohrer S, Rubel E W. Hear Res. 1992;57:166–174. doi: 10.1016/0378-5955(92)90149-h. [DOI] [PubMed] [Google Scholar]
- 3.Weisleder P, Rubel E W. J Comp Neurol. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- 4.Weisleder P, Tsue T T, Rubel E W. Hear Res. 1995;82:125–133. doi: 10.1016/0378-5955(94)00169-q. [DOI] [PubMed] [Google Scholar]
- 5.Jones T A, Nelson R C. Hear Res. 1992;62:181–186. doi: 10.1016/0378-5955(92)90184-o. [DOI] [PubMed] [Google Scholar]
- 6.Carey J P, Fuchs A F, Rubel E W. Ann NY Acad Sci. 1996;781:47–58. doi: 10.1111/j.1749-6632.1996.tb15692.x. [DOI] [PubMed] [Google Scholar]
- 7.Goode C T, Carey J P, Fuchs A, Rubel E. J Neurophysiol. 1999;81:1025–1035. doi: 10.1152/jn.1999.81.3.1025. [DOI] [PubMed] [Google Scholar]
- 8.Ruben R J. Acta Oto-Laryngol. 1967;220:1–44. [PubMed] [Google Scholar]
- 9.Warchol M E, Lambert P R, Goldstein B J, Forge A, Corwin J T. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 10.Forge A, Li L, Corwin J T, Nevill G. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- 11.Forge A, Li L, Nevill G. J Comp Neurol. 1998;397:69–88. [PubMed] [Google Scholar]
- 12.Zheng J L, Keller G, Gao W-Q. J Neurosci. 1999;19:2161–2170. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staecker H, Van De Water T R. Curr Opin Neurobiol. 1998;8:480–487. doi: 10.1016/s0959-4388(98)80035-4. [DOI] [PubMed] [Google Scholar]
- 14.Lambert P R. Laryngoscope. 1994;104:701–718. doi: 10.1288/00005537-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Oesterle E C, Tsue T T, Rubel E. J Comp Neurol. 1997;380:262–274. doi: 10.1002/(sici)1096-9861(19970407)380:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Zheng J L, Helbig C, Gao W G. J Neurosci. 1997;17:216–226. doi: 10.1523/JNEUROSCI.17-01-00216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita H, Oesterle E C. Proc Natl Acad Sci USA. 1995;92:3152–3155. doi: 10.1073/pnas.92.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenz D A, Liu W, Galinovic-Schwartz V, Van De Water T R. Teratology. 1996;53:292–303. doi: 10.1002/(SICI)1096-9926(199605)53:5<292::AID-TERA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Represa J, Sanchez J A, Miner C, Lewis J, Giraldez F. Development. 1990;110:1081–1090. doi: 10.1242/dev.110.4.1081. [DOI] [PubMed] [Google Scholar]
- 20.Kelley M W, Xu X M, Wagner M A, Warchol M E, Corwin J T. Development. 1993;119:1041–1053. doi: 10.1242/dev.119.4.1041. [DOI] [PubMed] [Google Scholar]
- 21.Kuntz A L, Oesterle E C. J Comp Neurol. 1998;399:413–423. [PubMed] [Google Scholar]
- 22.Kuntz A L, Oesterle E C. Otolaryngol Head Neck Surg. 1998;118:816–824. doi: 10.1016/S0194-5998(98)70275-X. [DOI] [PubMed] [Google Scholar]
- 23.Ernfors P, Van De Water T R, Loring J, Jaenisch R. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J L, Stewart R R, Gao W Q. J Neurobiol. 1995;28:330–340. doi: 10.1002/neu.480280306. [DOI] [PubMed] [Google Scholar]
- 25.Montcouquiol M, Valat J, Travo C, Sans A. Eur J Neurosci. 1998;10:598–606. doi: 10.1046/j.1460-9568.1998.00070.x. [DOI] [PubMed] [Google Scholar]
- 26.Brown N, Miller J, Altschuller R. Hear Res. 1993;70:167–172. doi: 10.1016/0378-5955(93)90155-t. [DOI] [PubMed] [Google Scholar]
- 27.Weibel E R. Practical Methods for Biological Morphometry. Vol. 1. London: Academic; 1979. pp. 101–159. [Google Scholar]
- 28.Lindeman H H, Reith A, Winther F O. Acta Oto-Laryngol. 1981;92:315–321. doi: 10.3109/00016488109133267. [DOI] [PubMed] [Google Scholar]
- 29.Lopez I, Honrubia V, Li S C, Schoeman G, Beykirch K. Int J Dev Neurosci. 1998;15:447–461. doi: 10.1016/s0736-5748(96)00103-7. [DOI] [PubMed] [Google Scholar]
- 30.Cochran W G. Sampling Techniques. 3rd Ed. New York: Wiley; 1977. [Google Scholar]
- 31.Andrews J C, Li J, Koyama S, Hoffman L F. Ann Otol Rhinol Laryngol. 1997;106:838–847. doi: 10.1177/000348949710601006. [DOI] [PubMed] [Google Scholar]
- 32.Escudero M, de Waele C, Vibert N, Berthoz A, Vidal P P. Exp Brain Res. 1993;97:254–262. doi: 10.1007/BF00228694. [DOI] [PubMed] [Google Scholar]
- 33.Dimitri P S, Wall C, Oas J G. Acta Oto-Laryngol. 1996;116:497–506. doi: 10.3109/00016489609137880. [DOI] [PubMed] [Google Scholar]
- 34.Low W, Dazert S, Baird A, Ryan A F. J Cell Physiol. 1996;167:443–450. doi: 10.1002/(SICI)1097-4652(199606)167:3<443::AID-JCP8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Staecker H, Dazert S, Malgrange B, Lefebvre P, Ryan A F, Van De Water T R. Int J Dev Neurosci. 1997;15:553–562. doi: 10.1016/s0736-5748(96)00110-4. [DOI] [PubMed] [Google Scholar]
- 36.Gabaizadeh R, Staecker H, Liu W, Van De Water T R. Mol Brain Res. 1997;50:71–78. doi: 10.1016/s0169-328x(97)00173-3. [DOI] [PubMed] [Google Scholar]
- 37.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van De Water T R. NeuroReport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 38.Duan M, Agerman K, Ernfors P, Canlon B. Proc Natl Acad Sci USA. 2000;97:7597–7602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobkowicz H M, August B K, Slapnick S M. Exp Neurol. 1992;115:55–49. doi: 10.1016/0014-4886(92)90219-g. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Nevill G, Forge A. J Comp Neurol. 1995;335:405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- 41.Zheng J L, Gao W-Q. J Neurosci. 1997;17:8270–8282. doi: 10.1523/JNEUROSCI.17-21-08270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez I, Honrubia V, Lee S, Li G, Beykirch K. Otolaryngol Head Neck Surg. 1998;119:255–262. doi: 10.1016/S0194-5998(98)70060-9. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Forge A. Int J Dev Neurosci. 1997;15:433–446. doi: 10.1016/s0736-5748(96)00102-5. [DOI] [PubMed] [Google Scholar]
- 44.Adler H J, Raphael Y. Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- 45.Steyger P S, Burton M, Hawkins J R, Schuff N R, Baird R A. Int J Dev Neurosci. 1997;15:417–432. doi: 10.1016/s0736-5748(96)00101-3. [DOI] [PubMed] [Google Scholar]
- 46.Baird R A, Steyger P S, Schuff N R. Ann NY Acad Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- 47.Lopez I, Honrubia V, Lee S, Li G, Beykirch K, Micevych P. Am J Otolaryngol. 1999;20:317–324. [PubMed] [Google Scholar]