Abstract
We examined α1A-adrenergic receptor (AR) mediation of preconditioning in a novel α1A-AR cardiac transgenic (TG) rat model (α1A-TG). Compared with nontransgenic littermates (NTLs), in conscious α1A-TG rats, heart rate was reduced, contractility [left ventricle (LV) +dP/dt, ejection fraction, end-systolic elastance] was significantly enhanced, and triple product (LV systolic wall stress × LV +dP/dt × heart rate) was unchanged. However, infarct size (IS)/area at risk (AAR) in response to ischemia-reperfusion (30 min coronary occlusion/3 h reperfusion) was reduced to 35 ± 4.6% in α1A-TGs vs. 52 ± 2.2% in NTLs (P < 0.05). Second window preconditioning reduced IS/AAR in NTLs to 29 ± 2.7% but did not afford further protection in α1A-TGs. In contrast, with first window preconditioning, IS/AAR was reduced to similar levels in both α1A-TGs (12 ± 1.4%) and NTLs (10 ± 1.1%). In untreated α1A-TGs, cardioprotection was associated with enhanced myocardial phosphorylated (p)-mitogen/extracellular signal-regulated kinase (MEK), p-extracellular signal-regulated kinase (ERK), and inducible nitric oxide synthase (iNOS) at the protein level, along with a 1.3-fold increase in total nitric oxide synthase activity like in second window preconditioning. Affymetrix microarrays revealed that few genes (4.6% of 3,172 upregulated; 8.8% of 3,498 downregulated) showed directionally similar changes in α1A-TGs vs. NTLs subjected to second window preconditioning. Thus, second, but not first, window cardioprotection is evident in α1A-TGs in the absence of ischemic preconditioning and is mediated by iNOS activation associated with MEK/ERK phosphorylation. Transcriptionally, however, second window preconditioning is considerably more complex than α1A-TG preconditioning, with the alteration of thousands of additional genes affording no further protection than that already available in α1A-TG rats.
Keywords: receptors, adrenergic, alpha, hemodynamics, ischemia, reperfusion, infarction
ischemic preconditioning (IPC) is a mechanism whereby a brief period of nonlethal ischemia protects against a following longer period of lethal ischemia. Two distinct components have been described: first window of IPC, which provides powerful protection against myocardial infarction within 2–3 h after preconditioning, and second window of IPC, which provides protection, 1–3 days after preconditioning. We have shown previously that second window preconditioning (SWOP) is mediated by cardiac nerves and α1-adrenergic receptors (ARs) (16). Although these findings are in agreement with other studies using pharmacological evaluations, the receptor subtype involved remains controversial. For example, the α1B-AR has been shown to be involved (5, 10, 14, 31) or not involved (6, 25) in cardioprotection. In contrast, work by Rorabaugh et al. (24, 25) has shown the α1A-AR to be protective against ischemia.
Accordingly, we elected to evaluate IPC in a novel transgenic rat model of cardiomyocyte-restricted α1A-AR overexpression. This model, in contrast to transgenic mice, allows evaluation of cardiac contractility and heart rate in the intact conscious animal, both of which are major determinants of myocardial oxygen consumption (MV̇o2) that can also contribute to cardioprotection. We further determined the cellular mechanism of protection against ischemia-reperfusion (I/R) and if any cardioprotection observed phenocopies first window preconditioning (FWOP) or SWOP. We show that cardiomyocyte-restricted α1A-AR overexpression in transgenic rats protects against I/R, that the degree of cardioprotection observed mimics second but not first window protection, and that cardioprotection is mediated by a mechanism involving activation of inducible nitric oxide synthase (iNOS) and mitogen/extracellular signal-regulated kinase (MEK)-extracellular signal-regulated kinase (ERK) signaling. Furthermore, cardiomyocyte Affymetrix microarray studies showed that, whereas the second window is associated with altered transcription of a plethora of genes, only a limited subset of these showed directionally similar alterations with α1A-AR overexpression. Thus, alterations in the thousands of additional genes regulated in SWOP do not provide further cardioprotection than that already available as a result of enhanced cardiac α1A-AR drive.
MATERIALS AND METHODS
Animal models.
Transgenic Sprague-Dawley rats expressing the rat α1A-AR under the control of the rat cardiac α-myosin heavy chain promoter were generated using a modification of the procedure described for the mouse (18). Two founder lines with 15- or 40-fold cardiac-specific receptor overexpression (determined from equilibrium radioligand binding studies), as well as their nontransgenic littermantes (NTLs), were used in this study. The α1A-AR specificity of ligand recognition was confirmed by competition binding studies, as reported previously for mice with cardiac α1A-AR overexpression. Litter sizes and postnatal development of the transgenic (TG) rats were indistinguishable from those of control NTLs. Development of the transgenic rats was performed with approval of the St. Vincent's Hospital Animal Ethics Committee, whereas the experiments performed at the University of Medicine and Dentistry of New Jersey were approved by their Institutional Animal Care and Use Committee, and all animals were maintained in accordance with guidelines for the Guide for the Care and Use of Laboratory Animals (NIH 83-23, revised 1996).
Hemodynamics.
Male rats, 300–450 g, were anesthetized with a mixture of ketamine and xylazine given intramuscularly, and catheterization using sterile surgical technique was performed as described previously (33). An 8-in. fluid-filled catheter was inserted via the right carotid artery into the left ventricle (LV) to measure LV pressure and LV dP/dt. A second catheter was inserted into a femoral artery to measure arterial pressure and a third into the jugular vein for drug administration. The catheters were tunneled under the skin, externalized at the back of the neck, and attached to a tether device (Kent Scientific) for conscious recordings of freely moving animals in their cage. The fluid-filled LV and femoral artery catheters were connected to a pressure transducer (DTX Plus; Becton-Dickinson), calibrated using a manometer, and the signals were amplified using an electronic filter setup. Data were recorded on LabChart 7 software (ADInstruments) with a Powerlab (ADInstruments) data acquisition system at a 1-KHz sampling rate. One day after rats recovered from surgery, baseline LV and arterial physiological measurements were recorded, and LV dP/dt and Weiss Tau were calculated with LabChart 7 software (ADInstruments).
Echocardiography.
Rats were anesthetized by injection of pentobarbital sodium (55 mg/kg ip). Transthoracic echocardiography was performed using a GE Vivid 7 Dimension ultrasound system with an 11-MHz linear transducer. LV internal dimensions and LV wall thickness were measured in systole and diastole using leading-edge methods and guidelines of the American Society of Echocardiography (26). LV systolic function was estimated from LV dimensions by the cubed method as a percentage of the LV ejection fraction (LVEF).
Pressure-volume loop analysis.
Rats were anesthetized with pentobarbital sodium (55 mg/kg ip), and a multisegment 2-Fr Millar pressure-volume (PV) loop catheter (SPR-932; Millar Instruments) was inserted via the right carotid artery into the LV. Adequate placement of the catheter was verified by the PV loop signals. A 5–0 silk suture was passed under the vena cava for inferior vena cava occlusion (IVCO). The abdominal incision was closed. Baseline hemodynamics were recorded followed by IVCO. End-systolic elastance (Ees; in mmHg/μl) was calculated from the linear end-systolic PV relationship. Time-varying maximum elastance (Emax) was obtained from the series of PV relationship regression curves at decreased preloads, and preload recruitable stroke work (PRSW) was calculated from linear LV stroke work and linear volume intercept (in μl).
Regional ischemia and reperfusion.
Male TG and NTL rats (350–450 g) were anesthetized with pentobarbital sodium (55 mg/kg ip). Rectal temperature was monitored and body temperature maintained at 37°C with an automatic heating lamp. A left thoracotomy was performed at the fourth intercostal space to allow ischemia induction by the temporary occlusion of the left anterior descending coronary artery for 30 min followed by reperfusion.
Ischemia protocol.
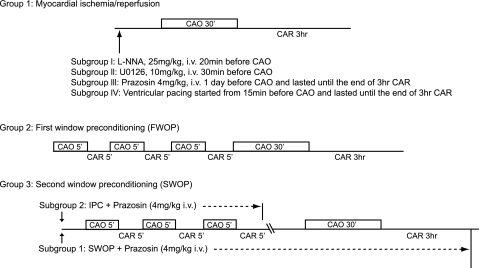
Three different studies were performed in both NTL and TG rats. The ischemia protocols are outlined in Fig. 1. Group 1 animals were subjected to I/R involving coronary artery occlusion (CAO) for 30 min, followed by 3 h coronary artery reperfusion (CAR), both in the absence and presence of the NOS inhibitor Nω-nitro-l-arginine (l-NNA, 25 mg/kg iv, 20 min before CAO; Sigma-Aldrich), the MEK inhibitor U-0126 (10 mg/kg iv, 30 min before CAO; LC Labs), the α1-AR inhibitor prazosin (4 mg/kg iv, 1 day before CAO; Sigma-Aldrich), or continuous ventricular pacing at 450 beats/min from 15 min before occlusion until the end of I/R. Group 2 animals were subjected to FWOP involving three episodes of 5 min CAO/5 min CAR followed by I/R (30 min CAO/3 h CAR). Group 3 animals were subjected to SWOP involving three similar episodes of 5 min CAO and 5 min CAR. Two subgroups in group 3 were treated with prazosin (4 mg/kg iv), a nonselective α1-AR blocker, every 6 h from 20 min before IPC. In brief, one subgroup (SWOP + prazosin) was treated with prazosin from 20 min before IPC until the end of the I/R period on the next day, and the other subgroup (IPC + prazosin) was treated with prazosin only during the preconditioning procedure (day 1). After three episodes of 5 min CAO/5 min CAR, the rats were allowed to recover for 24 h before being subjected to I/R (30 min CAO/3 h CAR).
Fig. 1.
Protocols for infarct size studies. Group 1 animals were subjected to coronary artery occlusion (CAO) for 30 min, followed by 3 h of coronary artery reperfusion (CAR). Three subgroups in this protocol received the following treatment: 1) Nω-nitro-l-arginine (25 mg/kg iv l-NNA; Sigma-Aldrich) given 20 min before CAO, 2) MEK inhibitor U-0126 (10 mg/kg iv; LC Labs) given 30 min before CAO, 3) α1-adrenergic receptor (AR) blocker prazosin (4 mg/kg iv; Sigma-Aldrich) given every 6 h from 1 day before CAO until the end of 3 h CAR, or 4) ventricular pacing at 450 beats/min. Group 2 animals were subjected to first window preconditioning (FWOP) involving 3 episodes of 5 min CAO/5 min CAR followed by 30 min CAO/3 h CAR. Group 3 animals with 2 subgroups were subjected to second window preconditioning (SWOP) involving 3 episodes of 5 min CAO and 5 min CAR and then subjected to 30 min CAO/3 h CAR. After the last 5-min episode of CAO, the rats were allowed to recover for 24 h before the prolonged ischemia-reperfusion (I/R). One subgroup (SWOP + prazosin) was treated with prazosin (4 mg/kg iv) every 6 h from 20 min before ischemic preconditioning (IPC) until the end of the 30-min CAO/3-h CAR on the next day. The other subgroup (IPC + prazosin) was treated with prazosin only during the IPC procedure on day 1.
Assessment of infarct size.
At the end of the reperfusion period, rats were killed, and hearts were arrested in diastole by right atrial injection with saturated KCl. The ascending aorta was cannulated and used to perfuse the heart with 1× PBS. After the original suture used for I/R was religated, the heart was perfused with 1% Evans Blue in 1× PBS. The LV was then sectioned into 1-mm sections from the apex to the base, followed by a 5-min incubation period in 1% 2,3,5-triphenyltetrazolium chloride at 37°C. Each section was imaged on both sides using a digital scanner at 300 dots per inch.
Terminal deoxynucleotide transferase-mediated dUTP nick end labeling staining.
Deparaffinized LV ring sections were treated with protease K (20 μg/ml; Sigma-Aldrich) for 30 min and then washed in 1× PBS. To discriminate apoptosis in nonmyocytes and myocytes, the sections were double stained for both DNA fragmentation with an in situ cell death detection kit (Roche Diagnostics) and for cell membranes with rhodamine-conjugated wheat germ agglutinin. Sections were mounted using Vectashield mounting medium containing 4,6-diamidino-2-phenylindole. Terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)-positive cardiomyocyte nuclei (22) were counted in 200 fields in both the ischemic and remote zones, at a magnification of ×60, to determine the number of apoptotic myocytes per square millimeter.
Western blotting.
LV samples were homogenized and resuspended in Laemmli buffer and fractionated by SDS-PAGE (6–12%). Proteins were electroblotted for 1.5 h at 4°C onto nitrocellulose membranes, which were then washed and incubated overnight with primary antibodies (all at 1:1,000 final dilution) against iNOS (Thermo Scientific), MEK, phosphorylated (p)-MEK, ERK, or p-ERK (Cell Signaling). After washing with TBS and 0.05% Tween 20, nonspecific protein binding was blocked with 2.5% milk for iNOS and 5% milk for MEK/p-MEK and ERK/p-ERK. Bound primary antibodies were detected with biotinylated secondary antibody (1:5,000) and Western Lightning Chemiluminescence Reagent (PerkinElmer Life Sciences).
Nitric oxide synthase assay.
Nitric oxide synthase (NOS) activity was determined using a NOS activity assay kit (Oxford Biomedical Research). Heart samples were homogenized with lysis buffer, and the protein concentration was determined. Samples (16 μg/μl) of the homogenate were mixed with reaction buffer (50 mM HEPES, 0.5 mM EDTA) and NADPH. After incubation for 4 h, reconstituted nitrate reductase was added, and the samples were incubated for an additional 20 min at room temperature. After incubation, the samples were centrifuged at 14,500 g for 5 min, and the supernatant was collected and mixed with color reagents [containing sulfanilamide in 3 N HCl and N-(1-naphthyl)ethylenediamine dihydrochloride] before spectrophotometric analysis at 540 nm.
Myocyte isolation.
Because receptor overexpression in the TG rats is cardiomyocyte-specific, we isolated these using a Langendorff perfusion system. The heart was first perfused with perfusion buffer (in mmol/l: 120.4 NaCl, 14.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4·7H2O, 4.6 NaHCO3, 10 Na-HEPES, 30 taurine, 10 BDM, and 5.5 glucose) for 4 min at 37°C at 4 ml/min and then with enzyme buffer [50 ml perfusion buffer containing 2.0 mg/ml collagenase II (Worthington)] for 12–15 min. At the end of the digestion procedure, live myocytes were collected.
RNA extraction.
Cardiomyocytes were homogenized in Trizol (1 ml/5 × 106 cells; Roche), and chloroform (0.2 ml) was added. After being vortexed and incubated at room temperature for 10 min, samples were centrifuged at 12,000 g at 4°C for 15 min. Isopropanol (1.5 ml) was added, and the resulting mixture was incubated at room temperature for 10 min before centrifugation at 12,000 g for 15 min. The supernatant was removed, and the pellet was washed with 1 ml of 75% ethanol. An additional centrifugation was performed at 10,000 g at room temperature for 10 min, and the resulting RNA pellet was dried and resuspended in nuclease-free water. RNA quality was tested using NanoDrop (Thermo Scientific) for the following criteria: concentration >1.0 μg/μl; ratio of absorbance at 260 to 280 nm >2.0. RNA integrity was confirmed by electrophoresis, with a 28S-to-18S ratio of 2:1 being considered satisfactory for microarray gene analysis.
Microarray analysis.
Gene expression was examined by Affymetrix GeneChip Rat Gene 1.0 ST Array, which contains probes for 27,342 genes. Microarray data were normalized using the Robust Multichip Average method. Genes detected above background (P < 0.05) for more than one-third of the probe sets were considered expressed. Significant genes were determined by t-test (P < 0.05). The Gene Ontology (GO) annotation of genes was obtained from the NCBI gene database. GO entries were tested for bias to upregulation and downregulation of expression using the hypergeometric test. We removed GO terms that shared >50% of associated genes with a more significant term.
Quantitative real-time reverse transcriptase PCR.
Total RNA from the same rat cardiomyocyte samples used in the microarray studies were subjected to quantitative real-time PCR. For each measurement, the mRNA of interest was reverse-transcribed from 1 μg of total RNA with the TaqMan RT kit (Applied Biosystems). The gene-specific cDNA was used for quantitative PCR (40 cycles of a 10-s step at 95°C and a 1-min step at 60°C) using the SYBR Green PCR mix (Applied Biosystems) on a 7700 ABI-Prizm sequence detector (Applied Biosystems). GAPDH was used as loading control to eliminate the variations due to loading differences. The values of the transcripts were reported as mRNA content relative to NTL.
Statistical analysis.
Data are expressed as means ± SE. Comparisons between hemodynamics in conscious and anesthetized animals were determined using one-way ANOVA. Comparisons of infarct size between I/R and IPC in NTL and TG animals were determined by two-way ANOVA. Statistical significance for PV loop data and other infarct size and protein expression levels were determined using Student's t-test. P < 0.05 was taken as a minimal level of significance.
RESULTS
Hemodynamics in conscious rats.
There were no differences in body weights or LV weights at baseline (Table 1), but there were major differences in heart rate and myocardial contractility (Table 2). Mean arterial pressure (110 ± 4.6 in NTLs vs. 111 ± 7.6 mmHg in TG) and LV pressure (142 ± 7.3 vs. 141 ± 6.4 mmHg) were similar in both groups. Heart rate was lower in TG rats (284 ± 8.6 beats/min) compared with NTLs (354 ± 8.3 beats/min, P < 0.01), and LV +dP/dt was higher in TGs (12,301 ± 746 mmHg/s) compared with NTLs (6,692 ± 377 mmHg/s, P < 0.01). LV diastolic function was also enhanced in the TG group with both lower LV −dP/dt (−6,722 ± 518 vs. −5,212 ± 299 mmHg/s in NTLs, P < 0.01) and Tau (13 ± 1.2 vs. 21 ± 2.5 ms in NTLs, P < 0.05) (Table 2).
Table 1.
Morphometry of TG rats and NTLs
| NTL (n = 4) | TG (n = 4) | |
|---|---|---|
| Body wt, g | 434 ± 18 | 454 ± 15 |
| LV wt, mg | 931 ± 33 | 879 ± 52 |
| LV wt/body wt, mg/g | 1.79 ± 0.04 | 1.80 ± 0.05 |
| Area at risk, % | 38 ± 2.0 | 41 ± 2.0 |
| Infarct size/area at risk, % | 52 ± 2.2 | 35 ± 4.6** |
Values are means ± SE; n, no. of rats. TG, transgenic; NTL, nontransgenic littermates; LV, left ventricle. NTL vs. TG:
P < 0.01.
Table 2.
Hemodynamics of conscious and anesthetized TG rats and NTLs
| Parameter | n | Conscious | n | Anesthetized |
|---|---|---|---|---|
| Heart rate, beats/min | ||||
| NTL | 8 | 354 ± 8.3 | 5 | 409 ± 21† |
| TG | 8 | 284 ± 8.6** | 5 | 347 ± 15*†† |
| LV systolic pressure, mmHg | ||||
| NTL | 8 | 142 ± 7.3 | 5 | 111 ± 5.5† |
| TG | 8 | 141 ± 6.4 | 5 | 113 ± 6.8† |
| End-diastolic pressure, mmHg | ||||
| NTL | 8 | 6.0 ± 1.2 | 5 | 8.3 ± 1.3 |
| TG | 8 | 6.3 ± 0.6 | 5 | 7.5 ± 1.4 |
| Systolic arterial pressure, mmHg | ||||
| NTL | 8 | 139 ± 4.9 | 5 | 117 ± 7.3† |
| TG | 8 | 139 ± 7.4 | 5 | 113 ± 4.1† |
| Diastolic arterial pressure, mmHg | ||||
| NTL | 8 | 91 ± 4.4 | 5 | 83 ± 7.5 |
| TG | 8 | 91 ± 6.9 | 5 | 80 ± 4.4 |
| Mean arterial pressure, mmHg/s | ||||
| NTL | 8 | 110 ± 4.6 | 5 | 96 ± 7.6 |
| TG | 8 | 111 ± 7.6 | 5 | 92 ± 4.4 |
| LV +dP/dt, mmHg/s | ||||
| NTL | 8 | 6,692 ± 377 | 5 | 6,087 ± 493 |
| TG | 8 | 12,301 ± 746** | 5 | 7,954 ± 526*†† |
| LV −dP/dt, mmHg/s | ||||
| NTL | 8 | −5,212 ± 299 | 5 | −4,718 ± 877 |
| TG | 8 | −6,722 ± 518* | 5 | −4,783 ± 712† |
| Tau, ms | ||||
| NTL | 8 | 21 ± 2.5 | 5 | 22 ± 3.5 |
| TG | 8 | 13 ± 1.2* | 5 | 15 ± 1.9 |
Values are means ± SE; n, no. of rats. Hemodynamics recorded by fluid catheter were compared between conscious and anesthetized NTLs and TG rats. TG vs. NTL:
P < 0.05 and
P < 0.01. Anesthetized vs. conscious:
P < 0.05 and
P < 0.01.
Hemodynamics in anesthetized rats before I/R.
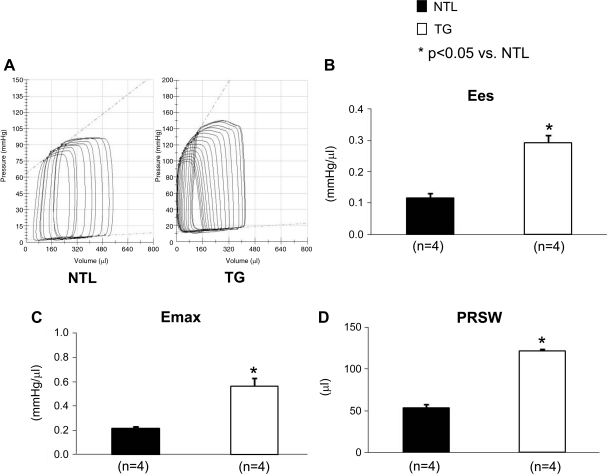
Rats were anesthetized with pentobarbital sodium, intubated, and ventilated. The chest was opened, and hemodynamic measurements were monitored. Mean arterial pressure (96 ± 7.6 vs. 92 ± 4.4 mmHg) and LV systolic pressure (111 ± 5.5 vs. 113 ± 6.8 mmHg) were similar in the NTL and TG rats, respectively. Heart rate was lower (347 ± 15 vs. 409 ± 21 beats/min, P < 0.05) and LV +dP/dt was higher (7,954 ± 526 vs. 6,087 ± 493 mmHg/s, P < 0.05) in the TG group compared with the NTLs, respectively. In TG rats, PV loop studies also showed significant increases (P < 0.05, Fig. 2) in Ees (0.12 ± 0.01 in NTLs vs. 0.29 ± 0.02 mmHg/μl in TGs), Emax (0.22 ± 0.01 in NTLs vs. 0.6 ± 0.1 in TGs), and PRSW (54 ± 3.9 in NTLs vs. 122 ± 2.1 in TGs) (Table 2).
Fig. 2.
Pressure-volume (PV) loop studies in anesthetized rats confirmed that the increase in left ventricle (LV) systolic function in α1A-transgenic (TG) rats is intrinsic and load independent. *Significant differences between the TGs and nontransgenic littermates (NTLs). The number of animals (n) is shown in parentheses. Values are means ± SE. A: example of PV loops in response to inferior vena cava constriction showing the linearity of the end-systolic PV relationship. B–D: end-systolic elastance (Ees), time-varying maximum elastance (Emax), and preload recruitable stroke work (PRSW), respectively.
Echocardiographic studies of TG rats (Table 3) also showed a significant increase in LVEF (83 ± 1.3 vs. 71 ± 0.7% in NTLs, P < 0.01) and left ventricular fractional shortening (45 ± 1.4 vs. 34 ± 0.5% in NTLs, P < 0.01). Both LV end-diastolic and LV end-systolic diameter were markedly decreased in the TG group compared with NTLs (LV end diastolic dimension: 6.3 ± 0.4 mm in TGs vs. 7.7 ± 0.3 in NTLs, P < 0.05; LV end systolic dimension: 3.5 ± 0.3 mm in TGs vs. 5.1 ± 0.2 in NTLs, P < 0.01, n = 4). LV triple product (HR × LV +dP/dt × LV systolic wall stress), an index of MV̇o2 was similar in both groups (1.8 × 108 ± 3.5 × 107 in TGs vs. 1.7 × 108 ± 2.8 × 107 in NTLs, P < 0.05).
Table 3.
Echocardiographically determined LV function of TG rats and NTLs
| NTL | TG | |
|---|---|---|
| LV ejection fraction, % | 71 ± 0.7 | 83 ± 1.3** |
| LV fractional shortening, % | 34 ± 0.5 | 45 ± 1.4** |
| LV end-diastolic diameter, mm | 7.7 ± 0.3 | 6.3 ± 0.4* |
| LV end-systolic diameter, mm | 5.1 ± 0.2 | 3.5 ± 0.3** |
| LV systolic wall stress, Kdyn/cm2 | 74 ± 3.2 | 46 ± 6.0** |
| LV triple product | 1.7 × 108 ± 2.8 × 107 | 1.8 × 108 ± 3.5 × 107 |
Values are means ± SE for n = 4 rats. TGs vs. NTLs:
P < 0.05 and
P < 0.01.
Infarct size and apoptotic myocytes were reduced in α1A-TG rats.
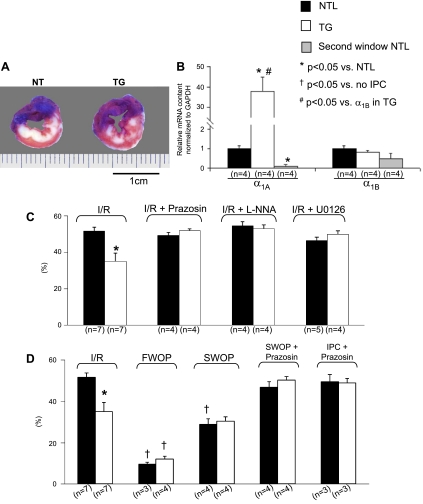
The 30-min CAO/3-h CAR I/R protocol resulted in a smaller infarct area despite a similar area at risk (Fig. 3A and Table 1) and a significant reduction in infarct size/area at risk (35 ± 4.6 vs. 52 ± 2.2%, P < 0.01) in the TG rats compared with the NTLs, respectively (Fig. 3C). There were also fewer TUNEL-positive myocytes in both the ischemic (127 ± 37, TGs vs. 340 ± 82 myocytes/mm2, NTLs, P < 0.05) and remote (0.8 ± 0.2, TGs vs. 2.4 ± 0.5 myocytes/mm2, NTLs, P < 0.05) zones of the TG rats (n = 5, data not shown). Cardiac protection resulting from α1A-AR overexpression was confirmed via α-AR blockade with prazosin. Thus, the previously seen reduction in infarct size in the TG group was abolished after prazosin treatment with infarct size now similar to that seen in NTLs (51.8 ± 1.1%, n = 4, P > 0.05), whereas infarct size in the NTL group remained unchanged (49.2 ± 1.7%, n = 4, P > 0. 05), (Fig. 3C).
Fig. 3.
Infarct size (IS) and α1A-AR mRNA levels in TGs, NTLs, and NTLs subjected to SWOP (Second window NTL). Symbols indicate statistical differences. The number of the animals (n) is shown in parentheses. Values are means ± SE. A: examples of infarcted hearts from both NTL and TG groups after ischemia-reperfusion (I/R) showing the area at risk (AAR; blue staining) and infarcted tissue [white areas that fail to stain with 2,3,5-triphenyltetrazolium chloride (TTC)]. Note that the infarct was more homogeneous in NTLs and more patchy in TGs, reflecting myocardial salvage in the latter. B: using quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) of cardiomyocyte RNA, compared with untreated NTLs, mRNA expression of the α1A-AR was increased ∼40-fold in TGs but was reduced 10-fold in second window preconditioned NTLs. However, expression of the α1B-AR was similar among NTL, TG, and second window preconditioned NTL groups. C: ratio of IS to AAR (expressed as a percentage and shown on the ordinate as %). In the absence of IPC, IS/AAR in response to I/R was lower in TGs. After administration of the α1-AR blocker prazosin, the NOS inhibitor l-NNA, or the MEK inhibitor U-0126, the cardioprotection from I/R observed in untreated TG rats was abolished, whereas IS/AAR was unchanged in prazosin-treated NTLs. D: IS/AAR after FWOP was reduced in both the NTL and TG groups, but, after SWOP, IS/AAR was only reduced in NTLs. In response to prazosin, given either at the time of preconditioning (IPC + prazosin) or throughout SWOP (SWOP + prazosin), the reduction in IS/AAR observed both in the TG rats and in the NTLs subjected to SWOP was abolished.
TG rats showed further reduction in infarct size after first, but not second, window IPC.
Based on the smaller infarct size and lesser cell death in the TG rats subjected to I/R, we reasoned that TG rats with α1A-AR overexpression may be preconditioned to injury from I/R. Therefore, we subjected TG rats and NTLs to both FWOP and SWOP. Following FWOP, infarct size was reduced further in TGs (12.1 ± 1.4%), to a level comparable to that obtained in the NTLs (10 ± 1.1%) subjected to first window precondition (Fig. 3D). With SWOP, infarct size decreased in NTLs (29 ± 2.7%) to a level that was now no longer different from that observed in TG rats subjected only to I/R (35 ± 4.6%). In contrast, infarct size remained unchanged in TG subjected to SWOP (Fig. 3D). These findings suggest that α1A-AR overexpression and delayed IPC activate common pathways of myocardial protection. To further test the role of α1A-AR in SWOP, α-ARs were blocked with prazosin, given either only during the ischemic precondition procedure or throughout the whole SWOP protocol. After prazosin, the protection resulting from SWOP was abolished in TG rats (50 ± 1.7% in the SWOP + prazosin group and 49 ± 2.1% in the IPC + prazosin group vs. 35 ± 4.6% in the I/R group) and was unchanged in NTLs (47 ± 2.6% in the SWOP + prazosin group and 50 ± 3.3% in the IPC + prazosin group vs. 52 ± 2.2% in the I/R group) (Fig. 3D). Interestingly, α1A-AR mRNA expression was increased by ∼40-fold, as expected, in TG rat hearts but was actually reduced with IPC in NTL rat hearts, whereas α1B-AR mRNA levels remained unchanged in both TGs and with SWOP of NTLs (Fig. 3B).
SWOP-related proteins in α1A-AR-overexpressed rats at baseline.
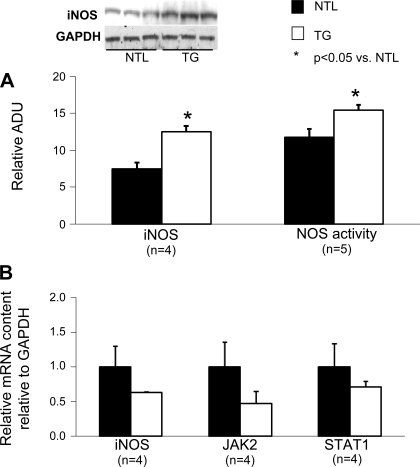
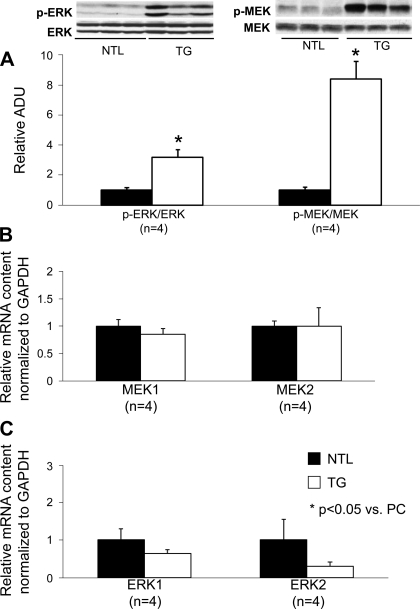
iNOS is a key signaling molecule mediating SWOP. We, thus, evaluated iNOS protein expression in the hearts of TG rats and NTLs. This revealed a 1.7-fold increase (P < 0.05) in iNOS protein expression in TG rats compared with NTLs (Fig. 4A), which was accompanied by a 1.3-fold increase in total NOS activity (P < 0.05, Fig. 4A). These increases are likely posttranscriptionally mediated as iNOS mRNA levels as well as related JAK2 and STAT1 transcripts were unaltered in the TGs (Fig. 4B). p-MEK and p-ERK were significantly increased in TG rats compared with NTLs (P < 0.05, Fig. 5A) because of posttranslational changes at the level of protein phosphorylation, since neither MEK1/2 nor ERK1/2 mRNA levels were altered in the TG rats (Fig. 5, B and C). Cyclooxygenase 2 protein expression was increased significantly (P < 0.05) in TG rats compared with NTLs (data not shown). In contrast, myocardial endothelial NOS expression was not significantly altered in TG rats (data not shown).
Fig. 4.
Protein, gene expression of inducible nitric oxide synthase (iNOS) signaling pathway, and activity of nitric oxide synthase (NOS) in NTL and α1A-TG rats. *Statistical differences between the groups. The number of animals (n) is shown in parentheses. Results shown are relative to NTL values. Values are means ± SE. A: protein expression level (Western blotting) of iNOS and total NOS activity were significantly increased in TGs compared with NTLs. B: gene expression level (determined by qRT-PCR of cardiomyocyte RNA) of iNOS and its downstream effectors, JAK2 and STAT1, were not significantly different between TGs and NTLs.
Fig. 5.
Protein and gene expression level of mitogen/extracellular signal-regulated kinase (MEK) 1/2 and extracellular signal-regulated kinase (ERK) 1/2 in NTL and α1A-TG rats. *Statistical differences between groups. The number of animals (n) used is shown in parentheses. Results shown in TG group are relative to NTL group. Data are means ± SE. A: protein expression levels of phosphorylated (p)-ERK/ERK and p-MEK/MEK were significantly increased in the TG group, although ERK and MEK levels were similar in both TG and NTL groups. MEK1/2 (B) and ERK1/2 (C) mRNA levels (determined by qRT-PCR of cardiomyocyte RNA) were not significantly different between the TG and NTL groups.
Cardioprotection abolished by NOS or MEK inhibition.
To further confirm the involvement of NOS and MEK in cardioprotection, infarct size was assessed in the presence of NOS or MEK inhibition. The nonselective NOS inhibitor l-NNA (25 mg/kg iv), administered 20 min before 30 min CAO/3 h CAR did not alter arterial pressure or heart rate (data not shown) and did not affect infarct size of the NTL rats (54 ± 2.4%, n = 4). However, NOS inhibition abrogated the cardioprotective effects of α1A-AR overexpression (P > 0.05) so that infarct size in the l-NNA-treated TG rats (53 ± 2.2%) was now similar to that seen in the NTLs (52 ± 2.2%) (Fig. 3C). After administration of the MEK inhibitor U-0126, infarct size in the TG rats (46 ± 0.5%, n = 5) was no longer significantly different from that in the NTLs (47 ± 1.8%, n = 4) (Fig. 3C).
Normalized heart rate did not alter the reduction in infarct size in TG rats.
To exclude the possibility that the lower heart rate in TG rats is responsible for the cardioprotection observed in these animals by an effect due to a reduction in MV̇o2, we paced the hearts of conscious TG rats at 450 beats/min, a rate similar to that observed in NTLs during I/R. However, infarct size was unchanged in the paced (33 ± 4.8%) vs. unpaced (35 ± 4.6%) animals (n = 3, data not shown).
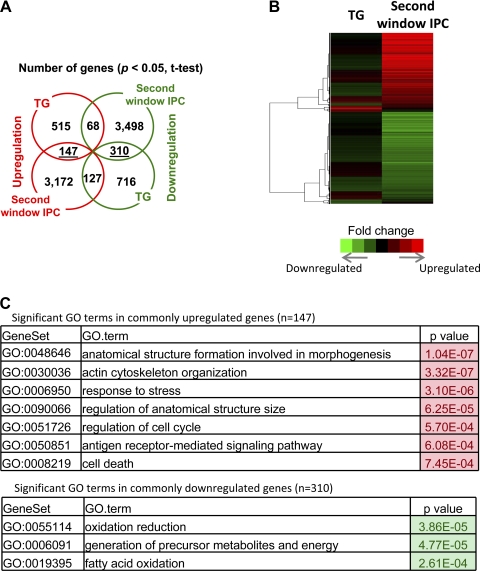
Similarities and differences in gene expression between α1A-AR overexpression and SWOP from microarray analysis.
Given the functional and mechanistic similarities between the cardioprotection observed with α1A-AR overexpression and that with SWOP, we investigated potential similarities in transcriptional responses by performing Affymetrix microarray analyses on RNA prepared from cardiomyocytes isolated from TGs, NTLs, and NTLs subjected to SWOP (n = 4 for each group). A heat map was generated from Affymetrix microarray data that compared changes in cardiomyocyte gene expression in NTLs subjected to SWOP vs. untreated NTLs, with gene changes in TGs vs. untreated NTLs. This showed highly significant changes in the expression of numerous genes in the NTLs subjected to SWOP, whereas in the TG group expression of far fewer genes was altered, although some similarities were noted (Fig. 6A). Compared with nonpreconditioned NTL cardiomyocytes, 3,172 genes were upregulated and 3,498 genes downregulated in the NTL SWOP group, whereas only 515 genes showed enhanced and 716 showed decreased expression in the TG cardiomyocytes (P < 0.05). Importantly, only 147 upregulated and 310 downregulated genes showed concordant regulation with both receptor overexpression and SWOP (Fig. 6B). Among the upregulated molecular and cellular functions, both α1A-AR overexpression and SWOP resulted in directionally similar alterations in the expression of a number of genes, such as those involved in cell cycle regulation, e.g., c-Fos and c-Jun, and stress responses, e.g., Cdkn1a and Gadd45 (Fig. 6C). The TG group also showed a greater increase in positive regulators of cellular metabolic process and transcription, such as insulin receptor substrate 1 and 2. In contrast, SWOP of NTLs did not enhance α1A-AR gene expression but altered the expression of a plethora of genes involved in processes such as responses to stress, cell proliferation, programmed cell death, signal transduction, and protein metabolic processes. Of interest, with SWOP, microarray profiling revealed a profound decrease in mitochondrial function with decreased expression of genes involved in complex I through complex V of the electron transport chain, while similar but less marked changes, involving only complex I to III, were observed in the TG group.
Fig. 6.
Affymetrix microarray studies of cardiomyocyte RNA prepared from α1A-TG rats and from second window preconditioned NTLs. A: heat map of gene expression (6.977 genes) profiling comparing TGs and second window preconditioned NTLs (in both cases relative to gene expression in untreated NTLs). Relative expression values (log ratios vs. control) were used for hierarchical clustering with Pearson correlation and the average linkage method and are represented according to the color scale shown in the graph. Cluster3.0 and Java TreeView were used for clustering and visualization, respectively. B: venn diagram comparing significantly regulated genes (P < 0.05) in untreated NTLs vs. TGs or in untreated NTLs vs. second window preconditioned NTLs. C: significant Biological Process terms associated with genes concordantly regulated in TGs and in second window preconditioned NTLs. P values are based on the hypergeometric test. Redundant Gene Ontology (GO) terms were eliminated.
Quantitative real-time reverse transcriptase polymerase chain reaction verification of genes related to cardiac protection.
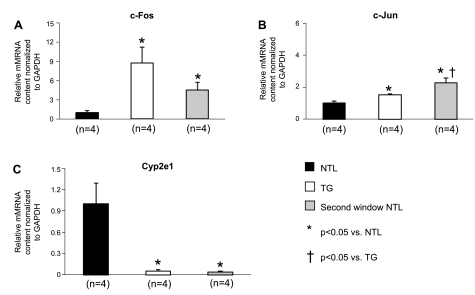
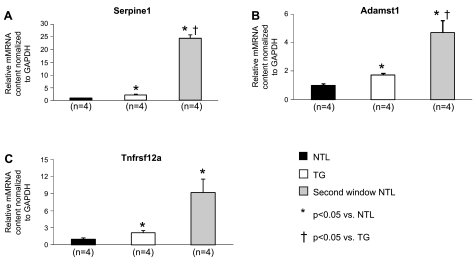
Quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) of cardiomyocyte RNA verified that the genes for Serpine1, c-Fos, c-Jun, and Tnfrsf12a, as well as the early immediate response gene Adamst 1, were all significantly upregulated in both the TG group and second window preconditioned NTL groups (P < 0.05 vs. NTL, n = 4) (Figs. 7 and 8), whereas the expression of the reactive oxygen species generator Cyp2e1 was significantly decreased in both TG and second window preconditioned NTL groups relative to its expression in untreated NTLs (P < 0.05, n = 4) (Fig. 7).
Fig. 7.
mRNA expression of various genes found to have altered expression by qRT-PCR. Statistical differences are indicated by the symbols, and the number of animals is shown beneath the bars. Values are means ± SE. c-Fos (A), c-Jun (B), and Cyp2e1 (C) mRNA expression levels (determined by qRT-PCR of cardiomyocyte RNA) were compared between untreated NTLs and TGs and between untreated NTLs and second window preconditioned NTLs. mRNA values were normalized to GAPDH and expressed relative to NTL levels.
Fig. 8.
mRNA expression of various genes found to have altered expression by qRT-PCR. Statistical differences are denoted by the symbols, and the number of animals is shown beneath the bars. Values are means ± SE. mRNA expression of Serpine1 (A), Adamst1 (B), and Tnfrsf12a (C) (determined by qRT-PCR of cardiomyocyte RNA) was compared between untreated NTLs and TGs and between untreated NTLs and second window preconditioned NTLs. mRNA values were normalized to GAPDH and expressed relative to NTL levels.
DISCUSSION
A novel feature of the current investigation was the use of a TG rat model of cardiac overexpression of the α1A-AR. This TG rat model exhibits marked improvements in systolic and diastolic cardiac function with a significant bradycardia at baseline. Thus, myocardial contractility was increased, as reflected by increases in LV +dP/dt, LVEF, and LV Ees. These changes are consistent with our previous findings in TG mice with cardiac-restricted α1A-AR overexpression (18). However, enhanced myocardial contractility was not observed in prior studies involving overexpression of a constitutively active mutant α1A-AR (25). Prior studies have also not found a decrease in heart rate; in fact, both prior studies in anesthetized mice in vivo (3, 18) and in isolated hearts (25) observed a tendency for heart rate to increase. Because the decreased heart rate we observed in the conscious rats is most likely autonomically mediated, the difference between our results and prior studies may be due either to the lack of anesthesia in this study, to the effects of the anesthetics used in the TG mice (3, 18), or to the lack of innervation of ex vivo hearts (25). Importantly, the rat model developed here allows ready determination of cardiac hemodynamics, including blood pressure and heart rate, in the conscious animal (Table 2).
Using this TG rat model, we show that, even in the absence of IPC, TG rats exhibit cardiac protection, and the magnitude and mechanisms of this protection are similar to that observed with SWOP of wild-type rats. Moreover, infarct size could not be further reduced by subjecting TG rats to SWOP. Taken together, these findings indicate that TG rats are already preconditioned through mechanisms similar to those mediating SWOP (Fig. 3D). This was in contrast to FWOP, which afforded additional cardioprotection, resulting in a greater reduction in infarct size in the TG rats. Based on previous studies showing that the α1-AR agonist phenylephrine can induce delayed IPC (16, 19, 31), we tested if enhanced α1-AR drive in the TG rats mediates SWOP. In these studies, animals were pretreated with the α1-AR blocker prazosin. Interestingly, although prazosin pretreatment did not alter infarct size in NTLs subjected to I/R (Fig. 3C), it completely abolished not only the protective effects of α1A-AR overexpression in TG rats but also that of SWOP in NTLs (Fig. 3D). These findings are of interest given the low myocardial expression of this AR (29) and suggest a critical role for α1A-AR signaling in the SWOP. Indeed, they are entirely consistent with an important prosurvival effect of cardiomyocyte α1A-AR signaling identified recently by Rorabaugh et al. (24) and Huang et al. (11) that appears to have direct relevance for the clinical management of heart disease in humans (12, 21).
Prior studies have not examined if α-AR expression is altered with IPC. In the current investigation, we were surprised to find that α1A-AR gene expression was actually reduced in NTLs subjected to IPC. Although this may be due to receptor downregulation associated with myocardial infarction-induced marked sympathetic activation, it was nonetheless not sufficient to completely abrogate receptor signaling, given the ability of prazosin to block SWOP in NTLs with native levels of cardiomyocyte α1A-AR expression.
Further evidence for the similarity between cardioprotection in the α1A-AR TG rat and second window protection in NTLs was the finding of upregulated iNOS expression, which, when blocked with l-NNA, eliminates the protection (16). This is exactly what we observed in the TG rats, where l-NNA completely blocked their cardioprotection in the absence of preconditioning (Fig. 3C). Prior studies have also demonstrated myocardial protection with pharmacological stimulation of α1-ARs (19, 31, 32), with overexpression of a constitutively active α1-AR mutant (25) and in TG mouse hearts (3). However, it is controversial if this protection is mediated by the α1A- (24, 25) or α1B- (5, 10, 14, 31) AR subtype. Importantly, the fact that increased α1A-AR drive phenocopies SWOP has not been reported previously and is a major finding of the current investigation. In addition, as indicated above, it could have direct clinical relevance (12) given the low levels of α1A-AR expression in the human heart (29), which may in fact facilitate increasing α1A-AR drive clinically to confer cardioprotection.
In our TG rats, p-MEK and p-ERK were both elevated compared with NTL rats, which could play a role in mediating their cardioprotection in TG animals. The cardioprotective role of MEK and ERK activation is well known (1, 4, 8, 17), and MEK/ERK signaling has been shown to be involved in both first (7, 13) and SWOP (1, 4, 13, 17). These signaling pathways inhibit apoptosis and lead to cell survival (8, 11). Furthermore, NOS activation by the MEK/ERK pathway is a downstream effector of cardiomyocyte α1A-AR signaling that can mediate receptor-coupled cardioprotective effects (9, 20, 23). As noted above, MEK/ERK activation protects the heart against ischemic stress (1, 4, 8, 17), and α1A-AR-mediated MEK/ERK activation has been shown to enhance cardiomyoctye survival following an ischemic stress in the isolated constitutively active α1-AR mutant mouse heart (24). Interestingly, Skyschally et al. (28) found that inhibition of MEK/ERK did not mediate the reduction in infarct size after ischemic postconditioning in pigs. One might conclude from that study (28) that there is a major species difference. However, this is not necessarily correct, since the MEK/ERK pathway has been shown to protect the ischemic heart both in pigs (30) and humans (27). The major difference among those studies is that our study and the studies showing a potential protective role for MEK/ERK focused on preconditioning (27, 30), whereas the one failing to show the protection was in a postconditioning model (28).
To evaluate a potential contribution of myocardial metabolic demands that may also affect infarct size, particularly the bradycardia of TG rats, which could, by itself, afford myocardial protection, since heart rate is a major determinant of MV̇o2 (2), we paced TG rat hearts to identical levels as in NTLs but found that this did not abolish myocardial protection in the TG rats. This was not entirely surprising, since heart rate is only one determinant of MV̇o2. Moreover, when the three major determinants of MV̇o2 were examined using LV triple product, this was found not to be different between TGs and NTLs, most likely because the marked increase in contractility is offset by the decreased heart rate in the TG.
We next compared Affymetrix microarrays of cardiomyocytes from the TG rat hearts without IPC with those from NTL rat hearts subjected to SWOP. Interestingly, the gene for iNOS was not upregulated in TG rat hearts even though these animals demonstrated SWOP and the ischemic protection in TGs was blocked by l-NNA. These findings are consistent with the regulation of the iNOS being predominantly via posttranscriptional mechanisms (15).
We also observed that several genes, which are also involved in cardioprotection, such as Fos, Jun, Gdf15, and Tnfrsf12a, were regulated similarly in both models, although in some cases to different levels (Figs. 7 and 8). Furthermore, decreased mitochondrial function was more extensive with SWOP involving decreased expression of the genes involved in complex I through complex V of the electron transport chain compared with only complex I through complex III in the TG group.
Despite the similarities between SWOP and TG rats, it is of interest that only 4.6% of 3,172 genes upregulated and 8.8% of 3,498 genes downregulated in NTLs subjected to SWOP relative to untreated NTLs showed similar directional changes to those resulting from α1A-AR overexpression alone. Indeed, only 28% of genes were upregulated and 43% of genes downregulated in common in the TGs (Fig. 6B).
In summary, 1) in conscious α1A-TG rats, heart rate is reduced compared with NTLs while myocardial contractility is significantly enhanced; 2) infarct size in response to I/R was significantly reduced; 3) cardioprotection in TG rats phenocopied second, but not first, window preconditioning and was mediated by iNOS activation, and 4) transcriptionally, SWOP is more complex than α1A-TG preconditioning, with the alteration of thousands of additional genes affording no further protection than already available in the TG rats with α1A-AR overexpression.
GRANTS
This study was funded by National Institutes of Health Grants 5P01HL-069020, 5R01HL-033107, 5P01AG-027211, 1R01HL-102472, 5T32HL-069752, 5R01HL-095888, 5R01HL-091781, 5R01HL-093415 and 5R01HL-093481, National Health and Medical Research Council of Australia Grants 526622 and 573732, and a grant from the Heart Foundation of Australia (G0954342).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.Z., D.E.V., R.M.G., and S.F.V. conception and design of research; X.Z., S.G., H.G., S.I., L.L., and B.T. performed experiments; X.Z., J.P., D.H., S.G., L.Y., H.G., and B.T. analyzed data; X.Z., D.H., S.G., L.Y., D.E.V., R.M.G., and S.F.V. interpreted results of experiments; X.Z. prepared figures; X.Z., D.H., and S.G. drafted manuscript; X.Z., D.H., L.Y., B.T., D.E.V., R.M.G., and S.F.V. edited and revised manuscript; X.Z., D.E.V., R.M.G., and S.F.V. approved final version of manuscript.
REFERENCES
- 1. Bolli R. The late phase of preconditioning. Circ Res 87: 972–983, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther 308: 236–240, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Du XJ, Gao XM, Kiriazis H, Moore XL, Ming Z, Su Y, Finch AM, Hannan RA, Dart AM, Graham RM. Transgenic alpha1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res 71: 735–743, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Fryer RM, Hsu AK, Gross GJ. ERK and p38 MAP kinase activation are components of opioid-induced delayed cardioprotection. Basic Res Cardiol 96: 136–142, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Gao H, Chen L, Yang HT. Activation of alpha1B-adrenoceptors alleviates ischemia/reperfusion injury by limitation of mitochondrial Ca2+ overload in cardiomyocytes. Cardiovasc Res 75: 584–595, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Gao XM, Wang BH, Woodcock E, Du XJ. Expression of active alpha(1B)-adrenergic receptors in the heart does not alleviate ischemic reperfusion injury. J Mol Cell Cardiol 32: 1679–1686, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288: H971–H976, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 61: 448–460, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 70: 240–253, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Hu K, Nattel S. Mechanisms of ischemic preconditioning in rat hearts. Involvement of alpha 1B-adrenoceptors, pertussis toxin-sensitive G proteins, and protein kinase C. Circulation 92: 2259–2265, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O'Connell TD. An alpha1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation 115: 763–772, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Jensen BC, O'Connell TD, Simpson PC. Alpha-1-adrenergic receptors: targets for agonist drugs to treat heart failure. J Mol Cell Cardiol 51: 518–528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joo JD, Kim M, Horst P, Kim J, D'Agati VD, Emala CW, Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol 293: F1847–F1857, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Kariya T, Minatoguchi S, Ohno T, Yamashita K, Uno Y, Arai M, Koshiji M, Fujiwara T, Fujiwara H. Infarct size-reducing effect of ischemic preconditioning is related to alpha1b-adrenoceptors but not to alpha1a-adrenoceptors in rabbits. J Cardiovasc Pharmacol 30: 437–445, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Korhonen R, Linker K, Pautz A, Forstermann U, Moilanen E, Kleinert H. Post-transcriptional regulation of human inducible nitric-oxide synthase expression by the Jun N-terminal kinase. Mol Pharmacol 71: 1427–1434, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Kudej RK, Shen YT, Peppas AP, Huang CH, Chen W, Yan L, Vatner DE, Vatner SF. Obligatory role of cardiac nerves and alpha1-adrenergic receptors for the second window of ischemic preconditioning in conscious pigs. Circ Res 99: 1270–1276, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Lasley RD, Keith BJ, Kristo G, Yoshimura Y, Mentzer RM., Jr Delayed adenosine A1 receptor preconditioning in rat myocardium is MAPK dependent but iNOS independent. Am J Physiol Heart Circ Physiol 289: H785–H791, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Lin F, Owens WA, Chen S, Stevens ME, Kesteven S, Arthur JF, Woodcock EA, Feneley MP, Graham RM. Targeted alpha(1A)-adrenergic receptor overexpression induces enhanced cardiac contractility but not hypertrophy. Circ Res 89: 343–350, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Naderi R, Imani A, Faghihi M. Phenylephrine produces late pharmacological preconditioning in the isolated rat heart. Eur J Pharmacol 627: 203–208, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Nandagopal K, Dawson TM, Dawson VL. Critical role for nitric oxide signaling in cardiac and neuronal ischemic preconditioning and tolerance. J Pharmacol Exp Ther 297: 474–478, 2001 [PubMed] [Google Scholar]
- 21. O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, Simpson PC. Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest 116: 1005–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park M, Shen YT, Gaussin V, Heyndrickx GR, Bartunek J, Resuello RR, Natividad FF, Kitsis RN, Vatner DE, Vatner SF. Apoptosis predominates in nonmyocytes in heart failure. Am J Physiol Heart Circ Physiol 297: H785–H791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Philipp S, Critz SD, Cui L, Solodushko V, Cohen MV, Downey JM. Localizing extracellular signal-regulated kinase (ERK) in pharmacological preconditioning's trigger pathway. Basic Res Cardiol 101: 159–167, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Rorabaugh BR, Handel EM, Waterson RE, Talbot JN, Perez DM. Alpha 1A-adrenergic receptor signaling protects the heart from ischemic injury through an ERK dependent mechanism. FASEB J 22: 1130–1137, 2008 [Google Scholar]
- 25. Rorabaugh BR, Ross SA, Gaivin RJ, Papay RS, McCune DF, Simpson PC, Perez DM. alpha1A- but not alpha1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrine-insensitive mechanism. Cardiovasc Res 65: 436–445, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 27. Sivaraman V, Mudalagiri NR, Di Salvo C, Kolvekar S, Hayward M, Yap J, Keogh B, Hausenloy DJ, Yellon DM. Postconditioning protects human atrial muscle through the activation of the RISK pathway. Basic Res Cardiol 102: 453–459, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104: 15–18, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Steinfath M, Chen YY, Lavicky J, Magnussen O, Nose M, Rosswag S, Schmitz W, Scholz H. Cardiac alpha 1-adrenoceptor densities in different mammalian species. Br J Pharmacol 107: 185–188, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strohm C, Barancik T, Bruhl ML, Kilian SA, Schaper W. Inhibition of the ER-kinase cascade by PD98059 and UO126 counteracts ischemic preconditioning in pig myocardium. J Cardiovasc Pharmacol 36: 218–229, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Tejero-Taldo MI, Gursoy E, Zhao TC, Kukreja RC. Alpha-adrenergic receptor stimulation produces late preconditioning through inducible nitric oxide synthase in mouse heart. J Mol Cell Cardiol 34: 185–195, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Tosaki A, Behjet NS, Engelman DT, Engelman RM, Das DK. Alpha-1 adrenergic receptor agonist-induced preconditioning in isolated working rat hearts. J Pharmacol Exp Ther 273: 689–694, 1995 [PubMed] [Google Scholar]
- 33. Xin Zhao D, Ho S, Gao C, Hong D, Vatner DE, Vatner SF. Arterial pressure monitoring in mice. Curr Protocols Mouse Biol 1: 105–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol 44: 1103–1110, 2004 [DOI] [PubMed] [Google Scholar]