Abstract
The purpose of this echocardiography study was to measure peak coronary blood flow velocity (CBVpeak) and left ventricular function (via tissue Doppler imaging) during separate and combined bouts of cold air inhalation (−14 ± 3°C) and isometric handgrip (30% maximum voluntary contraction). Thirteen young adults and thirteen older adults volunteered to participate in this study and underwent echocardiographic examination in the left lateral position. Cold air inhalation was 5 min in duration, and isometric handgrip (grip protocol) was 2 min in duration; a combined stimulus (cold + grip protocol) and a cold pressor test (hand in 1°C water) were also performed. Heart rate, blood pressure, O2 saturation, and inspired air temperature were monitored on a beat-by-beat basis. The rate-pressure product (RPP) was used as an index of myocardial O2 demand, and CBVpeak was used as an index of myocardial O2 supply. The RPP response to the grip protocol was significantly blunted in older subjects (Δ1,964 ± 396 beats·min−1·mmHg) compared with young subjects (Δ3,898 ± 452 beats·min−1·mmHg), and the change in CBVpeak was also blunted (Δ6.3 ± 1.2 vs. 11.2 ± 2.0 cm/s). Paired t-tests showed that older subjects had a greater change in the RPP during the cold + grip protocol [Δ2,697 ± 391 beats·min−1·mmHg compared with the grip protocol alone (Δ2,115 ± 375 beats·min−1·mmHg)]. An accentuated RPP response to the cold + grip protocol (compared with the grip protocol alone) without a concomitant increase in CBVpeak may suggest a dissociation between the O2 supply and demand in the coronary circulation. In conclusion, older adults have blunted coronary blood flow responses to isometric exercise.
Keywords: blood pressure, heart rate, sympathetic nervous system, oxygen consumption, tissue Doppler imaging
healthy aging is accompanied by several morphological and functional changes in the cardiovascular system. Specifically, older adults have elevated plasma catecholamines, higher resting blood pressure, and decreased cardiovagal baroreflex sensitivity compared with their young counterparts (8, 32, 41). In response to stress, these age-related changes may predispose older adults to adverse cardiac events. A better understanding of the factors that trigger cardiac ischemia in older adults may reduce the morbidity and mortality seen in this population.
Isometric exercise (e.g., static handgrip) activates the sympathetic nervous system and causes increases in heart rate (HR) and blood pressure in an attempt to increase O2 delivery to the working muscle. While it is clear that aging blunts the HR response to isometric handgrip (34), blood pressure responses are less consistent between young and older subjects. Furthermore, the effects of aging on coronary blood flow velocity (CBVpeak) and myocardial function have not been determined. It is important to study coronary blood flow responses to stress because acute exertion can trigger adverse events (3). If myocardial O2 demand [as assessed by the rate-pressure product (RPP)] outpaces myocardial O2 supply (CBVpeak), ischemia could predispose the person to chest discomfort, arrhythmias, and/or severe shortness of breath. Of note, older adults have blunted coronary hyperemic responses to both dipyridamole and skin surface cooling (6, 12, 45). Handgrip exercise [30% maximum voluntary contraction (MVC) for 2 min] causes coronary hyperemia in young people (33), and it is possible that a reduced hyperemia to a similar mechanical stimulus may be clinically meaningful in older adults.
Epidemiological studies (42, 47) have demonstrated that cardiovascular mortality peaks in the winter months, and inhalation of cold air might be a contributing factor. Cold air inhalation (cold air protocol) has been shown to increase muscle sympathetic nerve activity (MSNA) (19) and impair CBVpeak (33) in young adults, but it is unclear how aging might affect the CBVpeak response to this stimulus. Furthermore, combined isometric muscular work during cold air breathing (cold + grip protocol) is often confirmed by history in patients presenting with acute coronary events (i.e., snow shoveling) (9). Many of these patients are elderly (16). In response to the cold + grip protocol, the ratio of coronary supply to demand (i.e., the slope of the line: ΔCBVpeak/ΔRPP) would likely be attenuated compared with isometric handgrip (grip protocol) alone.
The purpose of this study was to determine how healthy aging affects the hemodynamic, coronary, and left ventricular function response to isometric handgrip and cold air inhalation. We hypothesized that compared with young adults, 1) older adults would have an attenuated CBV response to the cold air protocol, 2) older adults would have an attenuated CBV response to the grip protocol, and 3) older adults would have an attenuated CBV response to the cold + grip protocol.
METHODS
Subjects.
All study protocols were approved by the Institutional Review Board of the Penn State Milton S. Hershey Medical Center and conformed with the Declaration of Helsinki. In total, 31 subjects (17 young subjects and 14 older subjects) were recruited to participate in this study and provided written informed consent. Three individuals (one older man, one young man, and one young woman) did not meet the inclusion criteria and were excluded from further participation. One young woman and one young man also withdrew for personal reasons. The remaining subjects (6 young men, 7 young women, 6 older men, and 7 older women) were all normotensive, nonasthmatic, nonobese, nonsmokers, and not taking any medication and were in good health as determined by history and physical examination. Four of the young women were taking oral contraceptives. All of the older women were postmenopausal; none were on hormone replacement therapy. Most of the older subjects had worked in the education (n = 5), engineering (n = 4), and business (n = 3) fields, and approximately half of the subjects were retired at the time of this study. The younger subjects included two hospital employees, seven students, and four people whose work was mostly sedentary. All subjects reported being physically active but none were competitive athletes. After the consent process but before enrollment, the older adults underwent a modified Bruce protocol with 12-lead ECG monitoring. The test was then interpreted by a cardiologist to rule out coronary artery disease. All subjects refrained from caffeine, alcohol, and exercise for 24 h before the study and arrived to the laboratory in a semifasted state (i.e., 4–6 h after their last meal). It should be noted that data from 10 of the young subjects have been included in a previous report (33).
Experimental protocols.
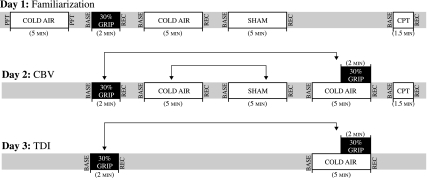
This study used a two treatment (cold inspired air vs. neutral inspired air temperature) by multiple time point (baseline, 1 min, 2 min, etc.) repeated-measures design with age (young, older) serving as a between-subjects factor. Consistent with our previous report (33), all subjects underwent a familiarization visit and then returned for two experimental trials on separate days [CBV visit and tissue Doppler imaging (TDI) visit]. In total, four isometric handgrip trials were performed (Fig. 1). Since TDI uses a different transducer (M4S) than CBV (7S) and since peak exercise responses must be captured within a 10- to 15-s window, we chose to bring subjects back to the laboratory instead of attempting to acquire all data in the same session. All CBV experiments (Fig. 1) were conducted in the left lateral position. MVC of the right hand was determined for all subjects during the familiarization visit. Thirty percent of this MVC was then calculated and used in all subsequent visits. The exercise bouts (grip and cold + grip protocols) were performed in a counterbalanced fashion and were separated by ∼1 h to minimize fatigue (37). The 30% MVC grip was always performed for the last 2 min of the 5-min cold + grip protocol protocol. To provide visual feedback to the subject, a handgrip dynamometer was interfaced to a custom device using an analog meter display. The cold air protocol and the neutral air (sham) protocol were each 5 min in duration and were counterbalanced. The cold pressor test (immersion of the hand into ice water, a cold stimulus that activates the sympathetic nervous system) was always performed last. On the TDI visit, only the grip and cold + grip protocols were performed. Throughout the experiment, the room temperature was 22–25°C.
Fig. 1.
Experimental timeline. All subjects underwent a 2-min bout of isometric handgrip at 30% maximal voluntary contraction (MVC; grip protocol), 5 min of cold air inhalation (cold air protocol), 5 min of thermoneutral air inhalation (sham protocol), and 5 min of cold air inhalation with a 2-min bout of isometric handgrip during minutes 4 and 5 (cold + grip protocol). The arrows signify that connected protocols were counterbalanced. The cold pressor test (CPT; hand in 1°C water) was always performed last. Coronary blood flow velocity (CBV; 7S transducer) was obtained on day 2; tissue Doppler imaging (TDI; M4S transducer) was obtained on day 3. PFT, pulmonary function test; Base, baseline; Rec, recovery.
Cold air breathing apparatus.
A custom system was used to deliver cold or neutral air to the subjects, as previously described (33). Briefly, a closed loop of copper coil was placed in an insulated box that was either empty (sham) or filled with liquid nitrogen (cold air protocol). Compressed medical air was attached to the distal end of the copper coil system, and subjects were encouraged to breathe normally. Breathing rate and air temperature were measured in real time using thermistors (TC-2000, Sable Systems) situated in the mouthpiece.
Measurements.
Blood pressure was recorded on a beat-to-beat basis from a finger via photoplethysmography (Finometer, FMS). Systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) from the Finometer were each corrected to match the blood pressure obtained with an automated sphygmomanometer (Philips SureSigns Vs3). A standard three-lead ECG (Cardiocap/5, General Electric Healthcare) was used to monitor HR. Basic pulmonary function was determined before and after cold air breathing with a MiniSpir device (Medical International Research). The variables of interest included forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC). Arterial O2 saturation was monitored by pulse oximetry on the finger (Philips SureSigns VM4). Ratings of thermal sensation of the body (where 1 = cold and 7 = hot) (10), perception of mouth coldness (where 0 = neutral/no sensation of cold and 11 = unbearably cold) (13), perception of mouth and hand pain (where 0 = no pain and 10 = unbearable pain) (18), and perceived exertion in the hand and forearm at the end of exercise (where 6 = very, very light and 20 = maximal exertion) (5) were also quantified.
Transthoracic echocardiography was performed using a commercially available system (Vivid 7, General Electric Healthcare) with an M4S probe. Left atrial diameter was measured in the parasternal long-axis view, and M-mode imaging was then obtained as previously described (40). Left ventricular end-diastolic diameter and end-systolic diameter were measured using Pro Solv 3.0, and fractional shortening was calculated. Pulsed Doppler transmitral early and late diastolic flow velocities were also measured, and the early-to-late diastolic flow velocity ratio was derived (35). We attempted to measure tricuspid regurgitation as a way to noninvasively assess right ventricular pressure (49), but this was only possible in ∼20% of our subjects.
CBV, an index of myocardial O2 supply, was obtained from the apical four-chamber view using the above system. The specific procedures used in our laboratory have been previously described (11, 30, 31). Briefly, a variable frequency phased-array transducer (7S) was positioned to explore the left ventricular apex. The imaging depth was set at 5 cm, and the focal zones were set at ∼2–3 cm. Color flow mapping was used, and the two-dimensional gain was adjusted to obtain the best blood flow signal of the left anterior descending coronary artery (LAD). Once this was obtained, a 2.0-mm sample volume was placed over the color signal, and CBV was recorded at end expiration. The transducer was held still throughout the protocols, and care was taken to obtain at least one 3-beat clip at the end of each minute. The Doppler tracing of the diastolic portion of each cardiac cycle was analyzed using Pro Solv 3.0 to obtain CBVpeak, the velocity time integral (VTI), flow duration, and relative perfusion time (also called coronary perfusion time fraction, flow duration/R-R interval) as previously described (33). CBVpeak is the most widely used measurement in the literature, but all these coronary flow parameters are considered indexes of myocardial O2 supply. Because of the limited spatial resolution and small vessel size, we did not attempt to measure LAD diameter. However, it has been documented that the percent increase in CBV measured via transthoracic Doppler echocardiography is similar to the percent increase in CBV measured by an intracoronary Doppler guidewire (30). Furthermore, intracoronary Doppler guidewire measurements of the percent increase in CBV significantly correlate with the percent increase in coronary blood flow (38).
TDI was used to measure myocardial tissue velocities during the last 15 s of each minute during the protocols. These velocities were obtained with the M4S transducer in the apical four-chamber view and the sampling volume at the septal mitral annular region. Measurements included the systolic myocardial velocity and early and late diastolic myocardial velocities at the mitral annulus at the end of the respiration. The TDI technique is relatively insensitive to changes in loading condition (1) and is able to detect both systolic and diastolic dysfunction within 5 s after an acute reduction in LAD blood flow (7) as well as impaired function in patients with myocardial infarction (2).
Data collection and statistical analysis.
Blood pressure, HR, air temperature, and grip workload were sampled at 200 Hz by a data-acquisition system (MacLab, AD Instruments). These data were collected on a beat-by-beat basis, and the final 15–20 s of each minute were averaged and reported (i.e., to match when CBV and TDI were obtained). The RPP, an index of myocardial O2 demand, was calculated as HR × SBP. Within the context of this noninvasive study, we chose RPP as an index of myocardial O2 demand because it can be obtained continuously regardless of the transducer being used. For each of the protocols, changes from baseline [change (Δ) = peak response − baseline] were calculated for SBP, DBP, MAP, HR, RPP, and CBVpeak. To characterize coronary responses for each individual subject, the ratio of coronary supply to demand was calculated (i.e., the slope of the line: ΔCBVpeak/ΔRPP).
All statistical analyses were conducted using IBM SPPS 19.0, and graphics were produced using Microsoft Excel and Adobe Illustrator CS5. Normality was confirmed by the Kolmogorov-Smirnov test (i.e., P > 0.05 for all physiological measurements). When the assumption of sphericity was violated, the Greenhouse-Geisser adjustment was used. For the first hypothesis, a two treatment (cold air protocol, sham protocol) by six time point (baseline, 1 min, 2 min, 3 min, 4 min, and 5 min) repeated-measures ANOVA was conducted for each dependent variable using SPPS General Linear Model. Age was entered as a between-subjects factor. There were no three-way interactions for any variable, so data presentation instead focused on between-groups differences across time (followed up with independent samples t-tests when appropriate). For hypotheses 2 and 3, separate three time point (baseline, 1 min, and 2 min) repeated-measures ANOVAs were conducted for each dependent variable, and age was again entered as a between-subjects factor. When a significant main effect was found, post hoc comparisons were made with independent-samples t-tests. Independent t-tests were also used to determine if the supply-to-demand ratio was different between groups in response to the cold air, grip, and cold + grip protocols. To further explore the effect of sex, a one-way ANOVA (young men, young women, older men, and older women) was conducted for ΔSBP, ΔDBP, ΔMAP, ΔHR, ΔRPP, ΔCBVpeak, and the coronary supply-to-demand ratio. Within each group (young subjects alone and older subjects alone), paired t-tests were used to compare the effect of inspired air temperature on the supply-to-demand ratio. The Mann-Whitney nonparametric test was used to determine group differences in the perceptual variables (ratings of thermal sensation, pain, and perceived exertion). Data are presented as means ± SE. P values of <0.05 were considered statistically significant.
RESULTS
The participants in this study were similar with regard to baseline body size (Table 1). Men were taller and heavier on average than women. MVC was very similar in young subjects (29 ± 2 kg) and older subjects (29 ± 3 kg), with men having higher MVC values than women (Table 2). None of the subjects had wall motion abnormalities, and neither left ventricular chamber size or wall thickness differed between groups. Pulmonary function was unchanged by the cold air protocol in both young subjects (ΔFEV1: 0.13 ± 0.16 liters and ΔFVC: 0.15 ± 0.18 liters) and older subjects (ΔFEV1: −0.02 ± 0.10 liters and ΔFVC: 0.13 ± 0.16 liters), but it should be noted that older adults had smaller lung volumes at baseline (Table 1). There were no detectable group differences in O2 saturation or inspired air temperature (young group: −14 ± 4°C and older group: −13 ± 3°C at 5 min of the cold air protocol). Considering the resting physiological data collected during this study, there were no significant effects of sex, so men and women were analyzed together.
Table 1.
Baseline anthropometric measurements and resting hemodynamics
| Young Group | Older Group | |
|---|---|---|
| Age, yr | 25 ± 1 | 63 ± 1† |
| Height, m | 1.75 ± 0.22 | 1.71 ± 0.20 |
| Weight, kg | 73 ± 3 | 75 ± 3 |
| Body mass index, kg/m2 | 23.7 ± 0.8 | 25.0 ± 0.7 |
| Forced expiratory volume in 1 s, liters | 4.17 ± 0.26 | 3.02 ± 0.21† |
| Forced vital capacity, liters | 5.33 ± 0.33 | 4.10 ± 0.25† |
| SBP, mmHg | 100 ± 2 | 113 ± 3† |
| DBP, mmHg | 57 ± 2 | 73 ± 3† |
| MAP, mmHg | 72 ± 2 | 86 ± 3† |
| HR, beats/min | 56 ± 2 | 56 ± 2 |
| Left atrial diameter, cm | 2.7 ± 0.1 | 3.1 ± 0.1* |
| Fractional shortening, % | 35 ± 3 | 35 ± 2 |
| E, cm/s | 92.4 ± 4.2 | 69.1 ± 3.9† |
| A, cm/s | 45.7 ± 2.9 | 58.1 ± 4.0* |
| E-to-A ratio | 2.06 ± 0.16 | 1.24 ± 0.10† |
Values are means ± SE; n = 13 subjects/group (7 women and 6 men). SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; E, early diastolic mitral inflow; A, late diastolic mitral inflow.
P < 0.05 between groups;
P < 0.001 between groups.
Table 2.
Hemodynamic responses to the grip and cold + grip protocols across age and sex
| Young Men | Young Women | Older Men | Older Women | |
|---|---|---|---|---|
| Sample size | 6 | 7 | 6 | 7 |
| Maximum voluntary contraction, kg | 32 ± 3 | 26 ± 3 | 38 ± 3 | 22 ± 3 |
| Grip protocol | ||||
| Workload, kg | 9.6 ± 0.9 | 7.8 ± 1.0 | 12.1 ± 1.0 | 7.3 ± 0.5 |
| ΔSBP, mmHg | 29 ± 5 | 19 ± 3 | 25 ± 3 | 21 ± 4 |
| ΔDBP, mmHg | 20 ± 2 | 19 ± 2 | 13 ± 2 | 15 ± 5 |
| ΔMAP, mmHg | 26 ± 3 | 23 ± 2 | 21 ± 4 | 18 ± 4 |
| ΔHR, beats/min | 12 ± 5 | 15 ± 2 | 7 ± 3 | 7 ± 2 |
| ΔRPP, beats·min−1·mmHg | 3,203 ± 944 | 2,872 ± 463 | 2,369 ± 560 | 2,167 ± 540 |
| ΔCBVpeak, cm/s | 7.8 ± 2.5 | 14.1 ± 2.8 | 7.9 ± 1.2 | 4.7 ± 1.7 |
| Supply-to-demand ratio | 2.7 ± 0.8 | 5.4 ± 1.0 | 4.2 ± 1.0 | 4.3 ± 1.8 |
| RPE | 14–17 | 14–17 | 13–17 | 11–17 |
| Cold + grip protocol | ||||
| Workload, kg | 9.6 ± 0.9 | 7.6 ± 0.9 | 11.9 ± 1.0 | 7.0 ± 0.7 |
| ΔSBP, mmHg | 27 ± 8 | 24 ± 5 | 35 ± 2 | 23 ± 4 |
| ΔDBP, mmHg | 21 ± 3 | 20 ± 3 | 16 ± 4 | 18 ± 6 |
| ΔMAP, mmHg | 25 ± 3 | 24 ± 3 | 25 ± 4 | 21 ± 5 |
| ΔHR, beats/min | 23 ± 4 | 18 ± 5 | 8 ± 2 | 6 ± 3 |
| ΔRPP, beats·min−1·mmHg | 4,276 ± 652 | 4,051 ± 889 | 3,334 ± 407 | 2,049 ± 582 |
| ΔCBVpeak, cm/s | 6.8 ± 2.9 | 6.9 ± 3.3 | 6.4 ± 1.5 | 4.5 ± 1.4 |
| Supply-to-demand ratio | 1.7 ± 0.7 | 1.3 ± 0.8 | 2.3 ± 0.7 | 3.5 ± 1.2 |
| RPE | 15–18 | 13–17 | 14–17 | 12–19 |
Physiological values are presented as changes (Δ) from the respective baseline; means ± SE. RPP, rate-pressure product; CBVpeak, peak of the coronary blood flow velocity trace. The supply-to-demand ratio is ΔCBVpeak/ΔRPP and is expressed in arbitrary units (actual units are in the text). The rating of perceived exertion (RPE) is presented as a range (where 6 = very, very light to 20 = maximal exertion).
Hypothesis 1: response to the cold air protocol.
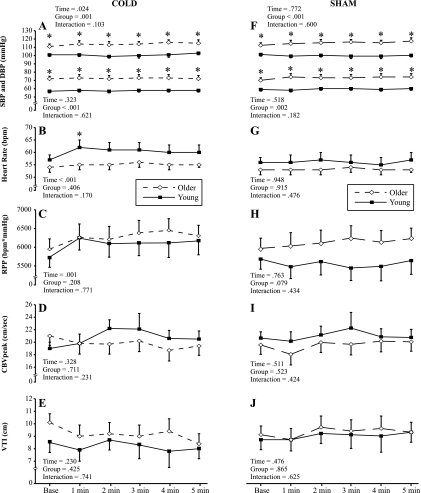
There were no significant three-way interactions, so Fig. 2 was constructed to separately show the cold air protocol (A–E) and sham protocol (F–J) with respect to group differences; the main effects and interactions are shown. Baseline SBP and DBP were significantly different between groups (P < 0.001), and these differences were maintained across time for both the cold air (Fig. 2A) and sham (Fig. 2F) protocols. Irrespective of group, the cold air protocol caused a significant increase in myocardial O2 demand (i.e., SBP, HR, and RPP, Fig. 2, A–C) across time. There was no detectable compensatory response in myocardial O2 supply (i.e., CBVpeak and VTI; Fig. 2, D and E). Flow duration also did not change across time and was comparable between groups at baseline (young group: 631 ± 43 ms vs. older group: 676 ± 33 ms) and 5 min of the cold air protocol (young group: 562 ± 44 ms vs. older group: 608 ± 41 ms). Similar results were found for relative perfusion time at baseline (young group: 59 ± 3% vs. older group: 58 ± 2%) and 5 min of the cold air protocol (young group: 57 ± 2% vs. older group: 54 ± 3%). The sham protocol had no significant effect on any of the measured variables across time.
Fig. 2.
Systemic hemodynamic and CBV responses to cold air inhalation (cold air protocol; A–E) and neutral air inhalation (sham protocol; F–J) in older (n = 11) and young (n = 12) subjects. A and F: systolic blood pressure (SBF) and diastolic blood pressure (DBP). B and G: heart rate [HR; in beats/min (bpm)]. C and H: rate-pressure product (RPP). D and I: peak of the CBV trace (CBVpeak). E and J: velocity time integral (VTI). *P < 0.05 between groups.
Hypothesis 2: response to the grip protocol.
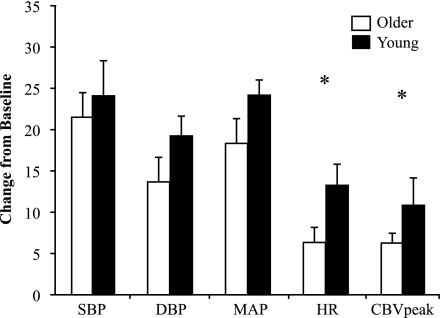
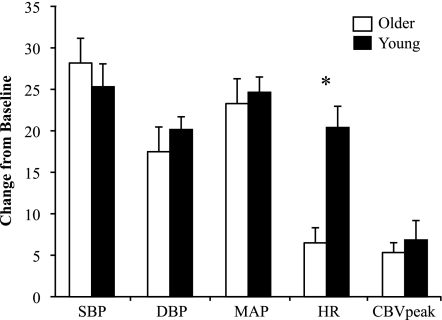
As shown in Fig. 3, SBP, DBP, and MAP responses to a 2-min bout of isometric handgrip at 30% MVC tended to be lower in older subjects compared with young subjects, but these comparisons did not reach statistical significance. The HR response was significantly blunted in older subjects (Δ: 7 ± 2 beats/min) compared with young subjects (Δ: 13 ± 2 beats/min, P = 0.033). The change in CBVpeak was also significantly blunted in the older group (Δ: 6.3 ± 1.2 vs. 11.2 ± 2.0 cm/s, P = 0.049), but the change in VTI (young group: 1.0 ± 1.2 cm vs. older group: 1.0 ± 0.5 cm) and flow duration (young group: −182 ± 62 ms vs. older group: −71 ± 38 ms) did not show group differences. Furthermore, the supply-to-demand ratio was not different between young and older subjects (4.15 ± 0.99 vs. 4.23 ± 0.75 × 10−3 cm·s−1·mmHg−1·beats·min−1; see Fig. 6A), and this ratio also did not differ among men and women (3.42 ± 0.66 vs. 4.91 ± 0.99 × 10−3 cm·s−1·mmHg−1·beats·min−1). As shown in Table 2, there were also no differences in grip workload or ratings of perceived exertion between ages.
Fig. 3.
Systemic hemodynamic and CBV responses to a 2-min bout of isometric handgrip at 30% MVC (grip protocol) in older (n = 13) and young (n = 13) subjects. All dependent variables are plotted on the same axis; units are mmHg for SBP, DBP, and mean arterial pressure (MAP), bpm for HR, and cm/s for CBVpeak. *P < 0.05 between groups.
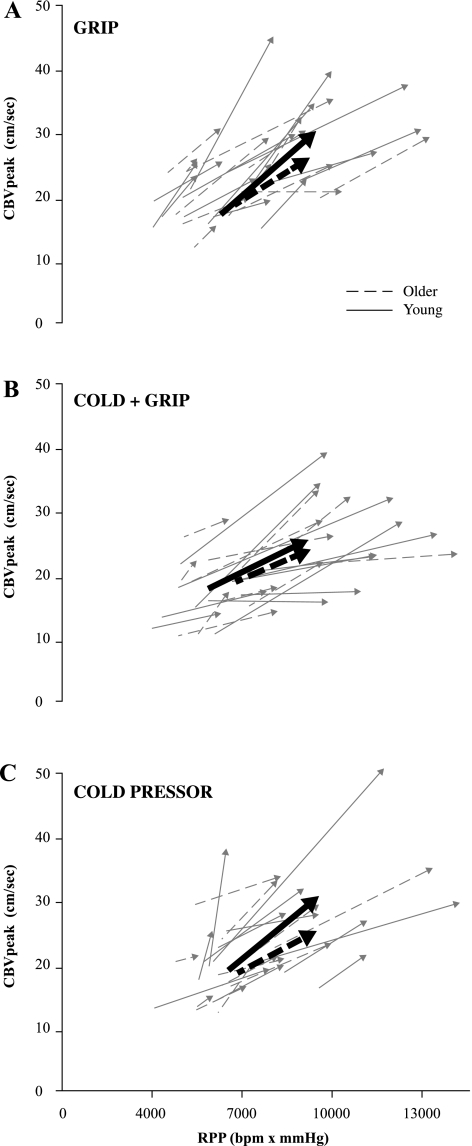
Fig. 6.
Individual coronary O2 demand (RPP, x-axis) and coronary O2 supply (CBVpeak, y-axes) responses to a 2-min bout of isometric handgrip at 30% MVC (grip protocol; A), 5 min of cold air inhalation with a 2-min bout of isometric handgrip during minutes 4 and 5 (cold + grip protocol; B) and a 90-s hand immersion in 1°C water protocol (CPT; C). The arrows point from baseline to the end of the interventions (peak responses). Young and older subjects are plotted together. The bold lines represent group averages. A large increase in RPP coupled with a small increase in CBVpeak (a shallower sloped line) is considered to be attenuated coronary hyperemia.
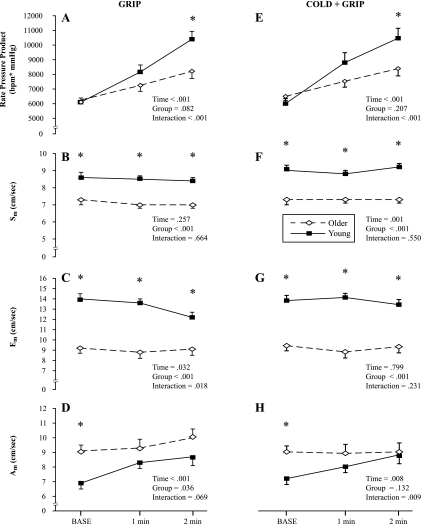
Separate repeated-measures ANOVAs revealed age-related differences in myocardial function (Fig. 4, A–D), coincident with a blunted RPP response. Systolic and early diastolic myocardial velocities were impaired in older adults at all time points (Fig. 4, B and C), whereas late diastolic velocity only showed baseline differences between groups (Fig. 4D).
Fig. 4.
Myocardial function was measured by TDI at baseline, 1 min, and 2 min of both the grip protocol (A–D) and cold + grip protocol (E–H). Systolic velocity (Sm), early diastolic velocity (Em), and late diastolic velocity (Am) were obtained in 10 older subjects and 11 young subjects. A and E: RPP. B and F: Sm. C and G: Em. D and H: Am. *P < 0.05 between groups at specific time points.
Hypothesis 3: response to the cold + grip protocol.
Similar to the grip protocol, older adults had blunted HR responses to the cold + grip protocol (Fig. 5), but the change in CBVpeak was not significantly different between groups (P = 0.595). The change in VTI (Δ: −0.9 ± 0.5 cm in the young group vs. 0.5 ± 0.7 cm in the older group) and flow duration (Δ: −271 ± 51 ms in the young group vs. −38 ± 71 ms in the older group) also did not show group differences. Furthermore, the supply-to-demand ratio was not different between young and older subjects (1.49 ± 0.48 vs. 2.84 ± 0.67 × 10−3 cm·s−1·mmHg−1·beats·min−1; Fig. 6B), and this ratio also did not differ among men and women (1.96 ± 0.46 vs. 2.32 ± 0.75 × 10−3 cm·s−1·mmHg−1·beats·min−1). TDI-derived measurements of myocardial function during the cold + grip protocol showed similar responses to the grip protocol protocol such that systolic and early diastolic velocities were slower in healthy older adults at all time points (Fig. 4, F and G), whereas the last diastolic velocity measurement was only different at baseline (Fig. 4H).
Fig. 5.
Systemic hemodynamic and CBV responses to a 2-min bout of isometric handgrip at 30% MVC performed while breathing cold air (cold + grip protocol) in older (n = 11) and young (n = 12) subjects. All dependent variables are plotted on the same axis; units are mmHg for SBP, DBP, and MAP, bpm for HR, and cm/s for CBVpeak. *P < 0.05 between groups.
Paired t-tests showed that older subjects had a greater change in SBP during the cold + grip protocol [Δ: 29 ± 3 compared with the grip protocol alone (Δ: 21 ± 2), P = 0.001] as well as RPP during the cold + grip protocol [Δ: 2,697 ± 391 compared with the grip protocol alone (Δ: 2,115 ± 375), P = 0.040]. During this same time, the change in HR (Δ: 6 ± 2 in the cold + grip protocol vs. 5 ± 2 in the grip protocol) and the change in CBVpeak (Δ: 3.5 ± 1.0 in the cold + grip protocol vs. 3.5 ± 1.0 in the grip protocol) were similar. Most importantly, in the older subjects, the supply-to-demand ratio was significantly attenuated during the cold + grip protocol compared with the grip protocol alone (2.84 ± 0.67 vs. 4.61 ± 1.01 × 10−3 cm·s−1·mmHg−1·beats·min−1). Similarly, paired analysis on the young subjects showed that the supply-to-demand ratio was significantly attenuated during the cold + grip protocol compared with the grip protocol alone (1.48 ± 0.48 vs. 4.32 ± 0.79 × 10−3 cm·s−1·mmHg−1·beats·min−1). Taken together, this indicates that myocardial O2 demand in older subjects was higher during the combined stimulus without a concomitant increase in myocardial O2 supply. This phenomenon is consistent with our previous work in young people (33).
One-way ANOVA was conducted to determine if the change in hemodynamics was different between the four groups (young men, young women, older men, and older women). Of all the comparisons (shown in Table 2), only ΔCBVpeak during the grip protocol was significant (P = 0.036), and followup independent t-tests showed that young women had significantly greater ΔCBVpeak compared with older women.
The cold pressor test elicited a significant increase in RPP that was not different between groups (Δ: 1,804 ± 433 beats·min−1·mmHg in young men, 3,728 ± 1,048 beats·min−1·mmHg in young women, 2,375 ± 698 beats·min−1·mmHg in older men, and 2,556 ± 567 beats·min−1·mmHg in older women). In a similar way, CBVpeak responses to the cold pressor test increased but were not different between groups (Δ: 5.6 ± 0.9 cm/s in young men, 14.6 ± 5.0 cm/s in young women, 5.7 ± 2.0 cm/s in older men, and 5.3 ± 1.3 cm/s in older women). In response to the cold pressor test, the supply-to-demand ratio was not different between young (9.2 ± 4.6 × 10−3 cm·s−1·mmHg−1·beats·min−1) and older (2.6 ± 0.5 × 10−3 cm·s−1·mmHg−1·beats·min−1) subjects (P = 0.164; Fig. 6C).
Figure 6 shows individual myocardial O2 supply (CBVpeak) and demand (RPP) responses to the grip protocol, the cold + grip protocol, and the cold pressor test (hand in 1°C water). Although there was considerable interindividual variability in our sample of healthy subjects, all three stressors caused an increase in CBVpeak as RPP increased (i.e., coronary hyperemia).
DISCUSSION
The purpose of this echocardiography study was to determine the effect of healthy aging on coronary blood flow and myocardial function during the cold air, grip, and cold + grip protocols. Our study using healthy young and healthy older adults provides four novel findings. First, we did not detect aging differences with respect to CBV despite a modest increase in RPP with the cold air protocol (hypothesis 1). Second, we determined that myocardial O2 supply and myocardial velocities were attenuated in response to the grip protocol in the older subjects but that there were no age differences in the coronary supply-to-demand ratio (hypothesis 2). Third, we showed that older adults have attenuated HR responses to the cold + grip protocol (hypothesis 3). Finally, we found that the cold + grip protocol in older adults caused a greater increase in SBP and RPP than the grip protocol alone and that the supply-to-demand ratio was attenuated in both groups of subjects under this same stressor.
It is clear that healthy human aging causes a number of structural and function changes in the cardiovascular system (24). However, little is known regarding the physiological responses to cold air inhalation in the aged. Two studies using clinical populations contained older subjects and warrant brief discussion. Hattenhauer and Neill (17) exposed 33 male patients with angina (age range: 42–66 yr) to −20°C air breathing and showed small but significant increases in HR (Δ: ∼3 beats/min), SBP (Δ: ∼12 mmHg), and coronary blood flow (Δ: ∼4 ml·100 g−1·min) via arteriography. Similarly, Lassvik and Areskog (25) studied 12 men (age range: 45–60 yr) with cold intolerance as they breathed −35°C air and showed that RPP was higher compared with breathing neutral air. Both of these reports studied subjects with coronary disease and did not include women, so generalization to the present study is challenging. Recent work has shown that skin cooling (non-noxious, nonhypothermic cold stress, which initiates thermoregulatory vasoconstriction) causes a greater O2 demand (RPP and left ventricular wall stress) in healthy older adults compared with young adults (20, 46). Despite this fact, older adults had attenuated coronary dilation to this systemic form of cold stress (12). Consistent with our previous experiments in young subjects, the present data suggest that local cooling of the mouth and upper airway increases SBP, HR, and RPP across the 5-min intervention without a similar increase in CBVpeak. The magnitude of effect (i.e., ΔO2 demand) is quite small but may be amplified in older adults with disease (e.g., obesity, diabetes, hypertension, or hypercholesterolemia).
The effect of aging on the response to handgrip exercise has been well studied in our laboratory (27) and others (34, 44). With regard to isometric handgrip exercise, the most profound difference between older and young subjects is the change in HR. This difference is seen with fatiguing isometric handgrip at 30% MVC (44) and 40% MVC (34) as well as 3-min bouts of isometric handgrip at 30% MVC (21, 23, 39). Rhythmic handgrip exercise at 30% MVC performed with progressive reduced limb perfusion (i.e., to accentuate the metaboreflex) also showed a blunted HR response in older subjects (27). MAP and MSNA responses to handgrip in this same study were also attenuated in the older subjects. While not a universal finding, the increase in blood pressure and MSNA tends to be smaller in healthy older adults (21, 23, 34), which may be related to higher baseline values (39) or lower aerobic fitness (23). Our data confirm these previous studies and furthers the literature by showing that CBVpeak is also attenuated. A blunted RPP response in the older subjects would lead to a relatively smaller O2 demand, and thus a blunted CBVpeak response would be physiologically appropriate (as evidenced by the supply-to-demand ratio being similar between groups). Other measurements of coronary blood flow (VTI, flow duration, and relative perfusion time) did not show group differences, and this is likely attributable to large interindividual variability and the fact that coronary blood flow occurs in diastole, which is relatively shorter at higher HRs.
For each subject, the cold + grip protocol used the same mechanical stimulus as the grip protocol. Therefore, it follows that the data shown in Figs. 3 and 5 demonstrate similar age differences. To our knowledge, there have been no other cold + grip protocol studies conducted with healthy older adults. Inhaling cold air during bicycle exercise (25, 36) and treadmill walking in a whole body cold chamber (22, 28) promotes higher O2 demand and reduced O2 supply in clinical populations (age range: 50–70 yr). We chose handgrip exercise because it allows for the measurement of coronary blood flow noninvasively and because some daily activities in the winter are isometric in nature (shoveling snow, carrying a briefcase, etc.). Because of our familiarization and control (sham) procedures, we do not feel this augmented response with the cold + grip protocol is due to participant anxiety or breathing compressed air through the mouthpiece. Furthermore, cold air inhalation did not change pulmonary function or O2 saturation in either group so it is most likely that these cardiovascular responses are due to a sympathetically mediated reflex originating in the oropharynx.
In our previous study (33) using young subjects, the percent increase in CBVpeak was significantly attenuated with the cold + grip protocol compared with the grip protocol, whereas the percent change in RPP was significantly accentuated. We interpreted this to indicate a supply-demand mismatch in the LAD. In the present study (using a paired analysis on just the older subject data), we also showed a significantly greater increase in RPP during the cold + grip protocol. To date, we have conducted 47 grip protocol trials and 47 cold + grip protocol trials in both young and older volunteers on separate days (CBV and TDI), and the total pool of subjects shows that the change in RPP was significantly greater with the cold + grip protocol (power = 0.646). The change in CBVpeak and TDI-derived myocardial function did not show clear differences in their response to the cold + grip protocol, but the supply-to-demand ratio was clearly attenuated in both young and older adults (compared with the grip protocol). An attenuated supply-to-demand ratio (i.e., a shallower slope) indicates a blunted coronary hyperemia to the cold + grip protocol relative to the grip protocol. Mechanistic (i.e., blockade) studies are needed to better explain the dissociation between changes in RPP and CBVpeak when cold air is added to static handgrip exercise.
Tissue Doppler-derived measurements of myocardial function did not change substantially across time in either group. This is in contrast to dynamic exercise studies, where systolic and early diastolic velocities increased nearly twofold as exercise intensity increased (43). Late diastolic velocity, indicative of atrial contribution to ventricular filling, generally increases during exercise until a HR level of ∼100 beats/min, when it fuses with the early diastolic wave (14, 43). In the present study, HR did not reach 100 beats/min. As shown in Fig. 4, there were clear age differences in myocardial function at baseline. These differences are consistent with a prior study (4) that showed that both older aerobically trained and untrained men had impaired early diastolic function compared with young aerobically trained and untrained men. In other words, aerobic fitness does not offset the healthy aging-induced decrements in myocardial function. The blunted RPP response to the grip and cold + grip protocols in older adults during the TDI visit confirms our own data from the CBV visit (i.e., mostly HR dependent and not SBP dependent). We suspect that the lower RPP in the older group is due to decreased β-adrenergic sensitivity compared with young people (24, 48).
Although not the initial purpose of this study, we have shown augmented CBVpeak responses to the grip protocol in young women compared with older women (Table 2). Since this comparison (n = 7 subjects/group) was not part of our a priori hypothesis, it could be criticized as being underpowered. Nevertheless, our data support a previous study (15) in which the intracoronary infusion of estradiol increased acetylcholine-stimulated coronary blood flow in estrogen-deficient women. Furthermore, postmenopausal women who took supplemental estrogen had improved flow-mediated dilation (26). Taken together, these prior studies suggest that the cardioprotective effects of estrogen act via a nitric oxide pathway. It is also interesting to note that young women had the greatest reduction in the coronary supply-to-demand ratio during the cold + grip protocol (Table 2). Whether this might predispose young women to angina during exertion in the cold is not clear but could be an avenue for future research.
We included the cold pressor test in this experiment because it is a nonspecific stimulus that evokes an increase in sympathetic nervous system activity (29). With regard to age, Ng et al. (34) showed that changes in HR and MAP were not different between young and older subjects in response to a cold pressor test. The present data support this and furthers the literature by showing that the changes in CBVpeak and the supply-to-demand ratio are also not significantly different between older and young people.
Limitations.
In this report, our older subjects were quite healthy, relative to the average American person in their early 60s. Thus, it could be argued that this group is subject to survival bias (i.e., ∼75% of our older subjects were fitness enthusiasts and would have likely not met the inclusion criteria had they been sedentary). The present data are also unable to identify how aerobic fitness might modify coronary blood flow responses because we did not determine maximal O2 consumption in these subjects. Future studies may allow for a more in-depth look at coronary regulation across a more diverse sample of aged subjects. Finally, the exercise mode and relatively low depth of breathing in our study may limit generalizability to dynamic whole body exercise. This too could be an area for future research.
Conclusions.
In summary, the present study has shown that healthy human aging results in attenuated responses to the grip and cold + grip protocols compared with young subjects. Specifically, the changes in HR and CBVpeak were less and baseline myocardial function (systolic myocardial and early diastolic velocities) was lower in older adults. However, the coronary supply-to-demand ratio was not different between groups, indicating that changes in coronary metabolism cause similar compensatory responses in coronary blood flow. Responses to a 5-min bout of cold air inhalation showed some group differences and a general increase across time, but aging did not modulate these responses (i.e., no interaction effect). Most importantly, in older adults, the coronary supply-to-demand ratio during the cold + grip protocol was attenuated compared with the grip protocol alone. Future experiments using pharmacological blockade will provide insights into age-related differences in cardiovascular responses to combined cold air inhalation and isometric exercise in humans.
GRANTS
This work was supported by National Institutes of Health Grants P01-HL-096570, R01-HL-070222, and UL1-RR-033184 (to L. I. Sinoway) as well as C06-RR-016499 and a Wilderness Medical Society Research in Training Grant (to M. D. Muller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.M., Z.G., J.L.M., C.A.B., R.C.D., U.A.L., and L.I.S. conception and design of research; M.D.M., Z.G., J.L.M., and C.A.B. performed experiments; M.D.M., Z.G., R.C.D., U.A.L., and L.I.S. analyzed data; M.D.M., Z.G., J.L.M., C.A.B., R.C.D., U.A.L., and L.I.S. interpreted results of experiments; M.D.M. prepared figures; M.D.M. drafted manuscript; M.D.M., Z.G., J.L.M., C.A.B., R.C.D., U.A.L., and L.I.S. edited and revised manuscript; M.D.M., Z.G., J.L.M., C.A.B., R.C.D., U.A.L., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are thankful to Dr. Michael Herr for the engineering contribution to this project and to Todd Nicklas, Charity Sauder, Amy Chabitnoy, and Josh Oman for assisting with data collection. Gratitude is also extended to Allen Kunselman for statistical support and Anne Muller for preparing the graphics for this study. Finally, the authors appreciate the administrative guidance of Kris Gray and Jen Stoner.
REFERENCES
- 1. Abali G, Tokgozoglu L, Ozcebe OI, Aytemir K, Nazli N. Which Doppler parameters are load independent? A study in normal volunteers after blood donation. J Am Soc Echocardiogr 18: 1260–1265, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Effects of first myocardial infarction on left ventricular systolic and diastolic function with the use of mitral annular velocity determined by pulsed wave doppler tissue imaging. J Am Soc Echocardiogr 13: 343–352, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med 343: 1355–1361, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Baldi JC, McFarlane K, Oxenham HC, Whalley GA, Walsh HJ, Doughty RN. Left ventricular diastolic filling and systolic function of young and older trained and untrained men. J Appl Physiol 95: 2570–2575, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
- 6. Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, Chen K, Chan A, Phelps ME, Schelbert HR. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 88: 62–69, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Derumeaux G, Ovize M, Loufoua J, Andre-Fouet X, Minaire Y, Cribier A, Letac B. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation 97: 1970–1977, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev; doi:10.1007/s10741-011-9270-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franklin BA, Hogan P, Bonzheim K, Bakalyar D, Terrien E, Gordon S, Timmis GC. Cardiac demands of heavy snow shoveling. JAMA 273: 880–882, 1995 [PubMed] [Google Scholar]
- 10. Gagge AP, Stolwijk JA, Saltin B. Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res 2: 209–229, 1969 [DOI] [PubMed] [Google Scholar]
- 11. Gao Z, Spilk S, Momen A, Muller MD, Leuenberger UA, Sinoway LI. Vitamin C prevents hyperoxia-mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol 112: 483–492, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol 302: H312–H318, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glickman-Weiss EL, Hearon CM, Nelson AG, Robertson RJ. A thermal perception scale for use during resting exposure to cold air. Percept Mot Skills 79: 547–560, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Goebel B, Arnold R, Koletzki E, Ulmer HE, Eichhorn J, Borggrefe M, Figulla HR, Poerner TC. Exercise tissue Doppler echocardiography with strain rate imaging in healthy young individuals: feasibility, normal values and reproducibility. Int J Cardiovasc Imaging 23: 149–155, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Guetta V, Quyyumi AA, Prasad A, Panza JA, Waclawiw M, Cannon RO., 3rd The role of nitric oxide in coronary vascular effects of estrogen in postmenopausal women. Circulation 96: 2795–2801, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Hammoudeh AJ, Haft JI. Coronary-plaque rupture in acute coronary syndromes triggered by snow shoveling. N Engl J Med 335: 2001, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Hattenhaur M, Neill WA. The effect of cold air inhalation on again pectoris and myocardial oxygen supply. Circulation 51: 1053–1058, 1975 [DOI] [PubMed] [Google Scholar]
- 18. Havenith G, van de Linde EJ, Heus R. Pain, thermal sensation and cooling rates of hands while touching cold materials. Eur J Appl Physiol Occup Physiol 65: 43–51, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Heindl S, Struck J, Wellhoner P, Sayk F, Dodt C. Effect of facial cooling and cold air inhalation on sympathetic nerve activity in men. Respir Physiol Neurobiol 142: 69–80, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol 107: 1076–1082, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Houssiere A, Najem B, Pathak A, Xhaet O, Naeije R, Van De Borne P. Chemoreflex and metaboreflex responses to static hypoxic exercise in aging humans. Med Sci Sports Exerc 38: 305–312, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Juneau M, Johnstone M, Dempsey E, Waters DD. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation 79: 1015–1020, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Krzeminski K, Cybulski G, Ziemba A, Nazar K. Cardiovascular and hormonal responses to static handgrip in young and older healthy men. Eur J Appl Physiol; doi:10.1007/s00421-011-2069-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lakatta EG. Changes in cardiovascular function with aging. Eur Heart J 11, Suppl C: 22–29, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Lassvik C, Areskog NH. Angina pectoris during inhalation of cold air. Reactions to exercise. Br Heart J 43: 661–667, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, Yeung AC, Creager MA. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Ann Intern Med 121: 936–941, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Markel TA, Daley JC, III, Hogeman CS, Herr MD, Khan MH, Gray KS, Kunselman AR, Sinoway LI. Aging and the exercise pressor reflex in humans. Circulation 107: 675–678, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Meyer P, Guiraud T, Curnier D, Juneau M, Gayda M, Nozza A, Nigam A. Exposure to extreme cold lowers the ischemic threshold in coronary artery disease patients. Can J Cardiol 26: e50–e53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. J Pain 5: 233–237, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Momen A, Kozak M, Leuenberger UA, Ettinger S, Blaha C, Mascarenhas V, Lendel V, Herr MD, Sinoway LI. Transthoracic Doppler echocardiography to non-invasively assess coronary vasoconstrictor and dilator responses in humans. Am J Physiol Heart Circ Physiol 298: H524–H529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer J, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol 296: H854–H861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 293: R3–R12, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Muller MD, Gao Z, Drew RC, Herr MD, Leuenberger UA, Sinoway LI. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol 111: 1694–1702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol Heart Circ Physiol 267: H344–H353, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28: 2539–2550, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Petersen CL, Hansen A, Frandsen E, Strange S, Jonassen O, Nielsen JR, Dige-Petersen H, Hesse B. Endothelin release and enhanced regional myocardial ischemia induced by cold-air inhalation in patients with stable angina. Am Heart J 128: 511–516, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Ray CA. Interaction between vestibulosympathetic and skeletal muscle reflexes on sympathetic activity in humans. J Appl Physiol 90: 242–247, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, Sopko G, Pepine CJ. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol 33: 1469–1475, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Roseguini BT, Alves CN, Chiappa GR, Stein R, Ribeiro JP. Muscle metaboreflex contribution to resting limb haemodynamic control is preserved in older subjects. Clin Physiol Funct Imaging 27: 335–339, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 41. Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol 528: 407–417, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol 33: 1916–1919, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Stoylen A, Wisloff U, Slordahl S. Left ventricular mechanics during exercise: a Doppler and tissue Doppler study. Eur J Echocardiogr 4: 286–291, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenal-circulatory regulation during sustained isometric exercise in young and older men. Am J Physiol Regul Integr Comp Physiol 261: R1061–R1069, 1991 [DOI] [PubMed] [Google Scholar]
- 45. Uren NG, Camici PG, Melin JA, Bol A, de Bruyne B, Radvan J, Olivotto I, Rosen SD, Impallomeni M, Wijns W. Effect of aging on myocardial perfusion reserve. J Nucl Med 36: 2032–2036, 1995 [PubMed] [Google Scholar]
- 46. Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol 298: R1627–R1633, 2010. PMCID: PMC2886703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, Hymer H, Wichmann HE, Peters A. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120: 735–742, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Xiao RP, Lakatta EG. Deterioration of β-adrenergic modulation of cardiovascular function with aging. Ann NY Acad Sci 673: 293–310, 1992 [DOI] [PubMed] [Google Scholar]
- 49. Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70: 657–662, 1984 [DOI] [PubMed] [Google Scholar]