Abstract
Doxorubicin is a highly effective chemotherapeutic agent used for treating a wide spectrum of tumors, but its usage is limited because of its dose-dependent cardiotoxicity, especially in pediatric patients. Accumulating evidence indicates that caspase-dependent apoptosis contributes to the cardiotoxicity of doxorubicin. However, less attention has been paid to the effects of age on doxorubicin-induced apoptosis signaling in myocardium. This study focused on investigating differential apoptotic sensitivity between neonatal and adult myocardium, in particular, between neonatal and adult cardiomyocytes in vivo. Our results show that caspase-3 activity in normal mouse hearts decreased by ≥20-fold within the first 3 wk after birth, associated with a rapid downregulation in the expression of key proapoptotic proteins in intrinsic and extrinsic pathways. This rapid downregulation of caspase-3 activity was confirmed by immunostaining for cleaved caspase-3 and terminal deoxynucleotidyl transferase dUTP-mediated nick-end label staining. Doxorubicin treatment induced a dose-dependent increase in caspase-3 activity and apoptosis in neonatal mouse hearts, and both caspase-8 and caspase-9 activations were involved. Using transgenic mice with a nuclear localized LacZ reporter gene to label cardiomyocytes in vivo, we observed a fourfold higher level of doxorubicin-induced cardiomyocyte apoptosis in 1-wk-old mice compared with that in 3-wk-old mice. This study points to a major difference in apoptotic signaling in doxorubicin cardiotoxicity between neonatal and adult mouse hearts and reveals a critical transition from high to low susceptibility to doxorubicin-induced apoptosis during postnatal heart maturation.
Keywords: anthracycline, caspase, cardiotoxicity, cardiomyocyte, chemotherapy
the anthracyclines, primarily doxorubicin, but also including daunomycin, epirubicin, and idarubicin, are among the most effective drugs to treat a wide spectrum of hematologic malignancies and solid tumors. However, their cumulative and dose-dependent cardiac toxicity has been a major concern of oncologists and cardiologists for decades (30). In addition to the cumulative dose, younger age at treatment is also a significant risk factor for anthracycline cardiotoxicity. Children and adolescents are particularly susceptible to the cardiotoxic effects of anthracycline chemotherapy, and there is no safe dose of anthracyclines in this population (14, 27). Children treated before the age of 4 yr appear to be especially vulnerable (14, 27). About one-half of the young adult survivors of childhood cancer have received anthracyclines at some point in their treatment, and long-term follow-up studies (14, 27) have shown that progressive cardiotoxicity will develop in the majority of those children treated with anthracyclines.
Despite intensive investigations on anthracycline cardiotoxicity, the underlying mechanisms, especially age-related sensitivity, have not yet been completely elucidated. Many mechanisms have been proposed, including free radical-induced oxidative stress, damage to nuclear DNA, dysregulation of calcium handling and cellular contractility, suppression of transcription factors that regulate cell survival and sarcomere protein synthesis, disruption of sarcomere stability, and mitochondrial degeneration in cardiomyocytes. Most of these cellular events eventually contribute to cardiomyocyte death. Indeed, numerous in vitro and in vivo studies implicate anthracycline-induced cardiomyocyte apoptosis as a primary cause of cardiac damage (9, 22, 33). However, very little attention has been paid to the effects of age on anthracycline-induced apoptosis signaling in myocardium.
The present study aimed at comparing the acute effects of doxorubicin on the induction of caspase-dependent apoptosis in the hearts of neonatal and adult mice. Multiple biochemical and histological approaches were used with a particular focus on quantifying critical apoptotic events during the postnatal growth period and on determining different apoptotic sensitivities between neonatal and adult cardiomyocytes in vivo. Transgenic mice with cardiac-specific expression of a nuclear localized LacZ reporter gene (26) were used to definitively identify cardiomyocytes in the heart. Our results demonstrated the presence of functionally intact caspase-dependent death machinery in neonatal hearts that is responsive to doxorubicin-induced apoptotic stimuli. In contrast, the adult heart is significantly more resistant to doxorubicin-induced apoptotic stimuli, most likely due to rapid postnatal silencing of numerous key apoptosis regulatory proteins.
MATERIALS AND METHODS
Animal model.
All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996) and were approved by the Institutional Animal Care and Use Committee at Indiana University School of Medicine. For all in vivo experiments, either wild-type FVB mice or transgenic mice [α-myosin heavy chain (αMHC)-nLacZ] carrying a nuclear localized LacZ-reporter gene under the control of the αMHC promoter (26) were used. A single dose of doxorubicin (Sigma-Aldrich) at 5, 10, 15, or 20 mg/kg was administered intraperitoneally to mice at different ages. For coadministration experiments, caspase-9 inhibitor (z-LEHD-fmk; 10 mg/kg), caspase-8 inhibitor (z-IETD-fmk; 10 mg/kg), cathepsin B inhibitor (CA-074Me; 5 mg/kg) (ENZO Life Sciences), or cathepsin D inhibitor (pepstatin A; 10 mg/kg; Sigma-Aldrich) were administered intraperitoneally 30 min before doxorubicin injection. Mice administered with the same volume of saline at the same timing served as controls. All mice except for those used for the determination of the time course of doxorubicin-induced cardiotoxicity were killed 24 h after treatment. Mice were anesthetized with inhalation anesthetic isoflurane (2%; Webster Veterinary Supply), and the hearts were removed immediately, rinsed in precooled PBS, and either flash frozen in liquid nitrogen for protein analysis or cryopreserved in optimum cutting temperature medium for cryosections.
Protein analysis.
Protein samples were prepared as previously described (23). Ventricular tissue fragments were disrupted with a PYREX Potter-Elvehjem tissue grinder on ice in lysis buffer containing 25 mM HEPES pH 7.5, 5 mM MgCl2, 1 mM EGTA, and 10 mM DTT, plus proteinase and phosphatase inhibitors (Roche). After being supplemented with detergents (0.5% TritonX-100 and 0.25% NP-40) and rotated at 4°C for 30 min, the homogenate was centrifuged at 15,000 g at 4°C for 15 min, and the supernatant was saved. Fresh lysates were used immediately for the determination of caspase-3 activity as described below. Western blot analysis was performed for detection of the expression level of apoptosis-related proteins in the ventricles. Primary antibodies included rabbit polyclonal antibodies to caspase-8 (sc-7890) (Santa Cruz), cathepsin B (06–480; Millipore), cathepsin D (GT15042; Neuromics), apoptotic-inducing factor (AIF; 551429; BD Biosciences), caspase-3 (9662; Cell Signaling), cleaved caspase-3 (9661), caspase-9 (9504), cleaved caspase-9 (9509), cleaved caspase-8 (9429), poly(ADP-ribose) polymerase (9542), Apaf-1 (4452), Bax (2772), Bak (3814), Bcl-2 (2872), Bcl-xL (2762), X-linked inhibitor of apoptosis (XIAP; 2042), Akt (9272), p-AktS473 (9271), cytochrome c (4272), and EndoG (4969). All blots were normalized to GAPDH (RDI Division of Fitzgerald Industries).
Subcellular fractionation.
Hearts were homogenized in ice-cold mitochondria buffer (5 mM MOPS pH 7.0, 225 mM mannitol, 1 mM EGTA, 75 mM sucrose, and 1 mM DTT) supplemented with proteinase and phosphatase inhibitors (Roche) and centrifuged twice at 700 g at 4°C for 10 min to pellet cell debris and nuclei. The supernatant was again centrifuged at 10,000 g at 4°C for 20 min, and the pellet was saved as the mitochondrial fraction. The supernatant was again centrifuged at 100,000 g at 4°C for 1 h, and the pellet was saved as the light membrane fraction; the supernatant was saved as the cytosolic fraction. After measurement of the protein concentration, samples with equal amounts of protein were analyzed by Western blot with specific antibodies. The purity of the subcellular fractionations was assessed by Western blot analysis with GAPDH (a cytosolic protein) and cytochrome c oxidase subunit IV (COX IV; a mitochondrial protein; Molecular Probes) as the markers, respectively.
Measurement of mitochondrial permeability transition.
Heart mitochondria were freshly isolated on the day of the experiments using the previously described methods (20). Briefly, hearts were homogenized in ice-cold mitochondrial isolation buffer (20 mM HEPES pH 7.4, 190 mM mannitol, and 70 mM sucrose) and then subjected to three rounds of differential centrifugation at 700 g, 900 g, and 1,000 g at 4°C for 10 min to remove cell debris and nuclei. The supernatant was again centrifuged at 10,000 g at 4°C for 20 min, and the pellet was saved as the mitochondrial fraction. The mitochondrial pellet was carefully resuspended in ice-cold isolation buffer and immediately used for the mitochondrial permeability transition (MPT) experiment after protein concentration measurement. MPT was measured spectrophotometrically by monitoring the decrease in absorbance at 540 nm with mitochondrial proteins. The swelling assays were performed in 120 mM KCl, 10 mM Tris pH 7.6, and 5 mM KH2PO4. CaCl2 (250 μM) was added at 1 min, and the absorbance change was measured per minute for 10 min. Swelling amplitude was calculated as the total decrease in absorbance from 0 to 10 min.
Caspase-3 activity assay.
Specific caspase-3 activity in tissue homogenates was measured using a caspase-3 immunosorbent enzyme assay kit (Roche) according to the manufacturer's instruction. To specifically quantify caspase-3 activity, it is necessary to first separate active caspase-3 from other proteases in tissue extracts by using a monoclonal anti-cleaved caspase-3 antibody. Briefly, caspase-3 is captured from tissue lysates by a monoclonal antibody coated onto an opaque 96-well plate. Following the washing step removing unbound proteases, caspase-3 fluorescent substrate Ac-DEVD-AFC was added, and the intensity of free fluorescent AFC liberated by proteolytic cleavage was periodically measured by excitation at 400 nm and emission at 505 nm. Tissue lysates preincubated with a pan-caspase inhibitor (z-VAD-fmk; EMD) at 40 μM for 1 h before being added into the wells for immunoadsorption served as the negative control. The caspase-3 activity is expressed as the net fluorescent intensity.
Histology analysis.
Eight-micrometer-thick cryosections were obtained from hearts of FVB mice or transgenic αMHC-nLacZ mice at progressive ages from neonatal to 5 wk old. Single or dual fluorescent staining to detect active caspase-3, LacZ, and terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL)-positive nuclei was performed. Fluorescent immunostaining to detect active caspase-3 was performed with FVB mouse heart sections using polyclonal anti-cleaved caspase-3 antibodies (Promega), followed by incubation with Alexa Fluor 488 or Alexa Fluor 555-conjugated goat anti-rabbit IgG (Molecular Probes). Sections were counterstained with DAPI (Molecular Probes) and mounted with Vectashield (Vector Laboratories). Apoptosis in mouse heart sections was assayed using the fluorescent ApopTag kit according to the manufacturer's instructions (Chemicon). These sections were also counterstained with DAPI (Molecular Probes) and mounted with Vectashield. To identify nuclei of cardiomyocytes in transgenic αMHC-nLacZ sections, they were first stained with anti-β-GAL (ab9361; Abcam) followed by incubation with Fluor 555 conjugated goat anti-chicken IgG (Molecular Probes). They were then stained with anti-cleaved caspase-3 antibodies (Promega) or TUNEL staining using the ApopTag in situ apoptosis detection kit (Chemicon). For all quantifications, a minimum of four cryosections from distinct regions of each heart sample and at least six hearts per group were analyzed.
Statistical analysis.
Data are reported as means ± SE. Comparisons between groups were analyzed by Student's t-test or ANOVA as appropriate, with P < 0.05 considered as significant.
RESULTS
Rapid downregulation of caspase-3 activation parallels the decreased expression of key proapoptotic proteins in postnatal hearts.
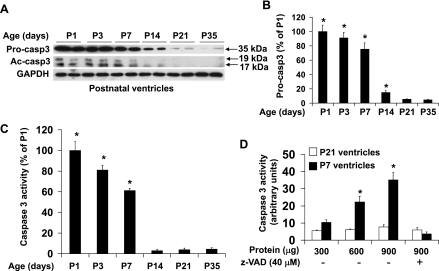
To determine apoptotic signaling in the postnatal heart, we first characterized the expression level of procaspase-3 together with that of cleaved caspase-3, a central marker for the activation of the caspase cascade, in postnatal ventricles under baseline conditions (Fig. 1). Procaspase-3 expression was rapidly downregulated from postnatal day 1 (P1) to 35 days by ∼20-fold, with the biggest fall from P7 to P14 (Fig. 1, A and B). Western blot analysis detected cleaved caspase-3 during the first 7 days after birth, which became undetectable after 14th postnatal day (Fig. 1A). These results indicate that both procaspase-3 and cleaved caspase-3 levels showed a concurrent drop between P7 and P14.
Fig. 1.
Downregulation of procaspase-3 expression, cleaved caspase-3, and caspase-3 activity in mouse ventricles during postnatal growth period. A: representative image of Western blot of procaspase-3 (Procasp3) and cleaved caspase-3 (Ac-casp3) with ventricular extracts at different ages as indicated. B: densitometry analysis of immunoreactive bands of procaspase-3. Expression of procaspase-3 was expressed as percent change relative to 1-day-old (P1) ventricles; n = 4–6 in each time point. *P < 0.05 vs. P35 (5-wk) of age. C: caspase-3 activity assay performed with ventricular extracts (600 μg) from 1-day-old (P1) to 35-day-old (P35) mice showing detectable specific caspase-3 activity in postnatal ventricles within the first week after birth. Caspase-3 activity was expressed as percent change relative to P1 ventricles. D: caspase-3 activity assay performed with different amounts of ventricular extracts from P7 and P21 mice. n = 6–8 in each time point. C and D: *P < 0.05 for vs. caspase inhibitor (z-VAD-fmk; 40 μM)-treated samples of same age.
In addition to the evaluation of cleaved caspase-3 protein levels by Western blot analysis, caspase-3 activity was also measured by immunosorbent enzyme assay (Fig. 1C). Consistent with the results of the Western blot, postnatal ventricles showed a detectable caspase-3 activity during the first 7 days after birth (Fig. 1C), which could be inhibited by preincubation with a pan-caspase inhibitor (Fig. 1D). The caspase-3 activity in neonatal ventricles was rapidly downregulated by >20-fold from P1 to P14 with a significant drop in signal after P7, becoming undetectable from P14 onward.
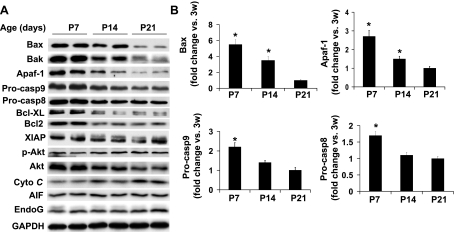
We next characterized the parallel downregulation of other key apoptotic-regulating proteins in postnatal ventricles (Fig. 2). The expression levels of Bax, Bak, Apaf-1, procaspase-9, and procaspase-8 were all downregulated from P7 to P14, by 1.6, 1.9, 1.8, 1.7, and 1.5-fold, respectively (Fig. 2, A and B). The concerted downregulation of procaspase-3, Bax, Bak, Apaf-1, procaspase-9, and procaspase-8 likely contributes to the rapid downregulation of caspase-3 activation during this time window. Moreover, the expression of several key anti-apoptotic proteins including Bcl-XL, Bcl2, XIAP, and phospho-Akt was either downregulated or unchanged during the postnatal period (Fig. 2A) indicating that downregulation of caspase-3 activation during the postnatal growth period is not due to the increased anti-apoptotic activity.
Fig. 2.
General downregulation of key proapoptotic proteins in mouse ventricles during postnatal growth period. A: representative image of Western blot of key proapoptotic and anti-apoptotic proteins in ventricular extracts at different ages as indicated. B: densitometry analysis of immunoreactive Bax, Apaf-1, procaspase-9 (Procasp9), and procaspase-8 (Procasp8). Expression of each protein at P7 or P14 was expressed as fold change relative to that of P21; n = 4–6 in each age point. AIF, apoptotic-inducing factor; Cyto C; cytochrome c; XIAP, X-linked inhibitor of apoptosis. *P < 0.05 vs. P21 group.
Activation of caspase-3 by doxorubicin was markedly attenuated during the postnatal growth period.
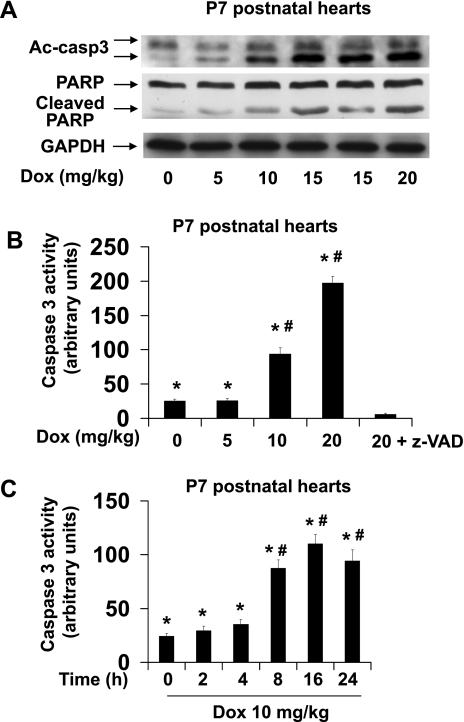
To determine if inducible activation of caspase-3 by cardiac injury is also downregulated during postnatal heart development, we investigated caspase-3 activation in postnatal mouse ventricles after acute doxorubicin treatment. Since doxorubicin has a half-life of ∼30 h in vivo in rat (2), its acute action on caspase-3 activation was examined 24 h after injection of various doses of doxorubicin (Fig. 3). Treatment of P7 neonatal mice elicited a dose-dependent increase of cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase, which is a nuclear caspase-3 substrate (Fig. 3A). There was also a dose-dependent increase in caspase-3 activity (Fig. 3B) with increasing doses of doxorubicin. A time-course study revealed that caspase-3 activity was markedly increased at 8 h after doxorubicin injection and remained at a similar level up to 24 h after injection (Fig. 3C).
Fig. 3.
Induction of caspase-3 activation by doxorubicin in neonatal mouse ventricles. A: representative image of Western blot of cleaved caspase-3 (Ac-casp3) and poly(ADP-ribose) polymerase (PARP) in ventricular extracts from P7 mouse hearts treated for 24 h with increasing dosages of doxorubicin (Dox) as indicated. B: caspase-3 activity assay performed with 600 μg of ventricular extracts from P7 mouse hearts treated for 24 h with increasing dosages of doxorubicin as indicated showing doxorubicin-induced caspase-3 activation. C: caspase-3 activity assay performed with 600 μg of ventricular extracts from P7 mouse hearts treated with 10 mg/kg doxorubicin for 0 to 24 h as indicated. n = 6–8 for each group. *P < 0.05 vs. samples pretreated with caspase inhibitor (z-VAD-fmk, 40 μM) before caspase activity assay. #P < 0.05 vs. saline-treated samples.
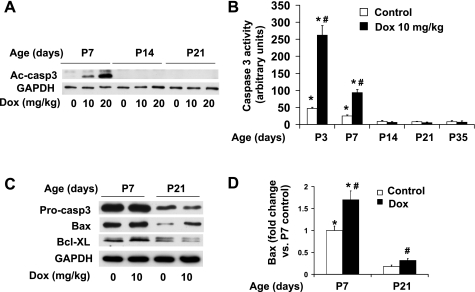
Although activation of caspase-3 by doxorubicin was apparent within the first 7 days after birth, treatment of mice from P14 could not induce detectable caspase-3 activation by these biochemical methods (Fig. 4, A and B). Our results indicate that the susceptibility of the postnatal mouse hearts to doxorubicin-induced caspase-3 activation closely parallels the time course of downregulation of basal caspase-3 activation and expression of key proapoptotic proteins during the postnatal period.
Fig. 4.
Acute effects of doxorubicin treatment on caspase-3 activation and expression of apoptotic regulatory proteins. A: representative image of Western blot of cleaved caspase-3 (Ac-caps3) in ventricular extracts from P7, P14, and P21 mice 24 h after injection of increasing dosages of doxorubicin as indicated. B: caspase-3 activity assay performed with 600 μg of ventricular extracts from postnatal mice at different ages as indicated; n = 4–6 in each group. *P < 0.05 vs. samples pretreated with caspase inhibitor (z-VAD-fmk, 40 μM) before caspase-3 activity assay. #P < 0.05 vs. saline-treated samples of same age. C: representative image of Western blot analysis of procasapse 3 (Procasp3), Bax, and Bcl-XL in ventricular extracts from P7 and P21 mice treated for 24 h with doxorubicin at 10 mg/kg. D: densitometry analysis of immunoreactive Bax, expressed as fold change relative to the baseline level at P7. *P < 0.05 vs. P21 group subjected to the same treatment. #P < 0.05 vs. saline-treated samples of same age.
To determine if apoptotic regulating proteins can be upregulated in response to doxorubicin-induced apoptotic stimuli, we next investigated the expression levels of key apoptotic regulating proteins (listed in Fig. 2) in postnatal mouse ventricles after doxorubicin treatment. Among them, a majority, including procaspase-3, Apaf-1, procaspase-9, and procaspase-8, remained unchanged and only Bax was upregulated in both P7 and P21 ventricles in response to doxorubicin treatment (Fig. 4C). Moreover, no significant upregulation of anti-apoptotic proteins including Bcl-XL (Fig. 4C) was observed after doxorubicin treatment. These results suggest that the lack of concerted induction of key apoptotic-regulating proteins in postnatal ventricles limits caspase activation by doxorubicin in an acute setting.
Downregulation of key proapoptotic proteins during the postnatal growth period increases resistance to mitochondrial apoptotic pathway.
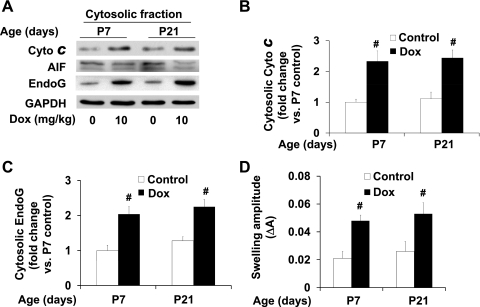
The release of cytochrome c and other proapoptotic proteins from mitochondria are key mediators of doxorubicin-induced apoptosis (9, 22, 33). While cytochrome c promotes caspase-dependent apoptosis (13), AIF and endonuclease EndoG are mitochondrial proteins that have been reported to promote caspase-independent apoptosis after their translocation to the cytosol in myocardium (3, 4). To examine if the release of these mitochondrial proteins is induced within the 24-h window after acute doxorubicin treatment, Western blotting was performed to determine their expression levels in ventricular cytosolic fractions. A similar increase in the cytosolic levels of cytochrome c and EndoG after doxorubicin treatment was detected in both P7 and P21 ventricles (Fig. 5, A-C), while AIF release was not significantly affected during this time window. In addition, mitochondrial swelling, indicative of susceptibility to calcium-induced MPT, was increased in both P7 and P21 ventricles by doxorubicin treatment (Fig. 5D). Altogether, these results indicate that acute doxorubicin treatment induces mitochondrial damage resulting in cytosolic translocation of mitochondrial proteins involved in caspase-dependent and -independent apoptosis in both P7 and P21 ventricles. These results also suggest that while increased mitochondrial release of cytochrome c was detected in both P7 and P21 ventricles, it has limited effects on caspase-3 activation in mouse hearts after the general downregulation of apoptotic proteins.
Fig. 5.
Effects of doxorubicin treatment on mitochondrial apoptotic protein release and on mitochondrial permeability transition (MPT) in mouse ventricles during postnatal growth period. A: representative image of Western blot of cytochrome c, AIF, and EndoG in cytosolic fraction of ventricular extracts from P7 and P21 mice treated for 24 h with doxorubicin at 10 mg/kg. B and C: densitometry analysis of immunoreactive cytosolic cytochrome c and EndoG expressed as fold change relative to the baseline level at P7; n = 4–6 in each group. D: mitochondrial swelling. MPT in mitochondria was measured by light transmission at 540 nm with 250 μM CaCl2 and mitochondrial proteins prepared from P7 and P21 mouse ventricles treated for 24 h with doxorubicin at 10 mg/kg. #P < 0.05 vs. saline-treated samples of same age.
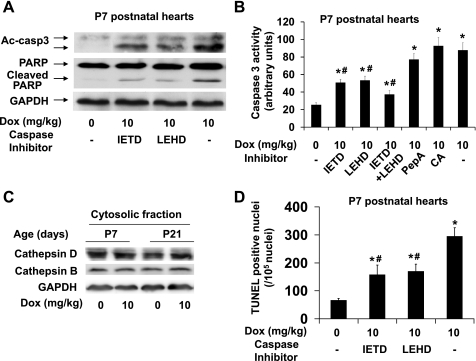
To determine if the mitochondrial apoptotic pathway is involved in caspase-3 activation in neonatal ventricles, we evaluated the effect of caspase-9 inhibitor (z-LEHD-fmk; 10 mg/kg). Treatment with caspase-9 inhibitor attenuated the doxorubicin-induced increase in cleaved caspase-3 protein level (Fig. 6A) and caspase-3 activity by ∼55% (Fig. 6B). To determine if caspase-8 activation is also involved in caspase-3 activation, we evaluated the effect of caspase-8 inhibitor (z-IETD-fmk; 10 mg/kg). Interestingly, treatment with caspase-8 inhibitor also attenuated the doxorubicin-induced increase in cleaved caspase-3 protein levels (Fig. 6A) and caspase-3 activity by ∼59% (Fig. 6B). These inhibitors at 10 mg/kg were previously reported to inhibit caspase-8 or -9 in vivo in mouse (24). In addition, treatment with both caspase-8 and caspase-9 inhibitors produced an additive effect (Fig. 6B) further supporting that both extrinsic and intrinsic apoptotic pathways are implicated in doxorubicin-induced caspase-3 activation in neonatal ventricles.
Fig. 6.
Effects of treatment with caspase-8, caspase-9, or cathepsin inhibitors on doxorubicin-induced caspase-3 activation in neonatal mouse ventricles. A: representative image of Western blot of cleaved caspase-3 and cleaved PARP in ventricular extracts from P7 neonatal mice treated with caspase-8 inhibitor (z-IETD-fmk; 10 mg/kg) or caspase-9 inhibitor (z-LEHD-fmk; 10 mg/kg) together with doxorubicin (10 mg/kg). B: caspase-3 activity assay was performed with 600 μg of ventricular lysates from P7 neonatal mice treated with caspase-8 and/or 9 inhibitors or with cathepsin D (PepA: pepstatin A; 10 mg/kg) or cathepsin B inhibitor (CA: CA-074Me; 5 mg/kg) together with doxorubicin (10 mg/kg). C: representative image of Western blot of cathepsin D and cathepsin B in cytosolic fraction of ventricular extracts from P7 and P21 mice. D: quantification of total terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL)-positive nuclei from ventricular sections; n = 6–8 in each group. *P < 0.05 vs. saline treated samples. #P < 0.05 vs. doxorubicin alone treated samples.
Since lysosomal protease release induced by doxorubicin could be involved in caspase activation, necrosis, and/or autophagy (5, 34), we examined if lysosomal enzyme release is increased by acute doxorubicin treatment (Fig. 6C). No significant induction of the cytosolic cathepsin D and cathepsin B levels was observed in both P7 and P21 ventricles by doxorubicin treatment. Moreover, treatment with pepstatin A, an inhibitor of cathepsin D, or with CA-074Me, an inhibitor of cathepsin B, did not attenuate the doxorubicin-induced increase in caspase-3 activity (Fig. 6B), suggesting that lysosomal enzyme release does not play a major role in caspase-3 activation within 24 h after doxorubicin treatment.
Induction of cleaved caspase-3-positive and/or TUNEL-positive cardiac cells by doxorubicin was markedly reduced during the postnatal growth period.
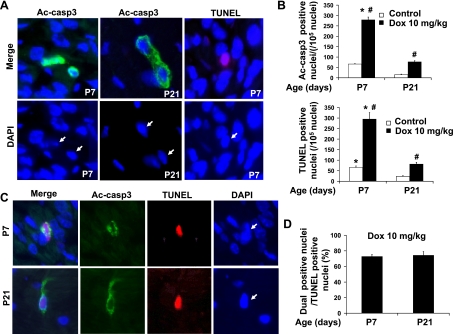
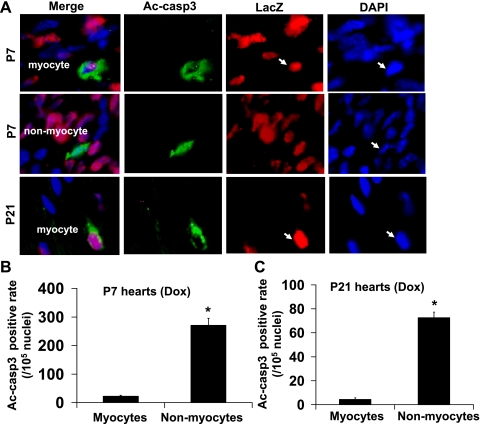
Immunohistological analysis was performed to quantify cleaved caspase-3-positive cells in ventricular cryosections by fluorescent microscopy (Fig. 7, A and B). There was a significant reduction of nuclear density in the ventricular sections from P7 or P21 mice (Fig. 7, A and C) due to the hypertrophic growth of cardiomyocytes occurring during this period. We observed downregulation of the number of cleaved caspase-3-positive cells during postnatal heart development at baseline condition and after doxorubicin treatment (Fig. 7, A and B; Table 1). These results were consistent with those of the Western blot and enzymatic activity assays. In addition, the incidence of cleaved caspase-3 positivity strongly correlated with that of TUNEL positivity (Fig. 7, A and B; Table 1) during postnatal heart development. Furthermore, inhibition of doxorubicin-induced caspase-3 activity by cotreatment with caspase-9 or caspase-8 inhibitors was associated with reduced TUNEL positivity in the P7 neonatal ventricles (Fig. 6D). It is worth noting that the majority of cleaved caspase-3-positive cells exhibited partial or complete overlap to staining with DAPI in the P7 neonatal ventricles (Fig. 7A), indicating perinuclear and nuclear accumulation of cleaved caspase-3. In contrast, P21 ventricles showed predominantly cytosolic staining of cleaved caspase-3 (Fig. 7A). After 2 wk of age, immunohistological staining could still detect apoptosis at baseline and its increase after doxorubicin treatment, whereas biochemical methods reached the limit of assay detection, most likely due to significant cardiomyocyte enlargement (and therefore an increase in total protein content) together with the downregulation of key proapoptotic proteins during this postnatal period.
Fig. 7.
Single and dual immunostaining of cleaved caspase-3 and TUNEL of postnatal ventricular sections. A: representative images of single cleaved caspase-3 (Ac-casp3) staining (green) or TUNEL staining (red) of ventricular sections from P7 or P21 mice treated for 24 h with doxorubicin at 10 mg/kg. Arrows point to the positive nuclei. B: quantification of total cleaved caspase-3-positive nuclei and total TUNEL-positive nuclei (n = 6–8 for each time point). *P < 0.05 vs. P21 group subjected to the same treatment. #P < 0.05 vs. saline treated samples of same age. C: representative images of dual staining of cleaved caspase-3 (green) and TUNEL (red) of ventricular sections from P7 or P21 mice. Arrows point to cleaved caspase-3 and TUNEL dual positive nuclei. D: quantitative analysis of dual-positive nuclei per total TUNEL-positive nuclei.
Table 1.
Summary of major apoptotic related events downregulated from 1 to 3 wk
| 1 wk |
3 wk |
|||
|---|---|---|---|---|
| Baseline | Dox* | Baseline | Dox* | |
| Procaspase 3 (WB) | 15-fold† | 15-fold† | 1‡ | 1† |
| Bax (WB) | 5.5-fold† | 9.4-fold† | 1‡ | 1.8† |
| Cleaved caspase 3 (WB) | Detectable | Detectable | Undetectable | Undetectable |
| Caspase 3 activity by enzymatic assay | Detectable | Detectable | Undetectable | Undetectable |
| >12-fold† | >44-fold† | |||
| Number of cleaved caspase 3-positive nuclei/total nuclei | 33/51,250 | 692/248,500 | 25/151,500 | 143/181,700 |
| (0.0643%) | (0.278%) | (0.0165%) | (0.0787%) | |
| 3.9-fold† | 16.8-fold† | 1‡ | 4.8-fold† | |
| Number of cleaved caspase 3-positive myocyte nuclei/total myocyte nuclei | 25/327,775 | 187/355,888 | 2/205,098 | 11/84,099 |
| (0.0076%) | (0.053%) | (0.00097%) | (0.0131%) | |
| 7.8-fold† | 54.6-fold† | 1‡ | 13.5-fold† | |
| Number of TUNEL-positive nuclei/total nuclei | 387/609,650 | 2,950/1,089,250 | 27/138,400 | 340/417,000 |
| (0.0635%) | (0.271%) | (0.0195%) | (0.0815%) | |
| 3.2-fold† | 13.9-fold† | 1‡ | 4.2-fold† | |
| Number of TUNEL-positive myocyte nuclei/total myocyte nuclei | 24/235,245 | 172/368,750 | 1/90,790 | 10/85,470 |
| (0.010%) | (0.0466%) | (0.0011%) | (0.0117%) | |
| 9.1-fold† | 42.4-fold† | 1‡ | 10.6-fold† | |
Dox: 24 h after intraperitoneal injection of 10 mg/kg of doxorubicin.
Expressed as fold change compared to the 3-wk group at baseline.
Value in the 3-wk group at baseline was defined as 1. WB, Western blot; TUNEL, terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling.
We performed dual immunostaining of cleaved caspase-3 and TUNEL (Fig. 7, C and D) in the P7 and P21 ventricles after acute doxorubicin treatment. About 74% of TUNEL-positive cells were also positive for cleaved caspase-3 staining in both P7 and P21 ventricles. This indicates a large portion of cleaved caspase-3-positive cells among the TUNEL-positive cells, representing caspase-3-dependent apoptosis in acute doxorubicin-induced cardiotoxicity in both P7 and P21 ventricles. TUNEL-positive but cleaved caspase-3-negative cells (∼26% of TUNEL-positive cells) may represent the cells at a late apoptotic stage or undergoing caspase-3-independent apoptosis. An induction of caspase-3-independent apoptosis was supported by increased cytosolic levels of EndoG detected after doxorubicin treatment in both P7 and P21 ventricles (Fig. 5, A and C), which may contribute to TUNEL-positive but cleaved caspase-3-negative cells detected by dual immunostaining.
Induction of cleaved caspase-3-positive or TUNEL-positive cardiomyocytes by doxorubicin was markedly downregulated during the postnatal growth period.
Because the results presented above do not distinguish the contributions of cardiomyocytes and noncardiomyocytes to the overall caspase-3 activation and apoptosis in mouse hearts under baseline and doxorubicin treatment conditions, we next determined the in vivo incidence of apoptosis in cardiomyocytes, which are believed to be the target of doxorubicin-induced cardiotoxicity. Transgenic mice with cardiac-specific expression of a nuclear localized LacZ reporter gene, which labels >99% of cardiomyocytes in the heart (26), were used in the subsequent experiments. We first confirmed that the time courses of postnatal downregulation of caspase-3 activation, expression of key proapoptotic proteins, and susceptibility to doxorubicin-induced caspase-3 activation were undistinguishable among the wild-type and transgenic mice (data not shown).
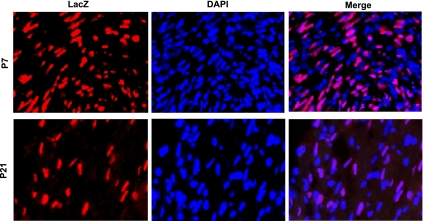
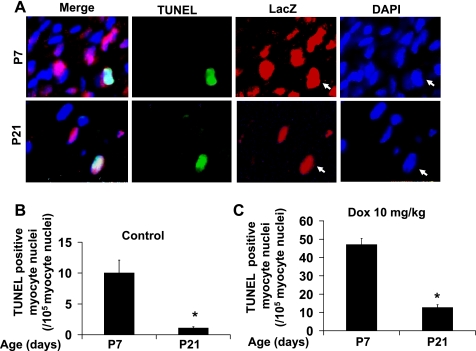
Anti-lacZ staining was used for cardiomyocyte nuclear identification in the transgenic mouse hearts (Fig. 8). Apoptotic cells were visualized by immunohistological analysis of cleaved-caspase-3 (Fig. 9A) or by TUNEL staining (Fig. 10A) of ventricular sections in combination with anti-lacZ staining. The majority of the apoptosis occurred in noncardiomyocytes as cleaved caspase-3-positive cardiomyocytes only accounted for ∼8.9% of total cardiac positivity in P7 and ∼5.5% in P21 ventricular sections from doxorubicin-treated mice (Fig. 9, B and C). The positivity for cleaved caspase-3 in cardiomyocytes and noncardiomyocytes was very similar to that of TUNEL (Table 1). Although doxorubicin can induce a similar degree of relative caspase-3 activation and increase in TUNEL positivity in 1- and 3-wk-old hearts, the incidences of baseline and doxorubicin-induced cleaved caspase-3 and TUNEL positivity were rapidly downregulated in cardiomyocytes during the postnatal growth period (Fig. 10, B and C; Table 1). The incidence of TUNEL-positive cardiomyocytes induced by doxorubicin was ∼0.0466% (of total cardiomyocytes) in P7 ventricles compared with 0.0117% in P21 ventricles (Table 1), corresponding to a fourfold decrease.
Fig. 8.
Representative images of the staining used to distinguish cardiomyocyte nuclei from noncardiomyocyte nuclei in ventricular sections from P7 or P21 mice. Sections were stained with anti-LacZ (red) to label the nuclei of the cardiomyocytes [α-myosin heavy chain (αMHC)-nLacZ reporter mice] followed by DAPI (blue).
Fig. 9.
Cleaved caspase-3-positive cells are predominantly noncardiomyocytes in mouse ventricles. A: representative images of the staining used to discriminate cleaved caspase-3 in cardiomyocytes and noncardiomyocytes in ventricular sections from P7 or P21 mice treated for 24 h with doxorubicin at 10 mg/kg. Sections were stained with anti-LacZ (red) to label the nuclei of the cardiomyocytes (αMHC-nLacZ reporter mice) followed by immunostaining with anti-cleaved caspase-3 antibodies (Ac-casp3, green) and DAPI (blue). Quantification of cleaved caspase-3-positive cardiomyocytes and noncardiomyocytes from P7 (B) and P21 (C) ventricles; n = 6–8 for each data point. *P < 0.05 vs. cardiomyocytes. Arrows point to cleaved caspase-3 positive nuclei.
Fig. 10.
Downregulation of doxorubicin-induced TUNEL positive cardiomyocytes in mouse ventricles during postnatal growth period. A: representative images of the staining used to identify TUNEL positive signal in cardiomyocytes. Sections were stained with anti-LacZ (Red) to label the nuclei of the cardiomyocytes (αMHC-nLacZ reporter mice) followed by immunostaining with TUNEL staining (green). Quantification of TUNEL-positive cardiomyocytes from P7 and P21 mouse hearts treated for 24 h with saline (B) or 10 mg/kg doxorubicin (C). n = 6–8 for each data point. *P < 0.05 vs. P21 group subjected to the same treatment. Arrows point to TUNEL-positive nuclei.
DISCUSSION
The present study clearly demonstrates age-dependent effects on the incidence of doxorubicin-induced apoptosis in the myocardium during postnatal mouse heart development (summarized in Table 1). Our experimental data support the presence of functionally intact caspase-dependent death machinery in neonatal hearts, which is rapidly silenced after birth. We observed a prominent drop of baseline caspase-3 activity by ≥20-fold in mouse hearts within the first 3 wk after birth, associated with a rapid downregulation of the expression of key proapoptotic proteins, in particular procaspase-3. These biochemical results are supported by immunohistological data showing a significant decrease in the positivity of cleaved caspase-3 staining and TUNEL assay during the first 3 wk of postnatal heart development. Our study further demonstrates the impact of the functionally intact death machinery in neonatal hearts on the acute apoptotic response to doxorubicin, revealing a critical transition from high to low susceptibility to doxorubicin-induced apoptosis in mouse heart during postnatal heart maturation. Moreover, our study provides in vivo evidence that neonatal cardiomyocytes are more susceptible to doxorubicin-induced apoptotic stimuli than terminally differentiated cardiomyocytes.
In this study, a significant effort was devoted to quantifying caspase-3 activation, which is a central event in the caspase cascade. Here, active caspase-3 was separated from other proteases in tissue lysates by immunoadsorption before the incubation with the fluorometric substrate. This assay detects active caspase-3 only and differs from other methods using direct incubation of heart lysates with fluorometric caspase-3 substrates, which measure caspase-3-like activity. This approach also differs from previous studies mainly focused on proapoptotic gene regulation, which have shown a general downregulation of key apoptotic-regulating factors at both the mRNA and protein levels in postmitotic tissues including heart, skeletal muscle, and brain during postnatal development (4, 16, 32).
To determine the direct effects of doxorubicin on apoptotic signaling, the present study focused on the acute apoptotic response within 24 h of doxorubicin administration, which differs from the majority of the previous studies mainly focused on the chronic effects of doxorubicin treatment in vivo. A much greater induction of caspase-3 activation was observed in neonatal mouse hearts within 1 wk after birth compared with more mature hearts after 3 wk of age. The induction of limited proapoptotic regulating proteins, such as Bax, by doxorubicin in mature hearts is not sufficient to restore their sensitivity. Although a similar increase in cytochrome c release from mitochondria induced by doxorubicin was observed in 1- and 3-wk-old hearts, its effect on caspase-3 activation remains limited in 3-wk-old hearts, most likely due to lack of upregulation of other caspase-dependent machinery proteins by doxorubicin, such as procaspase-3, Apaf-1, and procaspase-9. Our observation is consistent with previous reports demonstrating that adult cardiomyocytes were resistant to cytochrome c-induced apoptosis, which could not be rescued by overexpression of Apaf-1 or inhibition of XIAP due to a global downregulation of the whole caspase-dependent machinery (13), whereas cytochrome c-induced apoptosis in neonatal cardiomyocytes was sensitive to overexpression of Apaf-1 or inhibition of XIAP (13, 19, 21). Our in vivo observations, together with previous in vitro studies, support the concept that concerted downregulation of procaspase-3 along with other caspase-dependent machinery proteins during postnatal heart maturation is mainly responsible for resistance to cytochrome c-induced apoptosis in the mature heart and that the absence of their general induction by cardiac injuries contributes to the resistance of mature hearts against caspase activation.
Besides the caspase-3-dependent apoptosis, other forms of cell death have been reported in doxorubicin-induced cardiotoxicity such as caspase-3-independent apoptosis (3), necrosis (22), autophagy, and senescence (12, 15, 17). Our study supports the concept that caspase-3-dependent apoptosis predominates over caspase-3-independent apoptosis under acute doxorubicin treatment. First, we observed a strong correlation of the incidence of cleaved caspase-3 positivity with that of TUNEL positivity in both 1- and 3-wk-old heart sections. Second, dual staining for cleaved caspase-3 and TUNEL revealed that >70% of TUNEL-positive cells were also positive for cleaved caspase-3 in both 1- and 3-wk-old heart sections. Finally, treatment with caspase-9 or caspase-8 inhibitors not only attenuated doxorubicin-induced caspase-3 activation but also reduced TUNEL positivity in neonatal hearts. Nevertheless, mitochondrial damage occurred in both 1- and 3-wk-old hearts as indicated by increased release of mitochondrial proteins (cytochrome c and EndoG) and increased susceptibility to calcium-induced MPT, which may be involved in necrosis and autophagy in addition to apoptosis. In this respect, no significant increase in autophagy (data not shown) and in lyososomal enzyme release was detected in both 1- and 3-wk hearts within 24 h after doxorubicin treatment. These results suggest that apoptosis, predominantly caspase-3-dependent apoptosis, occurs during the acute time window tested in the present study and that reduced caspase activation in adult hearts may limit the impact of mitochondrial damage on acute induction of cell death, thus allowing a better cardiac recovery after the clearance of administrated doxorubicin. Future studies are needed to determine the cell type, timeline, incidence, and extent to which other forms of cell death contribute to the dose-dependent doxorubicin cardiotoxicity in neonatal vs. mature hearts.
Cardiomyocytes are believed to be a major target of doxorubicin-induced cardiotoxicity. Using the transgenic LacZ reporter gene mice (1, 26), our study reports a decrease of about fourfold in the in vivo incidence of cardiomyocyte apoptosis from 1 to 3 wk of age after acute doxorubicin treatment (0.0466% for 1-wk and 0.0117% for 3-wk ventricles after doxorubicin treatment). It is important to note that this in vivo incidence of apoptosis represents a hundredfold difference from in vitro apoptotic indexes observed in cardiomyocyte culture studies, which are frequently 10–40% after 24 h of doxorubicin stimulation (28), indicating the importance of the in vivo micro-environmental effects on the mechanism and amplitude of cardiomyocyte apoptosis. It is also worth noting that apoptosis predominates in noncardiomyocytes as >90% of apoptosis occurred in noncardiomyocytes under all conditions tested, consistent with a recent observation in a monkey model of heart failure (18). In addition to cardiomyocytes, noncardiomyocytes in 1-wk-old neonatal hearts are also more susceptible to doxorubicin-induced apoptotic stimuli than those in 3-wk-old hearts, indicating that multiple cell types in the growing myocardium are targets of doxorubicin-induced myocardial toxicity and are responsible for the increased susceptibility to this drug observed in neonatal mice.
Our results, together with others (4, 16, 32), indicate that the time course of the downregulation of basal and inducible apoptotic signaling in mouse hearts parallels that of cell cycle withdrawal during postnatal growth (25), suggesting decreased apoptotic potential is associated with terminal differentiation and cell cycle exit of cardiomyocytes. In addition, downregulation of the key apoptotic regulatory proteins has also been observed in other postmitotic cells such as skeletal muscle cells (6) and neuronal cells (29). Finally, apoptotic gene silencing has also been found in stem cells after cardiac-directed differentiation and cell cycle exit (8). The mechanisms of postnatal apoptotic gene silencing in cardiomyocytes and in other postmitotic cells remain largely unknown. The same factors such as E2F1 may be involved in the suppression of cell cycle and apoptotic gene silencing in cardiomyocytes (10). However, other studies (32) have challenged this concept. A recent study (31) suggested a role for polypyrimidine tract binding protein through translational regulation of apoptotic genes including Apaf-1 and caspase-3. Other regulators involved in the transcriptional regulation of apoptotic genes remain to be discovered.
The current study draws attention to the marked effects of age on the doxorubicin-induced cardiac apoptotic response with an emphasis on the critical postnatal developmental window, providing conceptual frameworks for future mechanistic analyses on the age-dependent effects of doxorubicin in the heart. This in vivo analysis of the acute apoptotic signaling using a combination of biochemical, histological, and transgenic reporter mouse approaches clearly demonstrates the presence of functional caspase-dependent death machinery in the neonatal hearts and supports the hypothesis that acute induction of caspase-dependent apoptosis in immature cardiomyocytes and other cardiac cells with each exposure to doxorubicin contributes to the cumulative toxicity of doxorubicin. Cardiac progenitor cells in younger hearts could also be more sensitive to doxorubicin-induced cytotoxicity resulting in impaired cardiac regenerative capacity (7, 11).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-085098 (to L. Wei and R. M. Payne) and by the Riley Children's Foundation and the Lilly Endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S. and L.W. conception and design of research; J.S., L.Z., Y.-W.Z., and M.S. performed experiments; J.S., L.Z., Y.-W.Z., M.S., and L.W. analyzed data; J.S. and L.W. interpreted results of experiments; J.S. and L.W. prepared figures; J.S. and L.W. drafted manuscript; J.S., R.M.P., and L.W. edited and revised manuscript; J.S., L.Z., Y.-W.Z., M.S., R.M.P., and L.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mica Gosnell for technical assistance. We also thank Dr. Loren J. Field for many insightful comments on this study and for providing the αMHC-nLacZ transgenic mice.
REFERENCES
- 1. Ang KL, Shenje LT, Reuter S, Soonpaa MH, Rubart M, Field LJ, Galinanes M. Limitations of conventional approaches to identify myocyte nuclei in histologic sections of the heart. Am J Physiol Cell Physiol 298: C1603–C1609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 60: 1789–1792, 2000 [PubMed] [Google Scholar]
- 3. Bae S, Siu PM, Choudhury S, Ke Q, Choi JH, Koh YY, Kang PM. Delayed activation of caspase-independent apoptosis during heart failure in transgenic mice overexpressing caspase inhibitor CrmA. Am J Physiol Heart Circ Physiol 299: H1374–H1381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem 281: 22943–22952, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bien S, Rimmbach C, Neumann H, Niessen J, Reimer E, Ritter CA, Rosskopf D, Cinatl J, Michaelis M, Schroeder HW, Kroemer HK. Doxorubicin-induced cell death requires cathepsin B in HeLa cells. Biochem Pharmacol 80: 1466–1477, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Burgess DH, Svensson M, Dandrea T, Gronlund K, Hammarquist F, Orrenius S, Cotgreave IA. Human skeletal muscle cytosols are refractory to cytochrome c-dependent activation of type-II caspases and lack APAF-1. Cell Death Differ 6: 256–261, 1999 [DOI] [PubMed] [Google Scholar]
- 7. De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H, Hosoda T, Rota M, Urbanek K, Kajstura J, Leri A, Rossi F, Anversa P. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 121: 276–292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doss MX, Winkler J, Chen S, Hippler-Altenburg R, Sotiriadou I, Halbach M, Pfannkuche K, Liang H, Schulz H, Hummel O, Hubner N, Rottscheidt R, Hescheler J, Sachinidis A. Global transcriptome analysis of murine embryonic stem cell-derived cardiomyocytes. Genome Biol 8: R56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation 111: 1601–1610, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Hauck L, Hansmann G, Dietz R, von Harsdorf R. Inhibition of hypoxia-induced apoptosis by modulation of retinoblastoma protein-dependent signaling in cardiomyocytes. Circ Res 91: 782–789, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Huang C, Zhang X, Ramil JM, Rikka S, Kim L, Lee Y, Gude NA, Thistlethwaite PA, Sussman MA, Gottlieb RA, Gustafsson AB. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation 121: 675–683, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem 285: 793–804, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Konorev EA, Vanamala S, Kalyanaraman B. Differences in doxorubicin-induced apoptotic signaling in adult and immature cardiomyocytes. Free Radic Biol Med 45: 1723–1728, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 23: 2629–2636, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Lu L, Wu W, Yan J, Li X, Yu H, Yu X. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int J Cardiol 134: 82–90, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Madden SD, Donovan M, Cotter TG. Key apoptosis regulating proteins are downregulated during postnatal tissue development. Int J Dev Biol 51: 415–423, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Maejima Y, Adachi S, Ito H, Hirao K, Isobe M. Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell 7: 125–136, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Park M, Shen YT, Gaussin V, Heyndrickx GR, Bartunek J, Resuello RR, Natividad FF, Kitsis RN, Vatner DE, Vatner SF. Apoptosis predominates in nonmyocytes in heart failure. Am J Physiol Heart Circ Physiol 297: H785–H791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Potts MB, Vaughn AE, McDonough H, Patterson C, Deshmukh M. Reduced Apaf-1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. J Cell Biol 171: 925–930, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rayapureddi JP, Tomamichel WJ, Walton ST, Payne RM. TAT fusion protein transduction into isolated mitochondria is accelerated by sodium channel inhibitors. Biochemistry 49: 9470–9479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchis D, Mayorga M, Ballester M, Comella JX. Lack of Apaf-1 expression confers resistance to cytochrome c-driven apoptosis in cardiomyocytes. Cell Death Differ 10: 977–986, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis 53: 105–113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi J, Zhang YW, Summers LJ, Dorn GW, 2nd, Wei L. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J Mol Cell Cardiol 44: 551–560, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silva EM, Guillermo LV, Ribeiro-Gomes FL, De Meis J, Pereira RM, Wu Z, Calegari-Silva TC, Seabra SH, Lopes UG, Siegel RM, Dosreis GA, Lopes MF. Caspase-8 activity prevents type 2 cytokine responses and is required for protective T cell-mediated immunity against Trypanosoma cruzi infection. J Immunol 174: 6314–6321, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol Heart Circ Physiol 271: H2183–H2189, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science 264: 98–101, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL, Lipshultz SE. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol 32: 342–353, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Wu S, Ko YS, Teng MS, Ko YL, Hsu LA, Hsueh C, Chou YY, Liew CC, Lee YS. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: in vitro and in vivo studies. J Mol Cell Cardiol 34: 1595–1607, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Yakovlev AG, Ota K, Wang G, Movsesyan V, Bao WL, Yoshihara K, Faden AI. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci 21: 7439–7446, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 53: 2231–2247, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Bahi N, Llovera M, Comella JX, Sanchis D. Polypyrimidine tract binding proteins (PTB) regulate the expression of apoptotic genes and susceptibility to caspase-dependent apoptosis in differentiating cardiomyocytes. Cell Death Differ 16: 1460–1468, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Zhang J, Bahi N, Zubiaga AM, Comella JX, Llovera M, Sanchis D. Developmental silencing and independency from E2F of apoptotic gene expression in postmitotic tissues. FEBS Lett 581: 5781–5786, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch Immunol Ther Exp (Warsz) 57: 435–445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng X, Chu F, Mirkin BL, Sudha T, Mousa SA, Rebbaa A. Role of the proteolytic hierarchy between cathepsin L, cathepsin D and caspase-3 in regulation of cellular susceptibility to apoptosis and autophagy. Biochim Biophys Acta 1783: 2294–2300, 2008 [DOI] [PubMed] [Google Scholar]