Abstract
Obesity is an independent risk factor for cardiovascular disease. Data from the Framingham Study have reported a higher incidence of heart failure in obese individuals compared with a normal cohort. The body initially copes with the abundance of fuel present in an obese milieu by storing it in adipose tissue. However, when the storage capacity is exceeded, the excess energy is taken up and stored ectopically as fat in vital organs such as the heart. Indeed, intramyocardial lipid overload is present in hearts of obese patients, as well as in hearts of animal models of obesity, and is associated with a distinct gene expression profile and cardiac dysfunction. By imposing a metabolic stress on the heart, obesity causes it to hypertrophy and ultimately to fail. Conventional measures to treat obesity include diet, exercise, and drugs. More recently, weight loss surgery (WLS) has achieved increasing prominence because of its ability to reduce the neurohumoral load, normalize metabolic dysregulation, and improve overall survival. The effects of WLS on systemic metabolic, neurohumoral, and hemodynamic parameters are well described and include an early normalization of serum glucose and insulin levels as well as reduction in blood pressure. WLS is also associated with reverse cardiac remodeling, regression of left ventricular hypertrophy, and improved left ventricular and right ventricular function. By targeting the source of the excess energy, we hypothesize that WLS improves contractile function by limiting exogenous substrate availability to the metabolically overloaded heart. These changes have also been found to be associated with increased levels of adiponectin and improved insulin sensitivity. Taken together, the sustained beneficial effects of WLS on left ventricular mass and function highlight the need to better understand the mechanism by which obesity regulates cardiovascular physiology.
Keywords: diabetes, left ventricular mass, obesity
this article is part of a collection on Cardiovascular Response to Obesity and Diabetes. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
The Concept of Cardiac Responses to Metabolic Load
In one of his last papers after a long career in metabolic research, Hans Krebs stated that “in a competitive environment the chances of survival are greatest if resources are optimal” (3). A fitting example is the “Thrifty Gene Hypothesis” of James Neel (58), which postulates that obesity and type 2 diabetes were an evolutionary advantage in times of starvation (7). However, in modern times, where there is an oversupply of fuels, the body's inability to have recognized and readjusted to environmental and lifestyle changes has made its ability to continually store energy a disadvantage.
An individual's predilection to obesity and development of associated complications is multifactoral and dictated by an individual's exposome, defined as the sum of all environmental exposures from conception onward. According to Rappaport and Smith (69), an individual's exposures include those that enter the body from the environment, such as food, as well as those subsequently generated in excess within the body by inflammation, oxidative stress, and metabolic dysregulation, such as circulating free fatty acids and adipokines. The cumulative impact of the exposome leads to deviation from the healthy state of homeostasis to a state of chronic disease such as obesity (69). In most instances, as is the case with obesity, the body attempts to reestablish homeostatic equilibrium, a process that is termed “allostasis” by McEwen (52).
The specific mechanisms that the body uses in an attempt to reestablish homeostasis are termed its “allostatic responses.” The body's initial allostatic response to excess fuel is to partition it into adipose tissue to reduce the metabolic burden on its major organs such as the heart. However, when the storage capacity of adipose tissue is exceeded, the excess energy is stored ectopically as fat in vital organs, including the heart (16, 83). Stated otherwise, excess body weight ultimately spills over to create a “lipotoxic” environment in non-adipose tissue. In hearts of women who have been obese for over a decade (12 ± 5 years), sustained increases in cardiac fatty acid utilization, measured with positron emission tomography imaging, is associated with increased myocardial oxygen consumption and decreased mechanical efficiency (65). Such studies have helped to establish obesity as an independent predictor of increased myocardial oxygen consumption and decreased cardiac efficiency. Not surprisingly, as the obese continue to get bigger (79), the risk of premature death and disability from cardiovascular disease increases as the heart is subjected to increases in hemodynamic, neurohumoral, and metabolic load (1, 7, 15, 23, 34, 54) (Fig. 1).
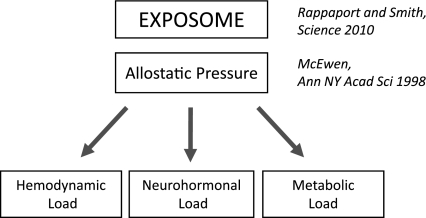
Fig. 1.
Proposed relationship of the exposome, susceptibility to allostatic pressure, and increased load on the heart in obesity. The exposome encompasses the genetic and epigenetic factors that dictate an individual's susceptibility to allostatic pressure. When an individual's ability to reestablish homeostatic equilibrium (allostasis) is exceeded, the heart is subjected to increased hemodynamic, neurohormonal, and metabolic load, and heart disease ensues.
In an effort to sustain efficiency and adequate tissue perfusion, the heart typically reverts to the energy sparing metabolic profile of the fetal gene program for energy substrate metabolism when stressed with an increase in load (19, 39). Unlike the healthy adult heart, which primarily metabolizes fatty acids for energy provision, the fetal heart increases its reliance on carbohydrates for energy provision. Cardiac efficiency, assessed as the ratio of left ventricular (LV) power output to myocardial oxygen consumption in isolated mouse hearts (perfused by the Langendorff technique), is greater with glucose than with fatty acids as substrates. Efficiency is decreased when fatty acid oxidation is increased (26). In the setting of diabetes, the heart's oversupply of fuel leads to an accumulation of metabolic signals that transcriptionally promote the adaptive fetal gene program in the heart (80) via the activation of transcription factors such as c-myc and/or Sp1 (87). This represents the heart's attempt to use the most efficient energy providing substrate in a diabetic milieu (10, 86). In obesity, increased adipose tissue lipolysis raises the supply of fatty acids to the heart while the heart's ability to oxidize glucose is impaired. In skeletal muscle, targeted metabolomic analyses have demonstrated that obesity-related insulin resistance contributes to metabolic dysfunction by impairing carbohydrate utilization and promoting excessive but incomplete β-oxidation, at the center of which is a depletion of Krebs cycle intermediates in the mitochondrial matrix (40). It is likely that a similar mechanism also exists in the heart. We have previously identified that intramyocardial lipid overload, measured by oil-red-O staining, is common in obese and diabetic patients with nonischemic heart failure and that it is associated with a distinct gene expression profile that is similar to an animal model of lipotoxicity and cardiac dysfunction (74, 81). Of the 27 patients with advanced heart failure, we found evidence of lipotoxicity in one-third of the hearts at their time of removal in the course of cardiac transplantation. All of these patients were either obese and/or insulin resistant (74). The mechanism underlying lipotoxic cardiac dysfunction involves the production of reactive oxygen species (74), impairment of calcium handling (43), and the buildup of toxic metabolites (27, 88) and is known to get progressively worse with time. Cardiac dysfunction progresses as obesity related complications impose further stress on the heart and activate other deleterious pathways.
In summary, the oversupply of energy-providing substrates in obesity subjects the heart to an increased metabolic load and disturbs the internal milieu of the healthy human heart. Sustained flooding with excess fuel exceeds the body's capacity to store the excess energy, which is ultimately shuttled into the peripheral organs such as the heart. Once the heart's capacity to cope with the fuel burden is exceeded, cardiac dysfunction and heart failure ensue. We now explore the potential for weight loss surgery (WLS) to metabolically unload the stressed heart in obesity.
Consequences of WLS for the Heart
Given the increasing number of WLSs being performed every year and the observed positive impact of weight loss on obesity, diabetes, and heart disease, we are in a position to evaluate the cardiac response to rapid weight loss. Studying the reversibility of the cardiac complications in response to weight loss will provide further understanding of the adaptive and maladaptive processes of the heart to changes in the internal environment.
It is widely recognized that weight loss improves cardiac function in obese patients and that WLS is the most effective means of significant and sustained weight loss. The two most common forms of WLS performed in the following studies include the adjustable gastric band and the proximal Roux-en-Y gastric bypass. The adjustable gastric band induces gastric restriction by wrapping a synthetic, inflatable band around the stomach laparoscopically, whereas the Roux-en-Y bypass procedure creates an anastamosis between the distal small intestine (Roux) to a small proximal gastric pouch formed by stapling the stomach (9). Both forms of WLS have demonstrated cardiovascular benefits in obese patients. The impact of WLS on cardiovascular risk factors was recently highlighted by a scientific statement of the American Heart Association (66). The most striking effects of WLS on systemic metabolic parameters include early normalization of insulin sensitivity and gut incretin levels in 12 obese patients within days after surgery, as assessed by measuring plasma concentrations of glucose, insulin, leptin, and gut peptide hormones before and for 180 min after mealtime. (14, 67). The early positive impact on these and other parameters, including cardiac parameters, is sustained even as patients stop losing weight and remain obese at the nadir (2, 6, 31, 48). Several studies highlight the association between weight loss and improved cardiac function. In a 10-year follow-up study of obese patients who failed to lose weight after WLS [body mass index (BMI), 42.5 kg/m2], cardiac function was less superior than those who lost weight after WLS even though they remained obese (BMI, 31.5 kg/m2) (36). The authors note that cardiac dysfunction was related to total body fat and visceral adiposity, whereas LV volume, stroke volume, and cardiac output were associated with lean body mass. In a prospective study comparing cardiac changes in obese patients at baseline and two years after WLS versus those with no surgery, WLS was associated with reverse cardiac remodeling and improved LV and right ventricular function (60). In this study, a significant reduction in LV mass (LVM) and LV hypertrophy (LVH) were noted by cardiac magnetic resonance imaging (MRI) as was a significant improvement in midwall fractional shortening by echocardiography two years after WLS, even though the group remained clinically obese (BMI, 32.2 kg/m2). In contrast to others (55, 70), LVM in this cohort was mostly associated with changes in BMI only, not visceral adiposity and waist circumference. Using MRI, Rider et al. (71) attributed LVH to lean body mass, LV stroke volume, and visceral fat mass and demonstrated that WLS- and diet-induced weight loss lead to partial regression of cardiac hypertrophy and reversal of diastolic dysfunction and aortic distensibility impairment as assessed by cardiac MRI. In our series of studies on severely obese women undergoing WLS, we showed that favorable changes in LV function occurred as early as three months after WLS and were associated with improvements in insulin sensitivity and decreases in serum glucose and insulin concentrations (47); nine months after WLS, normalization of LV diastolic function was accompanied by restored insulin sensitivity, a decline in serum leptin levels, a decrease in muscle fat, and a significant decrease in transcript levels of peroxisome proliferator-activated receptor (PPAR)-α and PPAR-α-regulated genes such as medium-chain acyl-CoA dehydrogenase (mcad), carnitine palmitoyltransferase 1 (cpt-1), and uncoupling protein 3 (ucp3) in skeletal muscle (Fig. 2)(48); after nine months, patients remained clinically obese, as changes in BMI, body composition, and metabolic markers of obesity plateaued, but LVM continued to decrease linearly throughout the 24-month study period (2). In the latter study, LVH was prominent preoperatively (average LVM/Ht2.7, 49.85 g/m2.7). LVH was normalized after three months (LVM/Ht2.7 46.73 g/m2.7) and continued to decrease at 9 and 24 months after surgery (LVM/Ht2.7, 37.76 g/m2.7at 24 months) even as weight loss reached a nadir (BMI, 32.4 kg/m2 at 24 months) (Fig. 3)(2). This impact of WLS on the heart is further exemplified by dramatic reversal of cardiac dysfunction of obese heart failure patients (32, 51, 72). In a retrospective study WLS was shown to be safe and effective in morbidly obese patients with heart failure, and improved heart function compared with diet and exercise matched controls (68).
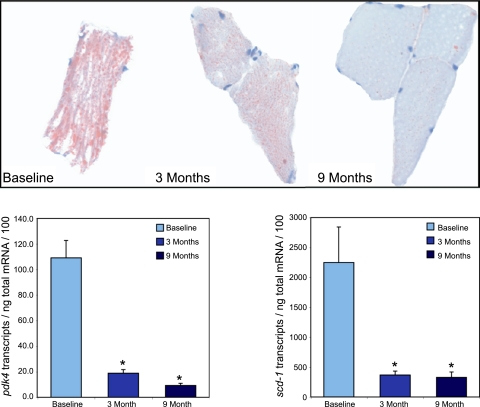
Fig. 2.
Representative oil-red-O stain of vastus lateralis biopsy sample and mRNA transcript levels of pyruvate dehydrogenase kinase 4 (pdk4) and human stearoyl CoA desaturase 1 (scd-1) in obese patients at baseline, 3 mo , and 9 mo after bariatric surgery. Top: oil-red-O staining at baseline and 3 and 9 mo after surgery (P < 0.009). Bottom: mRNA transcript levels of pdk4 and scd-1 at baseline and after surgery (P < 0.001). Permission was obtained to reuse figure art from Leichman et al. (48).
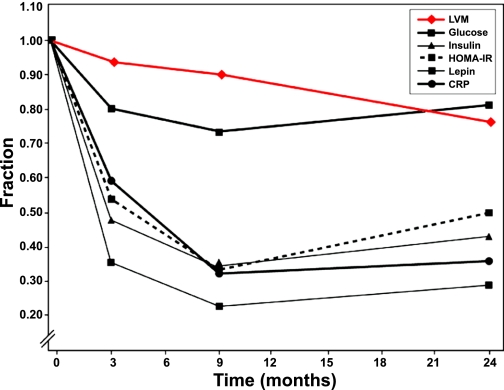
Fig. 3.
Fractional change in left ventricular mass (LVM) and metabolic markers of obesity up to 24 months after bariatric surgery. The decrease in LVM was linear, whereas the decrease in metabolic markers of obesity stabilized after 9 months. HOMA-IR, homeostasis model assessment of insulin resistance; CRP, C-reactive protein. Permission was obtained to reuse figure art from Algahim et al. (2).
The effect of WLS on the heart is evident. However, just as the cardiac response to obesity is complex and multifaceted, the impact of significant weight loss on the heart is more than a U-turn reversal of excess adiposity. The early effects of WLS on cardiac function and the associated metabolic changes before correction of body weight suggest that metabolic and neurohormonal changes precede and may regulate the improvements in systemic metabolism and cardiac function that have been observed. Systemically, WLS disrupts the gastrointestinal tract and causes weight loss by restricting food intake and/or causing malabsorption of calories and nutrients. The sudden decrease of caloric consumption leads to a state of starvation. Just as obesity can be considered a part of an allostatic response to maintain energy homeostasis in the face of excess caloric consumption, sudden and rapid depletion of energy after WLS may activate allostatic responses that oppose those activated by obesity by mobilizing the excess endogenous adipose tissue stores for energy. Aside from a subsequent decrease in weight and reversal of metabolic dysregulation, the decrease in caloric consumption likely activates biological mechanisms that protect against starvation and initially resist weight loss (46, 75). Although several studies have shown a paradoxical increase in satiety promoting gut hormones such as glucagon-like peptide 1 (GLP-1) and peptide YY (5, 44), particularly after Roux-en-Y gastric bypass surgery, this satiety feeling appears transient, as patients do not reach ideal body weight and many regain weight in the long term (73, 76). Nonetheless, the increase gastrointestinal hormones GLP-1 and peptide YY have been implicated in the resolution of insulin resistance and type 2 diabetes after WLS (14, 64). This begs the question, How does WLS improve insulin sensitivity? The endoplasmic reticulum (ER) has received considerable attention recently for its role in mediating obesity-induced insulin resistance both in cell culture and in mice fed a high-fat diet (61). In the normal adipocyte, the ER is responsible for balancing the nutritional status of the cell to the need for protein synthesis, energy storage, and continued nutrient sensing. When supplied with excess substrate, adipocyte ER undergo ER stress, which has been associated with the development of insulin resistance (22). Oral administration of ER stress relieving chemical chaperones 4-phenyl butyric acid and taurine-conjugated ursodeoxycholic acid, which have been shown to modulate the ER and increase protein folding capacity, systemically normalizes serum glucose levels, restores systemic insulin sensitivity, resolves fatty liver disease, and enhances insulin action in liver, muscle, and adipose tissues of ob/ob mice (62). In obese patients, WLS is associated with a reduction in ER stress in adipose tissue and liver, which directly correlates with reversal of insulin resistance (21). WLS also reduces substrate supply to the heart, and it is plausible that the beneficial cardiovascular effects may be mediated by a reduction in cardiac ER stress, as well.
Ultimately, WLS leads to forced weight loss and activation of biochemical pathways in an effort to reestablish energy homeostasis and alleviate the allostatic stress of obesity. This translates to decreased adipose tissue and reversal of metabolic and neurohormonal stress to unload the heart and reverse obesity related cardiac dysfunction. We now discuss the exogenous metabolic and humoral effectors that regulate both structure and function of the heart in response to WLS.
The Concept of Metabolically Unloading the Heart
We propose that improvements in cardiac metabolism and function after WLS are mediated by metabolic and neurohormonal factors. The rapid depletion in energy after WLS, compounded by the alterations of the gastrointestinal tract, activates biochemical pathways that seek to restore metabolic homeostasis. Excess endogenous adipose tissue stores are mobilized, fat mass decreases, and metabolic dysregulation is reversed. In the heart, cardiovascular hemodynamics are improved, and the associated improvements in cardiac structure and function in response to WLS are likely as complex and interrelated as the cardiac response to obesity. On a molecular level, obese Sprague-Dawley rats that undergo WLS demonstrate reduced hepatic steatosis and increased AMP-activated protein kinase (AMPK) phosphorylation after WLS (63). AMPK is known to be activated by caloric restriction (57). Caloric restriction has been shown to promote longevity in mice through the induction of autophagy, the catabolic process of degrading a cell's own components through the lysosomal machinery (25, 37, 42, 56). It is therefore plausible that on a molecular level the beneficial effects of WLS on the heart are also mediated by a reduction in substrate supply and may be dependent on AMPK activation and the induction of autophagy, although this has yet to be studied.
Tight coupling of substrate uptake and utilization is intimately related to contractile function and efficiency of the heart. As mentioned earlier, oversupply of energy substrates to the heart leads to a lipotoxic environment: myocellular metabolic dysregulation, lipid accumulation, and the induction of molecular processes that lead to decreased cardiac efficiency. We make the point here that by targeting the source of the excess energy, WLS improves contractile function by limiting exogenous substrate availability to the metabolically overloaded heart. Similarly, because insulin resistance prevents substrate uptake into peripheral organs such as the heart, it may in fact be cardioprotective itself and merely a marker, not a mediator, of cardiac disease. The rapid reversal of insulin resistance, the normalization of glucose metabolism, and the associated improvements in cardiac function early after WLS support this notion. We reported a sharp decline in metabolic markers of obesity and normalization of LVH within three months after WLS; LVM continued to decrease for 15 months after these markers stabilized in the normal range (Fig. 2) (2). On the contrary, insulin sensitizers, such as thiazolidinediones (TZDs), increase glucose uptake and oxidation in the insulin-responsive, metabolically overloaded heart. At the same time they decrease glucose, fatty acid, and triglyceride levels in the systemic circulation (18). Indeed, TZDs or TZD analogs are PPARγ agonists that promote adipocyte differentiation and lipogenesis; overexpression of PPARγ in cardiomyocytes of mice is associated with increased lipid and glycogen stores and development of dilated cardiomyopathy (77). It is possible that this contributes to explaining the increased risk of heart failure and adverse cardiac events associated with their use (11, 20, 49). Taken together, the data suggest that whereas systemic administration of TZDs may improve whole body metabolism, direct PPARγ activation in the heart may contribute to its reported adverse events. In contrast, the cardioprotective effects of metformin are associated with its decrease in substrate supply to the heart by lowering circulating nonesterified free fatty acids, inhibiting hepatic gluconeogenesis, improving peripheral glucose uptake, improving energy homeostasis (84), and enhancing cardiac autophagy through activation of AMPK (85). The cardioprotective effects of metformin are not demonstrated in an animal model of nondiabetic heart failure (4), highlighting the impact of metabolic stress on the heart and its reversibility with metformin.
Aside from the hormonal changes already mentioned, changes in adipokine levels have also been implicated in the reversal of the metabolic dysregulation and improvement of insulin sensitivity after WLS. Adiponectin is an anti-inflammatory cytokine important for cardiovascular structure and function (28); low levels of adiponectin have been identified as an independent risk factor for cardiovascular disease (17, 38) and insulin resistance in obesity. WLS increases levels of adiponectin that correlates with improved insulin sensitivity (24, 82), lower LVM (53), and lower parameters for LVH (12, 41, 59). The mechanism by which adiponectin affects the heart remains unclear, but in vitro experiments suggest that adiponectin accelerates fatty-acid oxidation in the heart (50). Leptin plays a critical role in lipid compartmentalization and prevention of ectopic fat deposition; it also promotes fatty-acid oxidation in nonadipose tissue, including the heart (45). WLS reverses leptin resistance, and decreased serum leptin levels are associated with weight loss, loss of fat mass, and decreased insulin resistance (30, 82), suggesting multiple mechanisms for improved cardiac metabolism and function secondary to leptin sensitization. Resistin is another adipokine of recent scrutiny; higher levels of resistin are associated with insulin resistance (78) and decreased LV systolic function (53). The impact of WLS on levels of resistin are conflicting and appear to be related to the type of surgery performed, the amount of weight lost, and the postoperative time of quantification (8, 13, 33). Much work remains to understand the role of resistin in obesity and reverse cardiac remodeling after WLS.
Aside from the improvements in cardiac metabolism, the decrease in excess body weight and sympathetic tone after WLS relieve the heart of pressure overload as well. In patients with heart failure and sleep apnea, correction of sleep disordered breathing has been shown to improve the LV ejection fraction, reduce basal heart rate, and lower blood pressure (35). These effects likely contribute to the improvement in cardiac function after WLS, as well. Indeed, improvements in LVH and cardiac function after WLS are also associated with improvement in aortic elasticity after WLS (29, 71).
Conclusions
WLS leads to the early adjustment of metabolic and neurohumoral pathways that seek to maintain energy homeostasis and subsequently lead to the reversal of obesity-related hemodynamic, metabolic, and cardiac dysfunction. We propose that a new framework of inquiry is needed to evaluate these factors and the cardiac response to the rapid and sudden changes in weight.
While WLS reverses obesity-related complications, it does not necessarily correct obesity per se. Consideration of the exposome and its impact on allostasis stresses the dynamic nature of the processes that govern the development (and reversal) of obesity and chronic disease. The sustained beneficial effects of WLS on LVM and function may be a new model for assessing the role of the environment on cardiovascular physiology.
GRANTS
Work from the authors' laboratory was supported by National Heart, Lung, and Blood Institute Grants R01-HL-073162 and R01-HL-61483 and National Institutes of Health T32 Training Program: Center for Clinical and Translational Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Roxy Tate for expert editorial help.
REFERENCES
- 1. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 355: 763–778, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Algahim MF, Lux TR, Leichman JG, Boyer AF, Miller CC, III, Laing ST, Wilson EB, Scarborough T, Yu S, Snyder B, Wolin-Riklin C, Kyle UG, Taegtmeyer H. Progressive regression of left ventricular hypertrophy two years after bariatric surgery: an unexpected dissociation with the body mass index. Am J Med 123: 549–555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baldwin JE, Krebs H. The evolution of metabolic cycles. Nature 291: 381–382, 1981 [DOI] [PubMed] [Google Scholar]
- 4. Benes J, Kazdova L, Drahota Z, Houstek J, Medrikova D, Kopecky J, Kovarova N, Vrbacky M, Sedmera D, Strnad H, Kolar M, Petrak J, Benada O, Skaroupkova P, Cervenka L, Melenovsky V. Effect of metformin therapy on cardiac function and survival in a volume-overload model of heart failure in rats. Clin Sci (Lond) 121: 29–41, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 93: 210–215, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Butner KL, Nickols-Richardson SM, Clark SF, Ramp WK, Herbert WG. A review of weight loss following Roux-en-Y gastric bypass vs restrictive bariatric surgery: impact on adiponectin and insulin. Obes Surg 20: 559–568, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr 26: 1–22, 2006 [DOI] [PubMed] [Google Scholar]
- 8. de Luis DA, Terroba MC, Cuellar L, Conde R, Primo D, Aller R, Sagrado MG, Izaola O. Resistin levels in morbid obese patients following the biliopancreatic diversion surgery. Horm Metab Res 43: 205–208, 2011 [DOI] [PubMed] [Google Scholar]
- 9. DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med 356: 2176–2183, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N, Davies PJ, Taegtmeyer H. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol 32: 985–996, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366: 1279–1289, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Ebinc H, Ebinc FA, Ozkurt ZN, Dogru MT, Tulmac M, Yilmaz M, Caglayan O. Impact of adiponectin on left ventricular mass index in non-complicated obese subjects. Endocr J 55: 523–528, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Edwards C, Hindle AK, Fu S, Brody F. Downregulation of leptin and resistin expression in blood following bariatric surgery. Surg Endosc 25: 1962–1968, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 96: 2227–2235, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 293: 1861–1867, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Friedman J. Fat in all the wrong places. Nature 415: 268–269, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab 92: 571–576, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Golfman LS, Wilson CR, Sharma S, Burgmaier M, Young ME, Guthrie PH, Van Arsdall M, Adrogue JV, Brown KK, Taegtmeyer H. Activation of PPARγ enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab 289: E328–E336, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem 273: 29530–29539, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 304: 411–418, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58: 693–700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gregor MG, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 48: 1905–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Gu D, He J, Duan X, Reynolds K, Wu X, Chen J, Huang G, Chen CS, Whelton PK. Body weight and mortality among men and women in China. JAMA 295: 776–783, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Guldstrand M, Ahren B, Adamson U. Improved β-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab 284: E557–E565, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281: 29776–29787, 2006 [DOI] [PubMed] [Google Scholar]
- 26. How OJ, Aasum E, Kunnathu S, Severson DL, Myhre ES, Larsen TS. Influence of substrate supply on cardiac efficiency, as measured by pressure-volume analysis in ex vivo mouse hearts. Am J Physiol Heart Circ Physiol 288: H2979–H2985, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 96: 1006–1013, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol 165: 574–90, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ikonomidis I, Mazarakis A, Papadopoulos C, Patsouras N, Kalfarentzos F, Lekakis J, Kremastinos DT, Alexopoulos D. Weight loss after bariatric surgery improves aortic elastic properties and left ventricular function in individuals with morbid obesity: a 3-year follow-up study. J Hypertens 25: 439–447, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Infanger D, Baldinger R, Branson R, Barbier T, Steffen R, Horber FF. Effect of significant intermediate-term weight loss on serum leptin levels and body composition in severely obese subjects. Obes Surg 13: 879–888, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Ippisch HM, Inge TH, Daniels SR, Wang B, Khoury PR, Witt SA, Glascock BJ, Garcia VF, Kimball TR. Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol 51: 1342–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Iyengar S, Leier CV. Rescue bariatric surgery for obesity-induced cardiomyopathy. Am J Med 119: e5–e6, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Jankiewicz-Wika J, Kolomecki K, Cywinski J, Piestrzeniewicz K, Swietoslawski J, Stepien H, Komorowski J. Impact of vertical banded gastroplasty on body weight, insulin resistance, adipocytokine, inflammation and metabolic syndrome markers in morbidly obese patients. Endokrynol Pol 62: 109–119, 2011 [PubMed] [Google Scholar]
- 34. Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, Samet JM. Body-mass index and mortality in Korean men and women. N Engl J Med 355: 779–787, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, Ando S, Bradley TD. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med 348: 1233–1241, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Kardassis D, Bech-Hanssen O, Schonander M, Sjostrom L, Petzold M, Karason K. Impact of body composition, fat distribution and sustained weight loss on cardiac function in obesity. 2011. March 2 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 290: 1717–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koenig W, Khuseyinova N, Baumert J, Meisinger C, Lowel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. J Am Coll Cardiol 48: 1369–1377, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol 278: H1345–H1351, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Koves T, Ussher J, Noland R, Slentz D, Mosedale M, Ikayeva O, Bain J, Stevens R, Dyck J, Newgard C, Lopaschuk G, Muoio D. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 5–6, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Kozakova M, Muscelli E, Flyvbjerg A, Frystyk J, Morizzo C, Palombo C, Ferrannini E. Adiponectin and left ventricular structure and function in healthy adults. J Clin Endocrinol Metab 93: 2811–2818, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature 432: 1032–1036, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Lagadic-Gossmann D, Buckler KJ, Le Prigent K, Feuvray D. Altered Ca2+ handling in ventricular myocytes isolated from diabetic rats. Am J Physiol Heart Circ Physiol 270: H1529–H1537, 1996 [DOI] [PubMed] [Google Scholar]
- 44. le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780–785, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH. Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J Biol Chem 276: 5629–5635, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Leichman JG, Aguilar D, King TM, Mehta S, Majka C, Scarborough T, Wilson EB, Taegtmeyer H. Improvements in systemic metabolism, anthropometrics, and left ventricular geometry 3 months after bariatric surgery. Surg Obes Relat Dis 2: 592–599, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leichman JG, Wilson EB, Scarborough T, Aguilar D, Miller CC, III, Yu S, Algahim MF, Reyes M, Moody FG, Taegtmeyer H. Dramatic reversal of derangements in muscle metabolism and diastolic left ventricular function after bariatric surgery. Am J Med 121: 966–973, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298: 1180–1188, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res 101: 335–347, 2007 [DOI] [PubMed] [Google Scholar]
- 51. McCloskey CA, Ramani GV, Mathier MA, Schauer PR, Eid GM, Mattar SG, Courcoulas AP, Ramanathan R. Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surg Obes Relat Dis 3: 503–507, 2007 [DOI] [PubMed] [Google Scholar]
- 52. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 338: 171–179, 1998 [DOI] [PubMed] [Google Scholar]
- 53. McManus DD, Lyass A, Ingelsson E, Massaro JM, Meigs JB, Aragam J, Benjamin EJ, Vasan RS. Relations of Circulating Resistin and Adiponectin and Cardiac Structure and Function: The Framingham Offspring Study. Obesity (Silver Spring) 2011. February 24 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mokdad A, Ford E, Bowman B, Dietz W, Vinicor F, Bales V, Marks J. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA 289: 76–79, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Morricone L, Malavazos AE, Coman C, Donati C, Hassan T, Caviezel F. Echocardiographic abnormalities in normotensive obese patients: relationship with visceral fat. Obes Res 10: 489–498, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis 1: e10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 10: 458–467, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 14: 353–362, 1962 [PMC free article] [PubMed] [Google Scholar]
- 59. Ohara T, Kim J, Asakura M, Asanuma H, Nakatani S, Hashimura K, Kanzaki H, Funahashi T, Tomoike H, Kitakaze M. Plasma adiponectin is associated with plasma brain natriuretic peptide and cardiac function in healthy subjects. Hypertens Res 31: 825–831, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, Gallagher J, Williams Z, Preece K, Gundersen N, Strong MB, Pendleton RC, Segerson N, Cloward TV, Walker JM, Farney RJ, Gress RE, Adams TD, Hunt SC, Litwin SE. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. J Am Coll Cardiol 57: 732–739, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peng Y, Rideout DA, Rakita SS, Gower WR, Jr, You M, Murr MM. Does LKB1 mediate activation of hepatic AMP-protein kinase (AMPK) and sirtuin1 (SIRT1) after Roux-en-Y gastric bypass in obese rats? J Gastrointest Surg 14: 221–228, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Perugini RA, Malkani S. Remission of type 2 diabetes mellitus following bariatric surgery: review of mechanisms and presentation of the concept of ‘reversibility’. Curr Opin Endocrinol Diabetes Obes 18: 119–128, 2011 [DOI] [PubMed] [Google Scholar]
- 65. Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109: 2191–2196, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Poirier P, Cornier MA, Mazzone T, Stiles S, Cummings S, Klein S, McCullough PA, Ren Fielding C, Franklin BA. Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation 123: 1683–1701, 2011 [DOI] [PubMed] [Google Scholar]
- 67. Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222: 339–352, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ramani GV, McCloskey C, Ramanathan RC, Mathier MA. Safety and efficacy of bariatric surgery in morbidly obese patients with severe systolic heart failure. Clin Cardiol 31: 516–520, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rappaport SM, Smith Epidemiology MT. Environment and disease risks. Science 330: 460–461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rider OJ, Francis JM, Ali MK, Byrne J, Clarke K, Neubauer S, Petersen SE. Determinants of left ventricular mass in obesity; a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 11: 9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rider OJ, Francis JM, Ali MK, Petersen SE, Robinson M, Robson MD, Byrne JP, Clarke K, Neubauer S. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol 54: 718–726, 2009 [DOI] [PubMed] [Google Scholar]
- 72. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail 14: 198–202, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab 91: 4223–4231, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18: 1692–1700, 2004 [DOI] [PubMed] [Google Scholar]
- 75. Sims EA, Danforth E., Jr Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 29: 457–496, 1973 [DOI] [PubMed] [Google Scholar]
- 76. Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693, 2004 [DOI] [PubMed] [Google Scholar]
- 77. Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest 117: 2791–2801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab 13: 18–23, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med 163: 2146–2148, 2003 [DOI] [PubMed] [Google Scholar]
- 80. Taegtmeyer H, Sen S, Vela D. Return to the fetal gene program: a suggested metabolic link to gene expression in the heart. Ann NY Acad Sci 1188: 191–198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Taylor M, Wallhaus T, DeGrado T, Russell D, Stanko P, Nickles R, Stone C. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid. J Nucl Med 42: 55–62, 2001 [PubMed] [Google Scholar]
- 82. Trakhtenbroit MA, Leichman JG, Algahim MF, Miller CC, 3rd, Moody FG, Lux TR, Taegtmeyer H. Body weight, insulin resistance, and serum adipokine levels 2 years after 2 types of bariatric surgery. Am J Med 122: 435–442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—an allostatic perspective. Biochim Biophys Acta 1801: 338–349, 2010 [DOI] [PubMed] [Google Scholar]
- 84. Wang X, Zhang J. Metformin improves cardiac functin in rats via activation of AMP-activated protein kinase. Clin Experm Pharmacol Physiol 38: 94–101, 2011 [DOI] [PubMed] [Google Scholar]
- 85. Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60: 1770–1778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol 34: 223–231, 2002 [DOI] [PubMed] [Google Scholar]
- 87. Young ME, Yan J, Razeghi P, Cooksey RC, Guthrie PH, Stepkowski SM, McClain DA, Tian R, Taegtmeyer H. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul Syst Biol 1: 251–262, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97: 1784–1789, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]