Abstract
Early afterdepolarizations (EADs) have been implicated in severe cardiac arrhythmias and sudden cardiac deaths. However, the mechanism(s) for EAD genesis, especially regarding the relative contribution of Ca2+ wave (CaW) vs. L-type Ca current (ICa,L), still remains controversial. In the present study, we simultaneously recorded action potentials (APs) and intracellular Ca2+ images in isolated rabbit ventricular myocytes and systematically compared the properties of EADs in the following two pharmacological models: 1) hydrogen peroxide (H2O2; 200 μM); and 2) isoproterenol (100 nM) and BayK 8644 (50 nM) (Iso + BayK). We assessed the rate dependency of EADs, the temporal relationship between EADs and corresponding CaWs, the distribution of EADs over voltage, and the effects of blockers of ICa,L, Na/Ca exchangers, and ryanodine receptors. The most convincing evidence came from the AP-clamp experiment, in which the cell membrane clamp was switched from current clamp to voltage clamp using a normal AP waveform without EAD; CaWs disappeared in the H2O2 model, but persisted in the Iso + BayK model. We postulate that, although CaWs and reactivation of ICa,L may act synergistically in either case, reactivation of ICa,L plays a predominant role in EAD genesis under oxidative stress (H2O2 model), while spontaneous CaWs are a predominant cause for EADs under Ca2+ overload condition (Iso + BayK model).
Keywords: reactive oxygen species, β-adrenergic stimulation, Ca2+ wave, L-type Ca2+ current, Na/Ca exchanger
early afterdepolarizations (EADs), delayed afterdepolarizations (DADs), and triggered activities (TAs) are implicated in arrhythmias and sudden cardiac deaths (4). Although afterdepolarizations and TAs have been extensively studied, their underlying mechanisms remain incompletely understood. While there is a consensus on the role of spontaneous sarcoplasmic reticulum (SR) Ca2+ release and Ca2+ waves (CaWs) in the generation of DADs (36), significant discrepancies exist regarding the mechanisms of EADs. For example, early experimental and computer model studies (14, 19, 44) suggested EADs are exclusively caused by the reactivation of L-type calcium current (ICa,L) without the involvement of CaWs. However, accumulating evidence obtained from recent experimental (33, 40) and computer simulation studies (13) support a mechanism involving CaW for EAD formation under Ca overload conditions. Thus it seems likely that EAD generation can be mediated by at least two mechanisms: 1) sarcolemma-dependent (or ICa,L-dominant) mechanism: enhancement of inward currents during repolarization, such as ICa,L; and 2) SR-dependent (or CaW-dominant) mechanism: Na/Ca exchange current (INCX) or transient inward current (Iti) initiated by spontaneous SR Ca2+ release through the ryanodine receptor (RyR) under Ca2+ overload conditions. Furthermore, these two mechanisms (i.e., ICa,L and INCX) are considered to be highly interactive and function synergistically to induce EADs (41). However, disputes still remain on the relative contribution of different mechanisms to EAD generation in different models (9, 31, 40). It is unquestionable that a better understanding of the factors playing a primary role in EAD formation may be helpful for developing therapeutic approaches.
In our previous studies, we have established the following two different EAD models in isolated rabbit ventricular myocytes: 1) hydrogen peroxide (H2O2) model: EADs induced by reactive oxygen species (ROS) and H2O2 (42); and 2) Iso + BayK model: EADs induced by isoproterenol (100 nM) in addition to BayK 8644 (50 nM) (43). Here, to further clarify the mechanisms accounting for EADs under different conditions, we make systematic comparisons between these two types of EADs. We have provided more convincing evidence suggesting that ICa,L reactivation and CaW may account for distinct predominant ionic mechanisms under different conditions, although they may act synergistically to generate EADs.
MATERIALS AND METHODS
Cell isolation.
Single ventricular myocytes were enzymatically isolated from adult rabbit hearts. Briefly, the hearts were removed from adult New Zealand White rabbits (2–3 kg), anesthetized with intravenous pentobarbital sodium, and hearts were perfused retrogradely in Langendorff fashion at 37°C with nominally Ca2+-free Tyrode solution containing ∼1.4 mg/ml collagenase (type II; Worthington) and 0.1 mg/ml protease (type XIV; Sigma) for 25–30 min. After the enzyme solution was washed out, the hearts were removed from the perfusion apparatus and swirled in a culture dish. The myocytes were isolated from left ventricles without specific separation from different layers. The Ca2+ concentration was slowly increased to 1.8 mM, and the cells were stored at room temperature and used within 8 h. The use and care of the animals in these experiments were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Medicine and Dentistry of New Jersey–New Jersey Medical School.
Patch-clamp methods.
Myocytes were patch clamped using the perforated whole cell configuration of the patch-clamp technique in the current-clamp or voltage-clamp mode. Recording pipettes (resistance, 2–4 MΩ) were filled with internal solution containing the following (in mM): 110 K+-aspartate, 30 KCl, 5 NaCl, 10 HEPES, 0.1 EGTA, 5 MgATP, 5 Na2-creatine phosphate, and 0.05 cAMP (pH 7.2, adjusted with KOH). Myocytes were superfused with normal Tyrode solution containing the following (in mM): 136 NaCl, 5.4 KCl, 0.33 Na2PO4, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (pH 7.4, adjusted with NaOH). Action potentials (APs) were elicited with 2-ms, 2- to 4-nA square pulses at various pacing cycle lengths (PCLs). In some cells, Ca2+ transients/waves were recorded under current-clamp or AP-clamp (voltage-clamp with a fixed AP waveform) conditions. Electrical signals were measured with an MultiClamp 700A patch-clamp amplifier, controlled by a personal computer using a Digidata 1322A interface, driven by pCLAMP 10 software.
Intracellular Ca2+ concentration measurement.
Myocytes were loaded with the Ca2+ indicator fluo-4 by incubating them for ∼30 min in bath solution containing 4 μM fluo-4 AM (Molecular Probe), after which the cells were washed and placed in a heated chamber on an inverted microscope. Intracellular Ca2+ concentration ([Ca2+]i) fluorescence was recorded using an Andor Ixon charge-coupled device camera (Andor Technology) operating at ∼100 frame/s with a spatial resolution of 500 × 400 pixels. Fluorescence intensity was measured as the ratio of fluorescence (F) over the basal diastolic fluorescence (F0).
Chemicals.
SEA0400 was synthesized by Taisho Pharmacutical (Saitama, Japan). Nifedipine and BayK 8644 were first dissolved in DMSO as stock solutions before being diluted into the bath solution to the final concentration. The maximum DMSO concentration was <0.2% (vol/vol). Chemicals and reagents were purchased from Sigma unless indicated. All experiments in the present study were carried out at 35–37°C.
Statistical analysis.
The incidence of EADs within groups of 20 consecutive AP recordings was analyzed by Fisher's exact test. All other data are shown as means ± SD. Statistical differences were evaluated with Student's paired or unpaired t-tests. P < 0.05 is considered as statistically significant.
RESULTS
Rate dependence of EADs in different models.
To compare systematically the properties and determine the mechanisms of EADs under different conditions, we have established the following two EAD models using different pharmacological interventions: 1) isoproterenol (Iso; 100 nM) + BayK 8644 (BayK; 50 nM); and 2) H2O2 (200 μM).
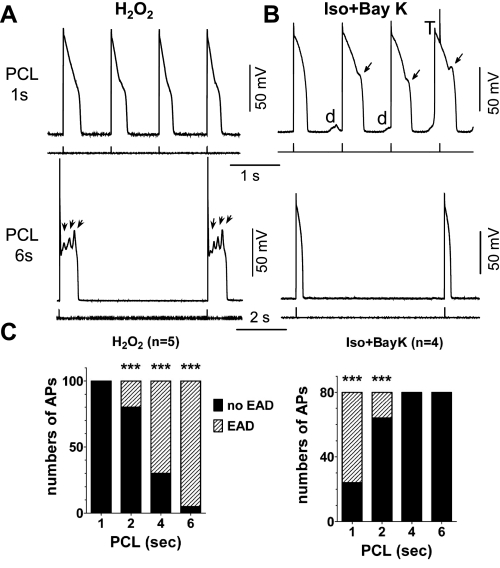
We first assessed the PCL dependence of EADs in these two models. In the H2O2 model, EADs were consistently observed at a PCL of 6 s after the cell was exposed to 200 μM H2O2 for >6 min, while no EADs were observed when the PCL was shortened to 1 s (Fig. 1A). Conversely, EADs were apparently induced by Iso + BayK at a PCL of 1 s but disappeared at a PCL of 6 s (Fig. 1B). The incidence of EAD was examined by counting the number of EADs within 20 consecutive APs and the PCL dependence of EAD incidence in the different models plotted in Fig. 1C. It was clearly shown that the EAD incidence rate was higher at a long PCL (or slow rate) in the H2O2 model but at a short PCL (or fast rate) in the Iso + BayK model. These results suggest that H2O2-induced EADs are slow-rate dependent, consistent with our previous report (29), while Iso + BayK-induced EADs are fast-rate dependent.
Fig. 1.
Pacing cycle length (PCL) dependence of early afterdepolarization (EAD) induction in different models. A: action potentials (APs) recorded from rabbit ventricular myocytes treated with 200 μM H2O2 (for 8 min in this case). Representative APs at PCL of 1 s (top) and 6 s (bottom) are shown. EADs were observed at long PCL of 6 s (arrows) but not at PCL of 1 s. B: APs recorded from myocytes treated with 100 nM isoproterenol and 50 nM BayK 8644 (Iso + BayK). EADs (arrows), delayed afterdepolarizations (DADs; d), and triggered AP (T) were induced at PCL of 1 s (top) but not at PCL of 6 s (bottom). C: summarized bar graphs showing the incidence of EADs within 20 APs at various PCLs in the 2 models (n ≥ 4 cells). The EAD incidence rate was higher at a low pacing rate (or long PCL) in the H2O2 model but at fast pacing rate (or short PCL) in Iso + BayK model. ***P < 0.0001, Fisher's exact test vs. no incidence of EADs.
Formation processes of EADs in different models.
Different pacing rates may affect the level of [Ca2+]i. For example, pacing a myocyte at a rapid rate is thought to facilitate [Ca2+]i overload (7, 13). Thus, in the following experiments, we simultaneously recorded [Ca2+]i transients (CaTs) and APs and compared the different behaviors of CaTs during EAD formation between the two models.
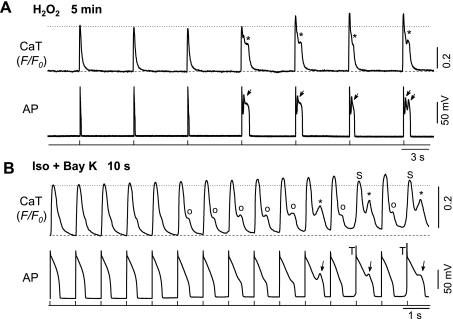
In the H2O2 model, EADs normally emerged 5–10 min after H2O2 (200 μM) perfusion. As shown in Fig. 2A, H2O2-induced spontaneous Ca transient (SCaT) occurred only when EADs were also observed in the corresponding APs. A significant increase of CaT amplitude was observed after the first EAD and SCaT occurred, suggesting sarcolemmal Ca2+ influx and SR Ca2+ content were enhanced during EADs, probably due to reactivation of ICa,L. Consistent with this notion, our and other's previous studies (32, 42) have clearly demonstrated that H2O2 activates ICa,L under either square pulse voltage clamp or AP waveform clamp.
Fig. 2.
Formation process of EADs in different models. A: simultaneous recordings of whole cell (global) Ca2+ transients (CaTs) and APs at 5 min after treatment with 200 μM H2O2. Note the increase of CaT amplitude (F/F0) following the emergence of EADs. B: same as A, except that the myocyte was treated with Iso + BayK (10 s after treatment). Note the Ca2+ accumulation before the emergence of EADs. Spontaneous Ca2+ transients or Ca2+ waves (SCaTs/CaWs) are indicated by ○ and *, which correspond to APD prolongation and EADs (arrows), respectively. Spontaneous CaT (S) and triggered APs (T) are also shown.
In the Iso + BayK model, apparent changes in CaT and AP were caused with a very fast time course. In the case shown in Fig. 2B, both diastolic Ca2+ level and CaT amplitude gradually increased at ∼10 s after Iso + BayK treatment. Particularly, apparent SCaTs were observed from the sixth CaT with their amplitudes increasing progressively, correlating to the gradual prolongation of APDs at first, and then frank EADs (2B, bottom) when the amplitudes of corresponding SCaT were higher. These results suggest Iso + BayK treatment leads to a progressive accumulation of [Ca2+]i before EAD emergence. The emergence of CaWs/SCaTs preceded Iso + BayK-induced EADs, indicating CaWs were generated from spontaneous SR Ca2+ release rather than ICa,L reactivation. CaWs then caused Iti, which contributed to EAD genesis in the Iso + BayK model.
Temporal relationship between EADs and corresponding SCaTs/CaWs.
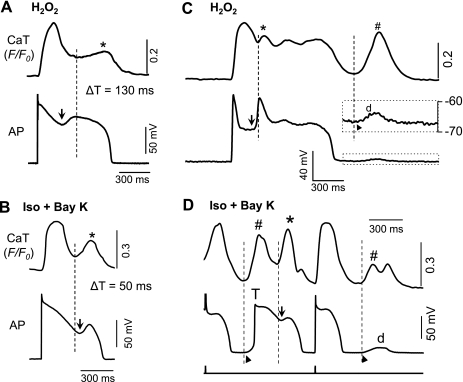
To further determine the primary cause (CaW vs. ICa,L) of EADs in each model, we next examined temporal correlation between EADs and SCaTs/CaWs by comparing the onsets of EAD upstrokes and the initiation of corresponding SCaTs/CaWs. It is conceivable that if an EAD is generated by a ICa,L-dominant mechanism, the corresponding SCaT will be the consequence of the EAD and therefore will occur subsequent to the EAD upstroke. On the other hand, if the EAD is generated by an SR-dependent mechanism, the spontaneous Ca release/CaW will be the cause of the EAD and would therefore precede the EAD upstroke. This rationale can be clearly discriminated, as illustrated by the results shown in Fig. 3.
Fig. 3.
Temporal relationship between EAD and DAD corresponding SCaTs/CaWs in different models. Whole cell CaTs and APs were simultaneously recorded to compare the initiation time of an EAD upstroke (arrows) and corresponding SCaT/CaW (dashed lines). A: representative recording from H2O2 model. EAD was 130 ms ahead of corresponding SCaT. B: in Iso + BayK model, the start of a CaW preceded the upstroke of the EAD with a time difference (ΔT) of 50 ms in this representative cell. C and D: temporal relationship between DAD and CaW in H2O2 and Iso + BayK models, respectively. While the temporal relationship between EAD and SCaT remained the same as A and B, the DADs (d) and DAD-triggered APs (T) in both models seemed to always occur (as indicated by ▴) behind the initiation of their corresponding CaW (#), suggesting Ca overload and Na/Ca exchange current (INCX) as a common mechanism for the DADs in both models.
In the H2O2 model (Fig. 3A), the start of the EAD upstroke was significantly earlier than the initiation of the corresponding SCaT. The difference in initiation time (ΔT) was 130 ms for this specific cell, and 113.5 ± 31.1 ms on average (means ± SD, n = 8). In the Iso + BayK model (Fig. 3B), however, the initiation of SCaT preceded corresponding EAD upstroke. The ΔT was 50 ms for this specific cell, and 44.2 ± 8.7 ms on average (n = 9).
As previously mentioned, DADs were also induced in both models but with different time courses. While DADs or DAD-induced TAs always occurred in association with EADs in the Iso + BayK model (see Figs. 2–7), H2O2-induced DADs were only observed occasionally, required prolonged treatment with H2O2, and arose much later than the emergence of EAD. In both the H2O2 and Iso + BayK models (Fig. 3, C and D), it is apparent that the DADs were initiated after the beginning of corresponding CaWs/SCaTs, suggesting that Ca2+ overload-induced INCX is most likely to account for the DAD generation in both cases.
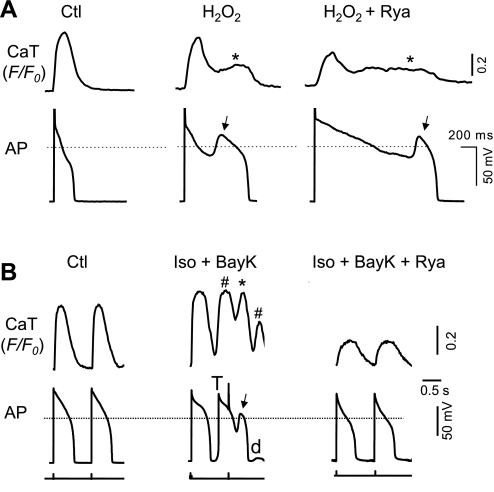
Fig. 7.
Effects of ryanodine (10 μM), a ryanodine receptor inhibitor, on EADs and corresponding SCaTs/CaWs. A: CaT and AP recording under control condition (left), after H2O2 treatment (middle) and the effect of ryanodine (Rya) on H2O2-induced EADs (arrows) and CaTs (*; right). B: CaT and AP recording under control condition (left), after Iso + BayK treatment (middle), and the effect of ryanodine (right). Iso + BayK-induced CaWs (* and #, corresponding to EAD and DAD, respectively), EADs (arrows), and DAD-triggered action potential (T) are indicated.
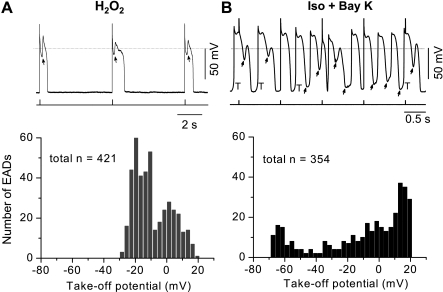
Distribution of EAD take-off potentials.
Since some ionic currents (e.g., ICa,L) highly depend on membrane voltages, the take-off potentials (TOPs) at which the EADs occurred were also compared between these two models. In the H2O2 model, EADs were consistently aroused at the end of phase 2 or early phase 3 of APs. The TOPs were within a narrow range (from +20 to −30 mV; Fig. 4A), which was consistent with that mediating the window current of ICa,L (14).
Fig. 4.
Distribution of take-off potentials (TOPs) of EADs in H2O2 and Iso + BayK models. A: representative recording of H2O2-induced EADs (top; stimulation marks are indicated below the trace) and a histogram graph showing distribution of their TOPs. Width of each bar is 3 mV. Data showed in the histogram were from 9 cells. B: same as A, except for Iso + BayK-induced EADs. Data showed in the histogram were from 7 cells.
On the other hand, the Iso + BayK-induced EADs occurred over a broader voltage range to a more negative level. As shown in Fig. 4B, the TOPs distributed over the whole AP repolarization phase (phases 2 and 3; from +20 to −60 mV). In addition, DADs and DAD-induced TAs (at phase 4) always appeared together in the same cell. These results suggest that in the Iso + BayK model, the charge carriers mediating EADs (as well as DADs) can be activated over a broad range of membrane potentials, in agreement with the voltage dependence of INCX.
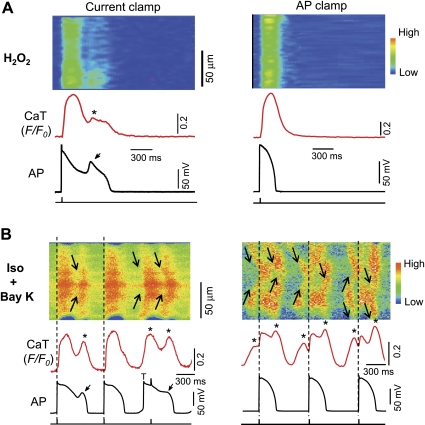
Relation between SCaTs/CaWs and EADs revealed by AP-clamp experiments.
As pointed out previously, a SCaT/CaW might be either the consequence (i.e., sarcolemma-dependent mechanism) or the cause (i.e., SR-dependent mechanism) of an EAD. The following rationales were tested in a setting of AP-clamp experiments: If a SCaT is the consequence of an EAD, it should be suppressed by preferentially eliminating the EAD. Conversely, if a SCaT/CaW is the cause of an EAD, it should remain even if the EAD is preferentially eliminated.
In either model, EADs and SCaTs/CaWs were initially elicited in a cell under current-clamp mode. Thereafter, the myocyte was switched to voltage-clamp mode and the membrane potential was clamped under a normal AP waveform without EAD, which was recorded previously from a control myocyte. In the H2O2 model, the SCaT was completely eliminated by switching current-clamp to AP-clamp mode with a normal AP morphology (without EAD; Fig. 5A), indicating that the SCaT was caused by the corresponding EADs. In other words, the sarcolemma-dependent (or ICa,L-dominant) mechanism plays a predominant role in EAD genesis in H2O2 model.
Fig. 5.
Behaviors of SCaTs/CaWs under AP-clamp condition. After EADs were induced under current-clamp configuration (left), the recording from the same cell were switched to voltage-clamp configuration under an AP morphology without EAD (right) in H2O2 (A) and Iso + BayK (B) model. AP was recorded previously from a control cell. Note the SCaTs/CaWs were completely eliminated under AP-clamp condition in H2O2 model, while they persisted in Iso + BayK model.
In the Iso + BayK model (Fig. 5B), however, the SCaTs/CaWs persisted even after the cell was switched to voltage-clamp under a normal AP without EADs, suggesting that SCaTs/CaWs occurred independently from membrane potential and seemed to be the primary cause for EAD formation under [Ca2+]i overload condition. These results have provided the most convincing evidence on the cause-effect relationship between EADs and SCaTs/CaWs under different conditions.
SR Ca2+ load and SCaT/CaW properties in the two EAD models.
To directly evaluate the SR Ca2+ content, the amplitudes of Ca transients induced by rapid exposure to 10 mM caffeine were measured. We observed SR Ca2+ was enhanced rapidly by Iso + BayK, while there was no significant change in the H2O2 model (Table 1), suggesting an intracellular Ca2+ overload status in the Iso + BayK model. Consistently, our results also revealed a significant elevation of CaT amplitude by Iso + BayK treatment but not by H2O2 treatment. The average amplitude of SCaT (or CaW) in the Iso + BayK model (F/F0 = 1.65 ± 0.24 was higher than that in H2O2 model at F/F0 = 1.26 ± 0.09; P < 0.01). It is also very interesting that while the SCaTs always had a lower amplitude compared with their preceding AP-elicited CaTs in H2O2 model, the amplitude of SCaTs in Iso + BayK model exhibited a rather wide range and were even higher than their preceding AP-elicited CaTs at certain beats (e.g., Figs. 6 and 7). These results suggest that the SCaT in the H2O2 model occur as a secondary event (triggered by reactivated ICa,L) when the SR Ca was partially depleted by the previous AP-elicited CaTs. On the contrary, the SCaTs/CaWs in the Iso + BayK model most likely represent primary SR Ca releases.
Table 1.
Comparison of Ca2+ handling properties in the two EAD models
| Model | CaT at Control | CaT After Treatment | Spontaneous CaT/CaW | SR Content at Control | SR Content After Treatment |
|---|---|---|---|---|---|
| H2O2 | 1.56 ± 0.27 (48) | 1.54 ± 0.32 (42) | 1.26 ± 0.09† (27) | 1.67 ± 0.57 (19) | 1.52 ± 0.35 (19) |
| Iso + BayK | 1.44 ± 0.20 (44) | 1.75 ± 0.37* (49) | 1.65 ± 0.24 (32) | 1.62 ± 0.35 (26) | 1.99 ± 0.54‡ (24) |
Values are means ± SD; numbers of calcium transients (CaTs) measured from multiple cells are indicated in the parenthesis. Amplitude (F/F0) of CaT, spontaneous CaT/calcium wave (CaW), and 10-mM caffeine-induced calcium release [sarcoplasmic reticulum (SR) content] are listed.
EAD, early afterdepolarization; Iso, isoproterenol; BayK, BayK 8644.
P < 0.01, compared with CaT at control;
P < 0.01, compared with CaT after treatment;
P < 0.01, compared with SR content at control.
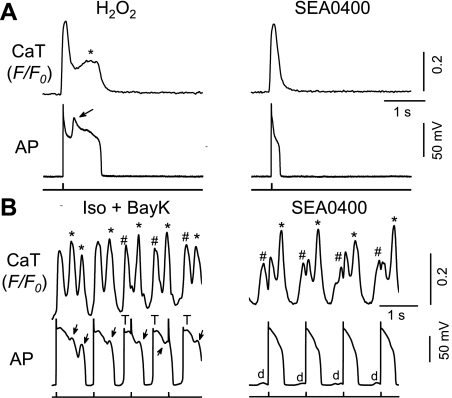
Fig. 6.
Effects of INCX inhibition on EADs and corresponding SCaTs/CaWs. A: effect of SEA0400, a selective INCX blocker, on H2O2-induced EADs (arrows) and CaTs (*). B: Iso + BayK-induced CaWs (* and #, corresponding to EAD and DAD, respectively), EADs (arrows), and DAD-triggered action potential (T; left) and the effect of SEA0400 (right). DADs (d) are indicated at right.
Effects of the INCX inhibitor SEA0400.
All above observations strongly suggest the contribution of ICa,L vs. INCX may vary (i.e., one of them is likely to play a predominant role) in EAD generation under different conditions. To determine directly the involvement of ICa,L and INCX, the effects of ICa,L and INCX inhibitors on the EADs and [Ca2+]i handling were evaluated in the two different EAD models.
SEA0400 has been used as a tool inhibitor of INCX (5, 20). In the H2O2 model (Fig. 6A), SEA0400 not only abolished EADs, but also eliminated SCaTs, resembling the AP-clamp experiment shown in Fig. 5A. This result indicates that, although the primary current accounting for EAD formation is suggested to be the reactivation of ICa,L in the H2O2 model, the inhibition of INCX may reduce the inward current that is necessary for the generation of EADs.
In the Iso + BayK model, EADs were also efficiently abolished by SEA0400 (2 μM). However, SEA0400 exerted less effect on [Ca2+]i behavior such that the SCaTs/CaWs persisted (Fig. 6B), suggesting SEA0400 functioned at the downstream targets (i.e., NCX) of CaWs.
SEA0400 is the most potent Na+/Ca2+ exchanger blocker available, although it is not completely specific and also inhibits L-type Ca2+ channels (5). While the level of SEA0400 we used (2 μM) may cause the maximal blockade of inward INCX (5), it only caused a small degree of inhibition of ICa,L (∼15%, from 1.56 ± 0.0.58 nA to 1.32 ± 0.38 nA; n = 7) , but this decrease was not significant by statistical standards (P = 0.13). Therefore, we assume that the effect of SEA can be mostly attributed to Na+/Ca2+ exchanger in the setting of our present study, although we cannot completely rule out the contribution of ICa,L (5). The different behaviors of SEA0400 in the two different EAD models (Fig. 6), particularly on CaWs, are consistent with its major role on INCX.
Effects of the ICa,L blocker nifedipine.
Next, we examined the effect of nifedipine, a selective ICa,L blocker on EADs and SCaTs/CaWs elicited in both H2O2 and Iso + BayK models. Nifedipine (10 μM) completely abolished both EADs and SCaTs in both models (data not shown). These results are conceivable since nifedipine can both suppress inward ICa,L and attenuate Cai overload by reducing Ca2+ influx.
Effects of the RyR blocker ryanodine.
Since spontaneous Ca2+ release from SR is mediated by the Ca release channel or RyR, we assessed the effect of ryanodine at 10 μM, a concentration that is thought to selectively inhibit RyR activities (15). As shown in Fig. 7, ryanodine suppressed CaTs in both H2O2 and Iso + BayK models, confirming its inhibitory effect on RyR and Ca2+-induced Ca2+ release. However, different effects on EADs were observed in the two models. In the H2O2 model, EADs were still present, although APDs were further prolonged, presumably due to reduced ICa,L inactivation (Fig. 7A). In the Iso + BayK model, however, both EADs and DADs were eliminated (while the corresponding CaWs were also removed) by ryanodine treatment.
DISCUSSION
EADs are abnormal voltage oscillations occurring during the repolarizing phase of cardiac APs and are thought to cause cardiac arrhythmias. It has been suggested that EADs occur under conditions of reduced repolarization reserves (3, 26). Thus either increased inward currents or reduced outward currents, or both, promote EAD generation. For example, activation of inward late sodium current (INa), ICa,L , INCX, chloride current (ICl), as well as blockage of outward IKr, IKs, or IK1 have all been reported to mediate EAD genesis. Among these ionic mechanisms, CaW-mediated INCX (SR-dependent mechanism) and reactivation of ICa,L (sarcolemma-dependent mechanism) have been suggested as two major contributors for EAD genesis. However, their relative contributions underlying different pathological conditions are still under debate. In the present study, we conducted systematic comparison of two cellular models and focused on investigating the relative role of ICa,L vs. CaW-induced INCX in generating EADs by simultaneously recording APs and CaTs. We have provided important clues to identify different EAD mechanisms. For example, one significant result was that abnormal SCaT accompanying EADs were abolished when the EADs were eliminated (by voltage clamp) in the H2O2 model. However, this was not the case for Iso + BayK-induced SCaT/CaW and EADs, i.e., CaWs persisted even when EADs were eliminated (Fig. 5). Our results provide convincing evidence for the relative contributions of CaW-induced INCX and ICa.L in different EAD models. These two mechanisms are not mutually exclusive. They function coordinately to cause EADs; however, one mechanism may play a predominant role under a certain pathological condition.
H2O2 model: ICa,L-predominant mechanism for EAD.
ROS (including H2O2) have been suggested to mediate disturbances in the cardiac rhythm under various circumstances, such as heart failure, aging, and ischemia/reperfusion (22, 37). In contrast to Iso + BayK, H2O2 seems to elicit EADs primarily by modulating membrane ionic channels. For example, we and others (32, 42) have shown that H2O2 (0.2–1 mM) increased both peak and plateau currents of ICa,L and late INa in rabbit ventricular myocytes. The activation of CaMKII, either directly via oxidative stress, or indirectly via elevated Cai, is critical for these effects, which contribute to the arrhythmogenic potential of oxidative stress.
Consistent to classical EADs caused by sarcolemma-dependent mechanism, H2O2-induced EADs have been shown to be dependent on slow pacing rate (bradycardia). A previous computer modeling study (16) suggests that the delayed rectifier potassium current (IKs), which has a long activation time course and slow deactivation process, plays a key role in facilitating EAD generation at a slow pacing rate. In our most recent study (Zhao Z, Xie Y, Wen H, Xiao D, Allen C, Fefelova N, Dun W, Boyden PA, Qu Z, Xie LH, unpublished observations), we have demonstrated that Ito is also an essential determinant for the slow rate dependence of EADs in rabbit ventricular myocytes due to its very slow recovery from inactivation. Ito, despite being a pure outward current, can potentiate EADs by lowering the AP plateau quickly into more negative voltages to allow ICa,L reactivation before IKs is fully activated to repolarize the myocyte, causing voltage oscillations in the plateau phase and thus EADs.
In addition to the difference in rate dependence, a difference in the TOPs of EAD upstrokes was also found between the two models. H2O2-induced EAD developed within a narrow voltage range (−30 to +20 mV), which is consistent with the ICa,L window current. Other evidence such as CaT amplitude increase after EAD emergence, the temporal precedence of EAD upstrokes, and elimination of CaWs by clamping cell membrane potential with a normal AP (without a EAD) all support the primary role of ICa,L-dominant mechanism in EAD generation in H2O2 model.
INCX functions predominantly in the forward mode, generating inward current during most of the AP repolarization. This is likely to lengthen the APD and form a “conditioning phase,” which facilitates reactivation of ICa,L and/or INa and generation of EAD upstroke (14, 40). It has been shown that various INCX blockers (e.g., SEA0400, SN-6, and inhibitory peptide) shorten APDs under regular condition (1, 24, 35). Our present study showed that SEA0400 also eliminate H2O2-induced (ICa,L-predominant) EADs, as well as accompanying SCaTs (different from Iso + BayK model), which further support the “conditioning phase” mechanism. The SCaTs in the H2O2 model were most likely formed by Ca2+ entry through reactivated ICa,L and SR Ca2+-induced Ca2+ release triggered by the ICa,L reactivation.
Iso + BayK model: CaW-predominant mechanism for EAD.
It is well accepted that INCX driven by CaW accounts for DAD formation (27, 34, 36). Recent experimental and simulation studies (13, 33) have demonstrated that CaW is also capable of evoking EADs. Pure β-adrenergic stimulation (Iso alone) has been used to cause EADs and DADs in dog myocytes (25, 39), and these could be facilitated by caffeine-enhanced SR Ca2+ leak (23). In rabbit myocytes, however, Iso alone cannot efficiently induce EADs/DADs. This may suggest a high threshold for SR Ca2+ spontaneous release in rabbit myocytes. In the present study, we were able consistently to generate frequent CaW and EADs/DADs by the application of Iso in the presence of BayK, as we demonstrated in our previous study (43). In this model, the SR Ca2+ content may be increased by the activation of both ICa,L and sarco(endo)plasmic reticulum Ca2+-ATPase activity. In addition, BayK has also been reported to increase RyR Ca2+ leak (17), perhaps through a functional linkage between the sarcolemmal dihydropyridine receptor and the SR ryanodine receptor (30). This may decrease the threshold for generation of CaW. In addition, the activation effect of BayK on ICa,L (via changing both voltage dependence and single channel gating; Refs. 2, 28) may also lead to additional Ca2+ overload. Therefore, spontaneous SR Ca2+ release/CaW-induced elevation of cytosolic Ca2+ may depolarize myocytes by the electrogenic INCX, which is most likely to primarily mediate EAD generation in Iso + BayK model.
Since intracellular Ca2+ overload is exaggerated by fast pacing, EADs caused by spontaneous Ca2+ release should be aggravated by fast pacing and tempered by slow pacing. This is proved to be true when we compared the EAD genesis at PCL of 6 vs. 1 s. It should be noted that Iso + Bay-induced EADs may be suppressed by further shortening PCL (i.e., from 800 to 300 ms), probably due to less ICa,L recovery from inactivation when pacing rate is so fast.
Several other lines of evidence, such as CaT changes before or after EAD emergence, the temporal precedence of SCaT/CaW, less voltage dependence of EAD distribution, and suppression of EADs and DADs by inhibiting RyR with ryanodine, support the primary role of CaWs in EAD generation under Iso + BayK condition. The most convincing evidence came from the AP-clamp experiment (see Relation between SCaTs/CaWs and EADs revealed by AP-clamp experiments), in which the CaWs remained to appear even if the cell membrane APs were clamped without EADs. In addition, Ca handling (e.g., SR Ca2+ content and SCaT amplitude) analysis provided direct evidence (see SR Ca2+ load and SCaT/CaW properties in the two EAD models) for intracellular Ca2+ overload in Iso + BayK model. Thus it is most likely that SCaT/CaWs-activated INCX function as a primary underlying mechanism for both EAD and DAD formation in the Iso + BayK model, which may share a similar ionic mechanism with that of catecholaminergic polymorphic ventricular tachycardia (6, 10, 23).
Synergy between ICa,L and INCX on EAD genesis.
Based on the above analysis, we suggest that the relative contributions of ICa,L and INCX may vary (i.e., one of them may play a primary role) in EAD generation under specific conditions. However, it is conceivable that the synergistic interactions between ICa,L and INCX are also present in the process of EAD generation [refer to a comprehensive review by Weiss et al. (41)]. For instance, reactivation of the ICa,L during the plateau is likely to be the primary cause of EADs in H2O2 model. Meanwhile, enhanced window ICa,L may trigger additional Ca2+ release from the SR and increase INCX. Conversely, under the Ca overload condition (e.g., Iso + BayK model), spontaneous Ca2+ release from the SR occurs to promote the forward mode of INCX, causing delay in repolarization, namely a conditional phase (44). This allows longer time for ICa,L to recover from inactivation, which in turn favors EAD formation.
Since strong synergies are present between ICa,L and INCX in the generation of EADs, reducing either current may be effective in suppressing EADs. This has been proven to be true in both the H2O2 and Iso + BayK models. Inhibition of ICa,L by nifedipine may suppress EADs in either model either by directly reducing the underlying inward Ca current or by secondarily attenuating the [Ca2+]i overload and thus INCX. SEA0400, the most selective agent available to inhibit INCX, suppressed both EADs and SCaTs in the H2O2 model, suggesting that INCX plays a facilitating role (conditional phase) in H2O2-induced EAD induction. The facilitated reactivation of ICa,L represents the ion carrier for both the formation of EAD and the trigger of SCaT in the H2O2 model. In the Iso + BayK model, however, SEA0400 inhibited EADs without affecting CaWs, suggesting a primary causal role of spontaneous SR Ca2+ release on the EAD generation under the Ca2+ overloaded condition. Several studies (11, 21) have shown that SEA0400 is effective to prevent/treat triggered activities and arrhythmias.
Relevance of EAD models.
One may raise concerns that the EAD models we used in the present study might be too complex. We have tried to establish other “simple” models. Unfortunately, neither elevating extracellular Ca2+ concentration (4–8 mM) nor Iso (up to 2 μM) alone successfully induced sustained EADs, although they might occasionally generate DADs. These results were consistent with previous reports showing that Iso alone was less efficient in causing sustained spontaneous Ca release and EADs/DADs (23, 38), since the reduction of SR threshold for spontaneous Ca2+ release is also required (10). Therefore, we consider that the two EAD models used in the present study are the most ideal and stable approaches that enable us to make systematic comparisons for the relative contribution of ICa,L vs. CaW.
It should be noted that similar approaches have been utilized in the whole heart setting to induce EADs and triggered arrhythmias (22, 23), indicating the relevance of the models. In addition, it seems that the H2O2 model is more relevant to some pathological conditions. The H2O2 level in human blood plasma may reach as high as ∼35 μM (12). It is also well known that ROS levels can increase under certain oxidative stressed conditions, such as chronic heart failure and ischemia-reperfusion (by as much as 100-fold; Ref. 8). Thus the concentrations (200 μM) used in most of our experiments are reasonably within its pathological range in situ.
H2O2-induced EADs were slow-rate dependent and were often observed at PCL >2 s. Accordingly, ROS-induced ventricular arrhythmias may be more readily implicated in the clinical setting of bradycardia (18), such as sinus-node dysfunction and atrial-ventricular conduction disturbances. It should be noted a heart rate at 10 beats/min is thought to be a very deep bradycardia and mostly incompatible with life.
Limitation.
One limitation is the complexity of the EAD models we used in the present study. While it is impossible to dissect one single current component that exclusively contributes to the generation of EAD, we have provided convincing evidence showing that either ICa,L or INCX may play a primary role in one specific EAD model vs. another.
Conclusion.
Our results have provided more convincing evidence for the heterogeneous mechanisms and their synergies for EAD generation. While EADs involve the complex interplay of several mechanisms, one mechanism (e.g., ICa,L reactivation vs. CaW-induced INCX) may play a primary role under a certain pathological condition.
The classification (or nomenclature) of afterdepolarizations have been largely based on the timing they appear on the different phases during APD, i.e., EADs occur at phase 2 or 3 of an AP and DADs at phase 4 (after complete repolarization). Since afterdepolarizations (in particular EADs) may result from distinct cellular and ionic mechanisms, it may be more meaningful to further classify them based on their predominant mechanisms. As for the EADs induced in H2O2 and Iso + BayK models shown in the present study, we call them sarcolemma-dependent and SR-dependent EADs, respectively.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.Z., H.W., N.F., C.A., and L.-H.X. performed experiments; Z.Z., H.W., N.F., C.A., and L.-H.X. analyzed data; Z.Z., A.B., T.M., and L.-H.X. interpreted results of experiments; Z.Z., H.W., N.F., C.A., and L.-H.X. prepared figures; Z.Z. and L.-H.X. drafted manuscript; Z.Z. and L.-H.X. edited and revised manuscript; Z.Z., H.W., N.F., C.A., A.B., T.M., and L.-H.X. approved final version of manuscript; L.-H.X. conception and design of research.
ACKNOWLEDGMENTS
This work was partially supported by a National Heart, Lung, and Blood Institute Grant R01-HL-097979 (to L.-H. Xie). We thank Andrew James (Bristol, UK) for insightful comments on the manuscript.
REFERENCES
- 1. Armoundas AA, Hobai IA, Tomaselli GF, Winslow RL, O'Rourke B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circ Res 93: 46–53, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bechem M, Hoffmann H. The molecular mode of action of the Ca agonist (−)BAY K 8644 on the cardiac Ca channel. Pflügers Arch 424: 343–353, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Biliczki P, Virag L, Iost N, Papp JG, Varro A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol 137: 361–368, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Binah O, Rosen MR. Mechanisms of ventricular arrhythmias. Circulation 85: I25–31, 1992 [PubMed] [Google Scholar]
- 5. Birinyi P, Acsai K, Banyasz T, Toth A, Horvath B, Virag L, Szentandrassy N, Magyar J, Varro A, Fulop F, Nanasi PP. Effects of SEA0400 and KB-R7943 on Na+/Ca2+ exchange current and L-type Ca2+ current in canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol 372: 63–70, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm 6: 1652–1659, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Coetzee WA, Opie LH, Saman S. Proposed role of energy supply in the genesis of delayed afterdepolarizations–implications for ischemic or reperfusion arrhythmias. J Mol Cell Cardiol 5: 13–21, 1987 [DOI] [PubMed] [Google Scholar]
- 8. Dhalla NS, Duhamel TA. The paradoxes of reperfusion in the ischemic heart. Heart Metab 37: 31–34, 2007 [Google Scholar]
- 9. Eckhardt LL, Teelin TC, January CT. Is ranolazine an antiarrhythmic drug? Am J Physiol Heart Circ Physiol 294: H1989–H1991, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Eisner DA, Kashimura T, O'Neill SC, Venetucci LA, Trafford AW. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol 46: 474–481, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart. Circ Res 103: 509–518, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett 486: 10–13, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Huffaker RB, Samade R, Weiss JN, Kogan B. Tachycardia-induced early afterdepolarizations: insights into potential ionic mechanisms from computer simulations. Comput Biol Med 38: 1140–1151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res 64: 977–990, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118: 2230–2245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. II. Afterdepolarizations, triggered activity, and potentiation. Circ Res 74: 1097–1113, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Mackiewicz U, Emanuel K, Lewartowski B. Agonist of dihydropyridine receptors, BayK8644 depresses excitation-contraction coupling in myocytes of guinea pig heart. J Physiol Pharmacol 52: 459–469, 2001 [PubMed] [Google Scholar]
- 18. Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N Engl J Med 342: 703–709, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest 78: 1185–1192, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, Takahashi K, Takahashi T, Suzuki T, Ota T, Hamano-Takahashi A, Onishi M, Tanaka Y, Kameo K, Baba A. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther 298: 249–256, 2001 [PubMed] [Google Scholar]
- 21. Milberg P, Pott C, Fink M, Frommeyer G, Matsuda T, Baba A, Osada N, Breithardt G, Noble D, Eckardt L. Inhibition of the Na+/Ca2+ exchanger suppresses torsades de pointes in an intact heart model of long QT syndrome-2 and long QT syndrome-3. Heart Rhythm 5: 1444–1452, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Morita N, Sovari AA, Xie Y, Fishbein MC, Mandel WJ, Garfinkel A, Lin SF, Chen PS, Xie LH, Chen F, Qu Z, Weiss JN, Karagueuzian HS. Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am J Physiol Heart Circ Physiol 297: H1594–H1605, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nam GB, Burashnikov A, Antzelevitch C. Cellular mechanisms underlying the development of catecholaminergic ventricular tachycardia. Circulation 111: 2727–2733, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niu CF, Watanabe Y, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Electrophysiological effects of SN-6, a novel Na+/Ca2+ exchange inhibitor on membrane currents in guinea pig ventricular myocytes. Ann NY Acad Sci 1099: 534–539, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Priori SG, Corr PB. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. Am J Physiol Heart Circ Physiol 258: H1796–H1805, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol 21: 1029–1034, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 115: 2305–2315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanguinetti MC, Krafte DS, Kass RS. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol 88: 369–392, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sato D, Xie LH, Sovari AA, Tran DX, Morita N, Xie F, Karagueuzian H, Garfinkel A, Weiss JN, Qu Z. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc Natl Acad Sci USA 106: 2983–2988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Satoh H, Katoh H, Velez P, Fill M, Bers DM. Bay K 8644 increases resting Ca2+ spark frequency in ferret ventricular myocytes independent of Ca influx: contrast with caffeine and ryanodine effects. Circ Res 83: 1192–1204, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol 294: H2031–H2039, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Song YH, Cho H, Ryu SY, Yoon JY, Park SH, Noh CI, Lee SH, Ho WK. L-type Ca2+ channel facilitation mediated by H2O2-induced activation of CaMKII in rat ventricular myocytes. J Mol Cell Cardiol 48: 773–780, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Spencer CI, Sham JS. Effects of Na+/Ca2+ exchange induced by SR Ca2+ release on action potentials and afterdepolarizations in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 285: H2552–H2562, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Sugai Y, Miura M, Hirose M, Wakayama Y, Endoh H, Nishio T, Watanabe J, ter Keurs HE, Shirato K, Shimokawa H. Contribution of Na+/Ca2+ exchange current to the formation of delayed afterdepolarizations in intact rat ventricular muscle. J Cardiovasc Pharmacol 53: 517–522, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Tanaka H, Namekata I, Takeda K, Kazama A, Shimizu Y, Moriwaki R, Hirayama W, Sato A, Kawanishi T, Shigenobu K. Unique excitation-contraction characteristics of mouse myocardium as revealed by SEA0400, a specific inhibitor of Na+-Ca2+ exchanger. Naunyn Schmiedebergs Arch Pharmacol 371: 526–534, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev 87: 457–506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tomaselli GF, Barth AS. Sudden cardio arrest: oxidative stress irritates the heart. Nat Med 16: 648–649, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res 100: 105–111, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Volders PG, Kulcsar A, Vos MA, Sipido KR, Wellens HJ, Lazzara R, Szabo B. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc Res 34: 348–359, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res 46: 376–392, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 7: 1891–1899, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104: 79–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie LH, Weiss JN. Arrhythmogenic consequences of intracellular calcium waves. Am J Physiol Heart Circ Physiol 297: H997–H1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J 68: 949–964, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]