Abstract
Myocarditis and dilated cardiomyopathy (DCM) are often caused by viral infections and occur more frequently in men than in women, but the reasons for the sex difference remain unclear. The aim of this study was to assess whether gene changes in the heart during coxsackievirus B3 (CVB3) myocarditis in male and female BALB/c mice predicted worse DCM in males. Although myocarditis (P = 4.2 × 10−5) and cardiac dilation (P = 0.008) were worse in males, there was no difference in viral replication in the heart. Fibrotic remodeling genes, such as tissue inhibitor of metalloproteinase (TIMP)-1 and serpin A 3n, were upregulated in males during myocarditis rather than during DCM. Using gonadectomy and testosterone replacement, we showed that testosterone increased cardiac TIMP-1 (P = 0.04), serpin A 3n (P = 0.007), and matrix metalloproteinase (MMP)-8 (P = 0.04) during myocarditis. Testosterone increased IL-1β levels in the heart (P = 0.02), a cytokine known to regulate cardiovascular remodeling, and IL-1β in turn increased cardiac serpin A 3n mRNA (P = 0.005). We found that 39 of 118 (33%) genes identified in acute DCM patients were significantly altered in the heart during CVB3 myocarditis in mice, including serpin A 3n (3.3-fold change, P = 0.0001). Recombinant serpin A 3n treatment induced cardiac fibrosis during CVB3 myocarditis (P = 0.0008) while decreasing MMP-3 (P = 0.04) and MMP-9 (P = 0.03) levels in the heart. Thus, serpin A 3n was identified as a gene associated with fibrotic cardiac remodeling and progression to DCM in male myocarditis patients and mice.
Keywords: myocarditis, dilated cardiomyopathy, cytokines, sex differences
myocarditis is a potentially life-threatening disease that is characterized by inflammation in the myocardium resulting in reduced systolic function that can progress to dilated cardiomyopathy (DCM) and heart failure in susceptible individuals (3). Around half of all DCM cases in the United States begin as myocarditis, with DCM being the most common form of cardiomyopathy requiring a heart transplant (39, 45). Myocarditis is considered to be an autoimmune disease that is caused or triggered by infections such as parvovirus B19, Borrelia burgdorferi, Trypansoma cruzi, or coxsackievirus B3 (CVB3) (5, 11, 17). Similar to coronary artery disease and heart failure, myocarditis and DCM occur more frequently in men than in women (11, 38, 39), but the reasons for the sex difference are not entirely clear.
Sex differences in cardiovascular diseases have largely been attributed to the effect of the sex steroid hormones estrogen and testosterone (38). A critical step in the progression from myocarditis to DCM and heart failure involves extracellular matrix (ECM) remodeling and fibrosis (21, 28). Cardiac remodeling is defined as structural and functional changes in the myocardium that result in left ventricular (LV) dilatation leading to heart failure (21). Alterations in the coordinated degradation and synthesis of ECM components such as collagen by matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) result in cardiac fibrosis (28). Serpin A 3n (also known as α1-anti-chymotrypsin) is a potent serine protease inhibitor of leukocyte-derived proteases that could influence ECM remodeling by altering MMP levels (25). In animal models, testosterone has been found to enhance myocardial inflammation and ECM remodeling after myocardial infarction (6), whereas estrogen is protective (36). In patients with myocarditis, men were found to be two times more likely to present with visual evidence of myocardial fibrosis compared with women based on cardiovascular MRI (10). However, the genes responsible for increased myocardial fibrosis in men were not investigated. Although a number of studies (12, 23, 42, 43, 46) have used genomic and proteomic strategies to identify gene/protein changes associated with myocarditis and DCM in patients or mouse models, these studies did not investigate sex differences.
In this study, we used an autoimmune model of CVB3-induced myocarditis in mice (14) to examine the role of gene changes in the heart during acute myocarditis that were associated with worse severity or progression of disease in males. To translate findings from the mouse model to the clinical setting, we compared our data with two separate meta-analyses conducted on 11 individual microarray studies of DCM patients to identify genes in common. We found that one gene in particular, serpin A 3n, was associated with the progression to DCM in male patients and mice. Here, we show, for the first time, that serpin A 3n levels in the heart are increased by testosterone and IL-1β and that serpin A 3n induces cardiac remodeling and fibrosis during acute CVB3 myocarditis in mice.
METHODS
Ethical approval.
All animal procedures were submitted to and approved by the Animal Care and Use Committee of the Johns Hopkins University.
Animal model.
Male or female 6- to 8-wk old BALB/c (BALB/cJ, stock no. 000651) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained under pathogen-free conditions in the animal facility at Johns Hopkins School of Medicine. Mice were inoculated intraperitoneally with 103 plaque-forming units of heart-passaged CVB3 (containing CVB3 and cardiac myosin) diluted in sterile PBS or PBS on day 0. CVB3 (Nancy strain) was originally obtained from the American Type Culture Collection (ATCC; Manassas, VA) and grown in Vero cells (ATCC), as previously described (14). Individual experiments using 7–10 mice/group were conducted 3–4 times unless stated otherwise.
Histology.
Hearts were cut longitudinally, fixed in 10% phosphate-buffered formalin, and embedded in paraffin for histological analysis. Sections (5 μm thick) were stained with hematoxylin and eosin or with Masson's trichrome to detect inflammation or fibrosis, respectively. Myocarditis and fibrosis were assessed as the percentage of the heart section with inflammation or fibrosis compared with the overall size of the heart section using an eyepiece grid at low power (×25), as previously described (9, 15, 16, 19, 37). Collagen deposition using Masson's trichrome stains an intensely “baby blue” color compared with inflammation, which appears as dark blue.
Echocardiography.
Cardiac function and ventricular dilation were assessed by transthoracic echocardiography (Acuson Sequoia C256, 15-MHz linear transducer, Siemens, Malvern, PA) in conscious mice, as previously described (9, 37). The M-mode LV end-systolic cross-sectional diameter was determined from an average of three to five cardiac cycles.
Gonadectomy.
Male BALB/c mice were bilaterally gonadectomized (Gdx) or received a sham operation under ketamine (80 mg/kg)-xylazine (8 mg/kg) anesthesia (Phoenex Pharmaceutical, St. Joseph, MO), as previously described (19). Testosterone (testosterone C-III, Sigma, St. Louis, MO) or control PBS capsules (5-mm capsules of testosterone powder) were implanted subcutaneously at the time of the Gdx operation for testosterone replacement, according to Ref. 29. Sera levels of testosterone were 12–15 ng/ml per 5-mm capsule inserted. Two weeks after Gdx, mice received CVB3 (day 0), and hearts were obtained at day 10 postinfection during acute myocarditis.
Recombinant IL-1β or recombinant serpin A 3n treatment.
Male BALB/c mice received 0.1 ml PBS or recombinant mouse IL-1β/IL-1F2 (catalog no. 401-ML, R&D Systems, 7.8 ng/0.1 ml diluted in PBS) or serpin A 3n (catalog no. 4709-PI, R&D Systems, 10 μg/ml diluted in PBS) postinfection every other day from day 1 to day 9 after infection with CVB3 on day 0, and hearts were collected on day 10 postinfection.
Plaque assay and cytokine measurements.
Hearts were homogenized at 10% (wt/vol) in 2% minimal essential medium and individual supernatants or sera used in ELISA to measure cytokines or in plaque assays to determine the level of infectious virus, as previously described (15,16, 19, 37).
RNA extraction.
TRIzol reagent and the PureLink Micro-to-Midi system (Invitrogen, Carlsbad, CA) were used for the extraction and purification of RNA, as previously described (37). Hearts were homogenized in 2 ml TRIzol reagent, and 1 ml of the homogenate was processed according to the manufacturer's protocol (Invitrogen). After elution of the purified RNA, quantification was performed using a NanoDrop spectrophotometer, and quality assessment was determined by RNA Nano LabChip analysis on an Agilent BioAnalyzer 2100 (Agilent Technologies, Santa Clara, CA). For each treatment group, processing and GeneChip analysis were performed in triplicate.
Real-time PCR.
cDNA was generated using Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA). Gene expression was measured using assay-on-demand probe sets and the ABI 7000 Taqman system according to the manufacturer's instructions (Applied Biosystems), as previously described (37). Hypoxanthine phosphoribosyltransferase was used for normalization.
Microarray.
Processing and GeneChip analysis for microarray were performed on three heart samples for each treatment group (4 treatment groups/time point: PBS-treated uninfected males vs. females and CVB3-treated males versus females on days 10 or 90 postinfection). Heart RNA (100 ng) was processed for hybridization to Affymetrix Mouse Gene ST 1.0 microarrays using the Affymetrix GeneChip Whole Transcript Sense Target Labeling Assay, according to the manufacturer's protocol and a previously published method (Affymetrix, Santa Clara, CA) (37). The Affymetrix Mouse GeneChip Gene 1.0 ST Array interrogates 28,853 well-annotated genes with 764,885 distinct probes. The expertise, facilities, and instrumentation for Affymetrix GeneChip experimentation and analyses were provided and supported by the Johns Hopkins Malaria Research Institute.
Analysis of microarray data was performed with Partek Genomics Suite (GS) (version 6.4). Gene expression patterns for each gene were normalized to the median array intensity for all chips, and data from infected animals were normalized to uninfected PBS controls (37). Microarray data were analyzed with Partek GS software by two-way ANOVA to look for significant differences between conditions (with sex and infection as factors), and P values and fold changes were then generated using Fisher's least-significant difference post hoc analysis for comparisons of diseased with undiseased or sex. False discovery rate corrections for multiple comparisons (Benjamini-Hochberg) were applied to reduce the total number of false positives. Genes were considered significant if they had a P value of <0.05. Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA) was used to generate network data by inputting microarray data analyzed with Partek GS. Ingenuity generates networks by identifying published gene relationships.
Microarray data accession number.
The Affymetrix gene expression data were deposited to the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) under Accession Number GSE35182.
Meta-analysis comparison.
We compared genes significantly altered in the myocardium of male mice during acute (day 10 postinfection) or chronic (day 90 postinfection) CVB3 myocarditis versus control undiseased male mice with myocardial genes found in a meta-analysis of seven microarray analyses by Asakura and Kitakaze (1) and a meta-analysis of four microarray analyses conducted by Barth et al. (2). Both meta-analyses were conducted mainly on male patients with DCM versus control patients, and so we compared their gene lists with genes in the mouse that were increased in males with myocarditis versus PBS control males. Asakura and Kitakaze (1) identified 91 genes that were identified in 2 of 7 microarray analyses of DCM versus control patients. Barth et al. (2) identified 27 genes common to 4 microarray analyses of DCM versus control patients.
Statistical analysis.
Data are expressed as means ± SE. Normally distributed data comparing two groups were analyzed by Student's t-test. Nonparametric data comparing two groups were analyzed using the Mann-Whitney U-test. Multiple comparisons were analyzed by two-way ANOVA with a Bonferroni correction. P values of <0.05 were considered significant.
Microarray data were analyzed with Partek GS software by two-way ANOVA to look for significant differences between conditions (with sex and infections as factors), and P values and fold changes were then generated using Fisher's least-significant difference post hoc analysis for the comparison of sex, as previously described (37). False discovery rate corrections for multiple comparisons (Benjamini-Hochberg) were applied to reduce the total number of false positives. Genes were considered significant if they had a P value of <0.05.
RESULTS
CVB3 myocarditis, DCM, and fibrosis are more severe in male versus female mice; this is not due to cardiac viral replication.
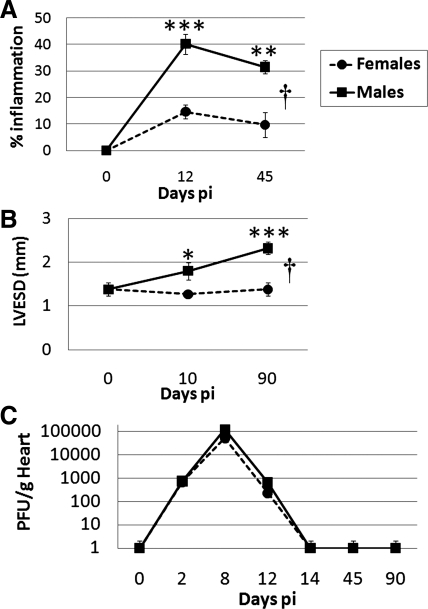
We have previously reported that male versus female BALB/c mice with acute CVB3 myocarditis (days 8–12 postinfection) develop more severe inflammation in the heart, have a lower ejection fraction (45% vs. 60% at day 10 postinfection), and develop DCM and fibrosis by day 35 postinfection (14, 16, 18, 37). The chronic stage of myocarditis/DCM occurs from day 35 postinfection to at least day 90 postinfection, but sex differences in the later stages of disease have not been previously examined. Here, we show that inflammation remained elevated in male versus female mice during the chronic stage of CVB3 myocarditis to at least day 45 postinfection (P = 0.001 by t-test, P = 4.19 × 10−5 by ANOVA; Fig. 1A) and that male mice continued to have DCM at day 90 postinfection, as assessed by echocardiography examining the LV end-systolic dimension (P = 0.008 by ANOVA; Fig. 1B). Inflammation during the chronic stage of CVB3 myocarditis is associated with fibrosis, which is higher in males (14, 19). Elevated myocarditis and DCM do not appear to be due to increased cardiac viral replication, because there were no significant sex differences in infectious virus levels in the heart or in viral clearance (Fig. 1C). These results show that males have more severe myocarditis and progress to DCM and fibrosis, whereas females remain largely protected.
Fig. 1.
Sex differences in coxsackievirus B3 (CVB3) myocarditis in relation to cardiac function and viral replication. Male and female BALB/c mice were inoculated intraperitoneally with 103 plaque-forming units (PFU) of heart-passaged CVB3 on day 0, and acute [days 10 or 12 postinfection (pi)] and chronic (days 45 or 90 postinfection) myocarditis (percent inflammation) and cardiac function [left ventricular end-systolic dimension (LVESD)] were assessed by histology (A) or echocardiography (B), respectively. C: viral replication in the heart was examined at various time points postinfection using a plaque assay. Student's t-test compared females with males at individual time points: *P < 0.05; **P < 0.01; ***P < 0.001. ANOVA compared females with males over time: †P = 4.12 × 10−5 in A and †P = 0.008 in B. Data show means ± SE of 7–10 mice/group.
Most genes associated with cardiac remodeling in diseased males are expressed during acute rather than chronic myocarditis.
To gain a better understanding of the cardiac remodeling gene changes that may contribute to the progression from acute CVB3 myocarditis to DCM in male BALB/c mice, we conducted microarray analysis of hearts during acute myocarditis at day 10 postinfection and chronic myocarditis/DCM at day 90 postinfection. We examined four groups for each time point: undiseased males versus females and diseased males versus females. Because males progress to DCM, we assessed gene changes that were associated with cardiac remodeling during acute (Fig. 2A) or chronic (Fig. 2B) CVB3 myocarditis in males compared with undiseased control males (Table 1). Ingenuity pathway analysis of the microarray data revealed that a network of genes associated with cardiovascular remodeling were significantly up- or downregulated during acute CVB3 myocarditis (day 10 postinfection) compared with undiseased males [25 of 35 (71.4%) genes in this network; Fig. 2A]. In contrast, only 2 of 35 (5.7%) genes in this cardiovascular remodeling network were upregulated during chronic myocarditis at day 90 postinfection in diseased males versus control males (Fig. 2B). We verified increased gene expression in males by RT-PCR during acute CVB3 myocarditis for several genes shown in Table 1, including TIMP-1 (PBS: 2.8 ± 0.4 vs. CVB3: 79.4 ± 8.7, P = 0.0001), serpin A 3n (PBS: 2.3 ± 0.2 vs. CVB3: 14.1 ± 2.0, P = 1.2 × 10−7), IL-33 (PBS: 3.5 ± 0.7 vs. CVB3: 7.8 ± 0.5, P = 0.0005), MMP-3 (PBS: 1.58 ± 0.14 vs. CVB3: 2.89 ± 0.17, P = 0.0001), and MMP-8 (PBS: 2.0 ± 0.3 vs. CVB3: 17.2 ± 2.3, P = 0.002). Our findings indicate that genes important in cardiac remodeling and fibrosis are induced in the heart of males as early as day 10 postinfection during acute myocarditis.
Fig. 2.
Cardiovascular remodeling genes increase during acute CVB3 myocarditis in male mice compared with undiseased control males. Male BALB/c mice were inoculated intraperitoneally with sterile PBS or CVB3 on day 0, and microarrays were conducted on individual hearts (n = 3 hearts/group) on day 10 postinfection (A; acute myocarditis) or day 90 postinfection (B; chronic myocarditis/dilated cardiomyopathy). Ingenuity pathway analysis of microarray data revealed that a major gene network associated with acute CVB3 myocarditis was “cardiovascular disease, organismal injury and abnormality, and tissue morphology.” Red/pink and green represent genes significantly up- or downregulated during acute or chronic myocarditis compared with undiseased controls, respectively.
Table 1.
Genes expressed more highly in male mice with myocarditis than in undiseased controls
| GenBank Accession Number | Gene | Gene Name | Fold Change | P Value |
|---|---|---|---|---|
| Day 10 postinfection: males with acute myocarditis versus undiseased controls | ||||
| NM_001044384 | Timp1 | Tissue inhibitor of metalloproteinase 1 | 7.05 | 0.0001 |
| NM_009252 | Serpina3n | Serpin A 3n | 3.31 | 0.0001 |
| NM_019429 | Il33 | Interleukin-33 | 3.10 | 0.00002 |
| NM_008491 | Lcn2 | Lipocalin 2 | 2.33 | 0.01 |
| NM_011246 | Rasgrp1 | RAS guanyl-releasing protein 1 | 2.14 | 0.0001 |
| NM_001163750 | TGFβ2 | Transforming growth factor-β2 | 2.11 | 0.02 |
| NM_008489 | Lbp | Lipopolysaccharide-binding protein | 1.75 | 0.0007 |
| NM_008611 | Mmp8 | Matrix metalloproteinase 8 | 1.74 | 0.0007 |
| NM_009636 | Aebp1 | Adipocyte enhancer-binding protein 1 | 1.69 | 0.0007 |
| NM_001136079 | Ptger4 | Prostaglandin E receptor 4 | 1.64 | 0.002 |
| NM_009696 | Apoe | Apolipoprotein E | 1.45 | 0.001 |
| NM_009255 | Serpine2 | Serpin E2 | 1.35 | 0.03 |
| NM_008591 | Met | Met protooncogene | 1.34 | 0.02 |
| NM_019971 | Pdgfc | Platelet derived growth factor C | 1.31 | 0.03 |
| Day 90 pi: males with chronic myocarditis/DCM versus undiseased controls | ||||
| NM_019429 | Il33 | Interleukin-33 | 1.65 | 0.02 |
| NM_008489 | Lbp | Lipopolysaccharide-binding protein | 1.45 | 0.005 |
P values compare fold changes in genes from microarray analysis of the heart of male BALB/c mice with acute coxsackievirus B3 (CVB3) myocarditis at day 10 postinfection with undiseased control males. Genes were found in the Ingenuity network of “cardiovascular disease, organismal injury and abnormalities, and tissue morphology.” DCM, dilated cardiomyopathy.
Testosterone upregulates genes associated with cardiac remodeling during acute CVB3 myocarditis in males.
To determine the effect of sex on gene expression during acute myocarditis, we analyzed our microarray data by comparing diseased males with diseased females at day 10 postinfection. Examination of the same cardiovascular remodeling network (Fig. 2) by sex revealed that 31 of 35 (88.6%) genes were significantly upregulated during acute myocarditis in diseased males versus diseased females (Table 2). We verified increased gene expression in males compared with females by RT-PCR during acute CVB3 myocarditis for TIMP-1 (females: 2.1 ± 0.3 vs. males: 7.3 ± 1.1, P = 0.02) and lipocalin-2 (females: 2.5 ± 0.3 vs. males: 4.0 ± 0.5, P = 0.002), for example. Although by RT-PCR there were no sex differences in the remodeling gene IL-1β, which is a part of the cardiovascular disease network (Fig. 2), by ELISA males had greater IL-1β levels than females (females: 386 ± 27 pg/g heart vs. males: 615 ± 61 pg/g heart, P = 0.009) (18). In contrast, MMP-3 mRNA was significantly decreased in the heart of males compared with females with CVB3 myocarditis by RT-PCR (P = 0.008). In general, male BALB/c mice had more upregulated genes associated with cardiovascular remodeling than female mice during acute CVB3 myocarditis.
Table 2.
Genes expressed more highly in male mice compared with female mice with acute CVB3 myocarditis (day 10 postinfection)
| GenBank Accession Number | Gene | Gene Name | Fold Change | P Value |
|---|---|---|---|---|
| NM_017370 | Hp | Haptoglobin | 5.70 | 0.006 |
| NM_011315 | Saa3 | Serum amyloid A3 | 3.93 | 4.6 × 10−5 |
| NM_009252 | Serpina3n | Serpin A 3n | 2.86 | 0.0004 |
| NM_019429 | Prss16 | Serine protease 16 | 2.84 | 0.02 |
| NM_021400 | Prg4 | Proteoglycan 4 | 2.78 | 5.8 × 10−5 |
| NM_001044384 | Timp1 | Tissue inhibitor of metalloproteinase 1 | 2.49 | 0.01 |
| NM_008491 | Lcn2 | Lipocalin 2 | 2.40 | 0.01 |
| NM_010728 | Lox | Lysloxidase | 2.33 | 0.02 |
| NM_011607 | Tnc | Tenascin C | 2.27 | 0.03 |
| NM_001002927 | Penk1 | Preproenkephalin 1 | 2.21 | 0.01 |
| NM_008458 | Serpina3c | Serpin A 3c | 2.06 | 0.01 |
| NM_011580 | Thbs1 | Thrombospondin 1 | 1.88 | 0.04 |
| NM_010809 | Mmp3 | Matrix metalloproteinase 3 | 1.66 | 0.00005 |
| NM_008611 | Mmp8 | Matrix metalloproteinase 8 | 1.64 | 0.001 |
| NM_009255 | Serpine2 | Serpin E2 | 1.56 | 0.005 |
| NM_013492 | Clu | Clusterin | 1.50 | 0.0007 |
| NM_001164311 | Loxl4 | Lysloxidase-like 4 | 1.49 | 0.04 |
| NM_009636 | Aebp1 | Adipocyte enhancer-binding protein 1 | 1.48 | 0.004 |
| NM_029796 | Lrg1 | Leucine-rich glycoprotein 1 | 1.46 | 0.01 |
| NM_009598 | Ace | Angiotensin-converting enzyme | 1.44 | 0.005 |
| NM_008215 | Has1 | Hyaluronan synthase 1 | 1.42 | 0.02 |
| NM_001042611 | Cp | Ceruloplasmin | 1.42 | 0.02 |
| NM_008591 | Met | Met protooncogene | 1.40 | 0.007 |
| NM_001136079 | Ptger4 | Prostaglandin E receptor 4 | 1.39 | 0.01 |
| NM_009139 | Ccl6 | Chemokine (C-C motif) ligand 6 | 1.37 | 0.003 |
| NM_008489 | Lbp | Lipopolysaccharide-binding protein | 1.34 | 0.02 |
| NM_019971 | Pdgfc | Platelet-derived growth factor C | 1.34 | 0.02 |
| NM_011246 | Rasgrp1 | RAS guanyl-releasing protein 1 | 1.33 | 0.03 |
| NM_009696 | Apoe | Apolipoprotein E | 1.28 | 0.009 |
| NM_001163750 | Spsb3 | SPRY domain-containing SOCs box 3 | 1.25 | 0.02 |
| NM_009378 | Thbd | Thrombomodulin | 1.25 | 0.001 |
P values compare fold changes in genes from microarray of the heart of male versus female BALB/c mice with acute CVB3 myocarditis at day 10 postinfection. Genes were found in the Ingenuity network of “cardiovascular disease, organismal injury and abnormalities, and tissue porphology.”
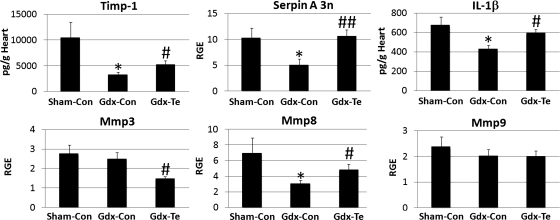
To determine whether testosterone was responsible for elevating remodeling genes in the heart of males during acute CVB3 myocarditis, we performed gonadectomy on male BALB/c mice with or without testosterone replacement. We found that gonadectomy significantly decreased TIMP-1 (P = 0.03), serpin A 3n (P = 0.04), IL-1β (P = 0.04), and MMP-8 (P = 0.04) expression in the heart (Fig. 3). Testosterone replacement in Gdx mice demonstrated that testosterone was responsible for increasing expression of these remodeling genes in the heart (TIMP-1: P = 0.04, serpin A 3n: P = 0.007, IL-1β: P = 0.02, and MMP-8: P = 0.04; Fig. 3). In contrast, Gdx and testosterone replacement significantly decreased MMP-3 levels compared with sham controls (P = 0.02; Fig. 3). Analysis of the three groups by ANOVA revealed significant differences for TIMP-1 (P = 0.04 by ANOVA), serpin A 3n (P = 0.02 by ANOVA), and IL-1β (P = 0.04 by ANOVA). Thus, we showed, for the first time, that testosterone elevates genes associated with cardiac remodeling in males during acute CVB3 myocarditis, including serpin A 3n.
Fig. 3.
Testosterone increases tissue inhibitor of metalloproteinase (Timp1), serpin A 3n, IL-1β, and matrix metalloproteinase (Mmp8) in the heart of male mice during acute CVB3 myocarditis. Male BALB/c mice received a sham operation and a control capsule without testosterone (Sham-Con mice), were gonadectomized and received a control capsule (Gdx-Con mice), or gonadectomized and received a testosterone replacement capsule (Gdx-Te mice). After recovering from the gonadectomy for 2 wk, mice received CVB3 on day 0, and the expression of cardiac remodeling genes was examined at day 10 postinfection by ELISA (pg/ml) or RT-PCR [relative gene expression (RGE)]. Data show means ± SE; n = 10 mice/group. *Gdx-Con or Gdx-Te mice compared with Sham-Con mice by Student's t-test; #Gdx-Te mice compared with Gdx-Con mice by Student's t-test; * or #P < 0.05; ##P < 0.01. The three groups were significantly different by two-way ANOVA for Timp1, serpin A 3n, and IL-1β (†P < 0.05).
Comparison of gene changes in male mice with CVB3 myocarditis versus control males to two meta-analyses of genes associated with DCM patients.
Asakura and Kitakaze (1) conducted a meta-analysis of 7 independent microarray analyses of the myocardium of acute (early stage) DCM versus control patients and identified 91 genes common to at least 2 of the 7 studies. Patients were described as having acute or early-stage cardiomyopathy if they had had symptoms for 6 mo or less (33). In a separate study, Barth et al. (2) conducted a meta-analysis of gene changes in the myocardium of 4 independent microarray data sets of acute DCM versus control patients and found 27 genes common to all 4 studies. These meta-analyses did not distinguish by sex (or whether genes were up- vs. downregulated), but the individual studies reported that the majority of the patients were men. For this reason, we compared their findings to our male mice versus undiseased control males. To determine whether the gene changes that we observed during CVB3 myocarditis in mice were similar to microarray analyses of the myocardium of patients with DCM, we examined whether the 91 (1) and 27 (2) genes or 118 total genes identified in the two meta-analyses were significantly altered during acute CVB3 myocarditis (day 10 postinfection) or chronic DCM (day 90 postinfection) in males versus undiseased males. We found that 39 of the 118 (33.1%) genes identified in the meta-analyses of DCM patients were also significantly altered in the heart during acute CVB3 myocarditis (day 10 postinfection) in male mice compared with uninfected control males (Table 3). In contrast, only 13 of the 118 (11%) genes were significantly altered in the heart of mice during chronic myocarditis/DCM at day 90 postinfection (data not shown). Of note, serpin A 3n and adipocyte enhancer-binding protein 1 were identified in DCM patients (1, 2), were elevated in the heart of male mice with acute CVB3 myocarditis versus undiseased males (Tables 1 and 3), and were elevated in the heart of male versus female mice with acute CVB3 myocarditis (Table 2). However, serpin A 3n was not significantly altered during chronic CVB3 myocarditis/DCM in mice. Thus, ∼33% of the genes that distinguish DCM patients from controls were significantly altered in the heart of male mice with acute CVB3 myocarditis, a time point when we found that most remodeling genes were altered (see Fig. 2). These data suggest that many of the DCM patients on which the meta-analyses were conducted represent acute, early-stage DCM rather than a later stage of disease.
Table 3.
Genes up- or downregulated during acute CVB3 myocarditis in male mice versus undiseased males that were identified in meta-analyses of DCM patients
| GenBank Accession Number | Gene | Gene Name | Fold Change | P Value |
|---|---|---|---|---|
| Upregulated genes | ||||
| NM_011333 | Ccl2 | Chemokine (C-C motif) ligand 2 | 7.94 | 0.0001 |
| NM_025961 | Gatm | Glycine amidinotransferase | 3.34 | 0.0007 |
| NM_009252 | Serpina3n | Serpin A 3n | 3.30 | 0.0001 |
| NM_009283 | Stat1 | STAT1 | 3.05 | 0.0001 |
| NM_001077361 | Fhl1 | 4-1/2 LIM domain protein 1 | 2.52 | 0.009 |
| NM_010846 | Mx1 | Myxovirus resistance 1 | 2.40 | 0.02 |
| NM_007498 | Atf3 | Activating transcription factor 3 | 2.36 | 0.04 |
| NM_008963 | Ptgds | Prostaglandin D2 synthase | 2.30 | 0.001 |
| NM_015784 | Postn | Periostin | 2.21 | 0.001 |
| NM_001034870 | Serpina3 h | Serpin A 3 h | 2.08 | 0.001 |
| NM_010233 | Fn1 | Fibronectin 1 | 2.00 | 0.002 |
| NM_009930 | Col3a1 | Collagen 3a1 | 1.92 | 0.006 |
| NM_001033335 | Serpina3F | Serpin A 3f | 1.90 | 0.006 |
| NM_009636 | Aebp1 | Adipocyte enhancer-binding protein 1 | 1.69 | 0.0007 |
| NM_007742 | Col1a1 | Collagen 1a1 | 1.65 | 0.02 |
| NM_016704 | C6 | Complement component 6 | 1.46 | 0.04 |
| NM_013471 | Anxa4 | Annexin A4 | 1.45 | 0.02 |
| NM_008252 | Hmgb2 | High-mobility group box 2 | 1.40 | 0.005 |
| NM_013650 | S100a8 | S100 calcium-binding protein A8 | 1.36 | 0.04 |
| NM_001159301 | Lgals9 | Galectin 9 | 1.33 | 0.003 |
| NM_013750 | Phlda1 | Pleckstrin homology-like domain, family A, member 1 | 1.32 | 0.02 |
| NM_011664 | Ubb | Ubiquitin B | 1.31 | 0.002 |
| NM_001110017 | Dzip3 | Daz-interacting protein 3 | 1.28 | 0.05 |
| NM_009061 | Rgs2 | Regulator of G protein s2 | 1.28 | 0.02 |
| NM_016844 | Rps28 | Ribosomal protein S28 | 1.27 | 0.009 |
| NM_029620 | Pcolce2 | Procollagen C-endopeptidase enhancer 2 | 1.08 | 0.02 |
| Downregulated genes | ||||
| NM_001122759 | Pde7a | Phosphodiesterase 7A | −1.39 | 0.01 |
| NM_146120 | Gsn | Gelsolin | −1.35 | 0.007 |
| NM_024197 | Ndufa10 | NADH dehydrogenase 1 | −1.33 | 0.006 |
| NM_172925 | Klhl3 | Kelch-like 3 | −1.32 | 0.0002 |
| NM_027275 | Ptcd3 | Pentatricopeptide domain 3 | −1.31 | 0.009 |
| NM_028177 | Ndufab1 | NADH dehydrogenase 1 | −1.31 | 0.02 |
| NM_024211 | Slc25a11 | Solute carrier family 25 | −1.29 | 0.007 |
| NM_027907 | Agxt2l1 | Alanine-glyoxylate aminotransferase 2-like 1 | −1.28 | 0.02 |
| NM_026444 | Cs | Citrate synthase | −1.27 | 0.004 |
| NM_023281 | Sdha | Succinate dehydrogenase | −1.27 | 0.01 |
| NM_025567 | Cyc1 | Cytochrome c1 | −1.25 | 0.006 |
| NM_010190 | Fcnb | Ficolin B | −1.19 | 0.03 |
| NM_010856 | Myh6 | Myosin, heavy polypeptide 6, cardiac | −1.11 | 0.01 |
Shown is a comparison of genes found to be up- or downregulated in a meta-analysis of 11 separate clinical studies of patients (mainly men) with DCM that were compared with undiseased men (1, 2) versus male mice with acute CVB3 myocarditis compared with PBS-inoculated controls (day 10 postinfection). Fold changes and P values are for male CVB3-infected mice versus PBS-inoculated male mice at day 10 postinoculation.
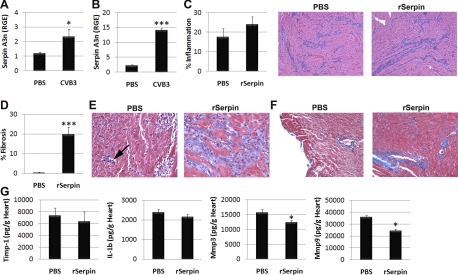
Recombinant serpin A 3n induces cardiac remodeling and fibrosis during acute CVB3 myocarditis by decreasing MMP-3 and MMP-9 expression.
Because serpin A 3n had been identified as increased in men with DCM and male mice with CVB3 myocarditis (Table 3 and Fig. 3), we examined the effect of administering recombinant serpin A 3n versus PBS intraperitoneally to male BALB/c mice on days 1, 3, 5, 7, and 9 postinfection and assessed acute CVB3 myocarditis at day 10 postinfection. Serpin A 3n-deficient mice have not been generated, and we could not find any previous studies that administered recombinant serpin A 3n intraperitoneally to rodents. We based the dose of recombinant serpin A 3n on a report (25) where recombinant serpin A 3n had been administered topically to mice during wound healing of the skin. We decided to administer recombinant serpin A 3n early after infection because we found that serpin A 3n mRNA was significantly upregulated in the heart as early as day 2 postinfection (Fig. 4A) and remained elevated during acute CVB3 myocarditis at day 10 postinfection (Fig. 4B). We found that the overall severity of acute CVB3 myocarditis (i.e., inflammation) was similar between PBS and recombinant serpin A 3n-treated mice (Fig. 4C). Recombinant serpin A 3n administration also did not alter CVB3 replication in the heart at day 10 postinfection compared with PBS controls (data not shown). However, fibrosis was significantly elevated in the heart of mice treated with recombinant serpin A 3n (Fig. 4, D–F). Areas of inflammation in recombinant serpin A 3n-treated mice stained bright blue using Masson's trichrome to detect collagen deposition (Fig. 4E, right), which was not present in myocardial foci of PBS-treated mice (Fig. 4E, left). The arrow shown in Fig. 4E points to a small area of fibrosis in PBS-treated mice in contrast to areas of inflammation largely devoid of fibrosis on the right side of the section. Note that immune cells stained dark blue using Masson's trichrome, but only the bright “sky blue” staining denotes collagen deposition (Fig. 4E). Additionally, recombinant serpin A 3n-treated mice had more severe perivascular fibrosis than PBS-treated controls (Fig. 4F).
Fig. 4.
Recombinant serpin A 3n (rSerpin) promotes cardiac remodeling during CVB3 myocarditis. Male BALB/c mice received sterile PBS or CVB3 intraperitoneally on day 0, and serpin A 3n levels were examined in the heart by RT-PCR on day 2 (A) or day 10 (B) postinfection. Male BALB/c mice were inoculated intraperitoneally with CVB3 on day 0, and either sterile PBS or rSerpin (10 μg/ml) was injected intraperitoneally every other day from day 1 to day 9 postinfection. Mice were examined at day 10 postinfection. C: summary of histology score (left) and representative hematoxylin and eosin staining for inflammation (purple; right). Magnification: ×64. D: percent fibrosis was calculated as the area with collagen deposition compared with the entire myocardial section using a microscope eyepiece grid. Masson's trichrome to detect collagen deposition (bright blue) showed areas of inflammation (E) or perivascular fibrosis (F). Magnification: ×250 in E and ×100 in F. E: small area of fibrosis (arrow) compared with nonfibrotic inflammation in PBS mice (left) versus widespread fibrosis associated with inflammation in rSerpin-treated mice (right). Data show means ± SE of 10 mice/group in A–C, D, and G. *P < 0.05; ***P < 0.001.
To assess how recombinant serpin A 3n affected cardiac remodeling, we examined TIMP-1, IL-1β, MMP-3, and MMP-9 levels in the heart of recombinant serpin A 3n-treated mice versus PBS controls at day 10 postinfection by ELISA. We found that recombinant serpin A 3n did not significantly alter TIMP-1 or IL-1β levels in the heart but significantly decreased MMP-3 (P = 0.04) and MMP-9 (P = 0.03; Fig. 4G). Because serpin A 3n is known to be expressed in tissues, the ECM, and immune cells (CD68+ macrophages) (25, 27, 44), we examined whether serpin A 3n was present in CD11b+ immune cells during myocarditis. Most of the immune cells in the heart of males express CD11b (18, 19). We separated CD11b+ immune cells from other cells in the heart of male mice during acute CVB3 myocarditis using magnetic beads and compared mRNA levels in CD11b versus “other” cells by RT-PCR. We found that serpin A 3n was primarily expressed in other cell types in the heart during acute CVB3 myocarditis (CD11b: 1.92 ± 0.92 relative gene expression vs. other: 22.53 ± 5.51 relative gene expression, P = 0.02). Here, we show, for the first time, that Serpin A 3n can contribute to cardiac remodeling in males during acute CVB3 myocarditis.
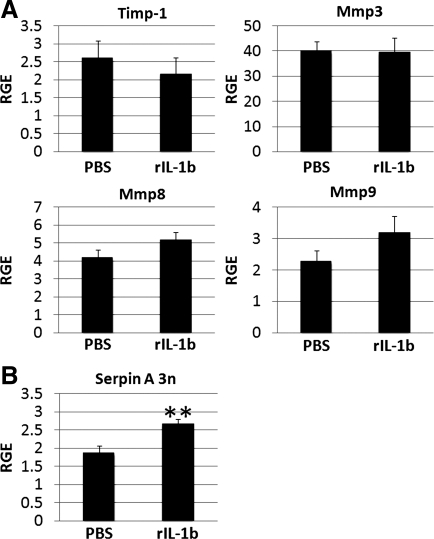
Recombinant IL-1β treatment increases serpin A 3n in the heart of male mice during acute CVB3 myocarditis.
Because several studies (7, 35) have reported that recombinant IL-1β administration is able to increase the expression of serpin A 3n in the pancreas and astrocytes of rodents, we investigated whether recombinant IL-1β was able to increase serpin A 3n expression in the heart of male mice during acute CVB3 myocarditis. We treated male BALB/c mice with recombinant IL-1β every other day from days 1 to 9 after CVB3 injection and examined mRNA levels by RT-PCR at day 10 postinfection. We also examined the effect of recombinant IL-1β treatment on TIMP-1, MMP-3, MMP-8, and MMP-9 because these genes were increased by testosterone or altered by recombinant serpin A 3n administration (Figs. 3 and 4). We found that recombinant IL-1β treatment did not significantly alter TIMP-1 (P = 0.53), MMP-3 (P = 0.94), MMP-8 (P = 0.43), or MMP-9 (P = 0.19; Fig. 5A) but significantly increased serpin A 3n (P = 0.005; Fig. 5B) mRNA in the heart at day 10 postinfection. Thus, we show, for the first time, that IL-1β can increase serpin A 3n in the heart during acute myocarditis in male mice (Fig. 5B).
Fig. 5.
Recombinant IL-1β (rIL-1b) increases serpin A 3n levels in the heart of males during CVB3 myocarditis. A and B: cardiac remodeling genes were assessed by RT-PCR during acute myocarditis (day 10 postinfection) in male BALB/c mice that had been treated with recombinant IL-1β from day 1 to day 9 postinfection (n = 10 mice/group). RGE, normalized to hypoxanthine phosphoribosyltransferase (HPRT), is shown as means ± SE of 10 mice/group. **P < 0.01.
DISCUSSION
The male sex is a major risk factor for the development of cardiovascular disease, including myocarditis and DCM (10, 11, 38, 39). Findings from a recent prospective multicenter study (33) found that men with myocarditis or acute cardiomyopathy had worse recovery and survival than women. Numerous studies (12, 23, 42, 43, 46) have examined gene profiles associated with myocarditis or DCM in patients and animal models, but few have investigated sex differences in gene expression (22). In animal models, testosterone has been found to enhance cardiac remodeling, whereas estrogen is protective, although the role of specific genes was not examined (6, 36). In this study, we show, for the first time, that the expression of remodeling genes, such as TIMP-1 and serpin A 3n, occurs during acute myocarditis rather than during chronic myocarditis/DCM. This finding is important because it indicates the need to study remodeling changes in patients as early as possible in the disease process. This point was reinforced by our finding that 33% of genes in our mouse model of CVB3 myocarditis/DCM were associated with DCM patients who primarily had acute or early-stage DCM and were male (1, 2).
Another novel finding from this study was that testosterone increases genes associated with cardiac remodeling during acute CVB3 myocarditis, including TIMP-1, serpin A 3n, IL-1β, and MMP-8. Previously, it has been shown using knockout mice that TIMP-1 exacerbates remodeling during CVB3 myocarditis by regulating MMP expression in the heart (13). We are the first to show that testosterone increases TIMP-1 expression in the heart during CVB3 myocarditis in male mice. Elevated cardiac TIMP-1 mRNA levels have been found to correlate with LV fibrosis in patients with chronic pressure overload (24), and elevated TIMP-1 levels in the sera predict the progression to heart failure in patients with coronary artery disease (31). These findings indicate that TIMP-1 contributes to cardiac remodeling in males, resulting in the progression to chronic cardiomyopathy.
MMPs have also been linked to the development of DCM and heart failure (4, 32). MMP-8 is a neutrophil collagenase that can be activated by MMP-3 (32). Various MMPs are able to cleave pro-IL-1β to its active form. Cardiac IL-1β is increased by testosterone in males with CVB3 myocarditis (Fig. 3), and we and others (9, 15–20) have shown that IL-1β is a strong inducer of proinflammatory and profibrotic responses during CVB3 myocarditis. A previous study (8) found that MMP-8 deficiency in male mice with CVB3 myocarditis did not significantly alter acute inflammation, fibrosis, or viral replication in the heart. In contrast, MMP-9 deficiency resulted in increased CVB3 myocarditis, fibrosis, and viral replication in male mice (8), indicating a protective role for MMP-9 during acute myocarditis. In this study, we found that MMP-9 was decreased in the heart after recombinant serpin A 3n administration, further suggesting a protective role for MMP-9 during myocarditis. In a separate study, Shen et al. (40) found that MMP-3 and MMP-9 levels were decreased when fibrosis was elevated during CVB3 myocarditis after treatment of mice with a hepatocyte growth factor inhibitor. Interestingly, we found that testosterone was responsible for decreasing MMP-3 expression in the heart of males with CVB3 myocarditis (Fig. 3), further confirming the negative effect of testosterone on cardiac remodeling. Overall, these findings suggest that lower cardiac expression of MMP-3 and MMP-9 during acute myocarditis contributes to the ECM remodeling in males that leads to DCM (Fig. 6).
Fig. 6.
Influence of testosterone (Te) on cardiac remodeling. Testosterone increases IL-1β and serpin A 3n levels in the heart during acute CVB3 myocarditis. IL-1β further elevates serpin A 3n levels, which contribute to cardiac remodeling and fibrosis by reducing Mmp3 and Mmp9.
We are the first to show that testosterone and IL-1β increase serpin A 3n levels in the heart of males during acute CVB3 myocarditis. Previously, IL-1β has been shown to increase serpin A 3n levels in the pancreas and astrocytes of rodents (7, 35), but its effect on serpin A 3n levels in the heart had not been examined. Serpin A 3n is known to be expressed in the heart of undiseased wild-type mice and is also highly expressed in the testis (26). It has been shown to protect Sertoli cells in the testis from granzyme B-mediated killing by immune cells (41). Thus, it is probably not surprising that testosterone regulates serpin A 3n levels in the heart, which is the major sex hormone-producing organ aside from the gonads (30, 34). However, our finding that administration of serpin A 3n increases remodeling during CVB3 myocarditis and alters MMP levels indicates that serpin A 3n is one of a panel of remodeling genes regulated by testosterone (i.e., TIMP-1, MMP-3, and IL-1β) that induce ECM remodeling during acute myocarditis leading to worse progression in males (Fig. 6). We found that IL-1β levels in the heart during CVB3 myocarditis are directly associated with inflammation (17, 18), indicating that immune cells are the primary source of IL-1β. Here, we show that during CVB3 myocarditis, serpin A 3n is primarily present in nonimmune cells. Thus, IL-1β production from cardiac inflammation likely increases testosterone-driven serpin A 3n production in the heart of males, further facilitating cardiac remodeling (Fig. 6).
Thus, our findings suggest that the regulation of IL-1β and serpin A 3n by testosterone in the heart plays a major role in the progression of males from myocarditis to DCM. The significance of this finding is emphasized by the observation that serpin A 3n is not only elevated in males in our mouse model of myocarditis but is also elevated in the heart of patients with DCM. Importantly, these remodeling genes are upregulated during acute myocarditis, indicating a critical window for treatments aimed at counteracting the damaging effects of IL-1β (such as the IL-1 receptor antagonist Anakinra) and serpin A 3n in the heart of males.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL087033 (to D. Fairweather).
DISCLAIMER
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.C. and D.F. conception and design of research; M.J.C., J.E.B., E.K., A.B., D.B., E.D.A., J.S., and D.F. performed experiments; M.J.C., J.E.B., E.K., A.B., D.B., E.D.A., J.S., K.L.G., and D.F. analyzed data; M.J.C., J.E.B., E.K., A.B., D.B., E.D.A., K.L.G., W.M., and D.F. interpreted results of experiments; M.J.C. and D.F. prepared figures; M.J.C. and D.F. drafted manuscript; M.J.C., K.L.G., and D.F. edited and revised manuscript; M.J.C., J.E.B., E.K., A.B., D.B., E.D.A., J.S., K.L.G., W.M., and D.F. approved final version of manuscript.
REFERENCES
- 1. Asakura M, Kitakaze M. Global gene expression profiling in the failing myocardium. Circ J 73: 1568– 1576, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Barth AS, Kuner R, Buness A, Ruschhaupt M, Merk S, Zwermann L, Kääb S, Kreuzer E, Steinbeck G, Mansmann U, Poustka A, Nabauer M, Sültmann H. Identification of a common gene expression signature in dilated cardiomyopathy across independent microarray studies. J Am Coll Cardiol 48: 1610– 1617, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis 52: 274– 288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brauer PR. MMPs–role in cardiovascular development and disease. Front Biosci 11: 447– 478, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Caforio ALP, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, Ramondo A, Carturan E, Iliceto S, Thiene G, Daliento L. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J 28: 1326– 1333, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol 290: H2043– H2050, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Chen MC, Schuit F, Pipeleers DG, Eizirik DL. IL-1β induces serine protease inhibitor 3 (Spi-3) gene expression in rat pancreatic β-cells. Detection by differential display of messenger RNA. Cytokine 11: 856– 862, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Cheung C, Marchant D, Walker EKY, Luo Z, Zhang J, Yanagawa B, Rahmani M, Cox J, Overall C, Senior RM, Luo H, McManus BM. Ablation of matrix metalloproteinase-9 increases severity of viral myocarditis in mice. Circulation 117: 1574– 1582, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Cihakova D, Barin JG, Afanasyeva M, Kimura M, Fairweather D, Berg M, Talor MV, Baldeviano GC, Frisancho-Kiss S, Gabrielson K, Bedja D, Rose NR. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am J Pathol 172: 1195– 1208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cocker MS, Abdel-Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: a cardiovascular magnetic resonance study. Heart 95: 1925– 1930, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Cooper LT., Jr Myocarditis. N Engl J Med 360: 1526– 1538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper LT, Jr, Onuma OK, Sagar S, Oberg AL, Mahoney DW, Asmann YW, Liu P. Genomic and proteomic analysis of myocarditis and dilated cardiomyopathy. Heart Fail Clin 6: 75– 85, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Crocker SJ, Frausto RF, Whitmire JK, Benning N, Milner R, Whitton JL. Amelioration of coxsackievirus B3-mediated myocarditis by inhibition of tissue inhibitors of matrix metalloproteinase-1. Am J Pathol 171: 1762– 1773, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods 41: 118– 122, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12Rβ1 and TLR4 increase IL-1β and IL-18-associated myocarditis and coxsackievirus replication. J Immunol 170: 4731– 4737, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Gatewood SJL, Davis SE, Njoku DB, Rose NR. IFN-γ protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines TGF-β1, IL-1β, and IL-4 in the heart. Am J Pathol 165: 1883– 1894, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fairweather D, Frisancho-Kiss S, Rose NR. Viruses as adjuvants for autoimmunity: evidence from coxsackievirus-induced myocarditis. Rev Med Virol 15: 17– 27, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Rose NR, Fairweather D. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol 178: 6710– 6714, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather D. Gonadectomy of male BALB/c mice increases Tim-3+ alternatively activated M2 macrophages, Tim-3+ T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun 23: 649– 657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fuse K, Chan G, Liu Y, Gudgeon P, Husain M, Chen M, Yeh WC, Akira S, Liu PP. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation 112: 2276– 2285, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level- risk factors, screening, and outcomes. Nat Rev Cardiol 8: 673– 685, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Haddad GE, Saunders LJ, Crosby SD, Carles M, del Monte F, King K, Bristow MR, Spinale FG, Macgillivray TE, Semigran MJ, Dec GW, Williams SA, Hajjar RJ, Gwathmey JK. Human cardiac-specific cDNA array for idiopathic dilated cardiomyopathy: sex-related differences. Physiol Genomics 33: 267– 277, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Heidecker B, Kittleson MM, Kasper EK, Wittstein IS, Champion HC, Rissell SD, Hruban RH, Rodriguez ER, Baughman KL, Hare JM. Transcriptomic biomarkers for the accurate diagnosis of myocarditis. Circulation 123: 1174– 1184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heymans S, Sschroen B, Vermeersch P, Milting H, Gao F, Kassner A, Gillijns H, Herijgers P, Flameng W, Carmeliet P, van de Werf F, Pinto YM, Janssens S. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibiter of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation 23: 1136– 1144, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Hoffmann DC, Textoris C, Oehme F, Klaassen T, Goppelt A, Romer A, Fugmann B, Davidson JM, Werner S, Krieg T, Eming SA. Pivotal role for α1-antichymotrypsin in skin repair. J Biol Chem 286: 28889– 28901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horvath AJ, Forsyth SL, Coughlin PB. Expression patterns of murine antichymotrypisn-like genes reflect evolutionary divergence at the Serpina3 locus. J Mol Evol 59: 488– 497, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Horvath AJ, Irving JA, Rossjohn J, Law RH, Bottomly SP, Quinsey NS, Pike RN, Coughlin PB, Whisstock JC. The murine orthologue of human antichymotrypsin: a structural paradigm for clade A3 serpins. J Biol Chem 280: 43168– 43178, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med 19: 247– 252, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Klein SL, Bird BH, Glass GE. Sex differences in Seoul virus infection are not related to adult sex steroid concentrations in Norway rats. J Virol 74: 8213– 8217, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krieg M, Smith K, Bartsch W. Demonstration of a specific androgen receptor in rat heart muscle: relationship between binding, metabolism, and tissue levels of androgens. Endocrinology 103: 1686– 1694, 1978 [DOI] [PubMed] [Google Scholar]
- 31. Lubos E, Schnabel R, Rupprecht HJ, Bickel C, Messow CM, Prigge S, Cambien F, Tiret L, Munzel T, Blankenberg S. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: results from the AtheroGene study. Eur Heart J 27: 150– 156, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Lui P, Sun M, Sader S. Matrix metalloproteinases in cardiovascular disease. Can J Cardiol 22, Suppl B: 25B– 30B, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, Gorcsan J, 3rd, Kip KE, Dec GW; IMAC Investigators Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol 58: 1112– 1118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melchert RB, Kennedy RH, Acosta D., Jr Cardiovascular effects of steroidal agents. In: Cardiovascular Toxicology, edited by Acosta D., Jr London: Taylor & Francis, 2001, p. 425– 475 [Google Scholar]
- 35.Morihara T, Teter B, Yang F, Lim GP, Boudinot S, Boudinot FD, Frautschy SA, Cole GM. Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic α1-antichymotrypsin to ameliorate β-amyloid (Aβ) pathology in Alzheimer's models. Neuropsychopharmacology 30: 1111– 1120, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Nikolic I, Liu D, Bell JA, Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-β-selective agonist is cardioprotective. J Mol Cell Cardiol 42: 769– 780, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Onyimba JA, Coronado MJ, Garton AE, Kim JB, Bucek A, Bedja D, Gabrielson KL, Guilarte TR, Fairweather D. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol Sex Differ 2: 2, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regitz-Zagrosek V, Oertelt-Prigione O, Seeland U, Hetser R. Sex and gender differences in myocardial hypertrophy and heart failure. Circ J 74: 1265– 1273, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, American Heart Association Statistics Committee, and Stroke Statistics Subcommittee Heart disease and stroke statistics–2011 update. Circulation 123: e18– e209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shen D, Tang Q, Huang Z, Chen Y, Xiong R, Wu H, Huang J, Feng S, Yan L, Bian Z. The effects of NK4 on viral myocarditis in mice. Cardiovasc Pathol 18: 323– 331, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Sipione S, Simmen KC, Lord SJ, Motyka B, Ewen C, Shostak I, Rayat GR, Dufour JM, Korbutt GS, Rajotte RV, Bleackley RC. Identification of a novel human granzyme B inhibitor secreted by cultured Sertoli cells. J Immunol 177: 5051– 5058, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Tang Q, Huang J, Qian H, Xiong R, Shen D, Wu H, Bian Z, Wei X. Microarray analysis reveals the role of matrix metalloproteinases in mouse experimental autoimmune myocarditis induced by cardiac myosin peptides. Cell Mol Biol Lett 12: 176– 191, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taylor LA, Carthy CM, Yang D, Saad K, Wong D, Schreiner G, Stanton LW, sMcManus BM. Host gene regulation during coxsackievirus B3 infection in mice: assessment by microarrays. Circ Res 87: 328– 334, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Jiang H, Dai Su M D, Martinka M, Brasher P, Zhang Y, McLean D, Zhang J, Ip W, Li G, Zhang X, Zhou Y. Alpha 1 antichymotrypsin is aberrantly expressed during melanoma progression and predicts poor survival for patients with metastatic melanoma. Pigment Cell Melanoma Res 23: 575– 578, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Wexler RK, Elton T, Pleister A, Feldman D. Cardiomyopathy: an overview. Am Fam Physician 79: 778– 784, 2009 [PMC free article] [PubMed] [Google Scholar]
- 46. Yung CK, Halperin VL, Tomaselli GF, Winslow RL. Gene expression profiles in end-stage human idiopathic dilated cardiomyopathy: altered expression of apoptotic and cytoskeletal genes. Genomics 83: 281– 297, 2004 [DOI] [PubMed] [Google Scholar]