Abstract
Pulmonary arterial hypertension (PAH) is a severe and progressive disease that usually culminates in right heart failure and death if left untreated. Although there have been substantial improvements in our understanding and significant advances in the management of this disease, there is a grim prognosis for patients in the advanced stages of PAH. A major cause of PAH is increased pulmonary vascular resistance, which results from sustained vasoconstriction, excessive pulmonary vascular remodeling, in situ thrombosis, and increased pulmonary vascular stiffness. In addition to other signal transduction pathways, Ca2+ signaling in pulmonary artery smooth muscle cells (PASMCs) plays a central role in the development and progression of PAH because of its involvement in both vasoconstriction, through its pivotal effect of PASMC contraction, and vascular remodeling, through its stimulatory effect on PASMC proliferation. Altered expression, function, and regulation of ion channels and transporters in PASMCs contribute to an increased cytosolic Ca2+ concentration and enhanced Ca2+ signaling in patients with PAH. This review will focus on the potential pathogenic role of Ca2+ mobilization, regulation, and signaling in the development and progression of PAH.
Keywords: vascular remodeling, vasoconstriction, calcium regulation, calcium channel, canonical transient receptor potential channel
this article is part of a collection on Hypertension and Novel Modulators of Vascular Tone. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
The lungs are the only organ that receives the entire cardiac output (CO), so the pulmonary circulation is maintained in a high-flow, low-pressure, and low-resistance state under normal physiological conditions. The human pulmonary vasculature is composed of pulmonary arteries, precapillary arterioles, capillaries, and pulmonary veins. The walls of pulmonary arteries and arterioles are thin, and the myogenic tone in pulmonary arteries is small compared with systemic arteries (e.g., in mesenteric, coronary, renal, and cerebral arteries). Therefore, pulmonary arterial distension and recruitment (opening of closed blood vessels) are two important mechanisms for reducing pulmonary vascular resistance (PVR) when blood flow or CO is increased (e.g., during heavy exercise). Under pathophysiological conditions, however, increased PVR is a major cause for the development of pulmonary hypertension. Elevated PVR increases the right heart afterload and over time results in right ventricular hypertrophy and eventually right heart failure and death.
Pulmonary arterial hypertension (PAH) is a disease that is rarely diagnosed during routine medical examinations. Instead, it is typically made from exclusion of other disorders such as congenital heart disease, emphysema, or pulmonary embolism. Although a noninvasive estimation of pulmonary arterial pressure (PAP) can be derived through echocardiography, there are limitations that make screening and diagnosis challenging (123). The gold standard for clinical diagnosis of pulmonary hypertension is by right heart catheterization. The mean PAP (mPAP) for a healthy adult is 10–20 mmHg, whereas mean systemic arterial pressure is 70–105 mmHg. Pulmonary hypertension is defined clinically as a mPAP ≥ 25 mmHg at rest or a mPAP ≥ 30 mmHg during exercise (6). Importantly, PAH can be distinguished from other forms of pulmonary hypertension with the additional criterion of a pulmonary wedge pressure ≤ 15 mmHg (6). Table 1 shows the clinical classifications set forth by the most recent World Symposium on Pulmonary Hypertension held in Dana Point, CA, in 2008. These conferences were instrumental in assigning categories based on shared underlying pathologies that allowed investigators to focus treatments on a well-defined group of patients. There are two widely used functional classification systems used by physicians for assessing patients with pulmonary hypertension: the New York Heart Association system and the World Health Organization system. Table 2 lists the four classes of functional assessment for each system with a brief description (98). Recent epidemiological data from the Registry to EValuate Early And Long-term pulmonary arterial hypertension disease management (REVEAL) study conducted from March 2006 to September 2007 in the United States reported that the most common age of patients with a diagnosis of PAH is between 45 and 54 years, with a mean age of 44.9 years. Additionally, PAH is more likely to affect women by a 3.6-to-1 ratio (7, 40). These results closely matched the French registry study with age and severity at time of diagnosis. However, the REVEAL data showed a preponderance of women and these patients had a tendency to be obese, whereas French patients with PAH were associated with human immunodeficiency virus (10, 40, 49).
Table 1.
WHO Classification of Pulmonary Hypertension: Dana Point, 2008
| 1. Pulmonary arterial hypertension |
| 1.1 Idiopathic PAH |
| 1.2 Heritable |
| 1) BMPR2 |
| 2) ALK1, endoglin (with or without hereditary hemorrhagic telangiectasia) |
| 3) Unknown |
| 1.3 Drug and toxin induced |
| 1.4 Associated with |
| 1) Connective tissue disease |
| 2) HIV infection |
| 3) Portal hypertension |
| 4) Congenital heart diseases |
| 5) Schistosomiasis |
| 6) Choric hemolytic anemia |
| 1.5 Persistent pulmonary hypertension of the newborn |
| 1.6 Pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary hemangiomatosis (PCH) |
| 2. Pulmonary hypertension due to left heart disease |
| 2.1 Systolic dysfunction |
| 2.2 Diastolic dysfunction |
| 2.3 Valvular disease |
| 3. Pulmonary hypertension due to lung diseases and/or hypoxia |
| 3.1 Chronic obstructive pulmonary disease |
| 3.2 Interstitial lung disease |
| 3.3 Other pulmonary diseases with mixed restrictive and obstructive pattern |
| 3.4 Sleep-disordered breathing |
| 3.5 Alveolar hypoventilation disorders |
| 3.6 Chronic exposure to high altitude |
| 3.7 Developmental abnormalities |
| 4. Chronic thromboembolic pulmonary hypertension (CTEPH) |
| 5. PH with unclear multifactorial mechanisms |
| 5.1 Hematologic disorders: myeloproliferative disorders, splenectomy |
| 5.2 Systemic disorders: sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis, neurofibromatosis, vasculitis |
| 5.3 Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders |
| 5.4 Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure (on dialysis) |
WHO, World Health Organization; PAH, pulmonary arterial hypertension; BMPR2, bone morphogenetic protein receptor 2. Reprinted from Simonneau et al. (104) with permission.
Table 2.
Functional Classification of Pulmonary Hypertension
| A. New York Heart Association functional classification |
| Class 1: No symptoms with ordinary physical activity. |
| Class 2: Symptoms with ordinary activity. Slight limitation of activity. |
| Class 3: Symptoms with less than ordinary activity. Marked limitation of activity. |
| Class 4: Symptoms with any activity or even at rest. |
| B. WHO functional assessment classification |
| Class I: Patients with PH but without resulting limitation of physical activity. Ordinary physical activity does not cause undue dyspnea or fatigue, chest pain, or near syncope. |
| Class II: Patients with PH resulting in slight limitation of physical activity. They are comfortable at rest. Ordinary physical activity causes undue dyspnea or fatigue, chest pain, or near syncope. |
| Class III: Patients with PH resulting in marked limitation of physical activity. There are comfortable at rest. Less than ordinary activity causes undue dyspnea or fatigue, chest pain, or near syncope. |
| Class IV: Patients with PH with inability to carry out any physical activity without symptoms. These patients manifest signs of right heart failure. Dyspnea and/or fatigue may even be present at rest. Discomfort is increase by any physical activity. |
PH, pulmonary hypertension. Rubin et al. (98) with permission from the American College of Chest Physicians.
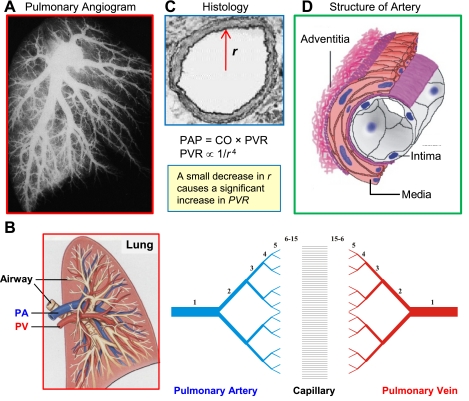
In humans, there are 15 orders of pulmonary arteries between the main pulmonary artery and the capillaries with 15 orders of pulmonary veins between the capillaries and the left atrium (Fig. 1, A and B) (47, 68). The pulmonary artery is composed of three layers: intima (endothelial cells), media (smooth muscle cells), and adventitia (fibroblasts) (Fig. 1D). The intraluminal diameter of the individual pulmonary arteries decreases exponentially from orders 1 to 15 with an exponential increase in pulmonary artery branches (Fig. 1C). This leaves a total area of ∼25% for large conducting arteries (diameter <0.6 mm), 44% for medium-sized (0.2–0.6 mm) and 30% for small vessels (diameter <0.2 mm). Although all branches of the pulmonary artery are involved in determining the level of PVR, changes in medium and small pulmonary arteries contribute more to the alteration of PVR in animals and patients with pulmonary hypertension.
Fig. 1.
Structure and architecture of the pulmonary vasculature. A: pulmonary angiogram of the human lung showing the structure of the vasculature tree from large conducting vessels down to higher generation branches (arterioles). B: scheme of the pulmonary vasculature depicts the 15 orders of pulmonary arteries and veins with capillaries in between. C: cross section of pulmonary artery showing a small decrease in radius (r) or diameter can lead to a significant increase in pulmonary vascular resistance (PVR) and, as a result, elevation of pulmonary arterial pressure (PAP). D: schematic demonstrating the 3-layer structure of an artery from the lumen to the outside of the vessel. PA, pulmonary artery; PV, pulmonary vein; CO, cardiac output.
PAP is a function of CO and PVR: PAP = CO × PVR. PVR is inversely proportional to the intraluminal radius (r) of pulmonary arteries (PVR ∝ 1/r4) (Fig. 1C). An important highlight of this equation is that a small change in the radius of a pulmonary blood vessel can have a dramatic impact on PVR since the radius is raised to the fourth power. As shown in Fig. 2, a small amount of pulmonary vasoconstriction induced by hypoxia, or an 18% decrease in the diameter of the medium or small pulmonary arteries, would cause a significant increase in mean PAP (from 16 to 39 mmHg or from 24 to 59 mmHg). Given the relationship between PVR and pulmonary artery intraluminal radius or diameter, total PVR can be significantly altered by small changes in contraction by these arteries or in the wall thickness of these arteries.
Fig. 2.
Morphological and functional changes of pulmonary arteries resulting from hypoxia. A: acute hypoxia induces pulmonary vasoconstriction (or decreased intraluminal diameter of pulmonary artery) leading to an increase in PVR with a corresponding increase in mean PAP (mPAP). The numbers shown in the box are the hypothetical prediction of the changes in mPAP before and after hypoxia based on the changes in pulmonary arterial diameter (top and middle). Arrows indicate the arterial branches that underwent significant pulmonary vasoconstriction during hypoxia. B: smaller arteries are also affected by hypoxia as indicated with vessel contraction in the smaller branches of the pulmonary tree. Images are reproduced with permission from Dawson (31).
Pathogenic Mechanisms of PAH
Regardless of the initial genetic or pathogenic trigger(s), the pathogenesis of PAH can be attributed to the combined effects of sustained vasoconstriction, concentric vascular remodeling, in situ thrombosis, and arterial wall stiffening, resulting in elevated PVR (Fig. 3) (75, 136). As shown in the angiogram (Fig. 3A), there is a striking contrast in the pulmonary vasculature of a normal subject versus a patient with PAH, which shows a significant decrease in the number of small vessels present. All patients with PAH share common pathological features such as precapillary arteriopathy; increased thickness of the intima, media and adventitia of peripheral arteries; muscularization of the precapillary arterioles and capillaries; and obliteration of small vessels (Fig. 3, A and C) (34, 112, 131).
Fig. 3.
Major causes for the elevated PVR in patients with pulmonary arterial hypertension (PAH): the effects of remodeling and corresponding changes in PVR. A: a significant loss of the small vessels of the pulmonary vasculature can be seen in an angiogram from a healthy (normal) patient versus a patient with PAH. Histological cross sections of small arteries reveal the extensive hypertrophy of the adventitia, media, and intima (or concentric pulmonary arterial remodeling) that occurs in patients with PAH [adapted by permission from BMJ Publishing Group Limited. Bedford et al. (8)]. B: list of the major causes leading to an increase PVR in patients with PAH. C: small arteries from a normal subject and 2 different patients with PAH show the change in architecture [adventitial and medial hypertrophy, intimal lesion, and intraluminal obliteration (top), as well as significant medial hypertrophy (bottom)] that occurs in the pulmonary vasculature causing elevated PVR. PASMC, pulmonary artery smooth muscle cell; PAEC, pulmonary artery endothelial cells.
The vasoconstriction induced by acute hypoxia, or hypoxic pulmonary vasoconstriction, is important to optimize ventilation-perfusion matching, diverting blood from poorly ventilated areas of the lung to the regions that are better ventilated for more efficient gas exchange (126, 127). However, persistent hypoxia causes pulmonary hypertension in animals and patients by inducing sustained pulmonary vasoconstriction and pulmonary vascular medial hypertrophy. Although PAH is disparate from hypoxia-induced pulmonary hypertension, sustained vasoconstriction and excessive pulmonary vascular remodeling are present in both forms of pulmonary hypertension.
The thickness and tissue mass of the pulmonary arterial walls are maintained by a balance between cell proliferation and apoptosis. A disruption of this balance in favor of proliferation can lead to thickening of the wall, narrowing, and eventually obliterating the vessel lumen. Pulmonary vascular remodeling refers to the structural changes that lead to hypertrophy and/or luminal occlusion (33, 111). An increase in cytosolic Ca2+ concentration ([Ca2+]cyt) in pulmonary artery smooth muscle cells (PASMCs) is a major trigger for pulmonary vasoconstriction, as well as an important stimulus for cell proliferation and migration, two major causes for pulmonary vascular remodeling. Thrombotic lesions are also often apparent along with pulmonary vascular remodeling and contribute to the increased PVR in patients with PAH (26, 52, 122). A hallmark of severe idiopathic PAH (IPAH) is the presence of plexiform lesions that can obstruct blood flow in small arteries and arterioles. Occlusion of the smaller blood vessels can arise from monoclonal proliferation of endothelial cells, smooth muscle cell migration, proliferation, and hyperplasia with an accumulation of circulating inflammatory, platelet, and progenitor cells (34, 121). Finally, decreased vascular wall compliance or increased wall stiffness, which has been ascribed to breakdown of extracellular matrix and increased collagen accumulation with endothelial and smooth muscle cell proliferation, is also a major cause of increased PVR (28, 83, 99). The elevated PVR in patients with PAH increases the workload (or afterload) on the right ventricle and leads to right heart failure with greater frequency (Fig. 4). This review focuses on the pathogenic role of Ca2+ signaling in PASMCs (involving Ca2+ channels and transporters) in the initiation and progression of PAH.
Fig. 4.
Potential pathogenic mechanisms involved in PAH. Flow chart demonstrating the 4 major causes of elevated PVR and how they lead to PAH and eventually right heart failure.
Ca2+ Is Required for Pulmonary Vasoconstriction and PASMC Proliferation
Both sustained pulmonary vasoconstriction and vascular remodeling are directly mediated by PASMC contraction and proliferation, respectively. Increased [Ca2+]cyt is a major stimulus for cellular proliferation (Fig. 5). Both nuclear and cytosolic Ca2+ pools promote proliferation by activating Ca2+-dependent kinases (e.g., CaMK), immediate early genes, and other transcription factors [e.g., c-Fos, nuclear factor of activated T-cells (NFAT), cAMP response element binding protein (CREB)], which are necessary for cell growth (Fig. 5A) (5, 11, 43, 102). Increases in cytoplasmic and nuclear [Ca2+] trigger Ca2+-dependent gene transcription in vascular smooth muscle cells (32, 45, 46, 76). Ca2+ can also affect gene expression through its interaction with protein kinase C (PKC) and calmodulin (CaM) or by activation of the proteins involved in the cell cycle (cyclins and cyclin dependent kinases). In addition to the stimulation of quiescent cells to enter the cell cycle (G0 to G1 transition), Ca2+ or Ca2+/CaM is also required for progression from G1 to S, from G2 to mitosis checkpoints, and through mitosis (Fig. 5A) (11, 23, 30, 56, 73, 103). In PASMCs specifically, both increased [Ca2+]cyt and intracellularly stored Ca2+ are essential for proliferation (42). In the presence of serum and growth factors, the removal of extracellular Ca2+ and the depletion of intracellularly stored Ca2+ inhibit proliferation of PASMCs, demonstrating the requirement for Ca2+ for the cell cycle progression and cell growth (Fig. 5C) (42).
Fig. 5.
An increase in cytosolic Ca2+ concentration ([Ca2+]cyt) in PASMCs is required for pulmonary vasoconstriction and plays an important role in cell proliferation and vascular remodeling. A: when the [Ca2+]cyt rises because of Ca2+ influx through different Ca2+ channels in the plasma membrane and Ca2+ mobilization from the intracellular stores [e.g., sarcoplasmic reticulum/endoplasmic reticulum (SR/ER)], Ca2+ binds calmodulin (CaM) which causes PASMC contraction by activating (via phosphorylation) myosin light chain (MLC) kinase (MLCK). Increased [Ca2+]cyt also activates CaM kinase (CaMK) and mitogen-activated protein kinase (MAPK), as well as other transcription factors [nuclear factor of activated T cells (NFAT), cAMP response element binding protein (CREB), activator protein-1 (AP-1), and NF-κB], to stimulate PASMC proliferation by propelling Ca2+-sensitive steps in the cell cycle progression. B: removal of extracellular Ca2+ (0 Ca) significantly inhibits vasoconstriction (determined by active tension) induced by 40 mM K+ (40 K) and phenylephrine (PE) in isolated rat pulmonary arterial rings (70). C: rat PASMC growth is significantly inhibited by chelation of extracellular Ca2+ with EGTA or by valinomycin (Val)-induced increase in K+ efflux (88). Cells are cultured in serum- and growth factor-contained media for 6 days in the absence of EGTA or Val (Cont), and the presence of 100 μM Val or 2 mM EGTA. MLCP, myosin light chain phosphatase; PAFB, pulmonary arterial fibroblasts; P, phosphorylated; G, M, S, phases of the cell cycle. **P < 0.01; ***P < 0.001.
In addition to stimulating proliferation, increased [Ca2+]cyt is necessary for contraction in PASMCs (Fig. 5A) (14, 17, 29, 70, 74, 77). Both Ca2+ influx through plasma membrane Ca2+ channels and Ca2+ release from intracellular stores [e.g., the sarcoplasmic reticulum (SR)] contribute to a rise in [Ca2+]cyt. In rat pulmonary arterial rings, the removal of extracellular Ca2+ prevents high K+-induced contraction (70), indicating that Ca2+ influx through plasmalemmal channels is necessary for contraction (Fig. 5B). When [Ca2+]cyt increases, it binds to CaM, which then activates myosin light chain kinase. Activated myosin light chain kinase phosphorylates the regulatory light chain of myosin, allowing for the activation of myosin ATPase. The ensuing hydrolysis of ATP provides the energy source needed for the cross-bridging cycles between myosin and actin filaments. These cross-bridging interactions constitute cellular contraction (106, 107) and, in the case of concerted contraction of PASMCs, pulmonary vasoconstriction. Sustained pulmonary vasoconstriction is thought to be partly responsible for the elevated PVR and PAP observed in some patients with IPAH. Thus a rise in [Ca2+]cyt in PASMCs due to Ca2+ influx through various Ca2+-permeable channels in the plasma membrane is required for agonist-induced pulmonary vasoconstriction and for serum and growth factor-mediated PASMC proliferation (which leads to pulmonary vascular medial hypertrophy and pulmonary vascular remodeling).
Regulation of [Ca2+]cyt in Normal PASMCs: Two Mechanisms
Voltage-dependent Ca2+ influx pathway.
The membrane potential (Em) in pulmonary vascular smooth muscle cells depends on Na+, K+, and Cl− concentration gradient across the plasma membrane and the relative ion permeabilities (P). The Goldman-Hodgkin-Katz voltage equation, or the Goldman equation, is used to determine the equilibrium potential across a cell membrane taking into account all of the ions that are permeable through the membrane. In resting vascular smooth muscle cells, Em is controlled primarily by K+ permeability and gradient, because PK is much greater than PCl, PNa, and PCa in excitable cells and many types of nonexcitable cells.
PASMCs maintain a negative Em, which is −40 to −50 mV in cultured and freshly dissociated PASMCs (measured by the patch-clamp technique) (3, 4, 138) and −50 to −65 mV in PASMCs of rat pulmonary arteries (measured by intracellular electrode) (114, 115). Although the Em in PASMCs is close to the calculated equilibrium potential for K+ (EK, approximately −85 mV, based on the Nernst equation), the 20–40-mV difference between the resting Em and the EK indicates that, in addition to K+, the plasma membrane of PASMCs may be permeable to other cations (e.g., Na+, Ca2+, H+, Mg2+) and anions (e.g., Cl−, HCO3−). In other words, the cation and anion currents through membrane channels other than K+ channels may also contribute to the regulation of the resting Em in PASMCs.
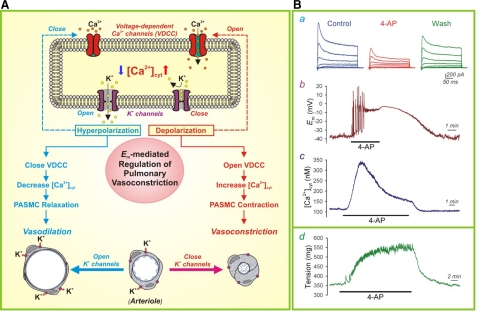
K+ permeability is directly related to the whole cell K+ current (IK), which is determined by the following equation: IK = N × i × Popen, where N is the number of membrane K+ channels, i is the amplitude of the single-channel K+ current, and Popen is the steady-state probability that the K+ channel is open. The activity of K+ channels in the membrane is thus important for the regulation of Em and plays a role in vascular contractility. Voltage-gated K+ (KV) channels, the most diverse group of K+ channels, are ubiquitously expressed in vascular smooth muscle cells (22, 87). When KV channels close, the membrane depolarizes, which leads to increased [Ca2+]cyt by inducing Ca2+ influx through voltage-dependent Ca2+ channels (VDCC) (Fig. 6A). Inhibition of KV channels with 4-aminopyridine reduces whole cell K+ currents (Fig. 6B,a), causes membrane depolarization (Fig. 6B,b), and results in increased [Ca2+]cyt in PASMCs. In isolated pulmonary arterial rings, inhibition of KV channels by 4-aminopyridine increases isometric tension as a result of PASMC contraction and vasoconstriction in response to membrane depolarization and Ca2+ influx through VDCC (Fig. 6B,d).
Fig. 6.
The role of K+ channels in membrane depolarization and pulmonary vasoconstriction. A: when K+ channels are closed (or K+ channel expression is downregulated), the resulting membrane depolarization opens voltage-dependent Ca2+ channel (VDCC), promotes Ca2+ influx, increases [Ca2+]cyt, and causes vasoconstriction. When K+ channels are activated (or K+ channel gene expression is upregulated), the resulting membrane hyperpolarization closes VDCC, inhibits agonist-mediated Ca2+ influx and causes vasodilation. Adapted from Jackson (57) and Makino et al. (67). B: representative recordings showing whole cell K+ currents (a), membrane potential (Em, b), and [Ca2+]cyt in rat PASMCs before, during, and after extracellular application of the KV channel blocker 4-aminopyridine (4-AP; 5 mM). A representative recording of tension measurement in an isolated mouse pulmonary arterial ring before, during and after treatment with 4-AP (d). Wash, washout. Reproduced from Yuan (137) with permission.
VDCC can be classified into six different subtypes based on their functional characteristics (39, 119, 120). However, in PASMCs L- and T-type channels are the important channels for voltage-gated Ca2+ entry involved in excitation-contraction coupling and cell proliferation (36, 61). The L-type VDCC are activated by high voltage, whereas inactivation is slow. The T-type channels are activated by low voltage, whereas inactivation is much faster than L-type channels. The activation threshold for L-type channels is approximately −30 to −20 mV, and the maximal activation often occurs at +10 to +15 mV. For T-type VDCC, the activation threshold and the maximal activation both shift to the left, with the former at approximately −40 to −30 mV and the latter at about −10 mV (35). Both types of VDCC are thought to exist in three states: resting (or closed), open, and inactivated conformations. Switching conformations from the resting or closed state to the open state is dependent on membrane depolarization (77). Ca2+ entry through T-type channels is linked to cell proliferation with the channel activity associated with G1-S boundary of cell-cycle progression in rat aortic smooth muscle cells (29, 78). It has been reported that T-type channels are needed in the early stages of skeletal muscle and cardiac development. Expression of T-type channels is lost as smooth muscle cells differentiate and lose their ability to proliferate; expression and function of T-type channels reappear in pathological cell proliferation in cancer cells and in cultured vascular smooth muscle cells (29). The L-type channel can be targeted indirectly through G protein-coupled receptors (GPCRs) by a host of cellular second messenger systems that are activated by various agonists including, but not limited to, norepinephrine, endothelin, angiotensin II, and 5-HT. Stimulation by protein kinases, such as protein kinase G, and nitric oxide can inhibit L-type channels, whereas protein kinase C (PKC) has been shown to potentiate L-type currents (25, 57). There is also evidence that nonreceptor tyrosine kinases, such as c-Src, enhance L-type currents (128).
Receptor- and store-operated Ca2+ influx pathways.
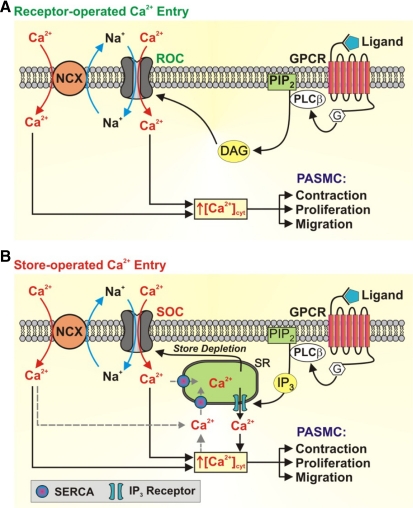
Stimulation of membrane receptors, such as GPCRs and receptor tyrosine kinases (RTKs), by their extracellular ligands results in the activation of phospholipase C and the production of two important second messengers, diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG can then open receptor-operated Ca2+ channels (ROC), leading to Ca2+ influx and increased [Ca2+]cyt (Fig. 7A). This process is referred to as receptor-operated Ca2+ entry (ROCE). Additionally, IP3 stimulates the IP3 receptor (IP3R), which is a Ca2+ release channel on the sarcoplasmic reticulum/endoplasmic reticulum (SR/ER) membrane, to release Ca2+ from the SR/ER to the cytosol. This leads to a depletion or a significant reduction of the SR/ER Ca2+ store. Upon depletion of Ca2+ from the SR/ER, a Ca2+ deficiency signal is transmitted to store-operated Ca2+ channels (SOC) on the plasma membrane causing SOC to open and allow Ca2+ to flow into the cytosol, this process is referred to as store-operated Ca2+ entry (SOCE). The cytosolic Ca2+ is then sequestered into the SR/ER by the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA), thus replenishing Ca2+ stores (Fig. 7B) (12, 24, 92). The exact cellular and molecular mechanisms of SOCE have been the research focus of many investigators since Putney et al. (93) first described SOCE, referred to then as capacitative Ca2+ entry. Although it is still controversial, many agree that the stromal interacting molecule (STIM) and Orai channels, as well as transient receptor potential (TRP) channels, are the essential components involved in SOCE, which plays an important role in increasing [Ca2+]cyt and refilling Ca2+ into the intracellular stores.
Fig. 7.
Receptor-mediated increase in [Ca2+]cyt in PASMC via receptor-operated Ca2+ entry (ROCE) and store-operated Ca2+ entry (SOCE). A: upon binding of ligands to membrane receptors [such as G protein-coupled receptor (GPRC) and receptor tyrosine kinase (RTK)], PLC is activated leading to the production of diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3). Receptor-operated Ca2+ (ROC) channels are activated by DAG. B: store-operated Ca2+ (SOC) channels are activated as a result of store depletion resulting from IP3-mediated Ca2+ release from the SR. The opening of ROC and SOC results in an influx of not only Ca2+ but also Na+ [canonical transient receptor potential (TRPC) channels are permeable to Ca2+ and Na+]. The locally increased [Na+] activates the reverse mode of the Na+/Ca2+ exchanger (NCX), which contributes to the increased [Ca2+]cyt and PASMC contraction, proliferation, and migration. PIP2, phosphatidylinositol 4,5-bisphosphate; SERCA, sarco(endo)plasmic reticulum Ca2+ ATPase; G, G protein. Adapted from Song et al. (109).
The precise components of ROC and SOC have remained a mystery and may be different among different types of cells. However, recent studies suggest that in vascular smooth muscle and endothelial cells, canonical TRP (TRPC) channels are involved in forming functional ROC and SOC. TRPC channels are members of the mammalian TRP family, which has 28 members that are divided into six structural subfamilies. TRPC channels multimerize to form homo- or heterotetramers that function as voltage-independent nonselective cation channels permeable to Ca2+, Na+, K+, Cs+, Li+, and Mg+ (13, 84, 110). TRPC1 is one of the pore-forming subunits of functional SOC in the pulmonary vasculature and is critical for pulmonary vasoconstriction and PASMC proliferation induced by SOCE (9, 18, 62, 79, 80, 116). TRPC1 is unlikely to form homomeric channels but has been shown to hetromultimerize with TRPC3, TRPC4, and TRPC5 (9). Interestingly, a novel mechanism of IP3-induced TRPC activation has been reported. In rabbit portal vein smooth muscle cells, IP3 generation can potentiate TRPC6/C7 activity by removing the inhibitory effect of phosphatidylinositol 4,5-bisphosphate independently of IP3R activation (55).
SOCE, or capacitative Ca2+ entry, was originally thought to be mainly a TRPC-dependent mechanism. More recent studies have demonstrated a role for Ca2+ release-activated Ca2+ (CRAC) channels in SOCE with STIM and Orai as major components. STIM and Orai were originally characterized in Drosophila, and shortly thereafter, mammalian homologs STIM1 and -2 and Orai1, -2, and -3 were identified (20, 21, 81, 96, 145). STIM1 is a 685 amino acid-long single-transmembrane protein that is mainly expressed on the SR/ER membrane (and also in plasma membrane) and contains an EF-hand domain near the NH2-terminus that senses the Ca2+ concentration in the SR/ER ([Ca2+]SR/ER). Under normal conditions, [Ca2+]SR/ER is ∼1 mM, and after IP3R activation the concentration can be depleted or significantly decreased to ∼300 μM. A decrease in the [Ca2+]SR/ER results in less Ca2+ bound to the EF-hand domain of STIM1 (Fig. 8). When Ca2+ is not bound to the EF-domain, STIM1 then undergoes a conformational change, which allows it to multimerize and translocate to the SR/ER-plasma membrane junction (or puncta) where it interacts with Orai1 homo- or heterotetramers on the plasma membrane, activates SOC, and induces SOCE (Fig. 8) (96, 145). Exactly how STIM migrates to the puncta to interact with Orai in the plasma membrane is not known, but as the details emerge, it may provide interesting insights on how STIM anchors to microtubules and on any additional scaffolding features that STIM has to keep the SR/ER intact.
Fig. 8.
The role of stromal-interacting molecule (STIM) and Orai in SOCE. The EF-hand domain in the NH2-terminus of STIM acts as a Ca2+ sensor and binds Ca2+ (red circles) when the SR/ER Ca2+ store is full or the intracellularly stored [Ca2+] in the SR/ER is around 1 mM. IP3-mediated Ca2+ store depletion causes Ca2+ to unbind from the low-affinity EF-hand domain of STIM and results in the oligomerization of Ca2+-depleted STIM dimers and their translocation to SR/ER-plasma membrane junctions. STIM accumulation in the vicinity of Orai channels induces Orai channels to open, allowing an influx of Ca2+. TKR, tyrosine kinase receptor; IP3R, IP3 receptor; N, NH2 terminal; PM, plasma membrane. Modified from Cahalan (20) with permission from Macmillan Publishers Ltd.
Orai1 is the pore-forming unit for CRAC channels in the plasma membrane (20, 86). STIM1 is capable of binding all three Orai homologs with the interaction first shown by coimmunoprecipitation (130). Many reports show that it is the cytosolic COOH-termini of STIM1 that acts as the effector to Orai and TRPC channels. When this domain is expressed on its own, it is sufficient to constitutively activate CRAC channels independently of Ca2+ store depletion (20). This suggests that STIM operates as an effector with some degree of promiscuity with pore-forming channels on the plasma membrane to mediate Ca2+ influx.
Both ROC and SOC, when activated, allow Ca2+ to flow into the cytosol. Resting [Ca2+]cyt is in the neighborhood of ∼100 nM, whereas the extracellular concentration is roughly 10,000-fold higher, near ∼2 mM. As a result, when ROC and SOC are open, Ca2+ influx is driven by the electrochemical gradient and [Ca2+]cyt rises without the expense of energy. Ca2+ can be pumped from the cytosol to extracellular or intercellular space against its electrochemical gradient. Extrusion of Ca2+ through the plasma membrane is mediated by the plasma membrane Ca2+-Mg2+ ATPase, or Ca2+ pump, and the Na+/Ca2+ exchanger (NCX). The action of these Ca2+ transporters are required for homeostatic levels of [Ca2+]cyt and are vital in maintaining the ∼100 nM [Ca2+]cyt (37, 68, 82, 142). The NCX has a larger capacity than the plasma membrane Ca2+-Mg2+ ATPase and is one of the important mechanisms for Ca2+ extrusion when [Ca2+]cyt is increased and cytosolic Na+ concentration ([Na+]cyt) is low. Mammalian cells maintain a low [Na+]cyt compared with the extracellular concentration of Na+. The transmembrane Na+ gradient can be used to energize the NCX, which moves three Na+ in per one Ca2+ out. Nevertheless, a rise in [Na+]cyt (especially in the local submembrane area) converts the NCX from the forward mode (Ca2+ out and Na+ in) to the reverse mode (Ca2+ in and Na+ out), which becomes an important mechanism to increase [Ca2+]cyt (Fig. 7). Studies have shown that the reverse mode of NCX contributes to the increased [Ca2+]cyt in pulmonary and systemic vascular smooth muscle cells, which can lead to pulmonary and systemic hypertension (50, 95, 118, 141). The inward transportation of Ca2+ through the reverse model of NCX is important for maintaining a high [Ca2+]cyt and refilling Ca2+ into the SR/ER via SERCA, when [Na+]cyt is increased locally by increased Na+ influx through TRPC channels (64).
The increase in [Ca2+]cyt due to SOCE also activates the Ca2+-activated Cl− (ClCa) channels in PASMCs (1, 38, 129). Since intracellular [Cl−] is relative high (up to 50 mM), the equilibrium potential for Cl− (ECl) is less negative than the resting Em. So the activation of ClCa channels by SOCE would induce Cl− efflux or inward currents and cause membrane depolarization, which subsequently opens VDCC and further increases [Ca2+]cyt in PASMCs. The functional interaction among SOCE, ClCa channel activation, membrane depolarization, and VDCC activation are actually an important mechanism involved in the development of sustained pulmonary vasoconstriction and vascular medial hypertrophy in animals and patients with pulmonary hypertension (1, 38, 71, 129).
Upregulated Expression and Enhanced Function of TRPC Channels in PASMCs from Patients with IPAH
The elevation of intracellular Ca2+ can activate signal transduction pathways important for the stimulation of transcription factors required for cell-cycle progression and proliferation, a key component of vascular remodeling (11, 46). TRPC channel function plays an important role in regulating [Ca2+]cyt during this process. In normal PASMCs, proliferation is associated with an increase in both mRNA and protein expression of TRPC1 and TRPC6 (42, 116, 133, 134). Consistent with upregulated TRPC expression, proliferating PASMCs have a significantly greater amplitude of SOCE than that of growth-arrested cells. Inhibition of TRPC expression with antisense oligonucleotides markedly decreases the amplitude of SOCE and significantly inhibits the proliferation of PASMCs (116).
In the PASMCs from patients with IPAH, the resting [Ca2+]cyt is higher than in cells from normal subjects and normotensive control patients. Additionally, the amplitude of SOCE, induced by store depletion using cyclopiazonic acid, is significantly greater in PASMCs from patients with IPAH than in cells from control patients (132, 133). Along with increased SOCE, the mRNA and protein expression of TRPC3 and TRPC6 are much greater in PASMCs from patients with IPAH than in PASMCs from control patients or those with secondary pulmonary hypertension (42). In addition to upregulated TRPC3/C6 channels, STIM2/Orai3 are upregulated in IPAH-PASMCs compared with normal PASMCs (108). The effect of increased expression levels of TRPC channels (and STIM2/Orai3) translates to enhanced growth and proliferation of IPAH-PASMCs compared with normal PASMCs (Fig. 9). Furthermore, when TRPC6 protein levels are dampened with small interfering RNA, the proliferative effect is significantly decreased (132). A recent report has identified a unique genetic variation associated with the TRPC6 gene in patients with IPAH. The “gain of function” single-nucleotide polymorphism in the promoter of TRPC6 gene may link the inflammatory mediators present before the onset of IPAH to the upregulation of TRPC6 channels and the augmented [Ca2+]cyt and proliferation of PASMCs in patients with IPAH (133). In addition, hypoxic treatment of PASMCs from normal humans subjects causes increase in basal [Ca2+]cyt that is SOC dependent and is accompanied by upregulation of TRPC1 (65). Enhanced [Ca2+]cyt resulting from SOC activation also leads to nuclear translocation of NFAT, which is involved in hypoxia-induced PASMC proliferation (124). It has been shown that sildenafil, a potent phosphodiesterase type-5 inhibitor, attenuates the hypoxic response and TRPC1 expression and decreases NFAT nuclear translocation, suggesting that sildenafil may interrupt this pathway and provide effective therapy in targeting the progression of pulmonary remodeling in PAH.
Fig. 9.
The pathogenic role of TRPC6 and NCX in idiopathic PAH (IPAH). A: genetic mutations and/or environmental stimulation leads to upregulation of TRPC6 resulting in an increased number of ROC and SOC channels in the plasma membrane and enhanced ROCE and SOCE. The enhanced ROCE/SOCE leads to not only increased [Ca2+]cyt but also to locally increased cytosolic Na+ concentration and activation of the reverse mode of NCX. NCX is also upregulated in patients with IPAH. These mechanisms contribute to increased [Ca2+]cyt and pulmonary vascular remodeling. B and C: representative currents (I) elicited by a ramp depolarization from −100 to +100 mV and [Ca2+]cyt in normal or control PASMCs and IPAH-PASMCs before and after treatment with 1-oleoyl-2-acetyl-sn-glycerol (OAG), a membrane permeable DAG analog. B and C reproduced from Yu et al. (133) with permission.
Upregulated NCX in PASMCs from Patients with IPAH
There have been a number of studies that have demonstrated that the plasma membrane NCX is implicated in the regulation of Ca2+ homeostasis in vascular smooth muscle cells (64, 66, 117). As mentioned earlier, TRPC channels are permeable to Ca2+ and Na+; the permeability of many TRPC channels to Na+ is greater than to Ca2+. Therefore, when SOC are opened upon store depletion or ROC are opened upon receptor activation, both Ca2+ influx and Na+ influx would occur. The Na+ influx through TRPC-formed SOC or ROC would increase [Na+]cyt and results in the transition of NCX from the forward mode (Ca2+ out and Na+ in) to the reverse mode (Ca2+ in and Na+ out), leading to the inward transportation of Ca2+ and increase in [Ca2+]cyt. The reverse mode of NCX has been shown to couple with TPRC6. Localized increases in [Na+]cyt generated by Na+ influx through TRPC6 channels drive the reversal NCX and mediate inward transportation of Ca2+ in smooth muscle cells (64). Additionally, in human bronchial smooth muscle cells, store depletion has been linked to NCX activation via STIM1 and plays an important role in Ca2+ homeostasis (66). We have shown that protein and mRNA expression of NCX is upregulated in PASMCs isolated from patients with IPAH and secondary pulmonary hypertension (Fig. 9) (142). Removal of the extracellular Na+, by decreasing transmembrane Na+ gradient, activates the reverse mode of NCX, resulting in a rapid increase in [Ca2+]cyt, which is significantly enhanced in IPAH-PASMCs (142, 144). When compared with controls, the increased expression of NCX leads to enhanced SOCE by the reverse mode but did not concomitantly accelerate Ca2+ efflux in the forward mode (144). These data indicate that upregulated NCX and enhanced SOCE due to the reverse mode of NCX are additional mechanisms responsible for the increased [Ca2+]cyt in PASMCs from patients with IPAH. Hence, NCX modulation may pose an interesting therapeutic target in treating PAH.
Upregulated Caveolin and Increased Number of Caveolae in PASMCs from Patients with IPAH
Caveolae are cholesterol- and sphingosine-rich invaginations in the plasma membrane, of which the main principal structural component is caveolin. They are found in various cell types and facilitate endocytosis, transcytosis, and Ca2+ mobilization in addition to acting as scaffold proteins to orchestrate signaling events (41, 94). There are three isoforms of caveolin: Cav1, Cav2, and Cav3 (Fig. 10). Cav2 is localized in the Golgi apparatus but can translocate to the plasma membrane. Cav3 is muscle specific and is more closely related to Cav1 in terms of amino acid sequence (101). Caveolae and caveolin have been implicated in both the human and mouse diseased states of PAH but with some contrasting features. Although there is a decrease in Cav1 expression in whole lung lysates from the pulmonary arteries of IPAH versus control patients, immunohistochemical studies on formalin-fixed lung sections show a substantial increase in Cav1 expression in the smooth muscle layer but not the endothelial layer of pulmonary arterioles from patients with IPAH. In agreement with these data, Western blot analysis demonstrates increased protein expression of both Cav1 and Cav2 in PASMCs from patients with IPAH compared with normal PASMCs (Fig. 10A). In addition, electron microscopy shows that IPAH-PASMCs express more Cav1 and form more caveolae than in normal PASMCs (Fig. 10) (85). An increase in caveolae contributes to a marked rise in SOCE and DNA synthesis. Downregulation of caveolin with Cav1 small interfering RNA or disruption of caveolae with disrupting agents (e.g., MβCD) attenuates SOCE and decreases DNA synthesis in IPAH-PASMCs (85, 100). Also, overexpressing Cav1 in normal PASMCs has the opposite effect and increases DNA synthesis and SOCE. However, these results contrast somewhat with the animal models of pulmonary hypertension. Cav1−/− mice show pathological features similar to a pulmonary hypertension phenotype such as right ventricle hypertrophy, increased medial thickness, and muscularization of distal pulmonary vessels (146, 147). In agreement with Cav1-deficient mice, rat models of pulmonary hypertension induced by monocrotaline or hypoxia exhibit a decrease in Cav1 expression, albeit mainly in endothelial cell isoform Cav1α. The reduced expression is noted as early as 48 h after monocrotaline challenge, but importantly the attenuated expression is predominantly found in the intimal layer (endothelium) of the pulmonary arteries (69). The above results highlight that caveolae and caveolin are critical regulators in the pathogenic mechanisms of pulmonary hypertension with some localization- and species-specific effects in humans versus rodents.
Fig. 10.
Functional coupling of TRPC channels and NCX in caveolae in PASMCs. A: electron micrograph (EM) images showing the increased number of caveolae (indicated by arrowheads) in PASMCs from patients with IPAH vs. PASMCs from normal subjects (Nor). Western blots demonstrate the differential expression levels of caveolin (Cav)-1, Cav-2, and Cav-3 in normal and IPAH PASMCs. Reproduced from Patel et al. (85) with permission. B: schematic diagram depicting the Cav-1 binding domains of TRPC channels and how the colocalization of NCX, TRPC, and membrane receptors, such as GPCR, in caveolae allows functional interactions between the components and enhances Ca2+ signaling. CBM, Cav-1-binding motif; PBD, protein 4-binding motif; CDS, caveolin-scaffolding domain.
Functional Coupling of ROC/SOC and NCX in Caveolae Is an Important Mechanism for Sustained Pulmonary Vasoconstriction and Medial Hypertrophy in Patients with IPAH
Caveolae are important for colocalizing receptors (e.g., GPCR and RTK) into microdomains and aid in an efficient activation of signal transduction pathways. Ligands accumulate in the caveolae and activate these receptors, leading to increased production of DAG and IP3. DAG activates ROC, and IP3 activates the IP3R on the SR/ER, releasing Ca2+ into the cytosol and depleting the store. Store depletion activates TRPC-formed SOC and/or Orail-formed SOC via STIM translocation and causes SOCE. Cav1 facilitates the localization of TRPC1 (and other TRPC isoforms) into caveolae through interaction with the caveolin-scaffolding domain found in the COOH-terminal of TRPC1 (Fig. 10B) (18, 63, 113). The activation of TRPC channels promotes Ca2+ and Na+ influx. The local accumulation of Na+ reverses the outward transportation of Ca2+ via NCX, which is also localized to caveolae (97), and further enhances Ca2+ entry. The close vicinity of the receptors and channels in the caveolae with the SR ensures efficient agonist-induced increase in [Ca2+]cyt, vasoconstriction, and pulmonary vascular wall thickening (Fig. 10B) (100). All of the components of this scenario (TRPC, Cav-1, caveolae, and NCX) are upregulated in PASMCs from patients with IPAH (85). These observations demonstrate that a functional coupling of GPCR, RTK, ROC/SOC, TRPC, and NCX in caveolae contributes to increased [Ca2+]cyt, resulting in increased proliferation of PASMCs and vasoconstriction in patients with IPAH.
Downregulated KV Channels, Membrane Depolarization and Opening of VDCC Contribute to Increases in [Ca2+]cyt in PASMCs from Patients with IPAH
Downregulated and dysfunctional KV channels have also been shown to impact Ca2+ signaling in patients with PAH. The amplitude of whole cell KV currents and the mRNA/protein expression of KV channels are significantly decreased in PASMCs from patients with IPAH compared with normotensive patients (135, 140). Additionally, PASMCs isolated from patients with IPAH have a depolarized resting membrane potential and a higher resting [Ca2+]cyt and display a blunted response to KV channel blockers (135). Decreased expression or compromised function of KV channels causes membrane depolarization, which activates VDCC, enhances voltage-dependent Ca2+ entry, and leads to PASMC contraction, migration, and proliferation (Fig. 11).
Fig. 11.
Decreased expression of K+ channels contribute to the development of PAH by increasing [Ca2+]cyt and inhibiting apoptosis in PASMCs. Flow chart depicting how downregulation of K+ channels causes membrane depolarization and increased [Ca2+]cyt through VDCC, leading to pulmonary vasoconstriction and PAH and how decreased K+ efflux leads to reducing apoptotic volume decrease (AVD) and apoptosis in PASMCs from patients with PAH. [K+]cyt, cytosolic K+ concentration.
In addition, changes in KV channel activity and expression have been implicated in acute hypoxia-mediated pulmonary vasoconstriction. Acute hypoxia inhibits KV channels (e.g., KV1.5, KV1.5/KV1.2, KCNQ, KV2.1, and KV3.1b), causing membrane depolarization and subsequent opening of L-type VDCCs (44, 53, 54, 90, 138, 139). The ensuing elevation of [Ca2+]cyt elicits PASMC contraction and pulmonary vasoconstriction. Chronic hypoxia also downregulates KV channels (125) and decreases whole cell KV currents in PASMCs (89, 105). Loss of function and reduced expression of KV1.5 and KV2.1 cause sustained increase in [Ca2+]cyt and contribute to the progression of pulmonary hypertension through sustained membrane depolarization, cell proliferation, and inhibition of apoptosis (2, 3, 125). Importantly, restoring the expression of KV1.5 through adenovirus in a chronic hypoxic pulmonary hypertension rodent model reduces the experimental pulmonary hypertension (91).
Downregulated KV Channels and Inhibited Apoptosis also Contribute to Pulmonary Vascular Medial Hypertrophy in PAH
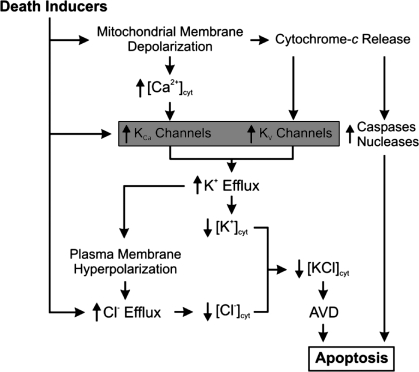
Another consequence of dysfunctional KV channels is a decrease in apoptosis. One of the earliest morphological changes seen in cells undergoing apoptosis is apoptotic volume decrease (16). In the early stages of apoptotic volume decrease, K+ and Cl− exit the cell along their electrochemical gradient through an increased number of open K+ and Cl− channels (Fig. 12). Water then leaves the cell through aquaporins in the plasma membrane to maintain the osmotic balance, thus causing cell shrinkage. Inhibition of KV channels in PASMCs from patients with IPAH and animals with chronic hypoxia-induced pulmonary hypertension inhibits apoptotic volume decrease and attenuates apoptosis (143).
Fig. 12.
The role of activity of K+ channels in AVD and apoptosis in PASMCs. In addition to regulating membrane potential, the activity of K+ channels also contributes to the regulation of AVD, an early hallmark of apoptosis. Decreased expression of K+ channels in PASMCs from patients with IPAH leads to decreased apoptosis. KCa, Ca2+-activaed K+ channel; KV, voltage-gated K+ channel; [KCl]cyt, cytosolic KCl concentration; [Cl−], cytosolic Cl− concentration.
Cytoplasmic K+ is an inhibitor of caspases and nucleases, and the maintenance of sufficient K+ in the cytosol due to decreased activity of K+ channels can inhibit apoptosis by attenuating the activity of intracellular caspases (19, 48, 58–60). In PASMCs from patients with IPAH, apoptosis induced by bone morphogenetic protein or staurosporine is inhibited compared with normal PASMCs (143). Additionally, an overexpression of KV1.5 in normal PASMCs accelerates staurosporine-mediated cell shrinkage and enhances apoptosis (19). These data suggest that downregulated KV channels in PASMCs from patients with IPAH contribute to decreased apoptosis as well as pulmonary vasoconstriction and increased PASMC proliferation because of increased [Ca2+] (Fig. 12).
Summary and Future Directions
PAH is a fatal disease that predominantly affects women. Sustained pulmonary vasoconstriction (due to PASMCs contraction) and excessive pulmonary vascular remodeling (due partially to PASMC migration and proliferation) are the major causes for the elevated PVR in patients with PAH. Increased PVR demands the right heart to work harder and can lead to right ventricular hypertrophy, right heart failure, and death. A reoccurring theme in the pathogenic mechanism of PAH is the involvement of Ca2+ signaling. Increased [Ca2+]cyt in PASMCs can stimulate vasoconstriction and vascular remodeling. A rise in [Ca2+]cyt due to the enhanced SOCE/ROCE and voltage-dependent Ca2+ entry in IPAH-PASMCs also activates many signal transduction proteins (e.g., CaMK, PKC, MAPK, and calcineurin) and transcription factors (e.g., activator protein-1, NFAT, CREB, and NF-κB), thereby affecting gene expression and promoting cell proliferation (Fig. 13). Ca2+ signaling activates cross talk between multiple genetic pathways and involves an interaction network of numerous genes and proteins. Several factors, such as increased expression of TPRC, Orai, STIM, and NCX, as well as the enhanced functional coupling of TRPC and NCX in an increased number of caveolae in PASMCs, have been identified in patients with IPAH. Furthermore, downregulated and/or dysfunctional KV channels have been implicated in PASMCs from patients with IPAH, which contribute to increasing [Ca2+]cyt by causing membrane depolarization and promoting voltage-dependent Ca2+ entry (Fig. 13). All these observations point to altered Ca2+ signaling as one of the major pathogenic mechanisms of PAH.
Fig. 13.
The positive-feedback loop hypothesis of Ca2+ signaling in PAH. Multiple Ca+ channels (ROC, SOC, VDCC, TRPC) contribute to regulating the [Ca2+]cyt in PASMC (A). An increase in [Ca2+]cyt, because of increased expression and/or function of Ca+ channels (or transporters), can lead to the activation of transcription factors and signaling pathways in a positive-feedback loop, which leads to a greater and persistent influx of Ca2+. The activated signaling pathways result in cross talk among many different genetic and signaling pathways and an interaction network of multiple genes and proteins (B). This ultimately leads to the processes (contraction, migration, imbalanced ratio of proliferation to apoptosis, misguided differentiation, dedifferentiation, transdifferentiation, partial reprogramming) in all cell types involved in the pulmonary vascular wall, which can contribute to the concentric pulmonary vascular remodeling (C) and, ultimately, PAH.
The proteins or subunits involved in forming functional SOC/ROC (e.g., TRPC and/or STIM/Orai) and VDCC (e.g., CACNA1C/Cav1.2), as well as the Kv channel α-subunits (e.g., KV1.5), are oppositely regulated by the Ca2+-dependent/sensitive transcription factors (Fig. 13). The resulting upregulation of SOC/ROC and VDCC and downregulation of Kv channels would enhance the Ca2+ influx and, ultimately, increase [Ca2+]cyt in PASMCs. The positive-feedback loop in the regulation of [Ca2+]cyt, shown in Fig. 3A, may be an important cellular (or pathogenic) mechanism responsible for the enhanced Ca2+ signaling (or increased [Ca2+]cyt) in PASMCs from patients and animals with PAH.
Increased activity or upregulation of the proteins involved in forming SOC/ROC and VDCC suggests that these channels may in fact be potentially valuable targets for developing novel therapeutic approaches using RNAi delivery or nanoparticle-driven inhibitors that antagonize functional coupling of these proteins. Additionally, restoring the function of KV channels could provide a reversal of apoptotic resistant PASMCs and ameliorate the medial hypertrophy associated with PAH (15, 72). Restoring protein function can be a challenging task in a diseased state, but as more is known regarding microRNA regulation of the players in PAH, potential targets may emerge (27).
As research continues, we hope to discover connections between Ca2+ signaling and other pathways to map out an interaction network to further our understanding of the pathogenic mechanisms of PAH with the goal of improving the prognosis for patients with PAH. Furthermore, it is important to reveal the molecular and cellular mechanisms involved in the upregulation of SOC/ROC (and the receptors that are functionally coupled to the ROC/SOC) and downregulation of KV channels and to identify the transcription factors responsible for the up- and downregulation of these proteins in PASMCs from patients and animals with PAH. Eventually, the goal is to identify a therapeutic regimen, or a therapeutic “cocktail,” targeting multiple channels in the Ca2+ signaling pathways, which is more effective and specific (than currently used VDCC blockers, such as nifedipine) for PAH.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants R01-HL-066012, P01-HL-098053, and P01-HL-098050.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.K.K. and J.X.-J.Y. prepared figures; F.K.K., M.Y.S., and J.X.-J.Y. drafted manuscript; F.K.K., K.A.S., M.Y.S., I.L., and J.X.-J.Y. edited and revised manuscript; F.K.K., K.A.S., and J.X.-J.Y. approved final version of manuscript; J.X.-J.Y. conception and design of research; J.X.-J.Y. analyzed data; J.X.-J.Y. interpreted results of experiments.
REFERENCES
- 1. Angermann JE, Sanguinetti AR, Kenyon JL, Leblanc N, Greenwood IA. Mechanism of the inhibition of Ca2+-activated Cl− currents by phosphorylation in pulmonary arterial smooth muscle cells. J Gen Physiol 128: 73–87, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation 102: 2781–2791, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv21, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 101: 2319–2330, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, McMurtry MS, Hashimoto K, Harry G, Michelakis ED. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res 95: 308–318, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science 253: 551–555, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 54: S55–S66, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137: 376–387, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Bedford DE, Evans W, Short DS. Solitary pulmonary hypertension. Br Heart J 19: 93–116, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium 33: 433–440, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD. The REVEAL risk score calculator in newly diagnosed patients with pulmonary arterial hypertension. Chest 2011. June 16 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11. Berridge MJ. Calcium signalling and cell proliferation. Bioessays 17: 491–500, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Berridge MJ. Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Ann NY Acad Sci 1047: 86–98, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JR, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-β/NFAT axis. Circulation 120: 1231–1240, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Bortner CD, Cidlowski JA. Volume regulation and ion transport during apoptosis. Methods Enzymol 322: 421–433, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 532–535, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem 278: 27208–27215, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brevnova EE, Platoshyn O, Zhang S, Yuan JX. Overexpression of human KCNA5 increases IK(V) and enhances apoptosis. Am J Physiol Cell Physiol 287: C715–C722, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Cahalan MD. STIMulating store-operated Ca2+ entry. Nat Cell Biol 11: 669–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium 42: 133–144, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of Kv1 channels in vasoregulation. Circ Res 99: 53–60, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Clapham DE. Intracellular calcium. Replenishing the stores. Nature 375: 634–635, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Clapp LH, Gurney AM. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflügers Arch 418: 462–470, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155: 411–419, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 208: 535–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest 105: 21–34, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cribbs LL. T-type Ca2+ channels in vascular smooth muscle: multiple functions. Cell Calcium 40: 221–230, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Cruzalegui FH, Means AR. Biochemical characterization of the multifunctional Ca2+/calmodulin-dependent protein kinase type IV expressed in insect cells. J Biol Chem 268: 26171–26178, 1993 [PubMed] [Google Scholar]
- 31. Dawson CA. Hypoxic pulmonary vasoconstriction: heterogeneity. In: Hypoxic Pumonary Vasoconstriction: Cellular and Molecular Mechanisms, edited by Yuan JX. Boston, MA: Kluwer Academic, 2004, p. 15–33 [Google Scholar]
- 32. Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Durmowicz AG, Parks WC, Hyde DM, Mecham RP, Stenmark KR. Persistence, re-expression, and induction of pulmonary arterial fibronectin, tropoelastin, and type I procollagen mRNA expression in neonatal hypoxic pulmonary hypertension. Am J Pathol 145: 1411–1420, 1994 [PMC free article] [PubMed] [Google Scholar]
- 34. Firth AL, Mandel J, Yuan JX. Idiopathic pulmonary arterial hypertension. Dis Model Mech 3: 268–273, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Firth AL, Remillard CV, Platoshyn O, Fantozzi I, Ko EA, Yuan JX. Functional ion channels in human pulmonary artery smooth muscle cells: Voltage-dependent cation channels. Pulm Circ 1: 48–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fleischmann BK, Murray RK, Kotlikoff MI. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci USA 91: 11914–11918, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Floyd R, Wray S. Calcium transporters and signalling in smooth muscles. Cell Calcium 42: 467–476, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Forrest AS, Angermann JE, Raghunathan R, Lachendro C, Greenwood IA, Leblanc N. Intricate interaction between store-operated calcium entry and calcium-activated chloride channels in pulmonary artery smooth muscle cells. Adv Exp Med Biol 661: 31–55, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Fox AP, Nowycky MC, Tsien RW. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol 394: 173–200, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, Feldkircher K, Turner M, McGoon MD. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 139: 128–137, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Gabella G. Quantitative morphological study of smooth muscle cells of the guinea-pig taenia coli. Cell Tissue Res 170: 161–186, 1976 [DOI] [PubMed] [Google Scholar]
- 42. Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca2+/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105: 863–875, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Gurney AM, Joshi S, Manoury B. KCNQ potassium channels: new targets for pulmonary vasodilator drugs? Adv Exp Med Biol 661: 405–417, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Hardingham GE, Bading H. Nuclear calcium: a key regulator of gene expression. Biometals 11: 345–358, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature 385: 260–265, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Huang W, Yen RT, McLaurine M, Bledsoe G. Morphometry of the human pulmonary vasculature. J Appl Physiol 81: 2123–2133, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Hughes FM, Jr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem 272: 30567–30576, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173: 1023–1030, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Jackson WF. Ion Channels and Vascular Tone. Hypertension 35: 173–178, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnson SR, Granton JT, Mehta S. Thrombotic arteriopathy and anticoagulation in pulmonary hypertension. Chest 130: 545–552, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res 7: 31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther 329: 368–376, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ju M, Shi J, Saleh SN, Albert AP, Large WA. Ins(1,4,5)P3 interacts with PIP2 to regulate activation of TRPC6/C7 channels by diacylglycerol in native vascular myocytes. J Physiol 588: 1419–1433, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev 24: 719–736, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca2+ channels (CaV1.2a,b) by protein kinases. Am J Physiol Cell Physiol 281: C1743–C1756, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Krick S, Platoshyn O, McDaniel SS, Rubin LJ, Yuan JX. Augmented K+ currents and mitochondrial membrane depolarization in pulmonary artery myocyte apoptosis. Am J Physiol Lung Cell Mol Physiol 281: L887–L894, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Krick S, Platoshyn O, Sweeney M, Kim H, Yuan JX. Activation of K+ channels induces apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol 280: C970–C979, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Krick S, Platoshyn O, Sweeney M, McDaniel SS, Zhang S, Rubin LJ, Yuan JX. Nitric oxide induces apoptosis by activating K+ channels in pulmonary vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 282: H184–H193, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Kuga T, Kobayashi S, Hirakawa Y, Kanaide H, Takeshita A. Cell cycle-dependent expression of L- and T-type Ca2+ currents in rat aortic smooth muscle cells in primary culture. Circ Res 79: 14–19, 1996 [DOI] [PubMed] [Google Scholar]
- 62. Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 287: L962–L969, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol 70: 1174–1183, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Lemos VS, Poburko D, Liao CH, Cole WC, van Breemen C. Na+ entry via TRPC6 causes Ca2+ entry via NCX reversal in ATP stimulated smooth muscle cells. Biochem Biophys Res Commun 352: 130–134, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Liu B, Peel SE, Fox J, Hall IP. Reverse mode Na+/Ca2+ exchange mediated by STIM1 contributes to Ca2+ influx in airway smooth muscle following agonist stimulation. Respir Res 11: 168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Makino A, Firth AL, Yuan JX. Endothelial and smooth muscle cell ion channels in pulmonary vasoconstriction and vascular remodeling. Compr Physiol 1: 1555–1602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res 68: 75–103, 2004 [DOI] [PubMed] [Google Scholar]
- 69. Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1α/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation 110: 1499–1506, 2004 [DOI] [PubMed] [Google Scholar]
- 70. McDaniel SS, Platoshyn O, Wang J, Yu Y, Sweeney M, Krick S, Rubin LJ, Yuan JX. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 280: L870–L880, 2001 [DOI] [PubMed] [Google Scholar]
- 71. McElroy SP, Drummond RM, Gurney AM. Regulation of store-operated Ca2+ entry in pulmonary artery smooth muscle cells. Cell Calcium 46: 99–106, 2009 [DOI] [PubMed] [Google Scholar]
- 72. McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 115: 1479–1491, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Means AR. Calcium, calmodulin and cell cycle regulation. FEBS Lett 347: 1–4, 1994 [DOI] [PubMed] [Google Scholar]
- 74. Mishra SK, Hermsmeyer K. Selective inhibition of T-type Ca2+ channels by Ro 40–5967. Circ Res 75: 144–148, 1994 [DOI] [PubMed] [Google Scholar]
- 75. Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Munaron L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review). Int J Mol Med 10: 671–676, 2002 [PubMed] [Google Scholar]
- 77. Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol 259: C3–C18, 1990 [DOI] [PubMed] [Google Scholar]
- 78. Neveu D, Quignard JF, Fernandez A, Richard S, Nargeot J. Differential β-adrenergic regulation and phenotypic modulation of voltage-gated calcium currents in rat aortic myocytes. J Physiol 479: 171–182, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ng LC, Airey JA, Hume JR. The contribution of TRPC1 and STIM1 to capacitative Ca2+ entry in pulmonary artery. Adv Exp Med Biol 661: 123–135, 2010 [DOI] [PubMed] [Google Scholar]
- 80. Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol 587: 2429–2442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ng LC, Ramduny D, Airey JA, Singer CA, Keller PS, Shen XM, Tian H, Valencik M, Hume JR. Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 299: C1079–C1090, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279: 33742–33750, 2004 [DOI] [PubMed] [Google Scholar]
- 83. Ooi CY, Wang Z, Tabima DM, Eickhoff JC, Chesler NC. The role of collagen in extralobar pulmonary artery stiffening in response to hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 299: H1823–H1831, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol 68: 685–717, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J 21: 2970–2979, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature 456: 116–120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, Chen T, Cole W. Heteromultimeric Kv1 channels contribute to myogenic control of arterial diameter. Circ Res 96: 216–224, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Platoshyn O, Golovina VA, Bailey CL, Limsuwan A, Krick S, Juhaszova M, Seiden JE, Rubin LJ, Yuan JX. Sustained membrane depolarization and pulmonary artery smooth muscle cell proliferation. Am J Physiol Cell Physiol 279: C1540–C1549, 2000 [DOI] [PubMed] [Google Scholar]
- 89. Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol 280: L801–L812, 2001 [DOI] [PubMed] [Google Scholar]
- 90. Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol Cell Physiol 262: C882–C890, 1992 [DOI] [PubMed] [Google Scholar]
- 91. Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 107: 2037–2044, 2003 [DOI] [PubMed] [Google Scholar]
- 92. Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev 231: 10–22, 2009 [DOI] [PubMed] [Google Scholar]
- 93. Putney JW, Jr, Poggioli J, Weiss SJ. Receptor regulation of calcium release and calcium permeability in parotid gland cells. Philos Trans R Soc Lond B Biol Sci 296: 37–45, 1981 [DOI] [PubMed] [Google Scholar]
- 94. Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev 54: 431–467, 2002 [DOI] [PubMed] [Google Scholar]
- 95. Ren C, Zhang J, Philipson KD, Kotlikoff MI, Blaustein MP, Matteson DR. Activation of L-type Ca2+ channels by protein kinase C is reduced in smooth muscle-specific Na+/Ca2+ exchanger knockout mice. Am J Physiol Heart Circ Physiol 298: H1484–H1491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rosker C, Graziani A, Lukas M, Eder P, Zhu MX, Romanin C, Groschner K. Ca2+ signaling by TRPC3 involves Na+ entry and local coupling to the Na+/Ca2+ exchanger. J Biol Chem 279: 13696–13704, 2004 [DOI] [PubMed] [Google Scholar]
- 98. Rubin LJ. Diagnosis and management of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 126: 7S–10S, 2004 [DOI] [PubMed] [Google Scholar]
- 99. Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet 361: 1533–1544, 2003 [DOI] [PubMed] [Google Scholar]
- 100. Schach C, Firth AL, Xu M, Remillard CV, Patel HH, Insel PA, Yuan JX. Regulation of pulmonary vasoconstriction by agonists and caveolae. Exp Lung Res 34: 195–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA 93: 131–135, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron 4: 571–582, 1990 [DOI] [PubMed] [Google Scholar]
- 103. Short AD, Bian J, Ghosh TK, Waldron RT, Rybak SL, Gill DL. Intracellular Ca2+ pool content is linked to control of cell growth. Proc Natl Acad Sci USA 90: 4986–4990, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 105. Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol Heart Circ Physiol 266: H365–H370, 1994 [DOI] [PubMed] [Google Scholar]
- 106. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 372: 231–236, 1994 [DOI] [PubMed] [Google Scholar]
- 107. Somlyo AP, Somlyo AV. Smooth muscle: excitation-contraction coupling, contractile regulation, and the cross-bridge cycle. Alcohol Clin Exp Res 18: 138–143, 1994 [DOI] [PubMed] [Google Scholar]
- 108. Song MY, Makino A, Yuan JX. STIM2 contributes to enhanced store-operated Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ 1: 84–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Song MY, Makino A, Yuan JX. Role of reactive oxygen species and redox in regulating the function of transient receptor potential channels. Antioxid Redox Signal 15: 1549–1565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Song MY, Yuan JX. Introduction to TRP channels: structure, function, and regulation. Adv Exp Med Biol 661: 99–108, 2010 [DOI] [PubMed] [Google Scholar]
- 111. Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology (Bethesda) 21: 134–145, 2006 [DOI] [PubMed] [Google Scholar]
- 112. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 113. Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB, Tiruppathi C. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol 296: C403–C413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Suzuki H, Twarog BM. Membrane properties of smooth muscle cells in pulmonary arteries of the rat. Am J Physiol Heart Circ Physiol 242: H900–H906, 1982 [DOI] [PubMed] [Google Scholar]
- 115. Suzuki H, Twarog BM. Membrane properties of smooth muscle cells in pulmonary hypertensive rats. Am J Physiol Heart Circ Physiol 242: H907–H915, 1982 [DOI] [PubMed] [Google Scholar]
- 116. Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L144–L155, 2002 [DOI] [PubMed] [Google Scholar]
- 117. Syyong HT, Poburko D, Fameli N, van Breemen C. ATP promotes NCX-reversal in aortic smooth muscle cells by DAG-activated Na+ entry. Biochem Biophys Res Commun 357: 1177–1182, 2007 [DOI] [PubMed] [Google Scholar]
- 118. Teubl M, Groschner K, Kohlwein SD, Mayer B, Schmidt K. Na+/Ca2+ exchange facilitates Ca2+-dependent activation of endothelial nitric-oxide synthase. J Biol Chem 274: 29529–29535, 1999 [DOI] [PubMed] [Google Scholar]
- 119. Thakali KM, Kharade SV, Sonkusare SK, Rhee SW, Stimers JR, Rusch NJ. Intracellular Ca2+ silences L-type Ca2+ channels in mesenteric veins: mechanism of venous smooth muscle resistance to calcium channel blockers. Circ Res 106: 739–747, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tsien RW, Tsien RY. Calcium channels, stores, oscillations. Annu Rev Cell Biol 6: 715–760, 1990 [DOI] [PubMed] [Google Scholar]
- 121. Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994 [PMC free article] [PubMed] [Google Scholar]
- 122. Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23–42, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vachiery JL, Brimioulle S, Crasset V, Naeije R. False-positive diagnosis of pulmonary hypertension by Doppler echocardiography. Eur Respir J 12: 1476–1478, 1998 [DOI] [PubMed] [Google Scholar]
- 124. Wang C, Li JF, Zhao L, Liu J, Wan J, Wang YX, Wang J, Wang C. Inhibition of SOC/Ca2+/NFAT pathway is involved in the anti-proliferative effect of sildenafil on pulmonary artery smooth muscle cells. Respir Res 10: 123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest 100: 2347–2353, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ward JP, McMurtry IF. Mechanisms of hypoxic pulmonary vasoconstriction and their roles in pulmonary hypertension: new findings for an old problem. Curr Opin Pharmacol 9: 287–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M., Ghofrani H.A, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA 103: 19093–19098, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wijetunge S, Lymn JS, Hughes AD. Effects of protein tyrosine kinase inhibitors on voltage-operated calcium channel currents in vascular smooth muscle cells and pp60 (c-src) kinase activity. Br J Pharmacol 129: 1347–1354, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wiwchar M, Ayon R, Greenwood IA, Leblanc N. Phosphorylation alters the pharmacology of Ca2+-activated Cl channels in rabbit pulmonary arterial smooth muscle cells. Br J Pharmacol 158: 1356–1365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443: 226–229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yi ES, Kim H, Ahn H, Strother J, Morris T, Masliah E, Hansen LA, Park K, Friedman PJ. Distribution of obstructive intimal lesions and their cellular phenotypes in chronic pulmonary hypertension. A morphometric and immunohistochemical study. Am J Respir Crit Care Med 162: 1577–1586, 2000 [DOI] [PubMed] [Google Scholar]
- 132. Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, Jiang W, Vangala N, Landsberg JW, Wang JY, Thistlethwaite PA, Channick RN, Robbins IM, Loyd JE, Ghofrani HA, Grimminger F, Schermuly RT, Cahalan MD, Rubin LJ, Yuan JX. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 119: 2313–2322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 135. Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, Orens JB, Rubin LJ. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation 98: 1400–1406, 1998 [DOI] [PubMed] [Google Scholar]
- 136. Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation 111: 534–538, 2005 [DOI] [PubMed] [Google Scholar]
- 137. Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res 77: 370–378, 1995 [DOI] [PubMed] [Google Scholar]
- 138. Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol Lung Cell Mol Physiol 264: L116–L123, 1993 [DOI] [PubMed] [Google Scholar]
- 139. Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. Contrasting effects of hypoxia on tension in rat pulmonary and mesenteric arteries. Am J Physiol Heart Circ Physiol 259: H281–H289, 1990 [DOI] [PubMed] [Google Scholar]
- 140. Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet 351: 726–727, 1998 [DOI] [PubMed] [Google Scholar]
- 141. Zhang J, Ren C, Chen L, Navedo MF, Antos LK, Kinsey SP, Iwamoto T, Philipson KD, Kotlikoff MI, Santana LF, Wier WG, Matteson DR, Blaustein MP. Knockout of Na+/Ca2+ exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2+ channel current and lowers blood pressure. Am J Physiol Heart Circ Physiol 298: H1472–H1483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang S, Dong H, Rubin LJ, Yuan JX. Upregulation of Na+/Ca2+ exchanger contributes to the enhanced Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol 292: C2297–C2305, 2007 [DOI] [PubMed] [Google Scholar]
- 143. Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, Kriett JM, Yung G, Rubin LJ, Yuan JX. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285: L740–L754, 2003 [DOI] [PubMed] [Google Scholar]
- 144. Zhang S, Yuan JX, Barrett KE, Dong H. Role of Na+/Ca2+ exchange in regulating cytosolic Ca2+ in cultured human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 288: C245–C252, 2005 [DOI] [PubMed] [Google Scholar]
- 145. Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA 99: 11375–11380, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest 119: 2009–2018, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]