Abstract
Monocarboxylate transporter 1 (MCT1) facilitates the transport of important metabolic fuels (lactate, pyruvate and ketone bodies) and possibly also acidic drugs such as valproic acid across the blood brain barrier. Because an impaired brain energy metabolism and resistance to antiepileptic drugs are common features of temporal lobe epilepsy (TLE), we sought to study the expression of MCT1 in the brain of patients with this disease. Immunohistochemistry and immunogold electron microscopy were used to assess the distribution of MCT1 in brain specimens from patients with TLE and concomitant hippocampal sclerosis (referred to as mesial TLE or MTLE (n = 15)), patients with TLE and no hippocampal sclerosis (non-MTLE, n = 13) and neurologically normal autopsy subjects (n = 8). MCT1 was present on an extensive network of microvessels throughout the hippocampal formation in autopsy controls and to a lesser degree in non-MTLE. Patients with MTLE were markedly deficient in MCT1 on microvessels in several areas of the hippocampal formation, especially CA1, which exhibited a 37 to 48% loss of MCT1 on the plasma membrane of endothelial cells when compared with non-MTLE. These findings suggest that the uptake of blood-derived monocarboxylate fuels and possibly also acidic drugs, such as valproic acid, is perturbed in the epileptogenic hippocampus, particularly in MTLE. We hypothesize that the loss of MCT1 on brain microvessels is mechanistically involved in the pathophysiology of drug-resistant TLE, and propose that re-expression of MCT1 may represent a novel therapeutic approach for this disease.
Keywords: blood-brain barrier, epilepsy, hippocampal sclerosis, ketogenic diet, mesial temporal sclerosis, valproic acid
Introduction
Despite recent advances in the treatment of epilepsy in humans, as many as 40% of patients suffering from temporal lobe epilepsy (TLE) cannot control their seizures with current antiepileptic drugs. The development of more efficacious therapies against TLE is requisite, and a better understanding of the underlying mechanisms of drug-resistant TLE is likely to facilitate the discovery of novel therapeutic targets for this disease.
TLE is characterized by recurrent episodes of partial seizures that may or may not be secondarily generalized. The seizures in TLE involve a network of temporal lobe and limbic structures such as the hippocampal formation, entorhinal cortex, amygdala, lateral temporal neocortex, medial thalamus and inferior frontal lobes (Spencer, 2002).
Approximately 70% of patients treated for drug-resistant TLE with surgical resection of the anteromedial temporal lobe, exhibit hippocampal sclerosis, which is characterized by astroglial proliferation and preferential loss of neurons particularly in CA1, CA3 and the dentate hilus of the hippocampal formation (de Lanerolle et al., 2003; Gloor, 1991; Sommer, 1880). Depth electrode recordings indicate that the sclerotic hippocampus is the focus of the seizures in patients with this pathology (Spencer, 1994), and surgical resection of the sclerotic hippocampus is associated with excellent seizure control in about 85% of patients (de Lanerolle et al., 2003). Patients with TLE and concomitant hippocampal sclerosis are here classified as mesial temporal lobe epilepsy (MTLE).
Approximately 30% of patients surgically treated for TLE do not exhibit hippocampal sclerosis (de Lanerolle et al., 2003). These “non-MTLE” patients either have a tumor, vascular malformation or dysplastic lesion in the temporal lobe or they have no remarkable findings by MRI or neuropathological examination. The seizure focus in non-MTLE is more variable than in MTLE and may involve areas other than the hippocampus. Finally, surgical resection of the hippocampus in non-MTLE is associated with poorer outcome than in MTLE (de Lanerolle et al., 2003).
There is increasing evidence to suggest that perturbations in the brain energy metabolism are involved in the pathophysiology of TLE. Firstly, studies by positron emission tomography and magnetic resonance spectroscopy performed interictally have shown that temporal lobe and limbic structures in TLE, particularly MTLE, are characterized by a slowed glucose metabolism (Theodore et al., 1983), a low concentration of N-acetyl aspartate (a marker of mitochondrial function and ATP synthesis rate) (Cendes et al., 1997; Connelly et al., 1994; Hetherington et al., 1995) and a decreased interictal phosphocreatine/ATP ratio, which indicates an impairment of the mitochondrial metabolism of ATP (Pan et al., 2005). Secondly, a high-fat, low-carbohydrate–i.e., ketogenic–diet improves the brain energy stores in patients with epilepsy (Pan et al., 1999), reduces the frequency of spontaneous recurrent seizures in many types of this disease (Hartman, 2008), and protects against neuronal loss in animal models of TLE (Acharya et al., 2008). Thirdly, treatment with the glycolysis inhibitor 2-deoxy-D-glucose (2DG) prevents epileptogensis and increases the seizure threshold in the kindling rat model of TLE (Garriga-Canut et al., 2006).
In order to better understand the brain metabolic pathways in TLE – especially those pathways involving non-glucose fuels – we assessed the distribution of monocarboxylate transporter 1 (MCT1) in the hippocampal formation in patients surgically treated for drug-resistant TLE and in neurologically normal autopsy control subjects. MCT1, which is preferentially localized on the plasma membrane of endothelial cells, is a key transporter of blood-derived monocarboxylates – i.e. pyruvate, lactate and the ketone bodies acetoacetate and betahydroxybutyrate – to the brain (Bergersen et al., 1999; Cornford et al., 1982; Cremer et al., 1976; Gerhart et al., 1997; Jackson et al., 1995; Koehler-Stec et al., 1998; Leino et al., 1999; Pellerin et al., 1998; Pierre et al., 2000; Price et al., 1998; Terasaki et al., 1991). Moreover, studies have indicated that MCT1 is a significant transporter of acidic antiepileptic drugs – such as valproic acid – across the blood-brain barrier (Fischer et al., 2008; Kang et al., 1990; Utoguchi and Audus, 2000). We hypothesize that the expression of MCT1 is perturbed in the hippocampal formation in TLE, and postulate that such perturbation, if present, may play a mechanistic role in the pathophysiology of drug-resistant TLE.
Materials and Methods
Human Subjects and Tissue Preparation
Patients with medically intractable TLE underwent phased presurgical evaluation at Yale-New Haven Hospital, and those elected for surgery had their hippocampal formation resected according to standard procedures (Spencer and Spencer, 1991). Informed consent from each patient and institutional approval were obtained for the surgery and the use of tissue for this study. Randomly selected hippocampal formations from 28 TLE patients were included in this study (Table 1). Fifteen patients exhibited hippocampal sclerosis and were classified as MTLE, 13 patients did not exhibit hippocampal sclerosis and were classified as non-MTLE. Patients with tumors, vascular malformations or obvious dysplastic lesions involving the hippocampal formation were excluded from the study. Hippocampal formations obtained at autopsy from 8 patients diagnosed with disorders other than epilepsy were also included.
Table 1.
Characteristics of Patients Selected for the Study
| Patient and classification | Sex | Age (years) | Time since first unprovoked seizure (years) | Antiepileptic drugs at surgery | MRI findings | Pathology |
|---|---|---|---|---|---|---|
|
Non-MTLE 1 |
F | 18 | 3 | Valproate, lamotrigine | Open lip schizencephaly, R hemisphere | Marked gliosis in cortex and white matter |
| 2 | F | 28 | 21 | Lamotrigine, zonisamide, phenytoin | Nonspecific, focal T2 signal hyperintensities in subcortical white matter, R frontal lobe | Mild to moderate neuronal loss with severe reactive gliosis. Abnormal lamination and clustering of neurons. |
| 3 | M | 4 | 2 | Oxcarbazepine | Abnormal signal of R amygdala, hippocampal head and parahippocampal gyrus with cyst near R hippocampal head | Oligodendroglioma |
| 4 | F | 49 | 13 | Carbamazepine XR, phenytoin, lamotrigine | Large mass in R frontal and R temporal lobes | Oligodendroglioma and ganglioneurocytoma |
| 5 | F | 38 | <1 | Levetiracetam | Nonenhancing R hippocampal lesion | Glioma |
| 6 | M | 13 | 9 | Phenytoin, levetiracetam, carbamazepine | Large porencephalic cavity in the R MCA distribution with surrounding gliosis. Compatible with remote R MCA infarct. | Frequent heterotopic neurons in molecular layer of cerebral cortex |
| 7 | M | 6 | Levetiracetam, lamotrigine | Abnormal gray matter with local mass-effect involving L frontal cortex. Possible localized hemimegalencephaly and cortical dysplasia. | Cortical dysplasia | |
| 8 | M | 69 | 7 | Phenytoin | Neoplastic lesion, R temporal lobe | Oligodendroglioma |
| 9 | F | 10 | 5 | Lamotrigine | R temporal tumor | Low-grade astrocytoma |
| 10 | M | 28 | 26 | Carbamazepine, acetazolamide | Ectopic gray matter adjacent to L anterior horn. High-signal by FLAIR in R posterior temporal lobe with corresponding low T1 signal. Possible bilateral hippocampal atrophy. | Heterotopic neurons in the molecular layer of the dentate gyrus. Subpial glial cell proliferation. |
| 11 | M | 40 | 35 | Valproate, levetiracetam | Normal | Hippocampus w/no significant neuronal loss. White matter shows increased number of glial cells. |
| 12 | F | 8 | 4 | Carbamazepine | L temporal tumor | Oligodendroglioma |
| 13 | M | 44 | 26 | Carbamazepine, clonazepam | Possible R mesial temporal sclerosis | Hippocampus w/mild, diffuse neuronal loss and mild to moderate increase in number of white matter glial cells. |
|
MTLE 14 |
F | 17 | 5 | Oxcarbazepine, lamotrigine, carbamazepine | Heterotopic gray matter in posterior R temporal lobe white matter with extension to the atrium and body of the right lateral ventricle. | Hippocampal sclerosis |
| 15 | M | 57 | 39 | Phenytoin, carbamazepine, topiramate, levetiracetam | Mesial temporal sclerosis | Hippocampal sclerosis |
| 16 | F | 51 | 48 | Levetiracetam, pregabalin | R mesial temporal sclerosis | Hippocampal sclerosis |
| 17 | F | 40 | 28 | Felbamate, topiramate, gabapentin, carbamazepine | L hippocampal sclerosis | Hippocampal sclerosis |
| 18 | M | 63 | 45 | Gabapentin, lamotrigine, levetiracetam | R hippocampal sclerosis | Hippocampal sclerosis |
| 19 | M | 28 | 6 | Valproate, phenytoin | Encephalomalacia, R anteromedial frontal lobe. Hemosiderin, R and L frontal and temporal lobes and L parietal lobe. Possible gliosis/scarring in white matter of R frontal lobe, L superior frontal gyrus and L parietal lobe. | Hippocampal sclerosis |
| 20 | F | 42 | 7 | Levetiracetam, oxcarbazepine | R hippocampal sclerosis | Hippocampal sclerosis |
| 21 | F | 40 | 37 | Carbamazepine, levetiracetam, zonisamide | L hippocampal sclerosis | Hippocampal sclerosis |
| 22 | M | 62 | 56 | Valproate, levetiracetam, phenytoin | L mesial temporal sclerosis | Hippocampal sclerosis |
| 23 | F | 40 | 27 | Carbamazepine, levetiracetam | Increased T2 signal, L hippocampus | Hippocampal sclerosis |
| 24 | F | 46 | 41 | Carbamazepine, primidone | L mesial temporal sclerosis | Hippocampal sclerosis |
| 25 | F | 30 | 22 | Zonisamide, carbamazepine XR | R mesial temporal sclerosis | Hippocampal sclerosis |
| 26 | F | 51 | 11 | Carbamazepine | L mesial temporal sclerosis | Hippocampal sclerosis |
| 27 | F | 37 | 11 | Primidone, Lamotrigine | R mesial temporal sclerosis | Hippocampal sclerosis |
| 28 | F | 15 | 5 | Carbamazepine XR, lamotrigine | L mesial temporal sclerosis | Hippocampal sclerosis |
| Patient and classification | Sex | Age (years) | Cause of death |
|---|---|---|---|
|
Autopsy 1 |
M | 33 | Adenocarcinoma of lung with widespread metastases |
| 2 | M | 52 | Myocardial infarct, coronary atherosclerosis |
| 3 | F | 49 | Ruptured berry aneurysm, R posterior inferior cerebellar artery |
| 4 | F | 76 | Peritonitis |
| 5 | M | 61 | Myocardial infarct, cardiovascular atherosclerosis |
| 6 | F | 62 | Dementia, Alzheimer’s disease, Parkinson’s disease |
| 7 | M | 87 | Aspiration pneumonia, severe atherosclerotic vascular disease |
| 8 | F | 47 | Asthma, morbid obesity |
Abbreviations: L, left; MCA, middle cerebral artery; R, right; XR, extended release
Immediately after resection of the hippocampus, a 5-mm-thick coronal slice was removed from the mid-anterior portion of the structure. The slice was immersed into a fixative containing 4% paraformaldehyde and 15% (vol/vol) saturated picric acid in 0.1 M phosphate buffer, pH 7.4 (PB) for 1 h, followed by immersion into 5% acrolein (Sigma Chemical Co, St. Louis, Mo) in PB for 3 h. Coronal sections were cut on a Vibratome and either stored in a cryoprotection solution (FD Neuro Technologies, Catonsville, Md) at −80°C until processed for Nissl staining and immunohistochemistry or processed immediately for freeze substitution (surgically resected tissue only).
Immunohistochemistry
Fifty-μm-thick Vibratome sections were incubated free floating in a solution of MCT1 antibody (AB1286, Millipore, diluted 1:1000, Billerica, Mass.) for 72 h at 4°C and processed according to the avidin biotin peroxidase method(Hsu et al., 1981) using the Vectastain Elite Kit (Vector Laboratories, Burlingame, Calif.) with diaminobenzidine as the chromogen. The immunostained sections were mounted on gelatin-coated glass slides and examined with a light microscope by two independent and experienced observers, who were blinded to the identity of the samples.
Freeze Substitution and Postembedding Immunogold Electron Microscopy
Small tissue blocks (0.3 by 0.5 by 1 mm3) of the CA1 area were dissected from 500-μm-thick Vibratome sections and subjected to freeze substitution according to the procedure described by Bergersen and colleagues (Bergersen et al., 2008). Immunogold labeling for MCT1 was performed on freeze-substituted ultrathin sections from 5 non-MTLE and 3 MTLE patients as described by Bergersen et al.(Bergersen et al., 2008). The primary antibody used was chicken anti-MCT1 (AB1286, diluted 1:300; Millipore). The secondary, colloidal gold-conjugated antibody was goat anti-chicken IgG 15 nm (Aurion, Wageningen, The Netherlands). The sections were counterstained with uranyl acetate and lead citrate after completion of the immunogold labeling procedure. Using an FEI Tecnai 12 transmission electron microscope (Hillsboro, Ore.), the linear densities of gold particles (gp) along the endothelial cell membranes were determined and expressed as gp/μm. Only particles detected on the membrane itself or those within 25 nm on the intracellular side were included (Bergersen et al., 2008).
Statistical Analysis
The Mann-Whitney U test was used for statistical comparisons. A P value of <0.05 was considered a statistically significant difference. Unless otherwise noted, the data are presented as mean ± SD.
Results
We first assessed the general histological features of the hippocampal formations included in this study, by analysis of Nissl stained sections. In agreement with prior reports, the hippocampal formations from autopsy subjects (Fig. 1A, B) and non-MTLE patients (Fig. 1C, D) were indistinguishable and did not exhibit significant neuronal loss or astroglial proliferation by visual examination (de Lanerolle et al., 2003). In contrast, the MTLE category revealed hippocampal sclerosis, as evident by a shrunken hippocampus which displayed astroglial proliferation and preferential loss of neurons in CA1, CA3 and the dentate hilus (Fig. 1E, F).
Fig. 1. Nissl-stained coronal sections of hippocampal formations from representative cases.
Compared to specimens from autopsy controls (A, B) and non-MTLE patients (C, D), there is loss of neurons (asterisks in E, inset in F) and glial proliferation (E, arrowheads inset in F) in the hilus, CA1 and CA3 in the hippocampal formation of MTLE patients, whereas neurons in the pyramidal layer of CA2 (upper arrows in E) and subiculum (lower arrows in E) are relatively preserved. The hippocampal formation in MTLE (E), vs. autopsy (A) and non-MTLE (C) is also atrophic, as expected in hippocampal sclerosis. Arrows, pyramidal neurons; arrowheads, glial cells. Panels B, D and F are high-power fields of the boxed areas in panels A, C and E respectively. Inset in B, D and E are high-powered fields of the boxed areas in B, D and F respectively. Abbreviations: CA1-3, subfields of hippocampus (cornu ammonis); hilus, dentate hilus; sub, subiculum. Scale bar: A–F, 1 mm; inset in B, D and F, 100 μm.
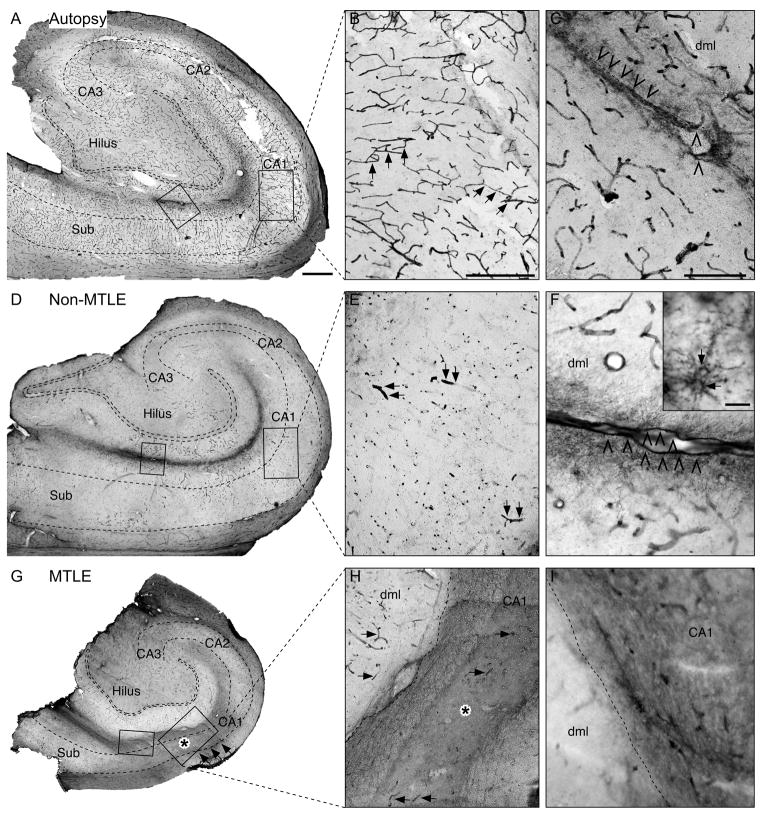
Using immunohistochemistry, we next assessed the distribution of MCT1 in the hippocampal formation in autopsy subjects (n = 8), patients with non-MTLE (n = 8) and patients with MTLE (n = 12). All of the autopsy brains revealed intense labeling for MCT1 on a widespread network of microvessels throughout the hippocampal formation (Fig. 2A–C). A similar labeling pattern was seen in the hippocampal formation in non-MTLE patients (Fig. 2D–F); however, the labeling of microvessels in non-MTLE was less intense than in autopsy. In contrast, the hippocampal formation in MTLE was characterized by a pronounced loss of MCT1 on microvessels in several areas, most notably CA1, CA3 and the hilus of the dentate gyrus (Fig. 2G–I).
Fig. 2. Immunohistochemical localization of MCT1 in coronal sections of hippocampal formations from representative cases.
Neurologically normal autopsy cases (A, B, C) are strongly and preferentially immunoreactive for MCT1 on microvessels in all hippocampal subfields and layers, such as the CA1 (arrows in B) and around the hippocampal fissure (arrowheads in C). This labeling pattern is altered in non-MTLE (D, E, F) and MTLE (G, H, I). In non-MTLE, staining for MCT1 is present on microvessels throughout the hippocampal subfields, including CA1 (arrows in E); however, the density of immunoreactive blood vessels is reduced in these patients (D, E) vs. autopsy (A, B). Notably, immunoreactivity for MCT1 is virtually absent on microvessels in several areas of the hippocampal formation in MTLE, particularly in CA1 (arrows in G, H, asterisk in H), but also in CA3 and in the dentate hilus (G). Other areas, such as the dentate molecular layer (dml) contain MCT1-positive microvessels (arrows in H). The lack of MCT1 labeling on microvessels in MTLE is replaced by a pattern of diffuse MCT1-labeling throughout the neuropil (asterisk in G, H). There is also a band of diffuse MCT1-labeling at the hippocampal fissure in all the patient categories (left boxed area in A, D, G and high power fields of these areas in C, F, I). This labeling, which is particularly distinct in autopsy (C) and non-MTLE (F), involves the subpial glial limiting membrane (arrowheads in C and F) and highly branched cells resembling astrocytes (arrows in inset in F). Panels B, E and H are high-power fields of the framed areas in CA1 in panels A, D and G respectively, and panels C, F and I are high-power fields of the framed areas at the hippocampal fissure in panels A, D and G respectively. Inset in F is from a different non-MTLE patient. Scale bars: A, D and G, 1 mm; B, E and H, 500 μm; C, F and I, 200 μm; Inset F, 25 μm.
Staining for MCT1 was also associated with structures other than microvessels. A band of MCT1 staining was present in the stratum lacunosum moleculare and along the hippocampal fissure in autopsy (Fig. 2C), non-MTLE (Fig. 2F) and MTLE specimens (Fig. 2I). Blood vessels, the subpial glial limiting membrane and small, branched cells resembling astrocytes were distinctly stained for MCT1 in this area in autopsy (Fig. 2C) and non-MTLE specimens (Fig. 2F), whereas the staining in MTLE was weaker and more diffusely distributed throughout the neuropil (Fig. 2I). Such diffuse labeling of the neuropil was also present in areas of the MTLE hippocampal formation that were deficient in microvessel-associated MCT1 staining, such as the neuron-depleted pyramidal layer of CA1 (asterisks in Fig. 2G, H).
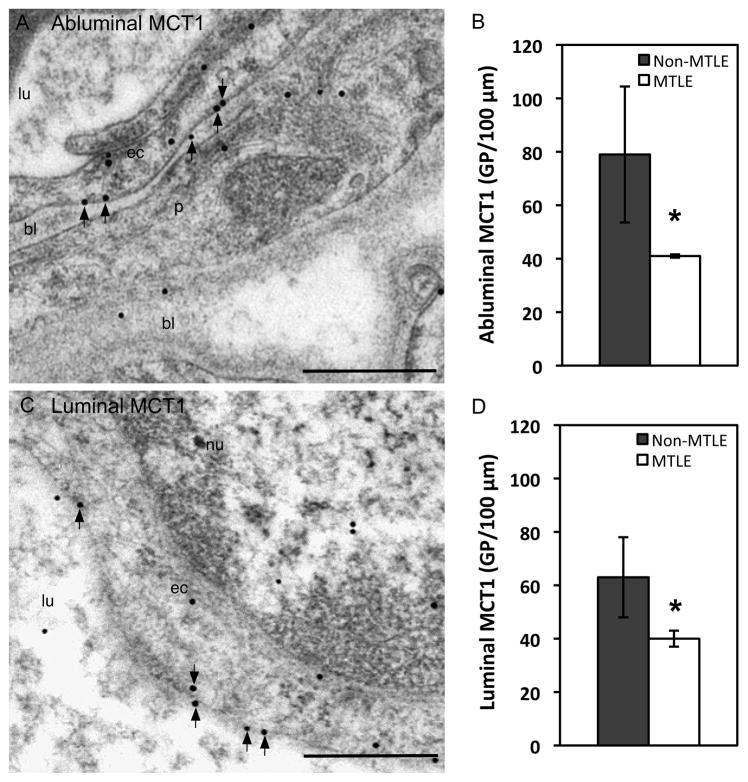
Using immunogold electron microscopy, we then determined the ultrastructural distribution of the microvessel-associated MCT1 in CA1 of non-MTLE (n = 5) and MTLE (n = 3) hippocampal formations. CA1 was chosen because this area exhibited pronounced loss of MCT1 in MTLE as assessed by prior light microscopy. Autopsy brains were not used for the immunogold studies due to the poorly preserved ultrastructure of such specimens. In non-MTLE and MTLE hippocampal formations, gold labeling for MCT1 was predominantly seen on the endothelial cell membranes facing the basal lamina (abluminal membrane, Fig. 3A) and blood vessel lumen (luminal membrane, Fig. 3C). There was a 48% reduction in gold particle density along the abluminal endothelial cell membrane in MTLE vs. non-MTLE (P < 0.05, Fig. 3B) and a 37% reduction in gold particle density along the luminal endothelial cell membrane in MTLE vs. non-MTLE (P < 0.05, Fig. 3D).
Fig. 3. Electron microscopic immunogold localization and quantification of MCT1 in hippocampal blood vessel endothelium.
Less gold particle labeling for MCT1 is found on CA1 microvessel plasma membranes in MTLE, compared to non-MTLE. The reduced particle density in MTLE is evident both at abluminal (arrows in A, B) and luminal (arrows in C, D) plasma membranes. Abbreviations: bl, basal lamina; ec, endothelial cell; lu, blood vessel lumen; nu, nucleus; p, pericyte. The bar diagrams depict mean ± SE, * P < 0.05. Scale bar: 500 nm.
Discussion
This study represents the first cellular and ultrastructural assessments of MCT1 in the hippocampus in patients with drug-resistant TLE. We have shown that MCT1 is severely deficient on the endothelium of microvessels in the hippocampal formation in MTLE vs. non-MTLE and neurologically normal autopsy subject. This deficiency is most pronounced in neuron-depleted areas of the MTLE hippocampal formation such as CA1, CA3 and the dentate hilus.
Blood monocarboxylates are critical for normal brain function. Even though glucose is the main energy substrate for the adult brain, lactate and the ketone bodies beta-hydroxybutyrate and acetoacetate are extensively used as metabolic fuels by the brain immediately after birth and during infancy (Cremer et al., 1979). A sustained shift in brain energy consumption from glucose to beta-hydroxybutyrate and acetoacetate also occurs in mature individuals during periods of ketosis, which is commonly caused by caloric restriction (Owen et al., 1967) or intake of a ketogenic diet (Hartman et al., 2007). Furthermore, data have shown that the mature brain imports and oxidizes blood-derived monocarboxylates, particularly lactate, in a concentration-dependent manner also during euglycemic conditions (van Hall et al., 2009). The abundance of MCT1 on microvessels in the hippocampal formation in normal subjects and to a lesser degree in non-MTLE patients, as shown in this study, suggests that the transport of monocarboxylates across the blood-brain barrier is important for the mature brain even in the absence of clinically significant ketosis.
Ketone bodies increase the brain energy stores, stabilize the neuronal membrane potential and enhance GABA-mediated inhibition. Studies have shown that a change in brain fuel consumption from glucose to ketone bodies results in a nearly 50% increase in mitochondrial density (Bough, 2008) and an elevated interictal phosphocreatine/creatine ratio (Pan et al., 1999). These changes are likely to improve the mitochondrial capacity for ATP production, thereby allowing the neurons to more readily restore the Na+/K+ transporters, thus stabilizing the membrane potential (Bough, 2008). Furthermore, ketone bodies rather than glucose may increase the energy stores preferentially in GABAergic neurons (Williamson et al., 2005) thus resulting in more sustained GABA-mediated inhibition (Cantello et al., 2007; Yudkoff et al., 2004). In addition, beta-hydroxybutyrate inhibits the enzymatic breakdown of GABA in astrocytes (Suzuki et al., 2009). Efficient transport of circulating ketone bodies across the blood-brain barrier is likely to be required for the above effects to take place.
The loss of MCT1 on microvessels may contribute to the energy impairment and seizure susceptibility in TLE and could, in part, explain the therapeutic efficacy of the ketogenic diet in this disease. The blood-brain barrier, particularly its expression of MCT1, appears to be the rate-limiting step in the utilization of circulating ketone bodies by the brain (Morris, 2005). It is therefore possible that the hippocampal formation in TLE, particularly MTLE, is deficient in ketone bodies under euglycemic conditions. This deficiency may lead to reduced brain energy stores, a destabilized neuronal membrane potential, loss of GABAergic inhibition and, hence, a lowered threshold for recurrent seizures. Induction of ketosis is likely to enhance the uptake of ketone bodies by the MCT1-deficient hippocampal formation in MTLE due to three different mechanisms. Firstly, the flux of monocarboxylates from the blood to the brain via MCTs is proportional to the monocarboxylate concentration in the blood (Bergersen, 2007; van Hall et al., 2009). Secondly, long-lasting ketosis increases the expression of MCT1 on brain microvessels, thereby further enhancing the uptake of ketone bodies by the brain (Leino et al., 2001). Thirdly, diet-induced ketosis increases the capillary density in rat brain cortex, suggesting that delivery of blood-derived fuels is enhanced during prolonged ketosis (Puchowicz et al., 2007). Thus, we hypothesize that long-lasting ketosis results in increased concentrations of ketone bodies in the hippocampal formation in MTLE via an upregulation of MCT1 on microvessels, and perhaps also via an increased density of brain microvessels. Elevated brain ketone concentrations may then improve the brain energy stores and reduce the frequency of recurrent seizures. Additional studies in humans and laboratory models are required to further explore this hypothesis.
MCT1 is critical also for the transport of other acidic molecules across the blood-brain-barrier, such as the antiepileptic drug valproic acid (Fischer et al., 2008; Kang et al., 1990; Utoguchi and Audus, 2000). Some patients with TLE are resistant to valproic acid, but the underlying mechanism of this resistance is not fully understood. However, the findings from the present study indicate that the resistance to valproic acid in patients with MTLE may be due to a loss of microvessel-associated MCT1 in the epileptogenic hippocampus. The observation that the loss of microvessel-associated MCT1 is more pronounced in MTLE than in non-MTLE also raises the possibility that the mechanism of pharmacoresistance is different between TLE patients with and without hippocampal sclerosis.
Multidrug resistance associated proteins (MDPs) facilitate the efflux of antiepileptic drugs from brain tissue and cerebrospinal fluid, thereby decreasing the bioavailability of such drugs at their target site (reviewed by (Cascorbi, 2010; Loscher, 2007; Marchi et al., 2010)). MDPs, particularly P-gp, are overexpressed on brain endothelial cells in patients with drug-resistant epilepsy (Aronica et al., 2005; Dombrowski et al., 2001). Moreover, it appears that overexpression of P-gp is more pronounced in the hippocampal formation in patients with MTLE vs. those with non-MTLE (Aronica et al., 2004; Kubota et al., 2006), suggesting a possible synergistic adverse effect between the presence of MDPs and lack of MCT1 in the MTLE brain. However, the exact functional relationship between MDPs and MCT1 remains to be investigated.
Why is MCT1 lost on microvessels in the MTLE hippocampus? Although very little is known about the regulation of MCT1, it is tempting to speculate that the transporter is lost due to the depletion of neurons in the MTLE hippocampal formation, and hence, a lesser demand for energy under interictal conditions. However, studies using combinations of magnetic resonance spectroscopy (MRS) and assessments of neuron densities have been unable to demonstrate a correlation between neuron loss and concentration of high-energy compounds in the hippocampal formation in MTLE (Kuzniecky et al., 2001; Theodore et al., 2001). Thus, depletion of neurons is unlikely to explain the loss of MCT1 on microvessels in the MTLE hippocampus. Another possibility is that MCT1 is lost on microvessels due to local tissue inflammation. Thibault and colleagues reported that the expression of MCT1 mRNA and protein was decreased on colonic cells in patients with inflammatory bowel disease (Thibault et al., 2007). The downregulation of MCT1 was closely associated with the intensity of colonic inflammation, suggesting an immune-mediated regulation of MCT1 expression. A similar mechanism may operate in MTLE as inflammatory changes such as microglial activation and increased expression of cytokines and chemokines are present in the epileptogenic hippocampus in patients with this disease and animal models of this disease (Beach et al., 1995; Kanemoto et al., 2003; Vezzani and Granata, 2005).
In summary, the present study suggests that MCT1 is mechanistically involved in the pathophysiology of drug-resistant TLE. Further investigations in human subjects and laboratory models are required to define the exact relationships among the MCT1 deficiency, brain metabolism, antiepileptic drug uptake, and recurrent seizures. Such investigations could facilitate the development of novel and more specific therapeutic approaches targeting MCT1 and brain metabolism in patients with drug-resistant TLE.
Research Highlights.
Monocarboxylate transporter 1 (MCT1) facilitates the transport of monocarboxylates (lactate, pyruvate, ketone bodies), certain amino acid, and acidic drugs such as valproic acid, across the blood brain barrier
The present study shows that MCT1 is severely deficient on microvessels in the hippocampal formation in patients with drug-resistant temporal lobe epilepsy (TLE) and concomitant hippocampal sclerosis
This finding suggests that the transport of monocarboxylates, certain amino acids and select antiepileptic drugs across the blood-brain barrier is impaired in patients with this disease.
The authors propose that MCT1 may represent a novel therapeutic target for patients with drug-resistant TLE
Acknowledgments
We thank Ms. Ilona Kovacs, Ms. Bjørg Riber and Ms. Karen Marie Gujord for excellent technical assistance; Dr. Max Larsson for developing the software used to quantify the immunogold labeling; Ms. Jennifer Bonito for managing the epilepsy patient database; Drs. Jullie Pan, Hoby Hetherington, Kjell Heuser and Jon Storm-Mathisen for helpful discussions; and Ms. Anne Sommer for editorial advice.
This work was supported by grants from the National Institutes of Health (NS058674 to T.E. and NS054038 to D.D.S.), the Swebelius Trust (to D.D.S.) and the Research Council of Norway (to F.L. and L.H.B.). Part of this work was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on Re-engineering the Clinical Research Enterprise can be obtained from the NIH website.
Abbreviations
- CA1-3
cornu ammonis subfields 1-3
- MCT1
monocarboxylate transporter 1
- MTLE
mesial temporal lobe epilepsy
- TLE
temporal lobe epilepsy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya MM, et al. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008;84:363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, et al. Expression and cellular distribution of multidrug resistance-related proteins in the hippocampus of patients with mesial temporal lobe epilepsy. Epilepsia. 2004;45:441–51. doi: 10.1111/j.0013-9580.2004.57703.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46:849–57. doi: 10.1111/j.1528-1167.2005.66604.x. [DOI] [PubMed] [Google Scholar]
- Beach TG, et al. Reactive microglia in hippocampal sclerosis associated with human temporal lobe epilepsy. Neurosci Lett. 1995;191:27–30. doi: 10.1016/0304-3940(94)11548-1. [DOI] [PubMed] [Google Scholar]
- Bergersen L, et al. Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience. 1999;90:319–331. doi: 10.1016/s0306-4522(98)00427-8. [DOI] [PubMed] [Google Scholar]
- Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–9. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- Bergersen LH, et al. Immunogold quantification of amino acids and proteins in complex subcellular compartments. Nat Protoc. 2008;3:144–52. doi: 10.1038/nprot.2007.525. [DOI] [PubMed] [Google Scholar]
- Bough K. Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):91–3. doi: 10.1111/j.1528-1167.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantello R, et al. Ketogenic diet: electrophysiological effects on the normal human cortex. Epilepsia. 2007;48:1756–63. doi: 10.1111/j.1528-1167.2007.01156.x. [DOI] [PubMed] [Google Scholar]
- Cascorbi I. ABC transporters in drug-refractory epilepsy: limited clinical significance of pharmacogenetics? Clin Pharmacol Ther. 2010;87:15–8. doi: 10.1038/clpt.2009.237. [DOI] [PubMed] [Google Scholar]
- Cendes F, et al. Proton magnetic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann Neurol. 1997;42:737–46. doi: 10.1002/ana.410420510. [DOI] [PubMed] [Google Scholar]
- Connelly A, et al. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology. 1994;44:1411–7. doi: 10.1212/wnl.44.8.1411. [DOI] [PubMed] [Google Scholar]
- Cornford EM, et al. Developmental modulations of blood-brain barrier permeability as an indicator of changing nutritional requirements in the brain. Pediatr Res. 1982;16:324–8. doi: 10.1203/00006450-198204000-00017. [DOI] [PubMed] [Google Scholar]
- Cremer JE, et al. Changes during development in transport processes of the blood-brain barrier. Biochim Biophys Acta. 1976;448:633–7. doi: 10.1016/0005-2736(76)90120-6. [DOI] [PubMed] [Google Scholar]
- Cremer JE, et al. Kinetics of blood-brain barrier transport of pyruvate, lactate, and glucose in suckling, weaning and adult. J Neurochem. 1979;33:439–445. doi: 10.1111/j.1471-4159.1979.tb05173.x. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, et al. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: Evidence for distinctive patient subcategories. Epilepsia. 2003;44:677–687. doi: 10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- Dombrowski SM, et al. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia. 2001;42:1501–6. doi: 10.1046/j.1528-1157.2001.12301.x. [DOI] [PubMed] [Google Scholar]
- Fischer W, et al. Transport of valproate at intestinal epithelial (Caco-2) and brain endothelial (RBE4) cells: mechanism and substrate specificity. Eur J Pharm Biopharm. 2008;70:486–92. doi: 10.1016/j.ejpb.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Garriga-Canut M, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–7. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, et al. Expression of monocarboxylate tranpsorter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273:207–213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- Gloor P. Mesial temporal sclerosis: Historical background and an overview from a modern perspective. In: Luders H, editor. Epilepsy Surgery. Raven Press; New York: 1991. pp. 6889–703. [Google Scholar]
- Hartman AL. Does the effectiveness of the ketogenic diet in different epilepsies yield insights into its mechanisms? Epilepsia. 2008;49(Suppl 8):53–6. doi: 10.1111/j.1528-1167.2008.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, et al. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–92. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington H, et al. Proton nuclear magnetic resonance spectroscopic imaging of human temporal lobe epilepsy at 4.1 T. Ann Neurol. 1995;38:396–404. doi: 10.1002/ana.410380309. [DOI] [PubMed] [Google Scholar]
- Hsu S, et al. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jackson VN, et al. cDNA cloning of MCT1, a monocarboxylate transporter from rat skeletal muscle. Biochim Biophys Acta. 1995;1238:193–6. doi: 10.1016/0005-2736(95)00160-5. [DOI] [PubMed] [Google Scholar]
- Kanemoto K, et al. Increased frequency of interleukin-1beta-511T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia. 2003;44:796–9. doi: 10.1046/j.1528-1157.2003.43302.x. [DOI] [PubMed] [Google Scholar]
- Kang YS, et al. Acidic drug transport in vivo through the blood-brain barrier. A role of the transport carrier for monocarboxylic acids. J Pharmacobiodyn. 1990;13:158–63. doi: 10.1248/bpb1978.13.158. [DOI] [PubMed] [Google Scholar]
- Koehler-Stec EM, et al. Monocarboxylate ransporter expression in mouse brain. Am J Physiol. 1998;275:E516–E524. doi: 10.1152/ajpendo.1998.275.3.E516. [DOI] [PubMed] [Google Scholar]
- Kubota H, et al. Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res. 2006;68:213–28. doi: 10.1016/j.eplepsyres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, et al. Magnetic resonance spectroscopic imaging in temporal lobe epilepsy: neuronal dysfunction or cell loss? Arch Neurol. 2001;58:2048–53. doi: 10.1001/archneur.58.12.2048. [DOI] [PubMed] [Google Scholar]
- Leino RL, et al. Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res Dev Brain Res. 1999;113:47–54. doi: 10.1016/s0165-3806(98)00188-6. [DOI] [PubMed] [Google Scholar]
- Leino RL, et al. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int. 2001;38:519–527. doi: 10.1016/s0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Loscher W. Drug transporters in the epileptic brain. Epilepsia. 2007;48(Suppl 1):8–13. doi: 10.1111/j.1528-1167.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- Marchi N, et al. Transporters in drug-refractory epilepsy: clinical significance. Clin Pharmacol Ther. 2010;87:13–5. doi: 10.1038/clpt.2009.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28:109–21. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- Owen OE, et al. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–95. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, et al. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia. 1999;40:703–7. doi: 10.1111/j.1528-1157.1999.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Pan JW, et al. Regional energetic dysfunction in hippocampal epilepsy. Acta Neurol Scand. 2005;111:218–24. doi: 10.1111/j.1600-0404.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- Pellerin L, et al. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci U S A. 1998;95:3990–5. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, et al. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience. 2000;100:617–27. doi: 10.1016/s0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- Price NT, et al. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329(Pt 2):321–8. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchowicz MA, et al. Diet-induced ketosis increases capillary density without altered blood flow in rat brain. Am J Physiol Endocrinol Metab. 2007;292:E1607–15. doi: 10.1152/ajpendo.00512.2006. [DOI] [PubMed] [Google Scholar]
- Sommer W. Erkrankung des Ammonshorns als aetiologisches Moment der Epilepsie. Arch Psychiatr Nervenkr. 1880;10:631–675. [Google Scholar]
- Spencer DD. Classifying the epilepsies by substrate. Clin Neurosci. 1994;2:104–109. [Google Scholar]
- Spencer DD, Spencer SS. Surgery for Epilepsy. Blackwell Scientific Publishers; Boston: 1991. [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–27. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, et al. Beta-hydroxybutyrate alters GABA-transaminase activity in cultured astrocytes. Brain Res. 2009;1268:17–23. doi: 10.1016/j.brainres.2009.02.074. [DOI] [PubMed] [Google Scholar]
- Terasaki T, et al. Transport of monocarboxylic acids at the blood-brain barrier: studies with monolayers of primary cultured bovine brain capillary endothelial cells. J Pharmacol Exp Ther. 1991;258:932–7. [PubMed] [Google Scholar]
- Theodore WH, et al. Hippocampal volume and glucose metabolism in temporal lobe epileptic foci. Epilepsia. 2001;42:130–2. doi: 10.1046/j.1528-1157.2001.080874.x. [DOI] [PubMed] [Google Scholar]
- Theodore WH, et al. [18F]fluorodeoxyglucose positron emission tomography in refractory complex partial seizures. Ann Neurol. 1983;14:429–37. doi: 10.1002/ana.410140406. [DOI] [PubMed] [Google Scholar]
- Thibault R, et al. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007;133:1916–27. doi: 10.1053/j.gastro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Utoguchi N, Audus KL. Carrier-mediated transport of valproic acid in BeWo cells, a human trophoblast cell line. Int J Pharm. 2000;195:115–24. doi: 10.1016/s0378-5173(99)00398-1. [DOI] [PubMed] [Google Scholar]
- van Hall G, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–9. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–43. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Williamson A, et al. Correlations between granule cell physiology and bioenergetics in human temporal lobe epilepsy. Brain. 2005;128:1199–208. doi: 10.1093/brain/awh444. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, et al. Ketogenic diet, brain glutamate metabolism and seizure control. Prostaglandins Leukot Essent Fatty Acids. 2004;70:277–85. doi: 10.1016/j.plefa.2003.07.005. [DOI] [PubMed] [Google Scholar]