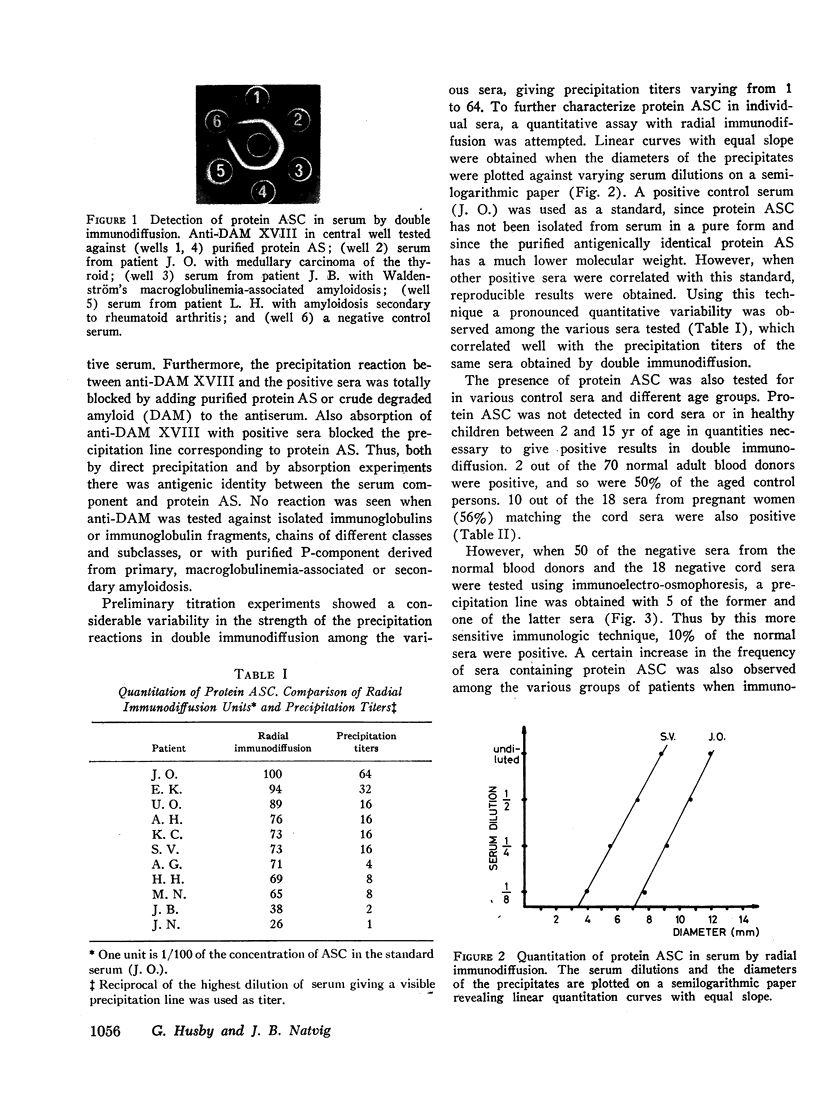
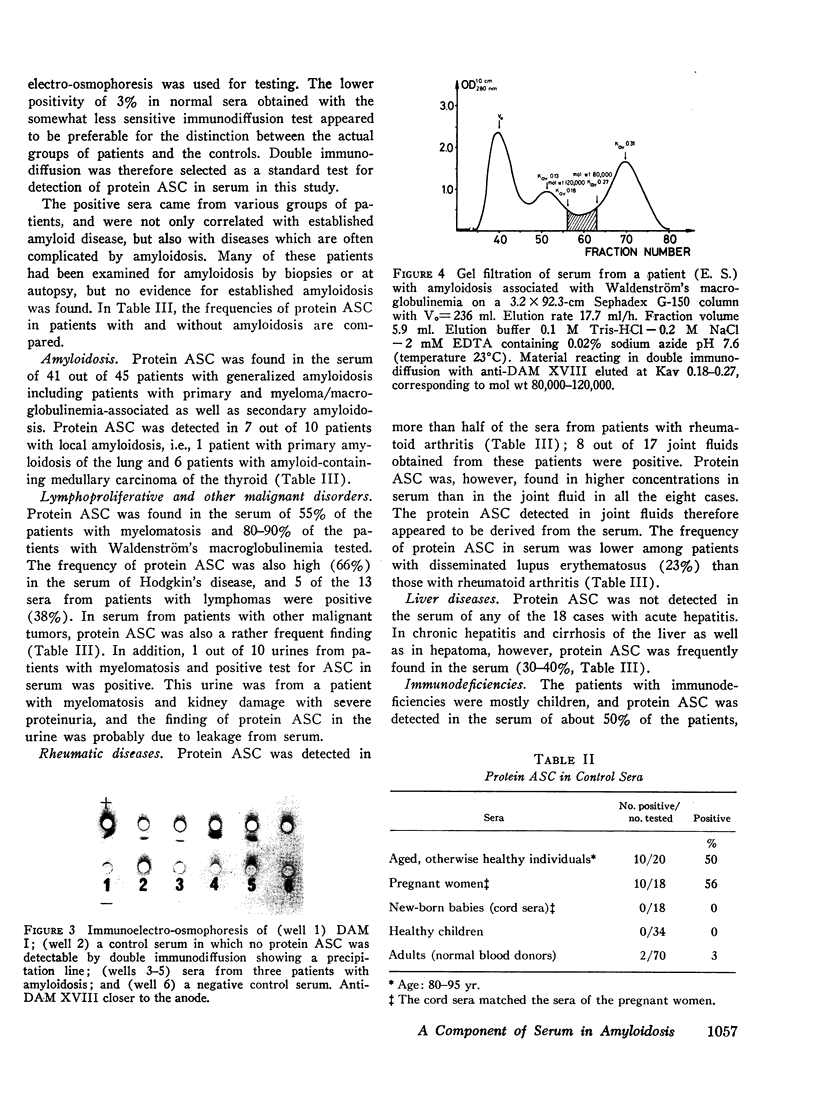
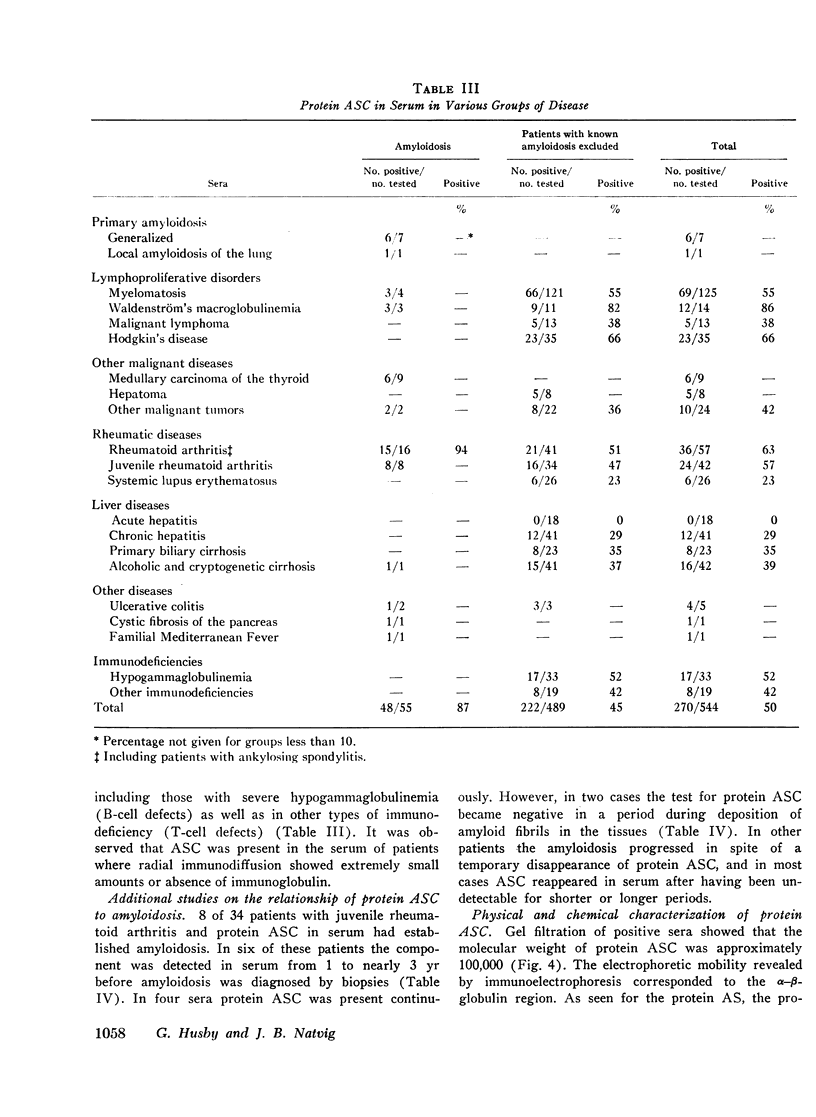
Abstract
A nonimmunoglobulin protein with the molecular weight of 9,145 (protein AS) has been shown to be a principal component of the amyloid fibrils in different clinical types of amyloidosis. A protein component, antigenically closely related to protein AS, was detected in human sera. The protein AS-related component (protein ASC) was found in the sera of many groups of patients, including 48 out of 55 patients with various clinical types of amyloidosis. No structural relationship of protein ASC to the plasma component of amyloid was found. Protein ASC was also present with high frequency in the serum of diseases known to be frequently complicated by amyloidosis. In some cases, ASC was found in the sera of patients 2-3 yr before the diagnosis of amyloidosis was established. Protein ASC was also frequently found in hypogammaglobulinemia. Among normal individuals, protein ASC was seldom detected in the serum by our techniques, but there was a noticeable increase with age and during pregnancy. Moreover, a more sensitive technique, immunoelectro-osmophoresis, revealed protein ASC in a higher number of sera from both patients and normal controls. Thus protein ASC was suggested to be a normal serum constituent, usually present only in minor quantities. Under certain conditions, protein ASC increases considerably in serum, and may in such instances act as a precursor for the deposition of amyloid fibrils in the tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N. Chemical classes of amyloid substance. Am J Pathol. 1971 Oct;65(1):231–252. [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Nylen M. U., Glenner G. G. The ultrastructure of human amyloid as revealed by the negative staining technique. J Ultrastruct Res. 1966 Mar;14(5):449–459. doi: 10.1016/s0022-5320(66)80075-8. [DOI] [PubMed] [Google Scholar]
- Cathcart E. S., Skinner M. S., Cohen A. S., Lawless O. J., Benson M. D. Antigenic determinants in amyloid deposits. Nature. 1970 Dec 12;228(5276):1090–1091. doi: 10.1038/2281090b0. [DOI] [PubMed] [Google Scholar]
- Cathcart E. S., Wollheim F. A., Cohen A. S. Immunoassay of P-component in amyloidotic sera. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1123–1125. doi: 10.3181/00379727-125-32292. [DOI] [PubMed] [Google Scholar]
- Cathcart E. S., Wollheim F. A., Cohen A. S. Plasma protein constituents of amyloid fibrils. J Immunol. 1967 Aug;99(2):376–385. [PubMed] [Google Scholar]
- Glenner G. G., Ein D., Eanes E. D., Bladen H. A., Terry W., Page D. L. Creation of "amyloid" fibrils from Bence Jones proteins in vitro. Science. 1971 Nov 12;174(4010):712–714. doi: 10.1126/science.174.4010.712. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Terry W. D., Isersky C. Amyloidosis: its nature and pathogenesis. Semin Hematol. 1973 Jan;10(1):65–86. [PubMed] [Google Scholar]
- Harada M., Isersky C., Cuatrecasas P., Page D., Bladen H. A., Eanes E. D., Keiser H. R., Glenner G. G. Human amyloid protein: chemical variability and homogeneity. J Histochem Cytochem. 1971 Jan;19(1):1–15. doi: 10.1177/19.1.1. [DOI] [PubMed] [Google Scholar]
- Husby G., Natvig J. B. Individual antigenic specificity and cross-reactions among amyloid preparations from different individuals. Clin Exp Immunol. 1972 Apr;10(4):635–647. [PMC free article] [PubMed] [Google Scholar]
- Husby G., Natvig J. B., Michaelsen T. E., Sletten K., Höst H. Unique amyloid protein subunit common to different types of amyloid fibril. Nature. 1973 Aug 10;244(5415):362–364. doi: 10.1038/244362a0. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K., Michaelsen T. E., Natvig J. B. Alternative, non-immunoglobulin origin of amyloid fibrils. Nat New Biol. 1972 Aug 9;238(84):187–187. doi: 10.1038/newbio238187a0. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K., Michaelsen T. E., Natvig J. B. Amyloid fibril protein subunit, "protein AS": distribution in tissue and serum in different clinical types of amyloidosis including that associated with myelomatosis and Waldenström's macroglobulinamia. Scand J Immunol. 1973;2(4):395–404. doi: 10.1111/j.1365-3083.1973.tb02048.x. [DOI] [PubMed] [Google Scholar]
- Husby G., Sletten K., Michaelsen T. E., Natvig J. B. Antigenic and chemical characterization of non-immunoglobulin amyloid proteins. Scand J Immunol. 1972;1(4):393–400. doi: 10.1111/j.1365-3083.1972.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mawas C., Sors C., Bernier J. J. Amyloidosis associated with primary agammaglobulinemia, severe diarrhea and familial hypogammaglobulinemia. Am J Med. 1969 Apr;46(4):624–634. doi: 10.1016/0002-9343(69)90081-3. [DOI] [PubMed] [Google Scholar]
- OSSERMAN E. F., TAKATSUKI K., TALAL N. MULTIPLE MYELOMA I. THE PATHOGENESIS OF "AMYLOIDOSIS. Semin Hematol. 1964 Jan;1:3–85. [PubMed] [Google Scholar]
- Pearse A. G., Ewen S. W., Polak J. M. The genesis of apudamyloid in endocrine polypeptide tumours: histochemical distinction from immunamyloid. Virchows Arch B Cell Pathol. 1972;10(2):93–107. doi: 10.1007/BF02899719. [DOI] [PubMed] [Google Scholar]
- Pras M., Reshef T. The acid-soluble fraction of amyloid--a fibril forming protein. Biochim Biophys Acta. 1972 Jun 22;271(1):193–203. doi: 10.1016/0005-2795(72)90147-x. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Zucker-Franklin D., Rimon A., Franklin E. C. Physical, chemical, and ultrastructural studies of water-soluble human amyloid fibrils. Comparative analyses of nine amyloid preparations. J Exp Med. 1969 Oct 1;130(4):777–796. doi: 10.1084/jem.130.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. M., Burke K. Serum hepatitis antigen (SH): rapid detection by high voltage immunoelectroosmophoresis. Science. 1970 Aug 7;169(3945):593–595. doi: 10.1126/science.169.3945.593. [DOI] [PubMed] [Google Scholar]
- Skinner M., Cohen A. S. P-component of amyloid: amino-terminal sequence. Biochem Biophys Res Commun. 1973 Sep 18;54(2):732–736. doi: 10.1016/0006-291x(73)91484-8. [DOI] [PubMed] [Google Scholar]
- Sletten K., Husby G. The complete amino-acid sequence of non-immunoglobulin amyloid fibril protein AS in rheumatoid arthritis. Eur J Biochem. 1974 Jan 3;41(1):117–125. doi: 10.1111/j.1432-1033.1974.tb03251.x. [DOI] [PubMed] [Google Scholar]
- Zuckerberg A., Gazith J., Rimon A., Reshef T., Gafni J. The structural subunit of amyloid. Isolation and characterization of a polypeptide capable of fibril formation. Eur J Biochem. 1972 Jul 13;28(2):161–165. doi: 10.1111/j.1432-1033.1972.tb01898.x. [DOI] [PubMed] [Google Scholar]