Abstract
The incomplete absorption of dietary cholesterol may represent an adaptive intestinal barrier that prevents hypercholesterolemia. To explore this mechanism, we compared cholesterol absorption in 15 normocholesterolemic and 6 hypercholesterolemic (type II) subjects fed background cholesterol-free formula diets with 40% of calories as fat. Each test meal consisted of a breakfast into which was incorporated scrambled egg yolk containing 300-500 mg of cholesterol and [4-14C]cholesterol (3-22 μCi), either naturally incorporated into the yolk cholesterol by previous isotope injection into the laying hen or added in peanut oil to the yolk of the test breakfast. In some instances [1α-3H]cholesterol was the radioactive marker.
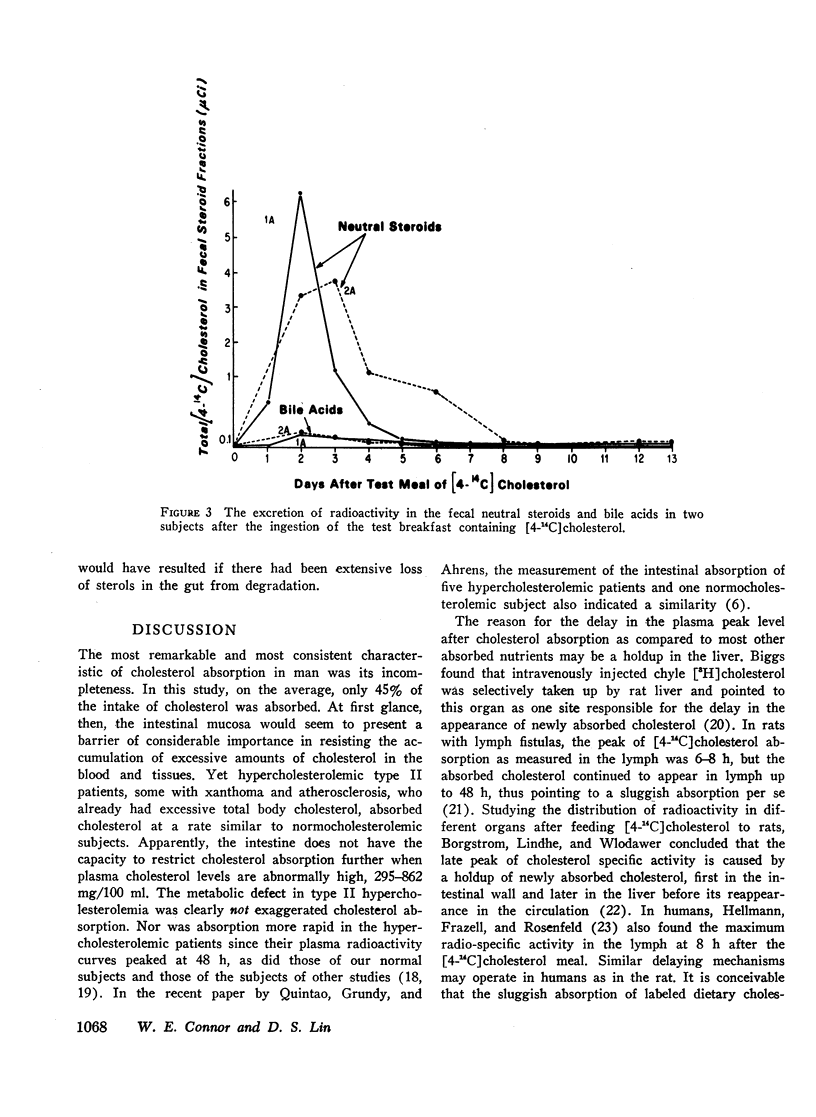
The radioactivity of the fecal neutral sterol fraction was determined in daily stool samples for the next 7 days to provide an estimate of unabsorbed dietary cholesterol. The amount of absorbed and reexcreted labeled cholesterol proved negligible. Most unabsorbed dietary cholesterol appeared in the stool on the second or third day after the meal, and 95% or more was recovered in the stool by 6 days. Plasma specific activity curves were usually maximal at 48 h. Normal subjects absorbed 44.5±9.3 (SD) of the administered cholesterol (range 25.9-60.3). Hypercholesterolemics absorbed the same percentage of cholesterol as normals: 47.6±12.6% (range 29.3-67.3). Absorption was similar whether the radiolabeled cholesterol was added to egg yolk or naturally incorporated in it (42.1±9.3 vs. 48.9±9.8%).
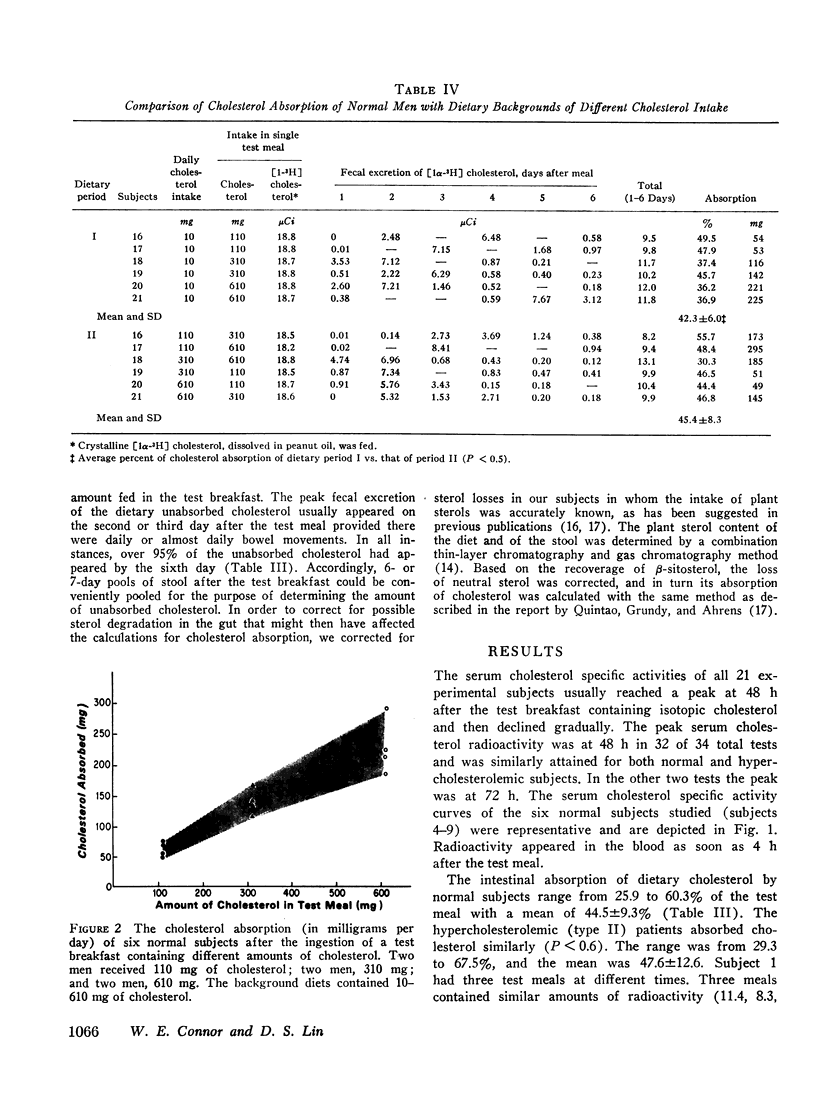
Six normal subjects were fed a cholesterol-free formula for 4 wk, and then different amounts of cholesterol (110-610 mg/day) were added for another 4 wk. At the end of each period, single test meals containing either 110, 310, or 610 mg of cholesterol and [1α-3H]cholesterol were administered. Cholesterol absorption was 42.3±6.0% and 45.4±8.3% for the two dietary periods, respectively. The absolute cholesterol absorption was linearly related to the amount of cholesterol in the test meal, and absorption was not affected by background diets high or low in cholesterol content.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- BIGGS M. W., KRITCHEVSKY D., COLMAN D., GOFMAN J. W., JONES H. B., LINDGREN F. T., HYDE G., LYON T. P. Observations on the fate of ingested cholesterol in man. Circulation. 1952 Sep;6(3):359–366. doi: 10.1161/01.cir.6.3.359. [DOI] [PubMed] [Google Scholar]
- BORGSTROM B., LINDHE B. A., WLODAWER P. Absorption and distribution of cholesterol-4-C14 in the rat. Proc Soc Exp Biol Med. 1958 Nov;99(2):365–368. doi: 10.3181/00379727-99-24352. [DOI] [PubMed] [Google Scholar]
- Borgstrom B. Quantification of cholesterol absorption in man by fecal analysis after the feeding of a single isotope-labeled meal. J Lipid Res. 1969 May;10(3):331–337. [PubMed] [Google Scholar]
- CONNOR W. E., HODGES R. E., BLEILER R. E. Effect of dietary cholesterol upon serum lipids in man. J Lab Clin Med. 1961 Mar;57:331–342. [PubMed] [Google Scholar]
- CONNOR W. E., HODGES R. E., BLEILER R. E. The serum lipids in men receiving high cholesterol and cholesterol-free diets. J Clin Invest. 1961 May;40:894–901. doi: 10.1172/JCI104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR W. E., OSBORNE J. W., MARION W. L. INCORPORATION OF PLASMA CHOLESTEROL-4-C14 INTO EGG YOLK CHOLESTEROL. Proc Soc Exp Biol Med. 1965 Mar;118:710–713. doi: 10.3181/00379727-118-29946. [DOI] [PubMed] [Google Scholar]
- CONNOR W. E., STONE D. B., HODGES R. E. THE INTERRELATED EFFECTS OF DIETARY CHOLESTEROL AND FAT UPON HUMAN SERUM LIPID LEVELS. J Clin Invest. 1964 Aug;43:1691–1696. doi: 10.1172/JCI105044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK R. P., EDWARDS D. C., RIDDELL C. Cholesterol metabolism. 7. Cholesterol absorption and excretion in man. Biochem J. 1956 Feb;62(2):225–234. doi: 10.1042/bj0620225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. I. N Engl J Med. 1970 May 14;282(20):1128–1138. doi: 10.1056/NEJM197005142822005. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GRUNDY S. M., AHRENS E. H., Jr, MIETTINEN T. A. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL FECAL BILE ACIDS. J Lipid Res. 1965 Jul;6:397–410. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res. 1969 May;10(3):304–315. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Dietary beta-sitosterol as an internal standard to correct for cholesterol losses in sterol balance studies. J Lipid Res. 1968 May;9(3):374–387. [PubMed] [Google Scholar]
- HELLMAN L., FRAZELL E. L., ROSENFELD R. S. Direct measurement of cholesterol absorption via the thoracic duct in man. J Clin Invest. 1960 Aug;39:1288–1294. doi: 10.1172/JCI104145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLMAN L., ROSENFELD R. S., EIDINOFF M. L., FUKUSHIMA D. K., GALLAGHER T. F., WANG C. I., ADLERSBERG D. Isotopic studies of plasma cholesterol of endogenous and exogenous origins. J Clin Invest. 1955 Jan;34(1):48–60. doi: 10.1172/JCI103062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN J. A., COX G. E., TAYLOR C. B. CHOLESTEROL METABOLISM IN MAN. STUDIES ON ABSORPTION. Arch Pathol. 1963 Oct;76:359–368. [PubMed] [Google Scholar]
- KARVINEN E., LIN T. M., IVY A. C. Capacity of human intestine to absorb exogenous cholesterol. J Appl Physiol. 1957 Sep;11(2):143–147. doi: 10.1152/jappl.1957.11.2.143. [DOI] [PubMed] [Google Scholar]
- Kudchodkar B. J., Sodhi H. S., Horlick L. Absorption of dietary cholesterol in man. Metabolism. 1973 Feb;22(2):155–163. doi: 10.1016/0026-0495(73)90266-7. [DOI] [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr An evaluation of four methods for measuring cholesterol absorption by the intestine in man. J Lipid Res. 1971 Mar;12(2):221–232. [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr Effects of dietary cholesterol on the regulation of total body cholesterol in man. J Lipid Res. 1971 Mar;12(2):233–247. [PubMed] [Google Scholar]
- SWELL L., TROUT E. C., Jr, HOPPER J. R., FIELD H., Jr, TREADWELL C. R. Mechanism of cholesterol absorption. I. Endogenous dilution and esterification of fed cholesterol-4-C14. J Biol Chem. 1958 May;232(1):1–8. [PubMed] [Google Scholar]
- Taylor C. B., Ho K. J. A review of human cholesterol metabolism. Arch Pathol. 1967 Jul;84(1):3–14. [PubMed] [Google Scholar]
- Wilson J. D., Lindsey C. A., Jr Studies on the influence of dietary cholesterol on cholesterol metabolism in the isotopic steady state in man. J Clin Invest. 1965 Nov;44(11):1805–1814. doi: 10.1172/JCI105288. [DOI] [PMC free article] [PubMed] [Google Scholar]



