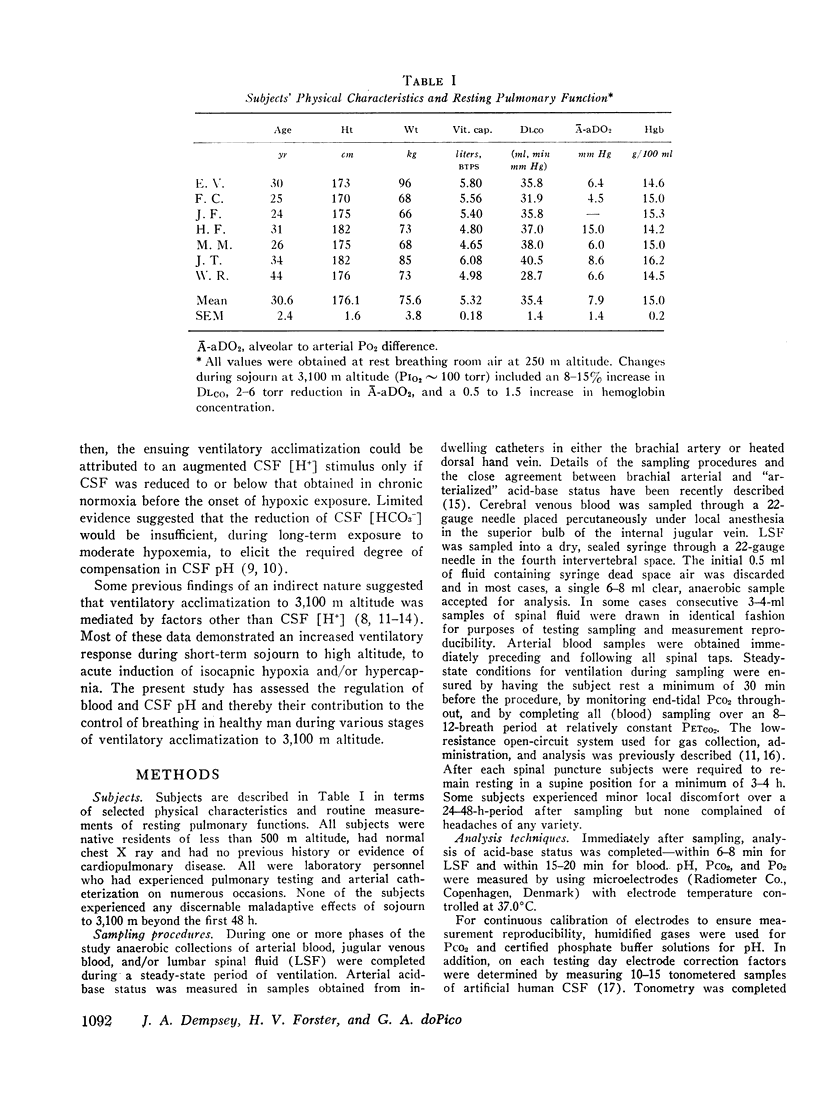
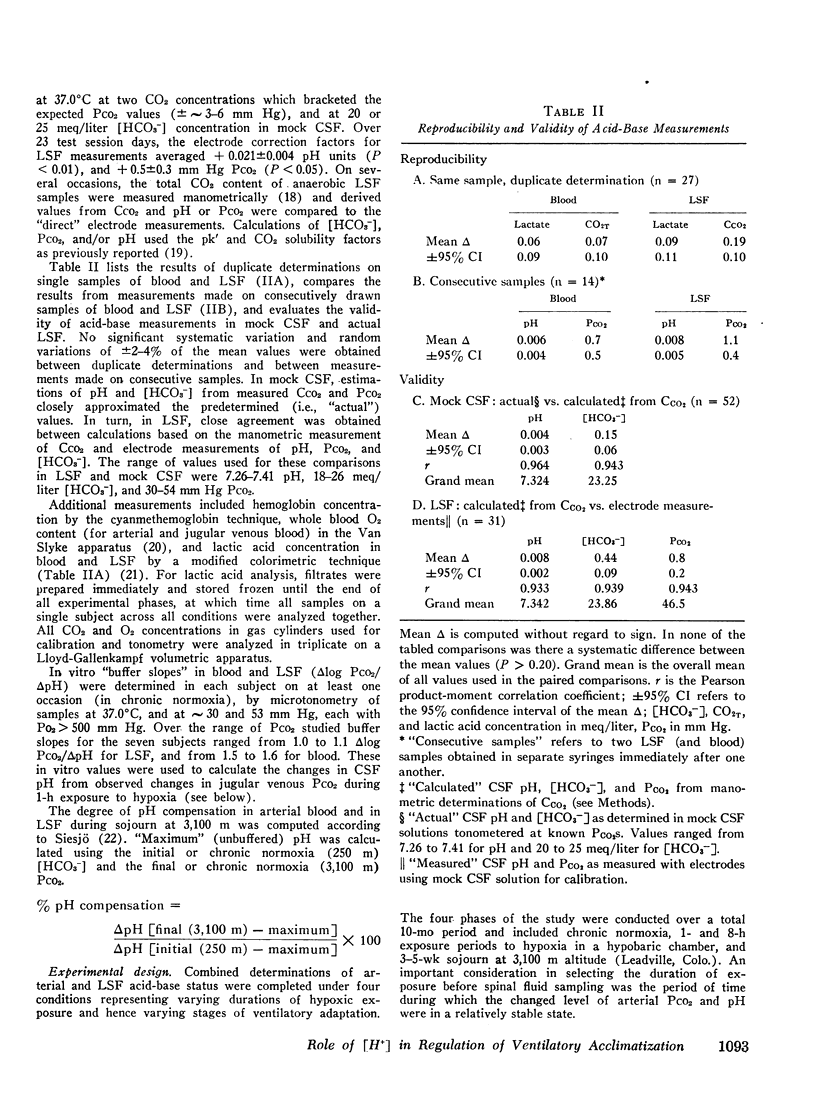
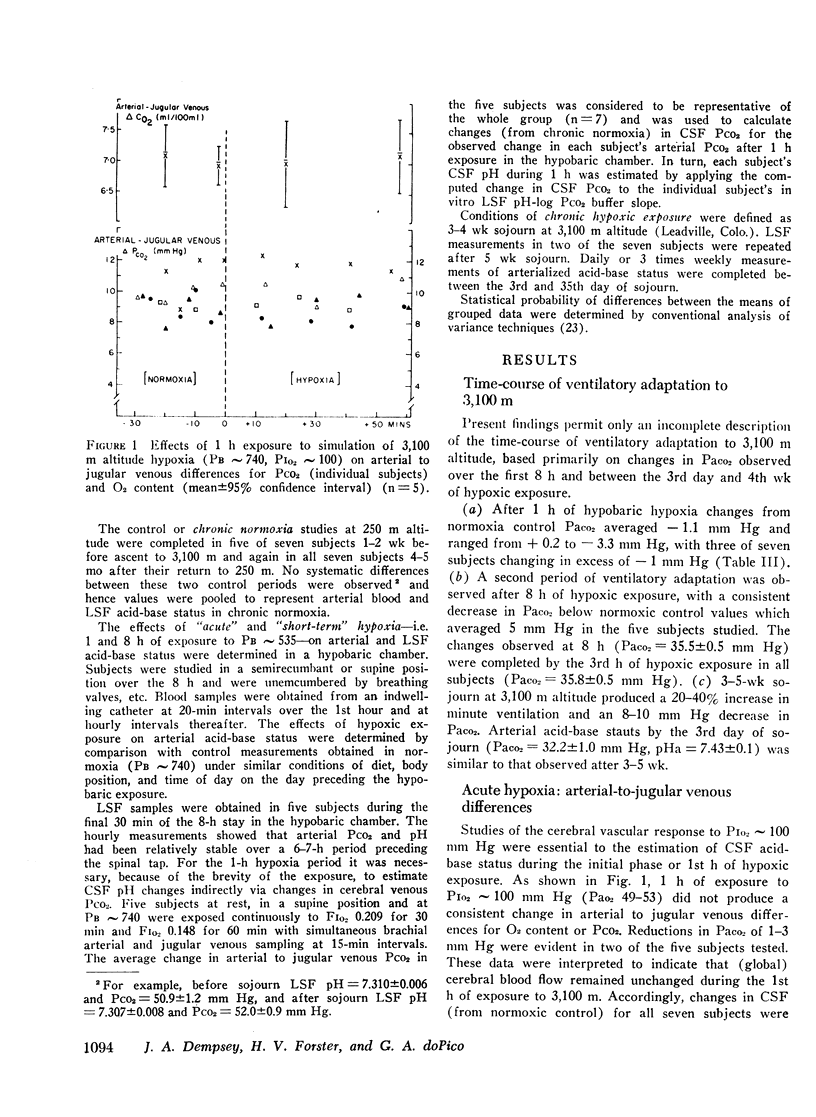
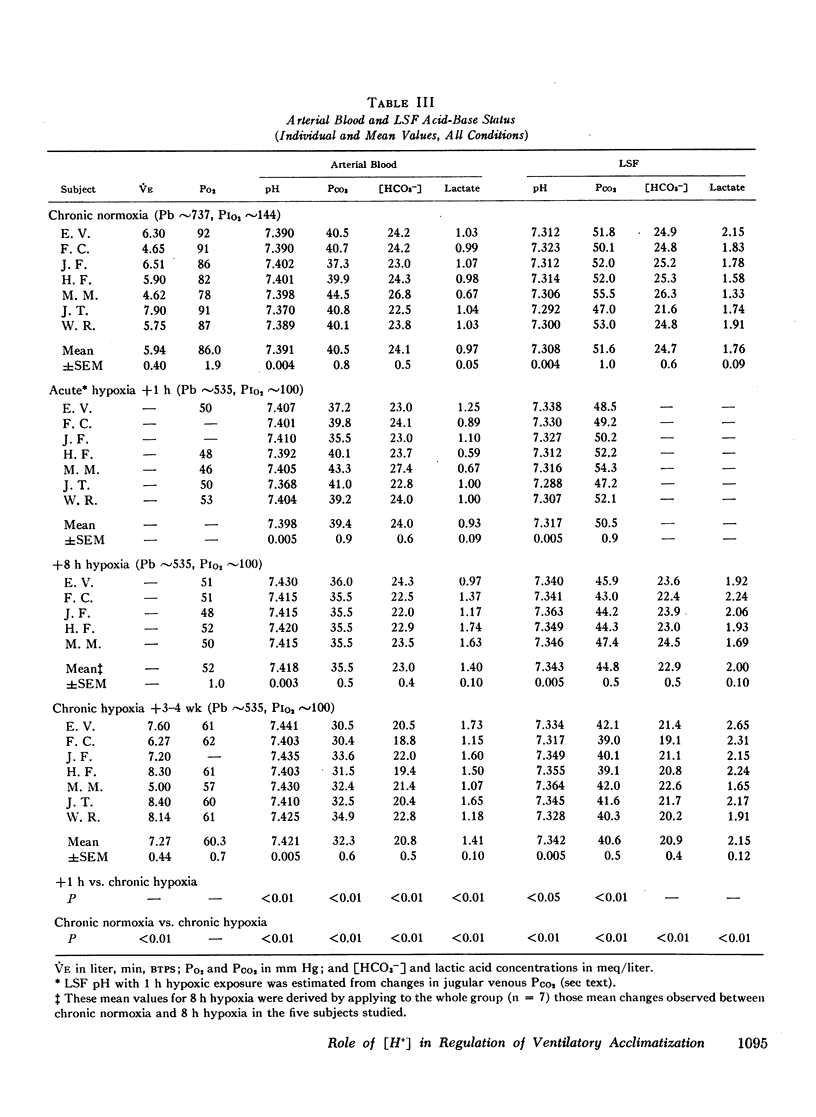
Abstract
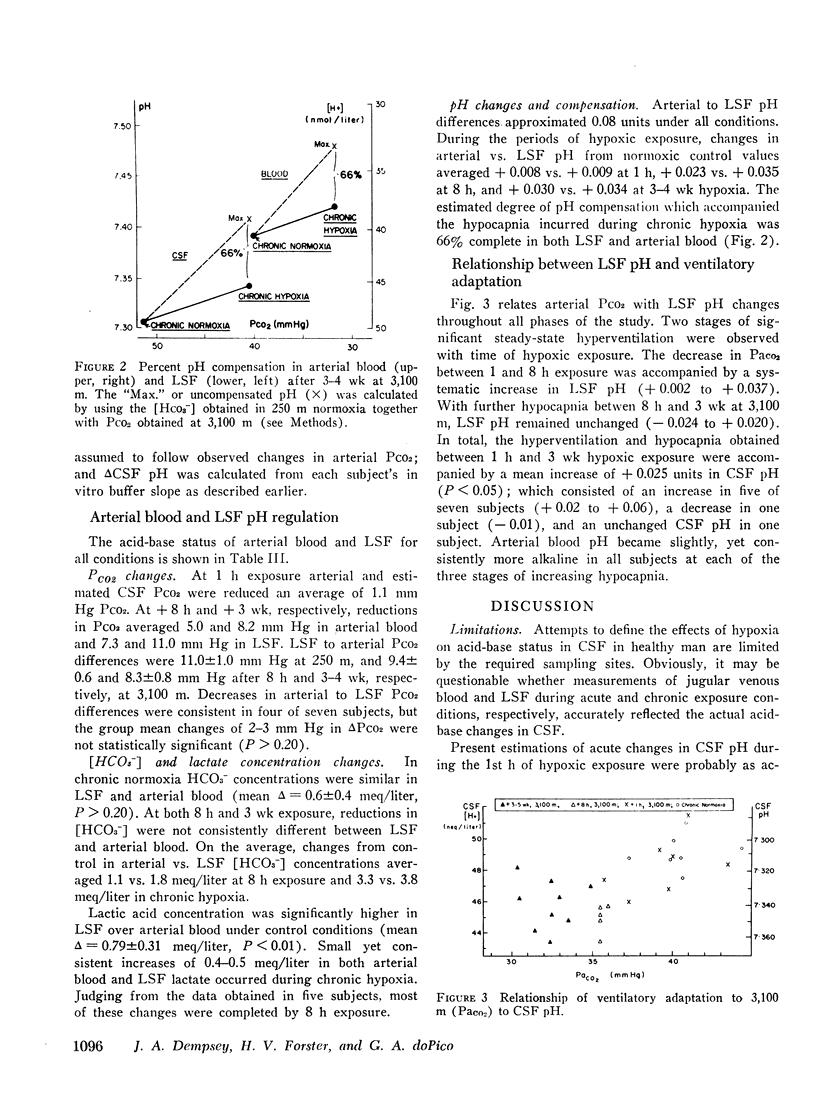
This study has assessed the regulation of arterial blood and cerebrospinal fluid (CSF) pH and thereby their contribution to the control of breathing in normal man during various stages of ventilatory acclimatization to 3,100 m altitude. CSF acid-base status was determined: (a) from measurements of lumbar spinal fluid during steady-state conditions of chronic normoxia (250 m altitude) and at + 8 h and + 3-4 wk of hypobaric hypoxia; and (b) from changes in cerebral venous PCO2 at + 1 h hypoxic exposure. After 3-4 wk at 3,100 m, CSF [H+] remained significantly alkaline to values obtained in either chronic normoxia or with 1 h hypoxic exposure and was compensated to the same extent (∼66%) as was arterial blood [H+]. Ventilatory acclimatization to 3,100 m bore no positive relationship to accompanying changes in arterial PO2 and pH and CSF pH: (a) CSF pH either increased or remained constant at 8 h and at 3-4 wk hypoxic exposure, respectively, coincident with significant, progressive reductions in PaCO2; (b) arterial PO2 and pH increased progressively with time of exposure; and (c) in the steady-state of acclimatization to 3,100 m the combination of chemical stimuli present, i.e. PaO2 = 60 mm Hg, pHa and pHCSF = + 0.03-0.04 > control, was insufficient to produce the observed hyperventilation (PaCO2 = 32 mm Hg). It was postulated that ventilatory acclimatization to 3,100 m altitude was mediated by factors other than CSF [H+] and that the combination of chronic hypoxemia and hypocapnia of moderate degrees provided no mechanisms for the specific regulation of CSF [HCO3−] and hence for homeostasis of CSF [H+].
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY R. D., SEMPLE S. J. A comparison of certain acidbase characteristics of arterial blood, jugular venous blood and cerebrospinal fluid in man, and the effect on them of some acute and chronic acid-base disturbances. J Physiol. 1962 Mar;160:381–391. doi: 10.1113/jphysiol.1962.sp006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey J. A., Forster H. V., Birnbaum M. L., Reddan W. G., Thoden J., Grover R. F., Rankin J. Control of exercise hyperpnea under varying durations of exposure to moderate hypoxia. Respir Physiol. 1972 Oct;16(2):213–231. doi: 10.1016/0034-5687(72)90052-7. [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Reddan W. G., Birnbaum M. L., Forster H. V., Thoden J. S., Grover R. F., Rankin J. Effects of acute through life-long hypoxic exposure on exercise pulmonary gas exchange. Respir Physiol. 1971 Oct;13(1):62–89. doi: 10.1016/0034-5687(71)90065-x. [DOI] [PubMed] [Google Scholar]
- FISHER V. J., CHRISTIANSON L. C. Cerebrospinal fluid acidbase balance during a changing ventilatory state in man. J Appl Physiol. 1963 Jul;18:712–716. doi: 10.1152/jappl.1963.18.4.712. [DOI] [PubMed] [Google Scholar]
- Fencl V., Miller T. B., Pappenheimer J. R. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol. 1966 Mar;210(3):459–472. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- Fencl V., Vale J. R., Broch J. A. Respiration and cerebral blood flow in metabolic acidosis and alkalosis in humans. J Appl Physiol. 1969 Jul;27(1):67–76. doi: 10.1152/jappl.1969.27.1.67. [DOI] [PubMed] [Google Scholar]
- Forster H. V., Dempsey J. A., Birnbaum M. L., Reddan W. G., Thoden J., Grover R. F., Rankin J. Effect of chronic exposure to hypoxia on ventilatory response to CO 2 and hypoxia. J Appl Physiol. 1971 Oct;31(4):586–592. doi: 10.1152/jappl.1971.31.4.586. [DOI] [PubMed] [Google Scholar]
- Forster H. V., Dempsey J. A., Thomson J., Vidruk E., DoPico G. A. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol. 1972 Jan;32(1):134–137. doi: 10.1152/jappl.1972.32.1.134. [DOI] [PubMed] [Google Scholar]
- Forster H. V., Klausen K. The effect of chronic metabolic acidosis and alkalosis on ventilation during exercise and hypoxia. Respir Physiol. 1973 Apr;17(3):336–346. doi: 10.1016/0034-5687(73)90008-x. [DOI] [PubMed] [Google Scholar]
- Kazemi H., Shannon D. C., Carvallo-Gil E. Brain CO2 buffering capacity in respiratory acidosis and alkalosis. J Appl Physiol. 1967 Feb;22(2):241–246. doi: 10.1152/jappl.1967.22.2.241. [DOI] [PubMed] [Google Scholar]
- Kazemi H., Shore N. S., Shih V. E., Shannon D. C. Brain organic buffers in respiratory acidosis and alkalosis. J Appl Physiol. 1973 Apr;34(4):478–482. doi: 10.1152/jappl.1973.34.4.478. [DOI] [PubMed] [Google Scholar]
- Kazemi H., Valenca L. M., Shannon D. C. Brain and cerebrospinal fluid lactate concentration in respiratory acidosis and alkalosis. Respir Physiol. 1969 Feb;6(2):178–186. doi: 10.1016/0034-5687(69)90056-5. [DOI] [PubMed] [Google Scholar]
- Kjällquist A., Nardini M., Siesjö B. K. The regulation of extra- and intracellular acid-base parameters in the rat brain during hyper- and hypocapnia. Acta Physiol Scand. 1969 Aug;76(4):485–494. doi: 10.1111/j.1748-1716.1969.tb04495.x. [DOI] [PubMed] [Google Scholar]
- Kogure K., Scheinberg P., Reinmuth O. M., Fujishima M., Busto R. Mechanisms of cerebral vasodilatation in hypoxia. J Appl Physiol. 1970 Aug;29(2):223–229. doi: 10.1152/jappl.1970.29.2.223. [DOI] [PubMed] [Google Scholar]
- Leusen I., Demeester G. Lactate and pyruvate in the brain of rats during hyperventilation. Arch Int Physiol Biochim. 1966 Feb;74(1):25–34. doi: 10.3109/13813456609059887. [DOI] [PubMed] [Google Scholar]
- MITCHELL R. A., HERBERT D. A., CARMAN C. T. ACID-BASE CONSTANTS AND TEMPERATURE COEFFICIENTS FOR CEREBROSPINAL FLUID. J Appl Physiol. 1965 Jan;20:27–30. doi: 10.1152/jappl.1965.20.1.27. [DOI] [PubMed] [Google Scholar]
- MacMillan V., Siesjö B. K. The effect of arterial hypoxemia upon acid-base parameters in arterial blood and cisternal cerebrospinal fluid of the rat. Acta Physiol Scand. 1971 Dec;83(4):454–462. doi: 10.1111/j.1748-1716.1971.tb05103.x. [DOI] [PubMed] [Google Scholar]
- Mines A. H., Morril C. G., Sorensen S. C. The effect of iso-carbic metabolic acidosis in blood on [H+] and [HCO3-] in CSF with deductions about the regulation of an active transport of H plus-HCO3-between blood and CSF. Acta Physiol Scand. 1971 Feb;81(2):234–245. doi: 10.1111/j.1748-1716.1971.tb04896.x. [DOI] [PubMed] [Google Scholar]
- Mines A. H., Sorensen S. C. Changes in the electrochemical potential difference for HCO3-between blood and cerebrospinal fluid and in cerebrospinal fluid lactate concentration during isocarbic hypoxia. Acta Physiol Scand. 1971 Feb;81(2):225–233. doi: 10.1111/j.1748-1716.1971.tb04895.x. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Carman C. T., Severinghaus J. W., Richardson B. W., Singer M. M., Shnider S. Stability of cerebrospinal fluid pH in chronic acid-base disturbances in blood. J Appl Physiol. 1965 May;20(3):443–452. doi: 10.1152/jappl.1965.20.3.443. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., FENCL V., HEISEY S. R., HELD D. ROLE OF CEREBRAL FLUIDS IN CONTROL OF RESPIRATION AS STUDIED IN UNANESTHETIZED GOATS. Am J Physiol. 1965 Mar;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- POSNER J. B., SWANSON A. G., PLUM F. ACID-BASE BALANCE IN CEREBROSPINAL FLUID. Arch Neurol. 1965 May;12:479–496. doi: 10.1001/archneur.1965.00460290035006. [DOI] [PubMed] [Google Scholar]
- Plum F., Posner J. B. Blood and cerebrospinal fluid lactate during hyperventilation. Am J Physiol. 1967 Apr;212(4):864–870. doi: 10.1152/ajplegacy.1967.212.4.864. [DOI] [PubMed] [Google Scholar]
- Pontén U., Siesjö B. K. Gradients of CO2 tension in the brain. Acta Physiol Scand. 1966 Jun;67(2):129–140. doi: 10.1111/j.1748-1716.1966.tb03294.x. [DOI] [PubMed] [Google Scholar]
- SEVERINGHAUS J. W., MITCHELL R. A., RICHARDSON B. W., SINGER M. M. RESPIRATORY CONTROL AT HIGH ALTITUDE SUGGESTING ACTIVE TRANSPORT REGULATION OF CSF PH. J Appl Physiol. 1963 Nov;18:1155–1166. doi: 10.1152/jappl.1963.18.6.1155. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W., Chiodi H., Eger E. I., 2nd, Brandstater B., Hornbein T. F. Cerebral blood flow in man at high altitude. Role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res. 1966 Aug;19(2):274–282. doi: 10.1161/01.res.19.2.274. [DOI] [PubMed] [Google Scholar]
- Shapiro W., Wasserman A. J., Baker J. P., Patterson J. L., Jr Cerebrovascular response to acute hypocapnic and eucapnic hypoxia in normal man. J Clin Invest. 1970 Dec;49(12):2362–2368. doi: 10.1172/JCI106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen S. C., Cruz J. C. Ventilatory response to a single breath of CO2 in O2 in normal man at sea level and high altitude. J Appl Physiol. 1969 Aug;27(2):186–190. doi: 10.1152/jappl.1969.27.2.186. [DOI] [PubMed] [Google Scholar]
- Sorensen S. C. The chemical control of ventilation. Acta Physiol Scand Suppl. 1971;361:1–72. [PubMed] [Google Scholar]
- Van Vaerenbergh P. J., Demeester G., Leusen I. Lactate in cerebrospinal fluid during hyperventilation. Arch Int Physiol Biochim. 1965 Dec;73(5):738–747. doi: 10.3109/13813456509084897. [DOI] [PubMed] [Google Scholar]
- van Heijst A. N., Maas A. H., Visser B. F. Comparison of the acid-base balance in cisternal and lumbar cerebrospinal fluid. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(3):242–246. doi: 10.1007/BF00362485. [DOI] [PubMed] [Google Scholar]



