Abstract
The extent and significance of spontaneous glucagon secretion in the immediate postnatal period were investigated in groups of normal infants studied cross-sectionally and longitudinally. Arginine-and alanine-stimulated glucagon secretion was also studied. Plasma glucagon concentrations were correlated with prevailing glucose and insulin concentrations.
The characteristic fall in blood glucose, reaching a nadir within hours of birth, was associated with a significant increase in glucagon concentration. Despite persistence of relative glucopenia, glucagon did not change appreciably between 2 and 24 h of life. A further significant elevation in glucagon concentration occurred from day 1 to day 3 of life associated with a return of glucose to euglycemic levels. In contrast to the sluggishness of pancreatic glucagon release, glucagon-like immunoreactivity rose markedly to mean levels of approximately 2,000 pg/ml after introduction of formula feeding. No significant changes in insulin levels were observed in these studies.
Arginine infusion via an umbilical vein catheter into six infants within 6 h of birth elicited a brisk, almost threefold increment in glucagon concentration (from 339±85 to 940±254 pg/ml) in blood obtained from, or close to, the portal circulation. Bolus injection of alanine (1 mmol/kg) into a peripheral vein to six infants resulted in significant increments in glucagon (mean maximal, 128 pg/ml) as well as glucose and insulin.
The observations suggest that spontaneous glucagon secretion may be an important factor in neonatal glucose homeostasis. Secretion seems more brisk in response to amino acid stimulation than to a falling glucose concentration.
Full text
PDF
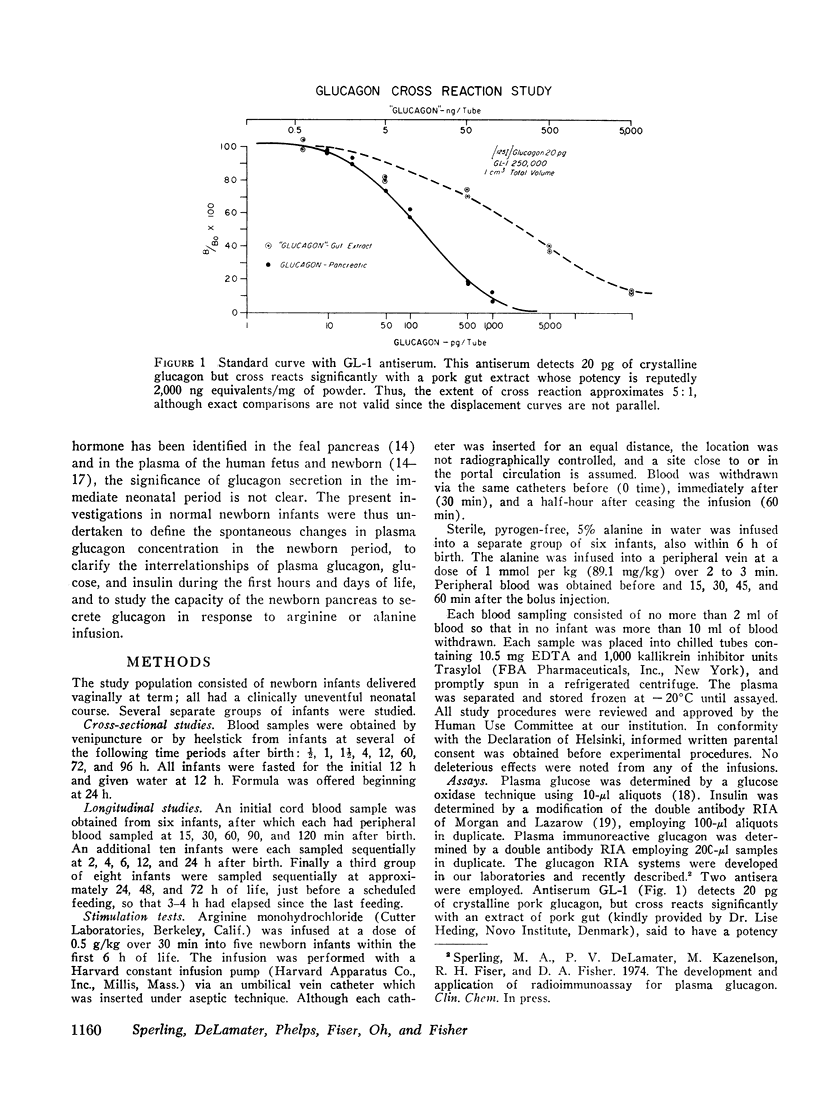
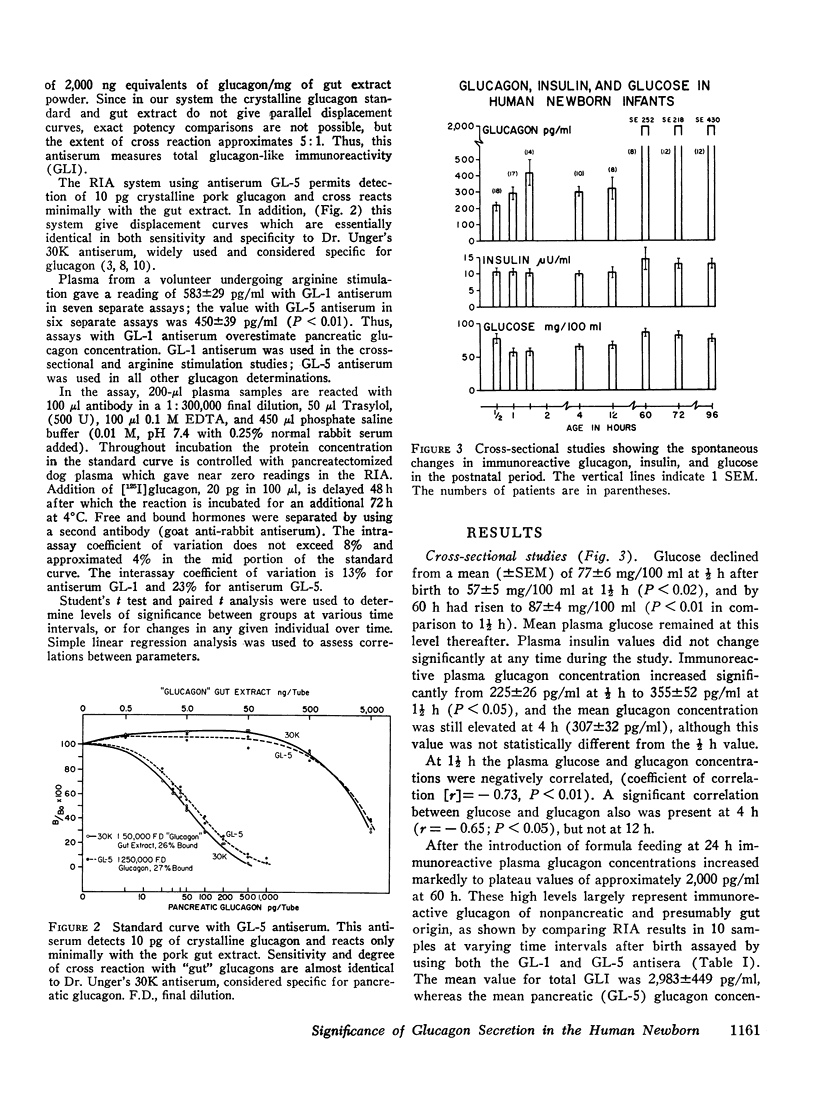
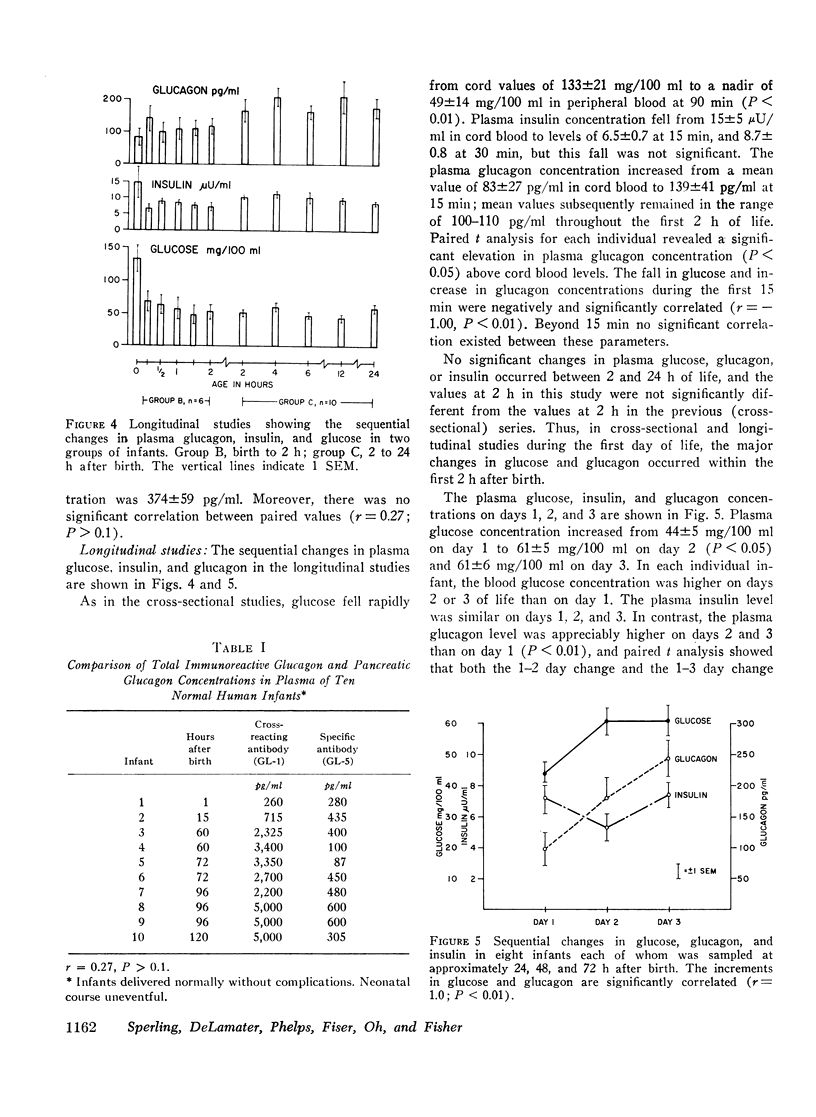

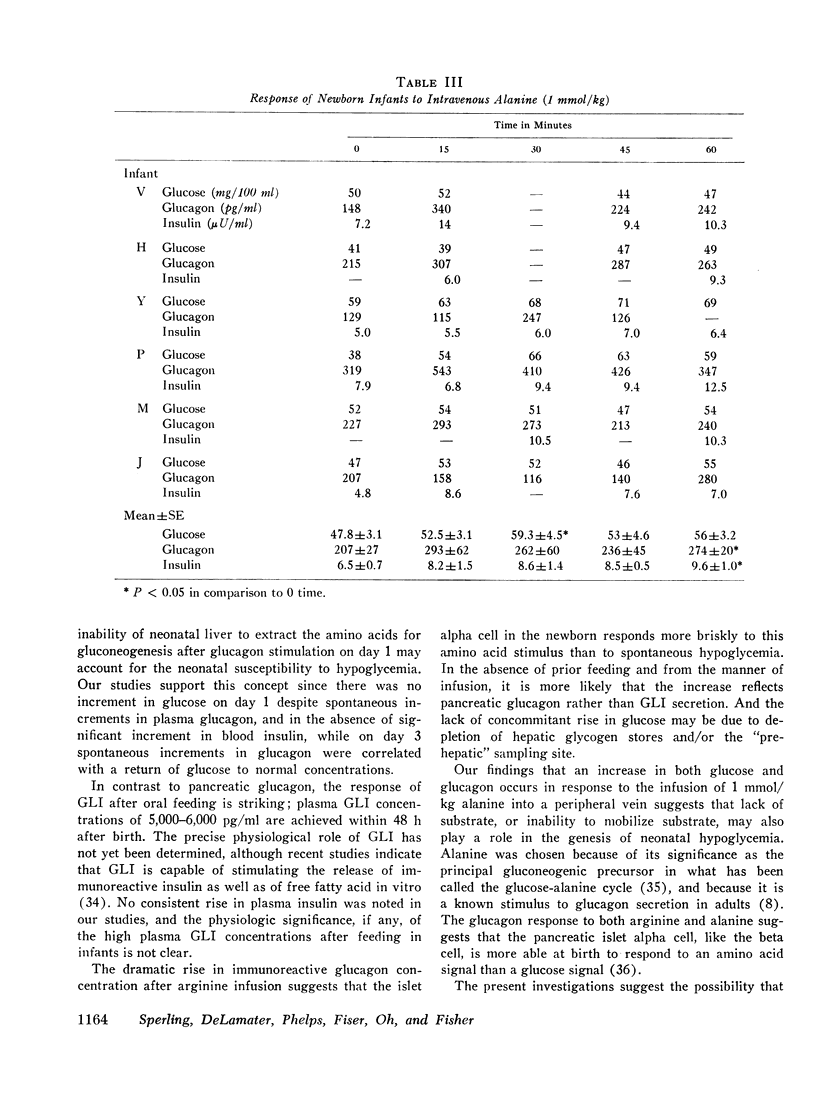


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A. Control of glucose metabolism in the human fetus and newborn infant. Adv Metab Disord. 1971;5:183–275. doi: 10.1016/b978-0-12-027305-8.50026-6. [DOI] [PubMed] [Google Scholar]
- Alexander D. P., Britton H. G., Cohen N. M., Nixon D. A. Plasma concentrations on insulin, glucose, free fatty acids and ketone bodies in the foetal and newborn sheep and the response to a glucose load before and after birth. Biol Neonat. 1969;14(3):178–193. doi: 10.1159/000240183. [DOI] [PubMed] [Google Scholar]
- BAIRD J. D., FARQUHAR J. W. Insulin-secreting capacity in newborn infants of normal and diabetic women. Lancet. 1962 Jan 13;1(7220):71–74. doi: 10.1016/s0140-6736(62)91720-8. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Daniel P. M., Johnston D. I., Ogawa O., Pratt O. E. Changes in glucagon level associated with anxiety or stress. Psychol Med. 1972 Nov;2(4):426–427. doi: 10.1017/s0033291700045256. [DOI] [PubMed] [Google Scholar]
- Bloom S. R., Johnston D. I. Failure of glucagon release in infants of diabetic mothers. Br Med J. 1972 Nov 25;4(5838):453–454. doi: 10.1136/bmj.4.5838.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAHILL G. F., Jr, EARLE A. S., ZOTTU S. In vivo effects of glucagon on hepatic glycogen, phosphorylase and glucose-6-phosphatase. Endocrinology. 1957 Feb;60(2):265–269. doi: 10.1210/endo-60-2-265. [DOI] [PubMed] [Google Scholar]
- CORNBLATH M., GANZON A. F., NICOLOPOULOS D., BAENS G. S., HOLLANDER R. J., GORDON M. H., GORDON H. H. Studies of carbohydrate metabolism in the newborn infant. III. Some factors influencing the capillary blood sugar and the response to glucagon during the first hours of life. Pediatrics. 1961 Mar;27:378–389. [PubMed] [Google Scholar]
- Chez R. A., Mintz D. H., Hutchinson D. L. Effect of theophylline on glucagon and glucose-mediated plasma insulin responses in subhuman primate fetus and neonate. Metabolism. 1971 Aug;20(8):805–815. doi: 10.1016/s0026-0495(71)80010-0. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Gluconeogenesis. Metabolism. 1972 Oct;21(10):945–990. doi: 10.1016/0026-0495(72)90028-5. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R., Ahlborg G. Plasma glucagon levels in exercising man. N Engl J Med. 1972 Jul 27;287(4):184–185. doi: 10.1056/NEJM197207272870412. [DOI] [PubMed] [Google Scholar]
- Girard J., Bal D., Assan R. Glucagon secretion during the early postnatal period in the rat. Horm Metab Res. 1972 May;4(3):168–170. doi: 10.1055/s-0028-1094093. [DOI] [PubMed] [Google Scholar]
- Grasso S., Messina A., Saporito N., Reitano G. Serum-insulin response to glucose and aminoacids in the premature infant. Lancet. 1968 Oct 5;2(7571):755–756. doi: 10.1016/s0140-6736(68)90954-9. [DOI] [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. Initiation by glucagon of the premature development of tyrosine aminotransferase, serine dehydratase, and glucose-6-phosphatase in fetal rat liver. J Biol Chem. 1967 Jun 25;242(12):2986–2991. [PubMed] [Google Scholar]
- Gutman R. A., Fink G., Voyles N., Selawry H., Penhos J. C., Lepp A., Recant L. Specific biologic effects of intestinal glucagon-like materials. J Clin Invest. 1973 May;52(5):1165–1175. doi: 10.1172/JCI107283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx A. S., Massi-Benedetti F., Falorni A., Lefebvre P. J. Presence of pancreatic glucagon in the portal plasma of human neonates. Differences in the insulin and glucagon responses to glucose between normal infants and infants from diabetic mothers. Diabetologia. 1972 Aug;8(4):296–300. doi: 10.1007/BF01225575. [DOI] [PubMed] [Google Scholar]
- MARKS V. An improved glucose-oxidase method for determining blood, C.S.F. and urine glucose levels. Clin Chim Acta. 1959 May;4(3):395–400. doi: 10.1016/0009-8981(59)90110-x. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Fekete M., Assan R. Glucagon, insulin, and growth hormone response to exchange transfusion in premature and term infants. Arch Dis Child. 1972 Apr;47(252):186–189. doi: 10.1136/adc.47.252.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of alanine on glucagon secretion. J Clin Invest. 1971 Oct;50(10):2215–2218. doi: 10.1172/JCI106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda A., Aguilar-Parada E., Eisentraut A. M., Unger R. H. Control of pancreatic glucagon secretion by glucose. Diabetes. 1969 Jan;18(1):1–10. doi: 10.2337/diab.18.1.1. [DOI] [PubMed] [Google Scholar]
- Pildes R. S., Hart R. J., Warrner R., Cornblath M. Plasma insulin response during oral glucose tolerance tests in newborns of normal and gestational diabetic mothers. Pediatrics. 1969 Jul;44(1):76–83. [PubMed] [Google Scholar]
- Raivio K. O. Neonatal hypoglycemia. II. A clinical study of 44 idiopathic cases with special reference to corticosteroid treatment. Acta Paediatr Scand. 1968 Nov;57(6):540–546. doi: 10.1111/j.1651-2227.1968.tb06977.x. [DOI] [PubMed] [Google Scholar]
- Reisner S. H., Aranda J. V., Colle E., Papageorgiou A., Schiff D., Scriver C. R., Stern L. The effect of intravenous glucagon on plasma amino acids in the newborn. Pediatr Res. 1973 Apr;7(4):184–191. doi: 10.1203/00006450-197304000-00021. [DOI] [PubMed] [Google Scholar]
- Rocha D. M., Santeusanio F., Faloona G. R., Unger R. H. Abnormal pancreatic alpha-cell function in bacterial infections. N Engl J Med. 1973 Apr 5;288(14):700–703. doi: 10.1056/NEJM197304052881402. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Glucagon physiology and pathophysiology. N Engl J Med. 1971 Aug 19;285(8):443–449. doi: 10.1056/NEJM197108192850806. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Aguilar-Parada E., Eisentraut A. M. The role of aminogenic glucagon secretion in blood glucose homeostasis. J Clin Invest. 1969 May;48(5):810–822. doi: 10.1172/JCI106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Evaluation of alpha-cell function by infusion of alanine in normal, diabetic and obese subjects. N Engl J Med. 1973 Mar 8;288(10):487–490. doi: 10.1056/NEJM197303082881003. [DOI] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Factors affecting the premature induction of phosphopyruvate carboxylase in neonatal rat liver. Biochem J. 1968 Jun;108(2):325–331. doi: 10.1042/bj1080325. [DOI] [PMC free article] [PubMed] [Google Scholar]



