Abstract
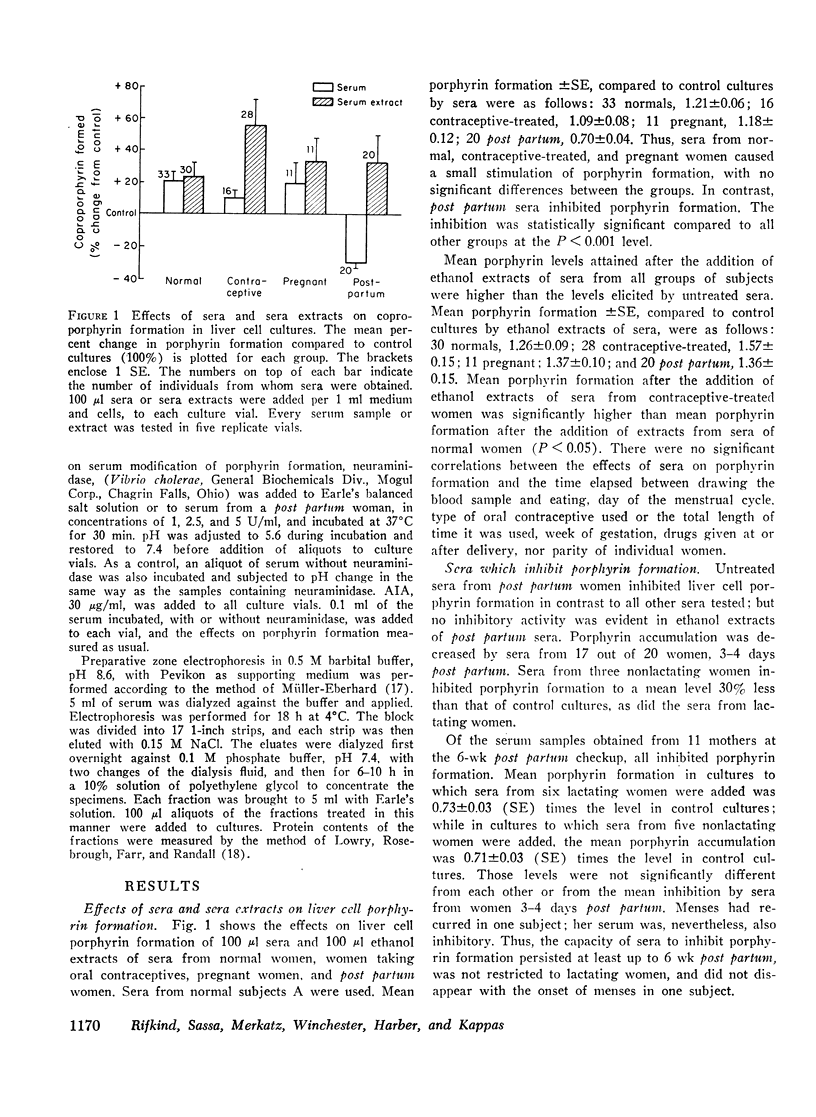
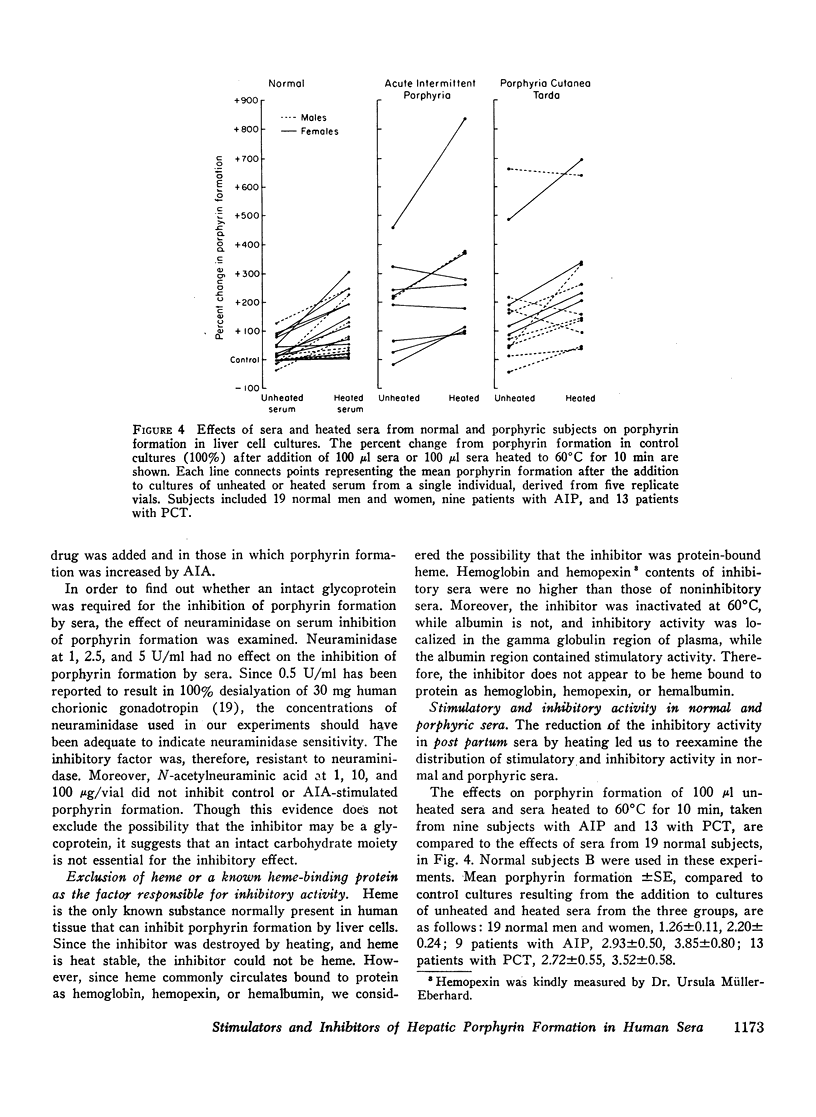
Human sera were found to contain factors that stimulate and factors that inhibit porphyrin formation by cultured avian liver cells. The capacity of sera to stimulate or inhibit porphyrin formation varied in different hormonal states and in the porphyrias. Sera from 31 post partum women, eight of whom were not lactating, inhibited porphyrin formation to a mean level 30% below the level in control cultures and also inhibited drug and steroid stimulation of porphyrin formation. In contrast, mean porphyrin formation compared to control cultures was increased between 9 and 21% by sera from 52 normal subjects, 16 women on oral contraceptives, and 11 pregnant women. It was increased 193% by sera from nine subjects with acute intermittent porphyria and 172% by sera from 13 subjects with porphyria cutanea tarda. Heated sera or ethanol extracts of sera from all groups of subjects further increased the mean porphyrin stimulation by sera and, for the post partum subjects, eliminated the inhibitory effect. Ethanol extracts of sera from 28 oral contraceptive-treated women caused significantly greater mean stimulation of porphyrin formation than did extracts of sera from 30 normal women. While sera from 17 out of 22 porphyric subjects contained both stimulatory and inhibitory factors, 5 out of 22 had no evidence of an inhibitory component. There appeared to be heterogeneity in the occurrence of the factors among porphyrics.
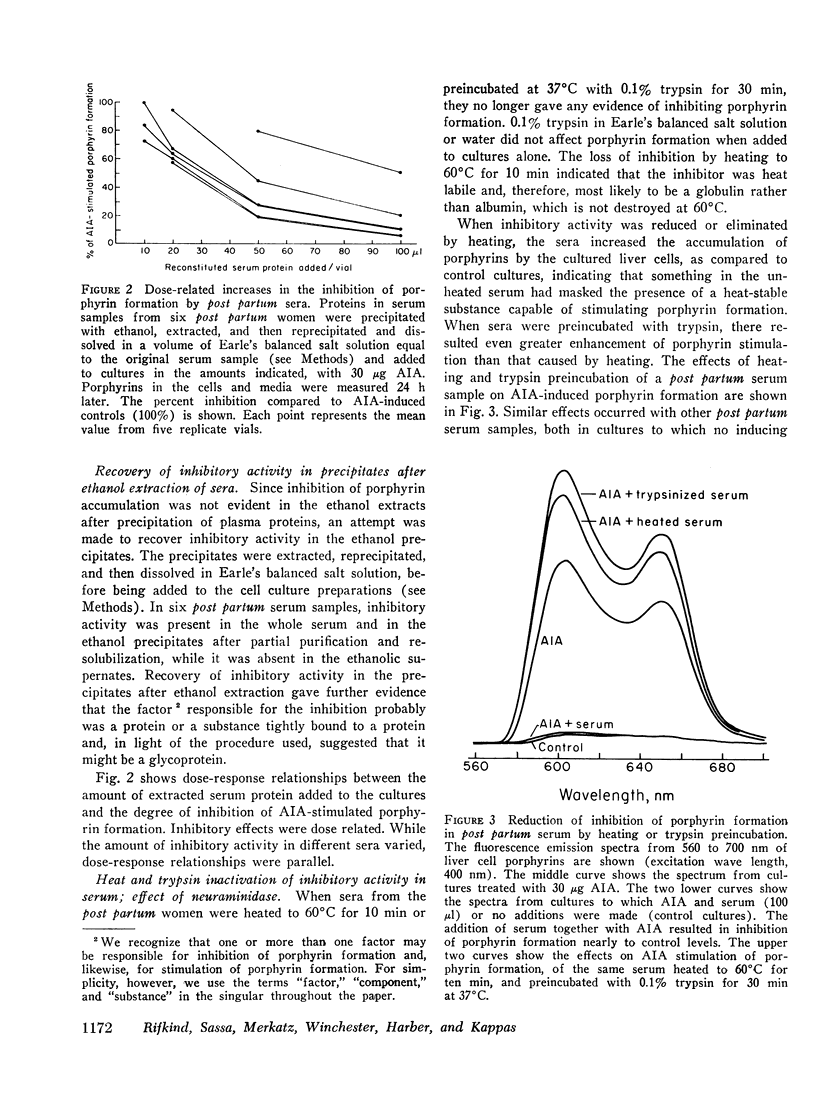
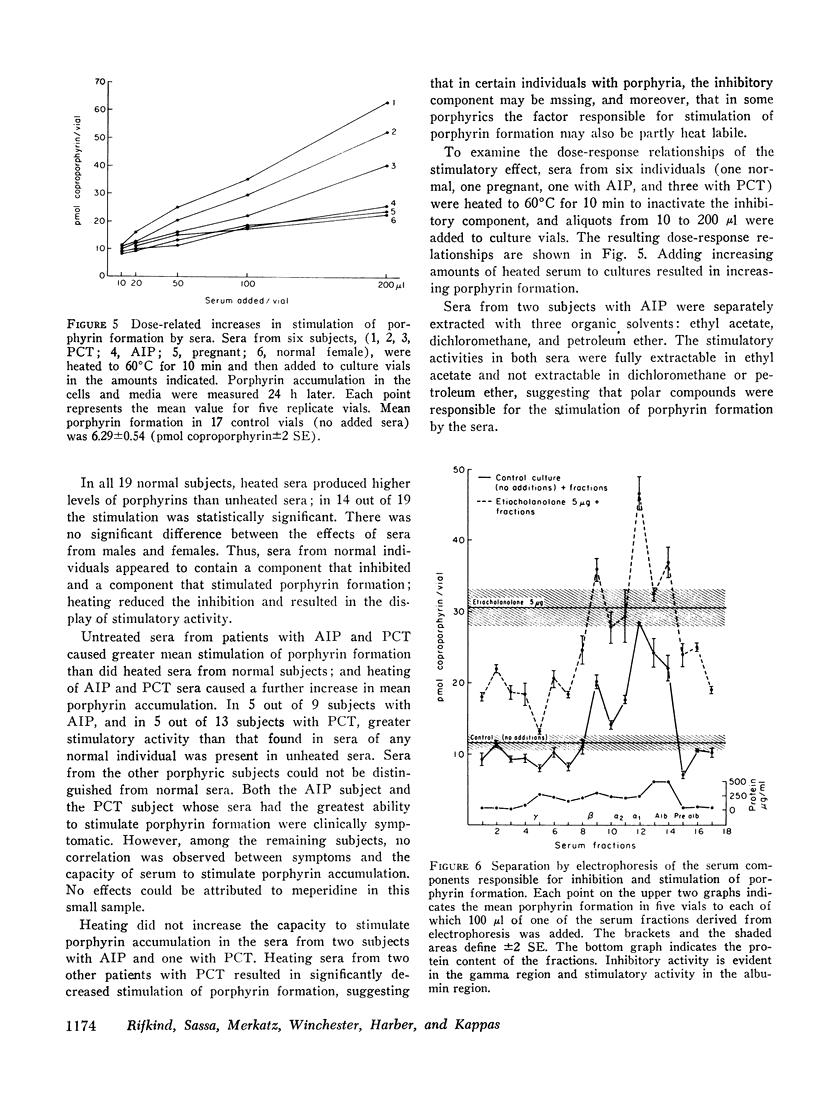
The factor(s) in sera responsible for porphyrin stimulation were heat-stable and insensitive to trypsin; were present in the supernates after ethanol precipitation of plasma proteins; were extractable in ethyl acetate and nondialyzable; and they migrated with the albumincontaining fraction of serum during electrophoresis. The factor(s) responsible for porphyrin inhibition were heat labile, sensitive to trypsin, and resistant to neuraminidase; were present in the ethanol precipitates of sera and were nondialyzable; and they migrated with the gamma globulin fraction of serum during electrophoresis. Inhibition of porphyrin formation was not attributable to heme, free or bound as hemoglobin, hemopexin, or hemalbumin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A., KOBI J., LEIFERMAN J., DERNER I. Purification of pituitary gonadotropin from urine of normal men. J Clin Endocrinol Metab. 1961 Jan;21:1–20. doi: 10.1210/jcem-21-1-1. [DOI] [PubMed] [Google Scholar]
- Erslev A. J., Kazal L. A. Inactivation of erythropoietin by tissue homogenates. Proc Soc Exp Biol Med. 1968 Dec;129(3):845–849. doi: 10.3181/00379727-129-33439. [DOI] [PubMed] [Google Scholar]
- Goldberg A., Moore M. R., Beattie A. D., Hall P. E., McCallum J., Grant J. K. Excessive urinary excretion of certain porphyrinogenic steroids in human acute intermittent porphyria. Lancet. 1969 Jan 18;1(7586):115–118. doi: 10.1016/s0140-6736(69)91134-9. [DOI] [PubMed] [Google Scholar]
- Granick S., Kappas A. Steroid induction of porphyrin synthesis in liver cell culture. I. Structural basis and possible physiological role in the control of heme formation. J Biol Chem. 1967 Oct 25;242(20):4587–4593. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Gusdon J. P., Jr Fetal and maternal immunoglobulin levels during pregnancy. Am J Obstet Gynecol. 1969 Apr 1;103(7):895–900. doi: 10.1016/s0002-9378(16)34434-9. [DOI] [PubMed] [Google Scholar]
- Hwang P., Guyda H., Friesen H. A radioimmunoassay for human prolactin. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1902–1906. doi: 10.1073/pnas.68.8.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kappas A., Bradlow H. L., Gillette P. N., Gallagher T. F. Studies in porphyria. I. A defect in the reductive transformation of natural steroid hormones in the hereditary liver disease, acute intermittent porphyria. J Exp Med. 1972 Nov 1;136(5):1043–1053. doi: 10.1084/jem.136.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Song C. S., Levere R. D., Sachson R. A., Granick S. THE INDUCTION OF delta-AMINOLEVULINIC ACID SYNTHETASE in vivo IN CHICK EMBRYO LIVER BY NATURAL STEROIDS. Proc Natl Acad Sci U S A. 1968 Oct;61(2):509–513. doi: 10.1073/pnas.61.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Song C. S., Sassa S., Levere R. D., Granick S. The occurrence of substances in human plasma capable of inducing the enzyme delta-aminolevulinate synthetase in liver cells. Proc Natl Acad Sci U S A. 1969 Oct;64(2):557–564. doi: 10.1073/pnas.64.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskelo P., Eisalo A., Toivonen I. Urinary excretion of porphyrin precursors and coproporphyrin in healthy females on oral contraceptives. Br Med J. 1966 Mar 12;1(5488):652–654. doi: 10.1136/bmj.1.5488.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz S. B., Kao V. Studies on red cell aplasia. I. Demonstration of a plasma inhibitor to heme synthesis and an antibody to erythroblast nuclei. Proc Natl Acad Sci U S A. 1967 Aug;58(2):493–500. doi: 10.1073/pnas.58.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levere R. D., Swerdlow F., Garavoy M. R. Measurement of human plasma hemoglobin by difference spectrophotometry. J Lab Clin Med. 1971 Jan;77(1):168–176. [PubMed] [Google Scholar]
- Molinari P. F., Menninger F. F., Jr, Rosenkrantz H. Detection of an inhibitor of hemoglobin synthesis in rat plasma. J Lab Clin Med. 1970 Sep;76(3):466–471. [PubMed] [Google Scholar]
- Poland A., Glover E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin: a potent inducer of -aminolevulinic acid synthetase. Science. 1973 Feb 2;179(4072):476–477. doi: 10.1126/science.179.4072.476. [DOI] [PubMed] [Google Scholar]
- REYNAFARJE C., RAMOS J., FAURA J., VILLAVICENCIO D. HUMORAL CONTROL OF ERYTHROPOIETIC ACTIVITY IN MAN DURING AND AFTER ALTITUDE EXPOSURE. Proc Soc Exp Biol Med. 1964 Jul;116:649–650. doi: 10.3181/00379727-116-29331. [DOI] [PubMed] [Google Scholar]
- Racz W. J., Marks G. S. Drug-induced porphyrin biosynthesis. IV. Investigation of the differences in response of isolated liver cells and the liver of the intact chick embryo to porphyria-inducing drugs. Biochem Pharmacol. 1972 Jan 15;21(2):143–151. doi: 10.1016/0006-2952(72)90264-x. [DOI] [PubMed] [Google Scholar]
- Rifkind A. B., Gillette P. N., Song C. S., Kappas A. Drug stimulation of -aminolevulinic acid synthetase and cytochrome P-450 in vivo in chick embryo liver. J Pharmacol Exp Ther. 1973 May;185(2):214–225. [PubMed] [Google Scholar]
- Rifkind A. B., Gillette P. N., Song C. S., Kappas A. Induction of hepatic delta-amino-levulinic acid synthetase by oral contraceptive steroids. J Clin Endocrinol Metab. 1970 Mar;30(3):330–335. doi: 10.1210/jcem-30-3-330. [DOI] [PubMed] [Google Scholar]
- Sardesai V. M., Orten J. M. Role of a plasma factor in porphyrin biosynthesis. Proc Soc Exp Biol Med. 1965 Nov;120(2):552–554. doi: 10.3181/00379727-120-30587. [DOI] [PubMed] [Google Scholar]
- Skjaelaaen P., Halvorsen S. Inhibition of erythropoiesis by plasma from newborn infants. Acta Paediatr Scand. 1971 May;60(3):301–308. doi: 10.1111/j.1651-2227.1971.tb06661.x. [DOI] [PubMed] [Google Scholar]
- Van Hall E. V., Vaitukaitis J. L., Ross G. T., Hickman J. W., Ashwell G. Immunological and biological activity of HCG following progressive desialylation. Endocrinology. 1971 Feb;88(2):456–464. doi: 10.1210/endo-88-2-456. [DOI] [PubMed] [Google Scholar]
- Wada O., Yano Y., Urata G., Nakao K. Behavior of hepatic microsomal cytochromes after treatment of mice with drugs known to disturb porphyrin metabolism in liver. Biochem Pharmacol. 1968 Apr;17(4):595–603. doi: 10.1016/0006-2952(68)90275-x. [DOI] [PubMed] [Google Scholar]
- Whitcomb W. H., Moore M. Z. The inhibitory effect of plasma from hypertransfused animals on erythrocyte iron incorporation in mice. J Lab Clin Med. 1965 Oct;66(4):641–651. [PubMed] [Google Scholar]
- Zalusky R., Zanjani E. D., Gidari A. S., Ross J. Site of action of a serum inhibitor of erythropoiesis. J Lab Clin Med. 1973 Jun;81(6):867–875. [PubMed] [Google Scholar]



