Abstract
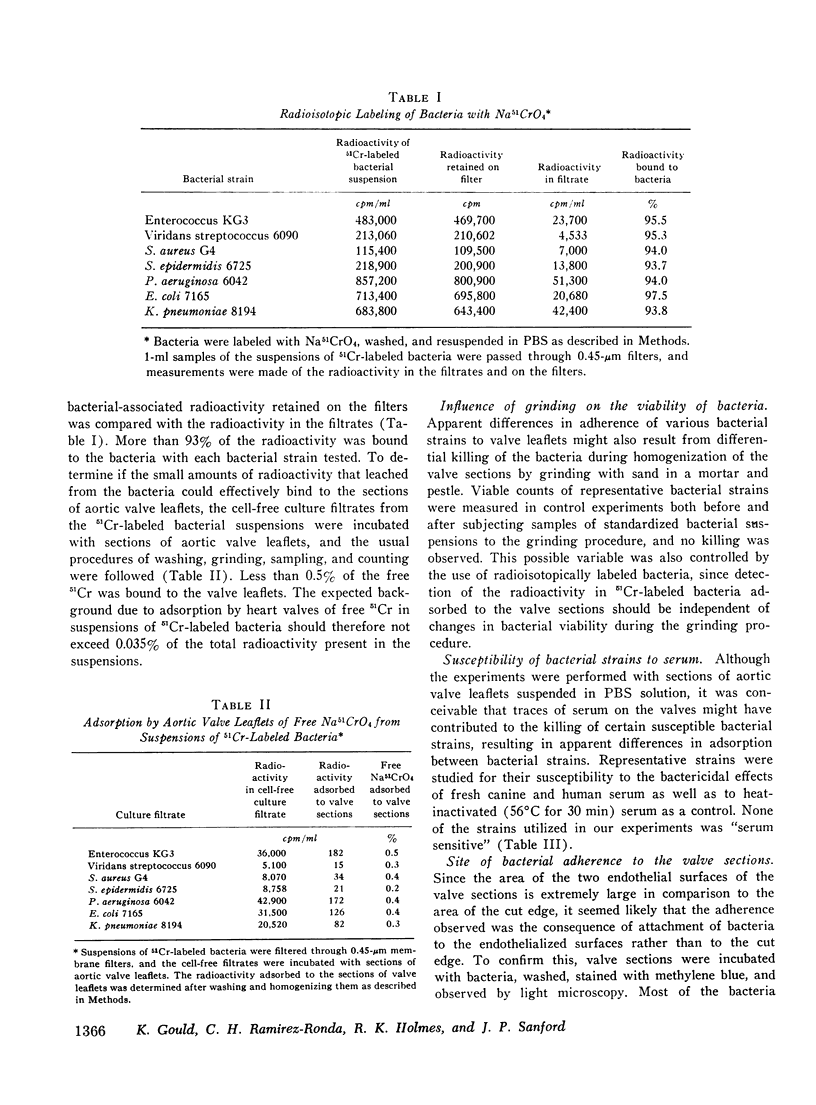
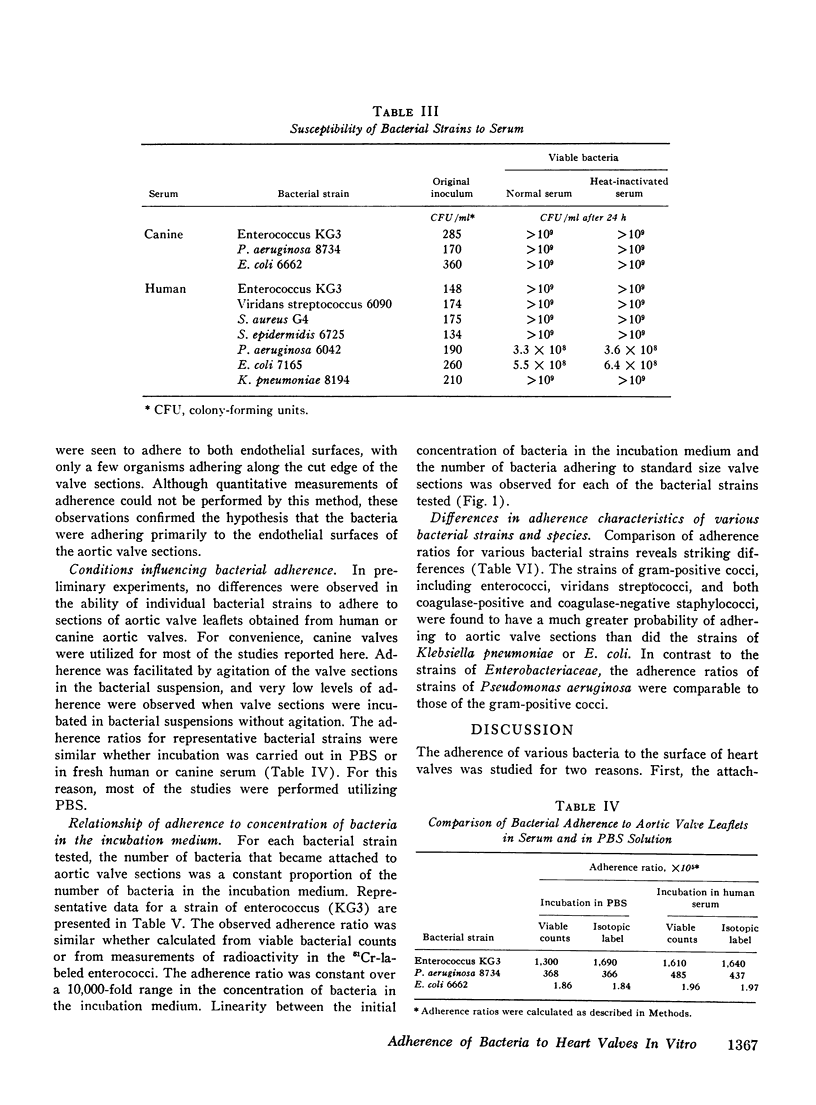
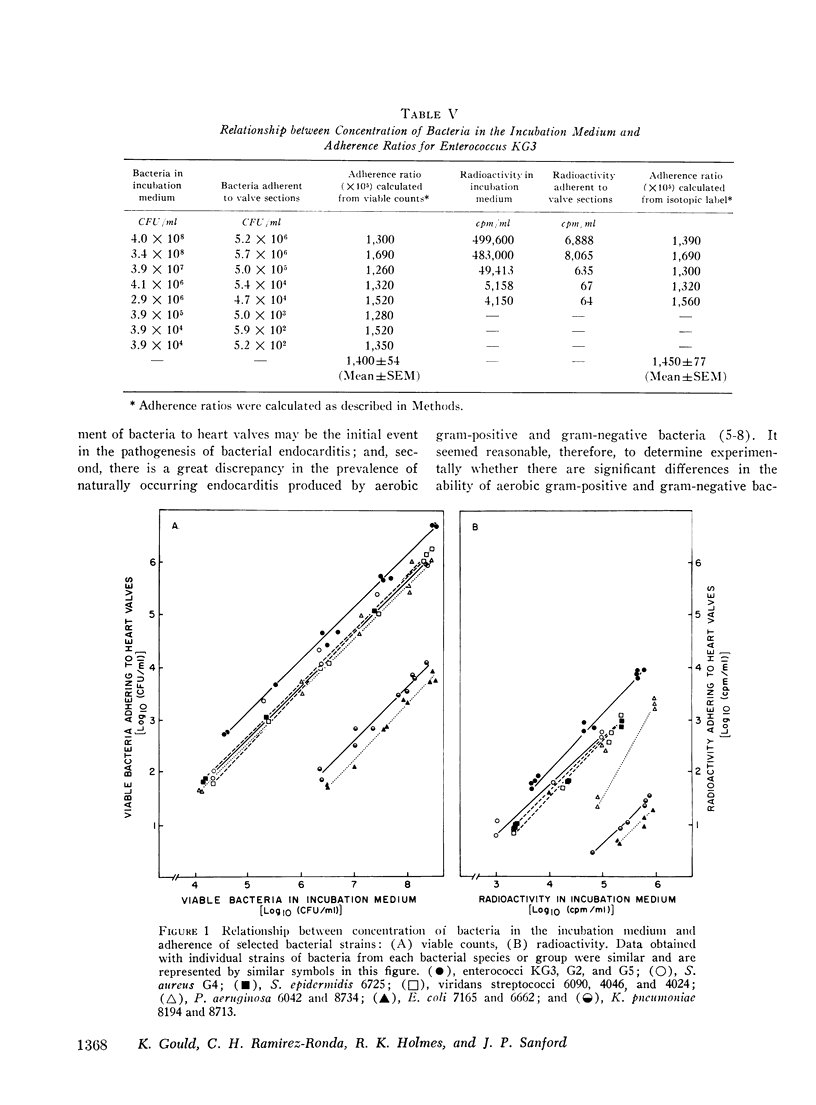
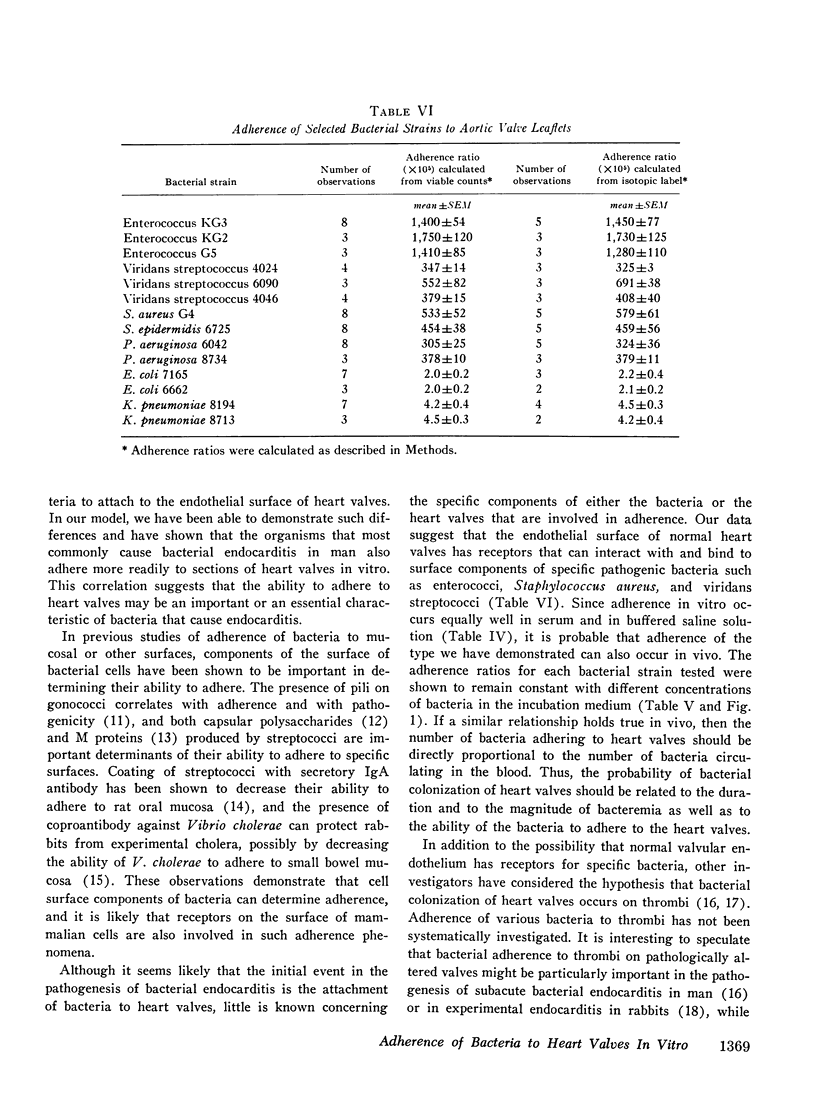
The abilities of 14 strains of aerobic gram-positive cocci and gram-negative bacilli to adhere in vitro to human or canine aortic valve leaflets were compared. 2-mm sections of excised valve leaflets were obtained by punch biopsy and were incubated under standardized conditions in suspensions of bacteria. Valve sections were subsequently washed and homogenized, and quantitative techniques were used to determine the proportions of bacteria from the initial suspensions that had adhered to the valve sections. Comparable results were obtained when these adherence ratios were determined by two independent methods based either on measurements of bacterial viability or of radioactivity in 51Cr-labeled bacteria. For each bacterial strain, the adherence ratio was constant over a wide range of concentrations of bacteria in the incubation medium. Strains of enterococci, viridans streptococci, coagulase-positive and coagulase-negative staphylococci and Pseudomonas aeruginosa (adherence ratios 0.003-0.017) were found to adhere more readily to valve sections than strains of Escherichia coli and Klebsiella pneumoniae (adherence ratios 0.00002-0.00004). The organisms that most frequently cause bacterial endocarditis were found to adhere best to heart valves in vitro, suggesting that the ability to adhere to valvular endothelium may be an important or essential charcteristic of bacteria that cause endocarditis in man.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGRIST A. A., OKA M. Pathogenesis of bacterial endocarditis. JAMA. 1963 Jan 26;183:249–252. doi: 10.1001/jama.1963.63700040009010b. [DOI] [PubMed] [Google Scholar]
- Cherubin C. E., Baden M., Kavaler F., Lerner S., Cline W. Infective endocarditis in narcotic addicts. Ann Intern Med. 1968 Dec;69(6):1091–1098. doi: 10.7326/0003-4819-69-6-1091. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer J., Finley F., Braude A. I. Release of 51Cr-endotoxin from bacteria as an assay of serum bactericidal activity. J Immunol. 1974 Jun;112(6):2184–2192. [PubMed] [Google Scholar]
- Finland M., Barnes M. W. Changing etiology of bacterial endocarditis in the antibacterial era. Experiences at Boston City Hospital 1933-1965. Ann Intern Med. 1970 Mar;72(3):341–348. doi: 10.7326/0003-4819-72-3-341. [DOI] [PubMed] [Google Scholar]
- Freter R. Parameters affecting the association of vibrios with the intestinal surface in experimental cholera. Infect Immun. 1972 Aug;6(2):134–141. doi: 10.1128/iai.6.2.134-141.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner P. I., Weinstein L. Infective endocarditis in the antibiotic era. N Engl J Med. 1966 Feb 3;274(5):259–contd. doi: 10.1056/NEJM196602032740506. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Jaumin P. M., Danielson G. K., Giuliani E. R., Washington JA I. I., Geraci J. E. Prosthetic valve endocarditis. Ann Intern Med. 1975 Jun;82(6):751–756. doi: 10.7326/0003-4819-82-6-751. [DOI] [PubMed] [Google Scholar]



